Long-Term Nitrogen Addition Stimulated Soil Respiration in a Rainfed Wheat Field on the Loess Plateau
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Measurement and Methods
2.3.1. Soil Respiration and Temporal Soil Temperature
2.3.2. Soil Water Content and Soil Nitrogen Measurements
2.3.3. Gas Exchange and Leaf Area Index
2.4. Statistical Analysis
3. Results
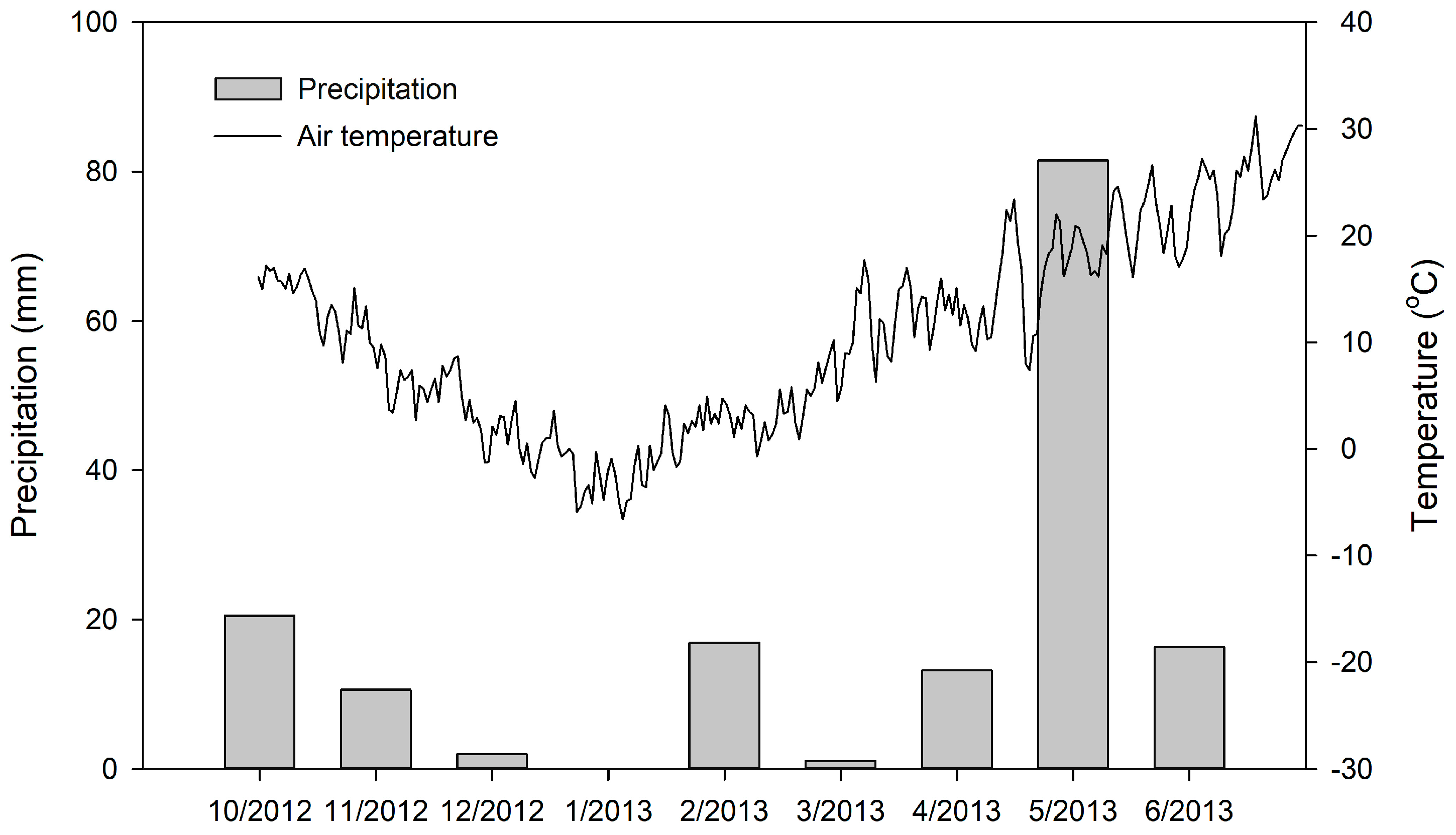
3.1. Variations in Annual Microclimatic, Biotic, and Abiotic Factors
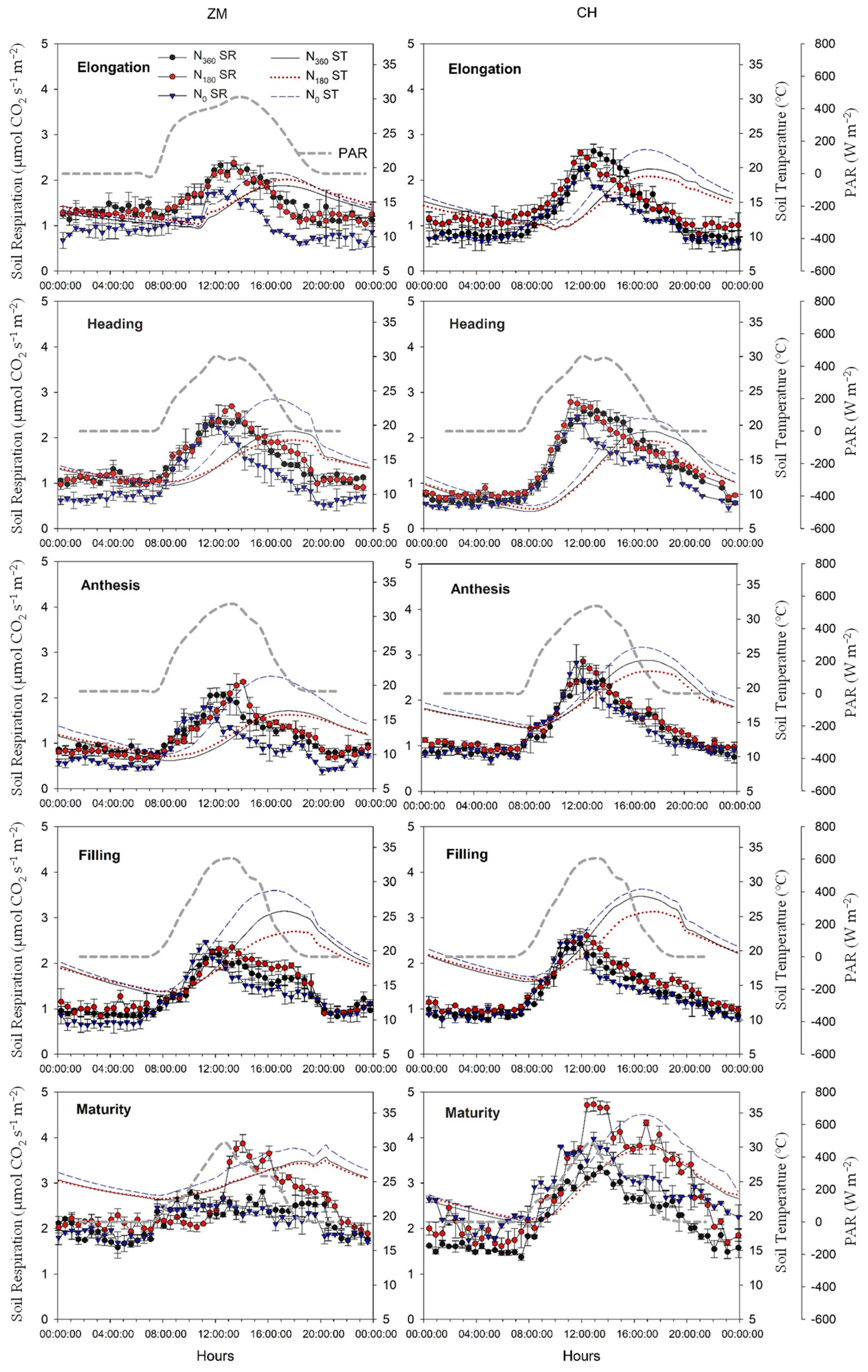
3.2. Soil Respiration Dynamics
3.3. Relationships of Soil Respiration with Biotic and Abiotic Factors
4. Discussion
4.1. Regulatory Mechanisms of Soil Respiration under N Application
4.2. Main Factors of Soil Respiration in Different Wheat Cultivars
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedback. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef]
- Ito, A. Global trends in land-atmosphere CO2 exchange fluxes: An analysis of a flux measurement dataset and comparison with terrestrial model simulations. J. Agric. Meteorol. 2021, 77, 211–223. [Google Scholar] [CrossRef]
- Jennifer, D.W.; Natali, S.M.; Minions, C.; Risk, D.; Arndt, K.; Zona, D.; Euskirchen, E.S.; Rocha, A.V.; Sonnentag, O.; Helbig, M.; et al. Soil respiration strongly offsets carbon uptake in Alaska and Northwest Canada. Environ. Res. Lett. 2021, 16, 084051. [Google Scholar] [CrossRef]
- Tu, L.H.; Hu, T.X.; Zhang, J.; Li, X.W.; Hu, H.L.; Liu, L.; Xiao, Y.L. Nitrogen addition stimulates different components of soil respiration in a subtropical bamboo ecosystem. Soil Biol. Biochem. 2013, 58, 255–264. [Google Scholar] [CrossRef]
- Diao, H.J.; Chen, X.P.; Wang, G.; Ning, Q.S.; Hu, S.Y.; Sun, W.; Dong, K.H.; Wang, C.H. The response of soil respiration to different N compounds addition in a saline–alkaline grassland of northern China. J. Plant Ecol. 2022, 15, 897–910. [Google Scholar] [CrossRef]
- Lu, D.; Lu, F.; Yan, P.; Cui, Z.; Chen, X. Elucidating population establishment associated with N management and cultivars for wheat production in China. Field Crop. Res. 2014, 163, 81–89. [Google Scholar] [CrossRef]
- Liu, Y.; Men, M.; Peng, Z.; Chen, H.Y.H.; Yang, Y.; Peng, Y. Spatially explicit estimate of nitrogen effects on soil respiration across the globe. Glob. Change Biol. 2023, 29, 3591–3600. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.X.; Deng, L.; Yang, Q.H.; Shangguan, Z.P. Nitrogen fertilization increase soil carbon dioxide efflux of winter wheat field: A case study in Northwest China. Soil Tillage Res. 2014, 143, 164–171. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Nitrogen fertilization inhibits soil microbial respiration regardless of the form of nitrogen applied. Soil Biol. Biochem. 2010, 42, 2336–2338. [Google Scholar] [CrossRef]
- Vinícius, A.O.; Brasil, E.P.F.; Teixeira, W.G.; Felipe, C.V.d.S.; Silva, A.R.D. Effects of Enhanced-Efficiency Nitrogen Fertilizers on Soil Microbial Biomass and Respiration in Tropical Soil Under Upland Rice Cultivation. J. Agric. Sci. 2021, 13, 31–42. [Google Scholar] [CrossRef]
- Lee, K.H.; Jose, S. Soil respiration, fine root production, and microbial biomass in cottonwood and loblolly pine plantations along a nitrogen fertilization gradient. For. Ecol. Manag. 2003, 185, 263–273. [Google Scholar] [CrossRef]
- Zhang, X.L.; Tan, Y.L.; Zhang, B.W.; Li, A.; Daryanto, S.; Wang, L.X.; Huang, J.H. The impacts of precipitation increase and nitrogen addition on soil respiration in a semiarid temperate steppe. Ecosphere 2017, 8, e01655. [Google Scholar] [CrossRef]
- Ryan, M.G.; Law, B.E. Interpreting, measuring, and modeling soil respiration. Biogeochemistry 2005, 73, 3–27. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Zhou, X.H.; Zhang, B.C.; Lu, M.; Luo, Y.Q.; Liu, L.L.; Li, B. Different responses of soil respiration and its components to nitrogen addition among biomes: A meta-analysis. Glob. Chang. Biol. 2013, 20, 2332–2343. [Google Scholar] [CrossRef]
- Carreiro, M.M.; Sinsabaugh, R.L.; Repert, D.A.; Parkhurst, D.F. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 2000, 81, 2359–2365. [Google Scholar] [CrossRef]
- Wei, L.; Su, J.; Jing, G.; Zhao, J.; Liu, J.; Cheng, J.; Jin, J. Nitrogen addition decreased soil respiration and its components in a long-term fenced grassland on the Loess Plateau. J. Arid Environ. 2018, 152, 37–44. [Google Scholar] [CrossRef]
- Widdig, M.; Schleuss, P.M.; Biederman, L.A.; Borer, E.T.; Spohn, M. Microbial carbon use efficiency in grassland soils subjected to nitrogen and phosphorus additions. Soil Biol. Biochem. 2020, 146, 107815. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Liu, L.L.; Ma, Y.C.; Yin, G.D.; Zhao, C.; Zhang, Y.; Piao, S.L. The effect of nitrogen addition on soil respiration from a nitrogen-limited forest soil. Agric. For. Meteorol. 2014, 197, 103–110. [Google Scholar] [CrossRef]
- Kivimäki, S.K.; Sheppard, L.J.; Leith, I.D.; Grace, J. Long-term enhanced nitrogen deposition increases ecosystem respiration and carbon loss from a Sphagnum bog in the Scottish Borders. Environ. Exp. Bot. 2013, 90, 53–61. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Janssens, I.; Dieleman, W.; Luyssaert, S.; Subke, J.-A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Bowden, R.D.; Davidson, E.; Savage, K.; Arabia, C.; Steudler, P. Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. For. Ecol. Manag. 2004, 196, 43–56. [Google Scholar] [CrossRef]
- Fan, T.L.; Stewart, B.A.; Wang, Y.; Luo, J.J.; Zhou, G.Y. Long-term fertilization effects on grain yield, water-use efficiency and soil fertility in the dryland of Loess Plateau in China. Agric. For. Meteorol. 2005, 106, 313–329. [Google Scholar] [CrossRef]
- Zhu, X.M.; Li, Y.S.; Peng, X.L.; Zhang, S.G. Soils of the Loess Plateau in China. Geoderma 1983, 29, 237–255. [Google Scholar] [CrossRef]
- Du, S.; Wang, Y.L.; Kume, T.; Zhang, J.G.; Otsuki, K.; Yamanaka, N.; Liu, G.B. Sapflow characteristics and climatic responses in three forest species in the semiarid Loess Plateau region of China. Agric. For. Meteorol. 2011, 151, 1–10. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Zhou, X.H.; Liu, R.Q.; Du, Z.G.; Zhou, L.Y.; Li, S.S.; Liu, H.Y.; Shao, J.J.; Wang, J.W.; Nie, Y.Y. Soil fungi and fine root biomass mediate drought-induced reductions in soil respiration. Funct. Ecol. 2020, 34, 2634–2643. [Google Scholar] [CrossRef]
- Liu, L.; Estiarte, M.; Bengtson, P.; Li, J.; Peuelas, J. Drought legacies on soil respiration and microbial community in a Mediterranean forest soil under different soil moisture and carbon inputs. Geoderma 2022, 405, 115425. [Google Scholar] [CrossRef]
- Ju, X.T.; Xing, G.X.; Chen, X.P.; Zhang, S.L.; Zhang, L.J.; Liu, X.J.; Cui, Z.L.; Yin, B.; Christie, P.; Zhu, Z.L. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc. Natl. Acad. Sci. USA 2009, 106, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yan, W.; Shangguan, Z. Soil carbon and nitrogen fractions in the soil profile and their response to long-term nitrogen fertilization in a wheat field. Catena 2015, 135, 38–46. [Google Scholar] [CrossRef]
- Zhong, Y.; Yan, W.; Shangguan, Z. Impact of long-term N additions upon coupling between soil microbial community structure and activity, and nutrient-use efficiencies. Soil Biol. Biochem. 2015, 91, 151–159. [Google Scholar] [CrossRef]
- Han, G.X.; Yu, J.B.; Li, H.B.; Yang, L.Q.; Wang, G.M.; Mao, P.L.; Gao, Y.J. Winter soil respiration from different vegetation patches in the Yellow River Delta, China. Environ. Manag. 2012, 50, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.Y.; Zhang, J.G.; Yan, M.J.; Yamanaka, N.; Du, S. Seasonal and diurnal dynamics of soil respiration fluxes in two typical forests on the semiarid Loess Plateau of China: Temperature sensitivities of autotrophs and heterotrophs and analyses of integrated driving factors. Soil Biol. Biochem. 2012, 52, 99–107. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Pokharel, P.; Liu, L.; Qiao, J.; Wang, Y.; An, S.; Chang, S.X. Global effects on soil respiration and its temperature sensitivity depend on nitrogen addition rate. Soil Biol. Biochem. 2022, 174, 108814. [Google Scholar] [CrossRef]
- Xu, W.H.; Wan, S.Q. Water-and plant-mediated responses of soil respiration to topography, fire, and nitrogen fertilization in a semiarid grassland in northern China. Soil Biol. Biochem. 2008, 40, 679–687. [Google Scholar] [CrossRef]
- Jia, H.; Fei, X.; Zhu, J.; Chen, W.; Chen, R.; Liao, Z.; Zhou, B.; Huang, Y.; Du, H.; Xu, P.; et al. Soil respiration and its response to climate change and anthropogenic factors in a karst plateau wetland, southwest China. Sci. Rep. 2024, 14, 8653. [Google Scholar] [CrossRef] [PubMed]
- Kopáček, J.; Cosby, B.J.; Evans, C.D.; Hruška, J.; Moldan, F.; Oulehle, F.; Šantrůčková, H.; Tahovská, K.; Wright, R.F. Nitrogen, organic carbon and sulphur cycling in terrestrial ecosystems: Linking nitrogen saturation to carbon limitation of soil microbial processes. Biogeochemistry 2013, 115, 33–51. [Google Scholar] [CrossRef]
- Wang, J.; Song, B.; Ma, F.; Tian, D.; Li, Y.; Yan, T.; Quan, Q.; Zhang, F.; Li, Z.; Wang, B.; et al. Nitrogen addition reduces soil respiration but increases the relative contribution of heterotrophic component in an alpine meadow. Funct. Ecol. 2019, 33, 2239–2253. [Google Scholar] [CrossRef]
- Du, W.; Li, Y.; He, P.; Zhang, J.; Jing, H.; Nie, C.; Liu, Y. Nitrogen Addition Decreases Soil Respiration without Changing the Temperature Sensitivity in a Semiarid Grassland. J. Resour. Ecol. 2020, 11, 129–139. [Google Scholar] [CrossRef]
- Yang, S.; Wu, H.; Wang, Z.; Semenov, M.; Ye, J.; Yin, L.; Wang, X.; Kravchenko, I.; Semenov, V.; Kuzyakov, Y. Linkages between the temperature sensitivity of soil respiration and microbial life strategy are dependent on sampling season. Soil Biol. Biochem. 2022, 172, 108758. [Google Scholar] [CrossRef]



| Total N (mg kg−1) | SWC (%) | ||||
|---|---|---|---|---|---|
| 2009 | 2013 | 2009 | 2013 | ||
| CH | N0 | 78.61 ± 3.57 c | 79.74 ± 3.29 c | 5.45 ± 0.73 c | 18.30 ± 1.83 b |
| N180 | 83.02 ± 3.04 b | 84.80 ± 2.75 b | 11.97 ± 0.95 b | 20.33 ± 1.38 b | |
| N360 | 86.25 ± 3.15 a | 88.72 ± 3.17 a | 24.08 ± 2.24 a | 27.89 ± 1.74 a | |
| ZM | N0 | 77.04 ± 4.03 b | 77.52 ± 3.02 b | 5.87 ± 0.69 c | 15.36 ± 1.94 b |
| N180 | 82.72 ± 4.19 a | 84.27 ± 4.26 a | 12.54 ± 1.58 b | 17.43 ± 1.67 b | |
| N360 | 83.24 ± 5.62 a | 85.34 ± 4.38 a | 20.32 ± 2.47 a | 20.11 ± 1.63 a | |
| Variety | Treatment | Fitted Equation | Q10 |
|---|---|---|---|
| ZM | N0 | SR = 9.80e0.38ST | 2.41 |
| N180 | SR = 7.07e0.56ST | 3.64 | |
| N360 | SR = 6.94e0.56ST | 3.66 | |
| CH | N0 | SR = 12.70e0.26ST | 1.81 |
| N180 | SR = 11.11e0.36ST | 2.28 | |
| N360 | SR = 10.20e0.30ST | 1.99 |
| Treatment | ST | PAR | |||
|---|---|---|---|---|---|
| R2 | p | R2 | p | ||
| ZM | N0 | 0.75 | 0.03 | 0.77 | 0.01 |
| N180 | 0.77 | 0.01 | 0.78 | 0.02 | |
| N360 | 0.70 | 0.04 | 0.79 | 0.03 | |
| CH | N0 | 0.59 | 0.01 | 0.82 | 0.01 |
| N180 | 0.42 | 0.05 | 0.86 | 0.02 | |
| N360 | 0.54 | 0.05 | 0.90 | 0.02 | |
| Treatment | LAI | ST | SWC | ||||
|---|---|---|---|---|---|---|---|
| R2 | p | R2 | p | R2 | p | ||
| ZM | N0 | 0.37 | 0.20 | 0.19 | 0.38 | 0.96 | 0.001 |
| N180 | 0.67 | 0.01 | 0.64 | 0.02 | 0.03 | 0.76 | |
| N360 | 0.85 | 0.01 | 0.89 | 0.00 | 0.15 | 0.45 | |
| total | 0.77 | 0.05 | 0.54 | 0.001 | 0.35 | 0.51 | |
| CH | N0 | 0.67 | 0.05 | 0.49 | 0.12 | 0.21 | 0.36 |
| N180 | 0.46 | 0.21 | 0.79 | 0.02 | 0.15 | 0.44 | |
| N360 | 0.49 | 0.12 | 0.35 | 0.21 | 0.10 | 0.57 | |
| total | 0.61 | 0.05 | 0.63 | 0.0004 | 0.18 | 0.40 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Song, X.; Yang, B.; Zhang, Y.; Zhang, H.; Zong, Y.; Shangguan, Z.; Hao, X. Long-Term Nitrogen Addition Stimulated Soil Respiration in a Rainfed Wheat Field on the Loess Plateau. Agronomy 2024, 14, 1136. https://doi.org/10.3390/agronomy14061136
Li C, Song X, Yang B, Zhang Y, Zhang H, Zong Y, Shangguan Z, Hao X. Long-Term Nitrogen Addition Stimulated Soil Respiration in a Rainfed Wheat Field on the Loess Plateau. Agronomy. 2024; 14(6):1136. https://doi.org/10.3390/agronomy14061136
Chicago/Turabian StyleLi, Chao, Xinli Song, Bosen Yang, Yan Zhang, Huirong Zhang, Yuzheng Zong, Zhouping Shangguan, and Xingyu Hao. 2024. "Long-Term Nitrogen Addition Stimulated Soil Respiration in a Rainfed Wheat Field on the Loess Plateau" Agronomy 14, no. 6: 1136. https://doi.org/10.3390/agronomy14061136




