Foliar Application of Selenium Enhances Drought Tolerance in Tomatoes by Modulating the Antioxidative System and Restoring Photosynthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Agricultural Cultivation Measures
2.3. Experimental Design
2.4. Observation Items and Methods
2.4.1. Plant Height and Stem Diameter
2.4.2. Biomass
2.4.3. Measurement of Photosynthesis Indices
2.4.4. The Levels of Peroxide and Osmotic Regulatory Substances in Tomato Leaves
2.4.5. Selenium Content of Aboveground Organs of Tomatoes
2.5. Statistical Analysis
3. Results
3.1. Growth Status of Tomato
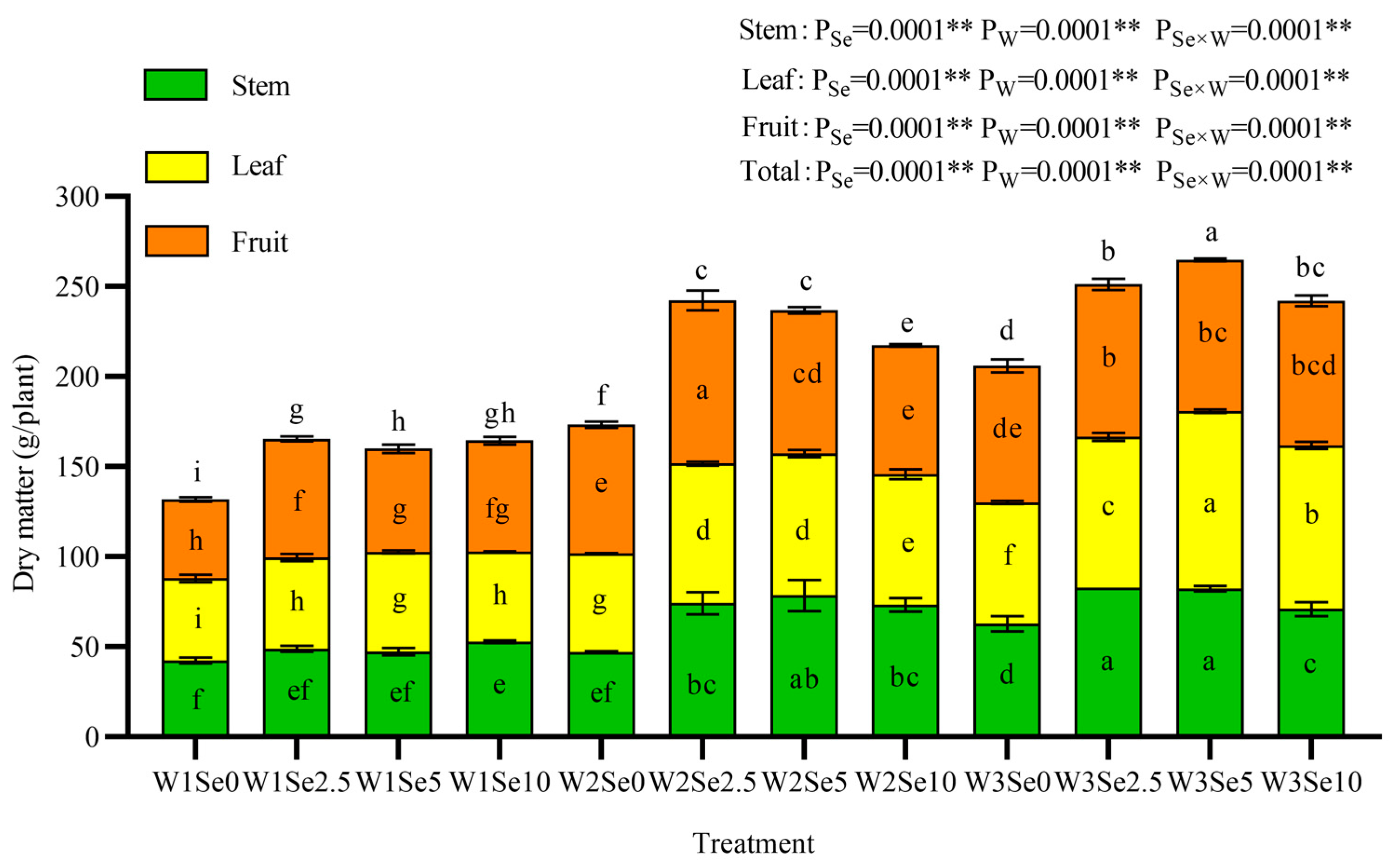
3.2. Aboveground Dry Matter of Tomato Plants
3.3. Physiological Characteristics of Tomato Plants
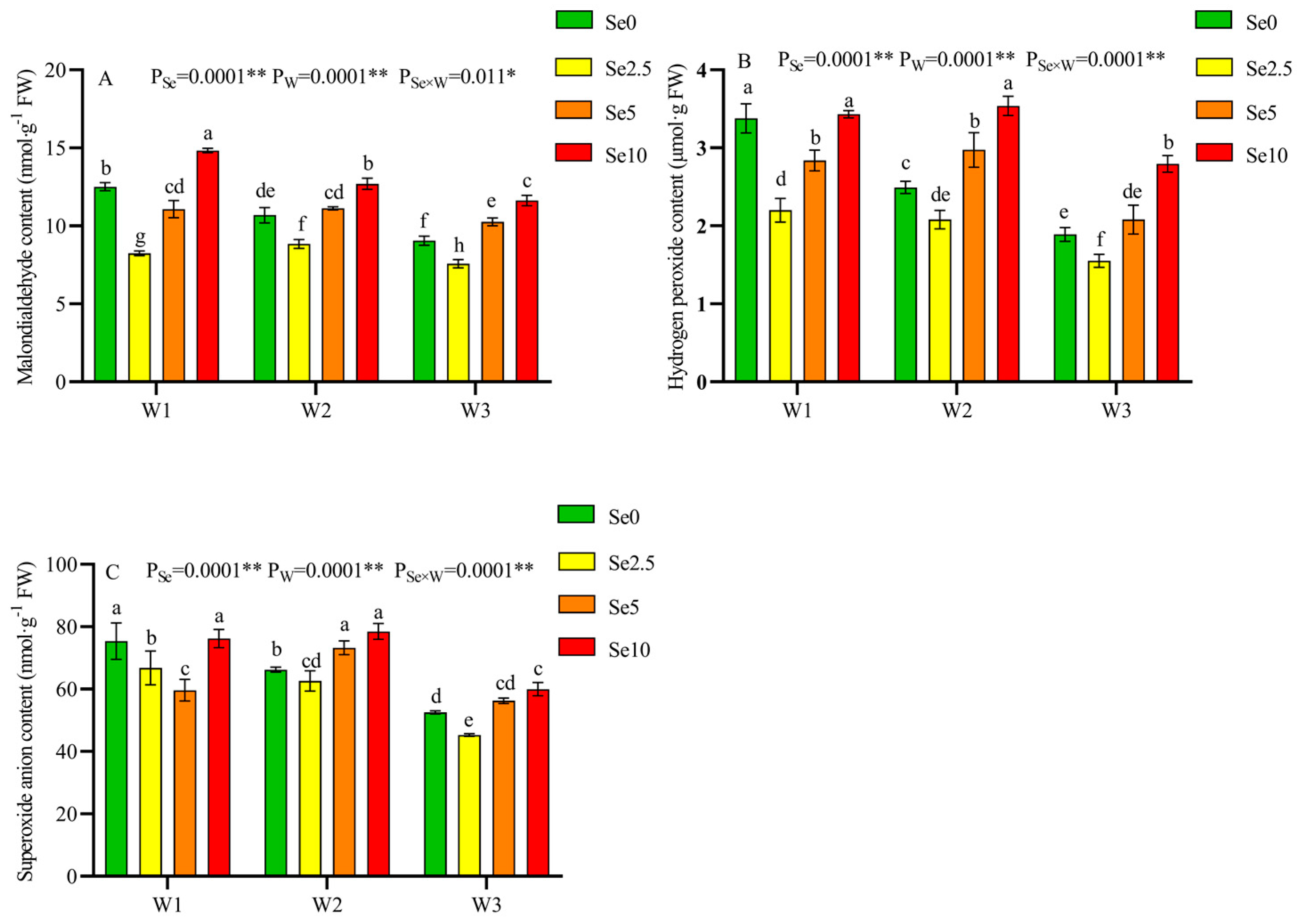
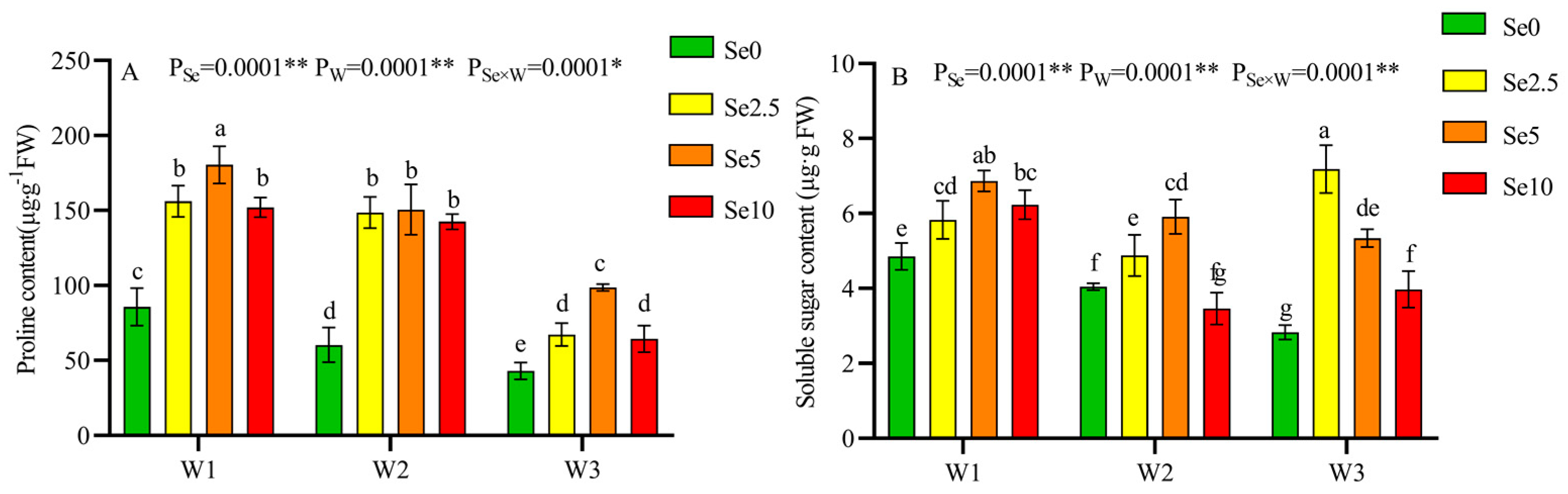
3.4. Antioxidant Capacity and Osmoregulation Substances of Tomato Leaves
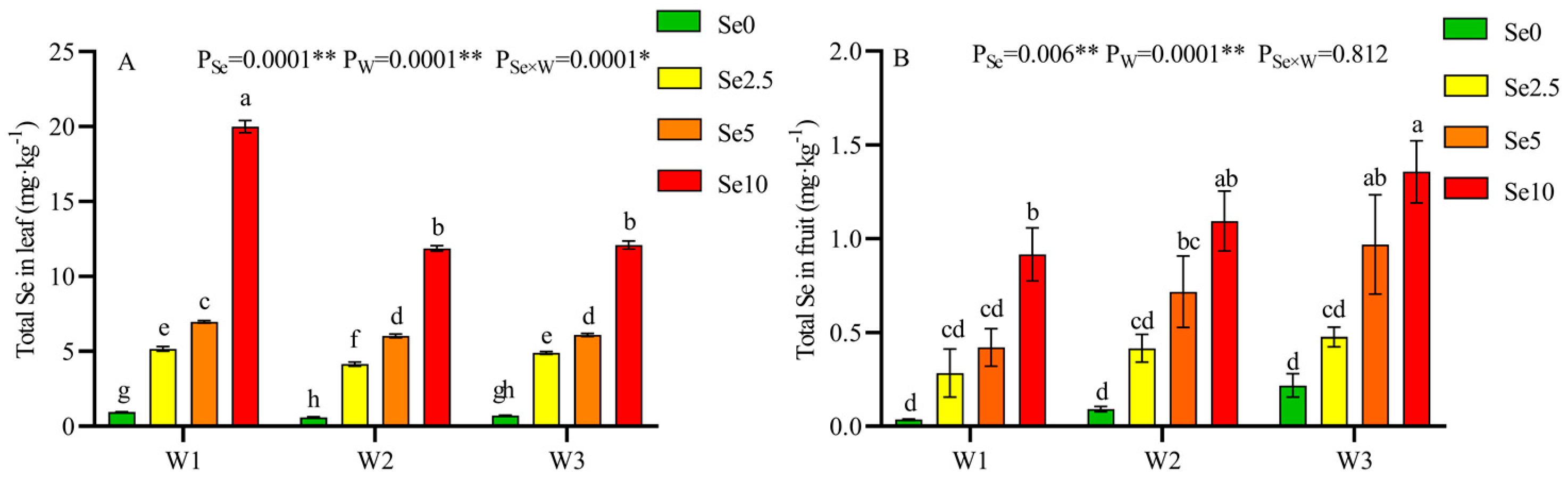
3.5. Content of Selenium in the Different Organs of the Tomato
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abatzoglou, J.T.; Williams, A.P. Impact of Anthropogenic Climate Change on Wildfire across Western US Forests. Proc. Natl. Acad. Sci. USA 2016, 113, 11770–11775. [Google Scholar] [CrossRef]
- El Habti, A.; Fleury, D.; Jewell, N.; Garnett, T.; Tricker, P.J. Tolerance of Combined Drought and Heat Stress Is Associated with Transpiration Maintenance and Water Soluble Carbohydrates in Wheat Grains. Front. Plant Sci. 2020, 11, 568693. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Elliott, J.; Deryng, D.; Ruane, A.C.; Müller, C.; Arneth, A.; Boote, K.J.; Folberth, C.; Glotter, M.; Khabarov, N.; et al. Assessing Agricultural Risks of Climate Change in the 21st Century in a Global Gridded Crop Model Intercomparison. Proc. Natl. Acad. Sci. USA 2014, 111, 3268–3273. [Google Scholar] [CrossRef]
- Fang, S.-L.; Cheng, Y.-J.; Tu, Y.-K.; Yao, M.-H.; Kuo, B.-J. Exploring Efficient Methods for Using Multiple Spectral Reflectance Indices to Establish a Prediction Model for Early Drought Stress Detection in Greenhouse Tomato. Horticulturae 2023, 9, 1317. [Google Scholar] [CrossRef]
- Shao, G.C.; Huang, D.D.; Cheng, X.; Cui, J.T.; Zhang, Z.H. Path Analysis of Sap Flow of Tomato under Rain Shelters in Response to Drought Stress. Int. J. Agric. Biol. Eng. 2016, 9, 54–62. [Google Scholar]
- Cogato, A.; Meggio, F.; De Antoni Migliorati, M.; Marinello, F. Extreme Weather Events in Agriculture: A Systematic Review. Sustainability 2019, 11, 2547. [Google Scholar] [CrossRef]
- Verslues, P.E.; Juenger, T.E. Drought, Metabolites, and Arabidopsis Natural Variation: A Promising Combination for Understanding Adaptation to Water-Limited Environments. Curr. Opin. Plant Biol. 2011, 14, 240–245. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A.H. The Fascinating Facets of Plant Selenium Accumulation—Biochemistry, Physiology, Evolution and Ecology. New Phytol. 2017, 213, 1582–1596. [Google Scholar] [CrossRef]
- White, P.J. Selenium Metabolism in Plants. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef]
- Wang, M.; Ali, F.; Qi, M.; Peng, Q.; Wang, M.; Bañuelos, G.S.; Miao, S.; Li, Z.; Dinh, Q.T.; Liang, D. Insights into Uptake, Accumulation, and Subcellular Distribution of Selenium among Eight Wheat (Triticum aestivum L.) Cultivars Supplied with Selenite and Selenate. Ecotoxicol. Environ. Saf. 2021, 207, 111544. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, C.; Chen, F.; Yue, L.; Cao, X.; Liu, X.; Yao, Y.; Wang, Z.; Xing, B. Multiomics Understanding of Improved Quality in Cherry Radish (Raphanus sativus L. Var. radculus pers) after Foliar Application of Selenium Nanomaterials. Sci. Total Environ. 2022, 824, 153712. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The Roles of Selenium in Protecting Plants against Abiotic Stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium Distribution in the Chinese Environment and Its Relationship with Human Health: A Review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Wang, M.; Tran, T.A.T.; Zhou, F.; Wang, D.; Zhai, H.; Peng, Q.; Xue, M.; Du, Z.; Bañuelos, G.S.; et al. Bioavailability of Selenium in Soil-Plant System and a Regulatory Approach. Crit. Rev. Environ. Sci. Technol. 2019, 49, 443–517. [Google Scholar] [CrossRef]
- Winkel, L.H.E.; Johnson, C.A.; Lenz, M.; Grundl, T.; Leupin, O.X.; Amini, M.; Charlet, L. Environmental Selenium Research: From Microscopic Processes to Global Understanding. Environ. Sci. Technol. 2012, 46, 571–579. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Biological Activity of Selenium in Plants: Physiological and Biochemical Mechanisms of Phytotoxicity and Tolerance. In Selenium and Nano-Selenium in Environmental Stress Management and Crop Quality Improvement; Hossain, M.A., Ahammed, G.J., Kolbert, Z., El-Ramady, H., Islam, T., Schiavon, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 341–363. [Google Scholar] [CrossRef]
- Habibi, G. Effect of Drought Stress and Selenium Spraying on Photosynthesis and Antioxidant Activity of Spring Barley. Acta Agric. Slov. 2013, 101, 31–39. [Google Scholar] [CrossRef]
- Bocchini, M.; D’Amato, R.; Ciancaleoni, S.; Fontanella, M.C.; Palmerini, C.A.; Beone, G.M.; Onofri, A.; Negri, V.; Marconi, G.; Albertini, E.; et al. Soil Selenium (Se) Biofortification Changes the Physiological, Biochemical and Epigenetic Responses to Water Stress in Zea mays L. by Inducing a Higher Drought Tolerance. Front. Plant Sci. 2018, 9, 329575. [Google Scholar] [CrossRef]
- Hemmati, M.; Delkhosh, B.; Rad, A.H.S.; Mohammadi, G.N. Effect of the Application of Foliar Selenium on Canola Cultivars as Influenced by Different Irrigation Regimes. Tarim Bilim. Derg. 2019, 25, 309–318. [Google Scholar] [CrossRef]
- Sieprawska, A.; Kornaś, A.; Filek, M. Involvement of Selenium in Protective Mechanisms of Plants under Environmental Stress Conditions—Review. Acta Biol. Cracoviensia Ser. Bot. 2015, 57, 9–20. [Google Scholar] [CrossRef]
- Sattar, A.; Naveed, M.; Ali, M.; Zahir, Z.A.; Nadeem, S.M.; Yaseen, M.; Meena, V.S.; Farooq, M.; Singh, R.; Rahman, M.; et al. Perspectives of Potassium Solubilizing Microbes in Sustainable Food Production System: A Review. Appl. Soil Ecol. 2019, 133, 146–159. [Google Scholar] [CrossRef]
- Moloi, M.J.; Khoza, B.M. The Effect of Selenium Foliar Application on the Physiological Responses of Edamame under Different Water Treatments. Agronomy 2022, 12, 2400. [Google Scholar] [CrossRef]
- Haghighi, M.; Abolghasemi, R.; Teixeira da Silva, J.A. Low and High Temperature Stress Affect the Growth Characteristics of Tomato in Hydroponic Culture with Se and Nano-Se Amendment. Sci. Hortic. 2014, 178, 231–240. [Google Scholar] [CrossRef]
- Ramasamy, S.; Ganesh Thiruvengadam Nandagopal, J.; Balasubramanian, M.; Girija, S. Effect of Abscisic Acid and Selenium Foliar Sprays on Drought Mitigation in Tomato (Solanum lycopersicum L.). Mater. Today Proc. 2022, 48, 191–195. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.E.; Gadallah, F.M.; Semida, W.M. Selenium Application in Two Methods Promotes Drought Tolerance in Solanum lycopersicum Plant by Inducing the Antioxidant Defense System. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q. Novel Mechanistic Insights of Selenium Induced Microscopic, Histochemical and Physio-Biochemical Changes in Tomato (Solanum lycopersicum L.) Plant. An Account of Beneficiality or Toxicity. J. Hazard. Mater. 2022, 434, 128830. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Sun, X.; Xing, S.; Cong, W.; Liu, X. Study on Dormancy Mechanism and Breaking Dormancy Method of Viburnum Sargentii Seeds. Am. J. Plant Sci. 2019, 10, 65–78. [Google Scholar] [CrossRef]
- Semida, W.M.; Abd El-Mageed, T.A.; Gyushi, M.A.H.; Abd El-Mageed, S.A.; Rady, M.M.; Abdelkhalik, A.; Merah, O.; Sabagh, A.E.; El-Metwally, I.M.; Sadak, M.S.; et al. Exogenous Selenium Improves Physio-Biochemical and Performance of Drought-Stressed Phaseolus Vulgaris Seeded in Saline Soil. Soil Syst. 2023, 7, 67. [Google Scholar] [CrossRef]
- Gong, L.; Xiong, J.; Yu, Y. Determination of organic selenium in food by inductively coupled plasma mass spectrometry. Food Mach. 2017, 33, 62–65. [Google Scholar] [CrossRef]
- Andrade, F.R.; da Silva, G.N.; Guimarães, K.C.; Barreto, H.B.F.; de Souza, K.R.D.; Guilherme, L.R.G.; Faquin, V.; dos Reis, A.R. Selenium Protects Rice Plants from Water Deficit Stress. Ecotoxicol. Environ. Saf. 2018, 164, 562–570. [Google Scholar] [CrossRef]
- Lanza, M.G.D.B.; dos Reis, A.R. Roles of Selenium in Mineral Plant Nutrition: ROS Scavenging Responses against Abiotic Stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef]
- Hartikainen, H.; Xue, T.; Piironen, V. Selenium as an Anti-Oxidant and pro-Oxidant in Ryegrass. Plant Soil 2000, 225, 193–200. [Google Scholar] [CrossRef]
- Hussain, S.; Ahmed, S.; Akram, W.; Li, G.; Yasin, N.A. Selenium Seed Priming Enhanced the Growth of Salt-Stressed Brassica rapa L. through Improving Plant Nutrition and the Antioxidant System. Front. Plant Sci. 2023, 13, 1050359. [Google Scholar] [CrossRef]
- Kolbert, Z.; Lehotai, N.; Molnár, Á.; Feigl, G. “The Roots” of Selenium Toxicity: A New Concept. Plant Signal. Behav. 2016, 11, e1241935. [Google Scholar] [CrossRef]
- Lehotai, N.; Kolbert, Z.; Pető, A.; Feigl, G.; Ördög, A.; Kumar, D.; Tari, I.; Erdei, L. Selenite-Induced Hormonal and Signalling Mechanisms during Root Growth of Arabidopsis thaliana L. J. Exp. Bot. 2012, 63, 5677–5687. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of Drought Stress on Photosynthesis and Photosynthetic Electron Transport Chain in Young Apple Tree Leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Akram, N.A.; Ashraf, M. Osmoprotection in Plants under Abiotic Stresses: New Insights into a Classical Phenomenon. Planta 2019, 251, 3. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and Sulfur Influence Ethylene Formation and Alleviate Cadmium-Induced Oxidative Stress by Improving Proline and Glutathione Production in Wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef]
- Nawaz, F.; Ahmad, R.; Ashraf, M.Y.; Waraich, E.A.; Khan, S.Z. Effect of Selenium Foliar Spray on Physiological and Biochemical Processes and Chemical Constituents of Wheat under Drought Stress. Ecotoxicol. Environ. Saf. 2015, 113, 191–200. [Google Scholar] [CrossRef]
- Regni, L.; Palmerini, C.A.; Del Pino, A.M.; Businelli, D.; D’Amato, R.; Mairech, H.; Marmottini, F.; Micheli, M.; Pacheco, P.H.; Proietti, P. Effects of Selenium Supplementation on Olive under Salt Stress Conditions. Sci. Hortic. 2021, 278, 109866. [Google Scholar] [CrossRef]
- Ahmad, S.; Muhammad, I.; Wang, G.Y.; Zeeshan, M.; Yang, L.; Ali, I.; Zhou, X.B. Ameliorative Effect of Melatonin Improves Drought Tolerance by Regulating Growth, Photosynthetic Traits and Leaf Ultrastructure of Maize Seedlings. BMC Plant Biol. 2021, 21, 368. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of Plants to Water Stress. Front. Plant Sci. 2014, 5, 76566. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Akbar, A.; Parveen, A.; Rasheed, R.; Hussain, I.; Iqbal, M. Phenological Application of Selenium Differentially Improves Growth, Oxidative Defense and Ion Homeostasis in Maize under Salinity Stress. Plant Physiol. Biochem. 2018, 123, 268–280. [Google Scholar] [CrossRef]
- Han, D.; Tu, S.; Dai, Z.; Huang, W.; Jia, W.; Xu, Z.; Shao, H. Comparison of Selenite and Selenate in Alleviation of Drought Stress in Nicotiana tabacum L. Chemosphere 2022, 287, 132136. [Google Scholar] [CrossRef]
- Bai, B.; Wang, Z.; Gao, L.; Chen, W.; Shen, Y. Effects of Selenite on the Growth of Alfalfa (Medicago sativa L. Cv. Sadie 7) and Related Physiological Mechanisms. Acta Physiol. Plant 2019, 41, 78. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Hao, J.; Fan, S.; Dong, R.; Zeng, H.; Liu, C.; Han, Y. Effects of Selenate and Selenite on Selenium Accumulation and Speciation in Lettuce. Plant Physiol. Biochem. 2022, 192, 162–171. [Google Scholar] [CrossRef]
- de Sousa, G.F.; Silva, M.A.; de Morais, E.G.; Van Opbergen, G.A.Z.; Van Opbergen, G.G.A.Z.; de Oliveira, R.R.; Amaral, D.; Brown, P.; Chalfun-Junior, A.; Guilherme, L.R.G. Selenium Enhances Chilling Stress Tolerance in Coffee Species by Modulating Nutrient, Carbohydrates, and Amino Acids Content. Front. Plant Sci. 2022, 13, 1000430. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, S.; Huang, X.; Zhang, F.; Pang, Y.; Huang, Q.; Yi, Q. Selenium Uptake, Dynamic Changes in Selenium Content and Its Influence on Photosynthesis and Chlorophyll Fluorescence in Rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, M.; Xu, J.; Xu, F.; Zhang, W. The Application of Organic Selenium (SeMet) Improve the Photosynthetic Characteristics, Yield and Quality of Hybrid rice. Plant Physiol. Biochem. 2024, 208, 108457. [Google Scholar] [CrossRef]
- Hondal, R.J.; Marino, S.M.; Gladyshev, V.N. Selenocysteine in Thiol/Disulfide-Like Exchange Reactions. Antioxid. Redox Signal. 2013, 18, 1675–1689. [Google Scholar] [CrossRef] [PubMed]
- Balk, J.; Pilon, M. Ancient and Essential: The Assembly of Iron–Sulfur Clusters in Plants. Trends Plant Sci. 2011, 16, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 234638. [Google Scholar] [CrossRef]
- Valkama, E.; Kivimäenpää, M.; Hartikainen, H.; Wulff, A. The Combined Effects of Enhanced UV-B Radiation and Selenium on Growth, Chlorophyll Fluorescence and Ultrastructure in Strawberry (Fragaria × ananassa) and Barley (Hordeum vulgare) Treated in the Field. Agric. For. Meteorol. 2003, 120, 267–278. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under Drought and Salt Stress: Regulation Mechanisms from Whole Plant to Cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Heyneke, E.; Fernie, A.R. Metabolic Regulation of Photosynthesis. Biochem. Soc. Trans. 2018, 46, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The Dual Effects of Two Inorganic Selenium Forms on the Growth, Selected Physiological Parameters and Macronutrients Accumulation in Cucumber Plants. Acta Physiol. Plant 2015, 37, 41. [Google Scholar] [CrossRef]
- GH/T 1135-2017 Selenium-Enriched Agricultural Products. Available online: https://hbba.sacinfo.org.cn/stdDetail/e5d423cc91f2d589a0b44fb1205af0022f180f4930fc379a11767642003e5219 (accessed on 22 May 2024).
- Lei, H.L.; Cong, W.Y.; Cai, Z.L.; Abdisamad, M.; Zhao, J.Y.; Wang, X.G.; Gao, G.Y.; Wang, Y.Q.; Zhang, R. Main process and factors affecting selenium absorption by plant roots and leaves. J. Plant Nutr. Fertil. 2021, 27, 1456–1467. [Google Scholar] [CrossRef]
- Kannan, S. Mechanisms of Foliar Uptake of Plant Nutrients: Accomplishments and Prospects. J. Plant Nutr. 1980, 2, 717–735. [Google Scholar] [CrossRef]
- Eichert, T.; Goldbach, H.E. Equivalent Pore Radii of Hydrophilic Foliar Uptake Routes in Stomatous and Astomatous Leaf Surfaces—Further Evidence for a Stomatal Pathway. Physiol. Plant. 2008, 132, 491–502. [Google Scholar] [CrossRef]
- Fan, S.; Wu, H.; Gong, H.; Guo, J. The Salicylic Acid Mediates Selenium-Induced Tolerance to Drought Stress in Tomato Plants. Sci. Hortic. 2022, 300, 111092. [Google Scholar] [CrossRef]
- de Souza Silveira, A.; Pinheiro, D.T.; de Oliveira, R.M.; dos Santos Dias, D.C.F.; da Silva, L.J. Osmopriming with Selenium: Physical and Physiological Quality of Tomato Seeds in Response to Water Deficit. J. Seed Sci. 2023, 45, e202345012. [Google Scholar] [CrossRef]
| Treatments | Exogenous Selenium Se Concentration (mg·L−1) | Water Content (%) |
|---|---|---|
| W1Se0 | 0 | 50% field capacity |
| W1Se2.5 | 2.5 | |
| W1Se5 | 5 | |
| W1Se10 | 10 | |
| W2Se0 | 0 | 65% field capacity |
| W2Se2.5 | 2.5 | |
| W2Se5 | 5 | |
| W2Se10 | 10 | |
| W3Se0 | 0 | 80% field capacity |
| W3Se2.5 | 2.5 | |
| W3Se5 | 5 | |
| W3Se10 | 10 |
| Treatments | Height (cm) | Stem Diameter (mm) |
|---|---|---|
| W1Se0 | 98.6 ± 2.7 ef | 8.1 ± 0.1 ef |
| W1Se2.5 | 99.8 ± 1.7 def | 8.5 ± 0.3 def |
| W1Se5 | 95.7 ± 2.1 f | 8.0 ± 0.1 f |
| W1Se10 | 97.5 ± 6.8 f | 8.0 ± 0.2 f |
| W2Se0 | 107.2 ± 1.9 bcd | 8.7 ± 0.3 cde |
| W2Se2.5 | 105.8 ± 6.3 cde | 8.8 ± 0.7 cd |
| W2Se5 | 106.2 ± 6.8 cde | 9.4 ± 0.3 bc |
| W2Se10 | 111.3 ± 5.0 bc | 8.5 ± 0.4 def |
| W3Se0 | 115.2 ± 2.7 ab | 9.2 ± 0.1 bc |
| W3Se2.5 | 120.7 ± 2.0 a | 10.0 ± 0.2 a |
| W3Se5 | 115.3 ± 5.7 ab | 9.5 ± 0.7 ab |
| W3Se10 | 108.7 ± 4.9 bc | 8.4 ± 0.2 def |
| W | ** | ** |
| Se | ns | ** |
| W × Se | ns | ** |
| Treatments | Pn (μmol CO2 m−2 s−1) | Gs (mol H2O m−2 s−1) | Ci (μmol mol−1) | Tr (mol H2O m−2 s−1) | SPAD |
|---|---|---|---|---|---|
| W1Se0 | 4.82 ± 0.47 f | 0.05 ± 0.00 g | 208.47 ± 8.30 g | 1.87 ± 0.15 f | 62.03 ± 0.49 f |
| W1Se2.5 | 14.70 ± 1.63 bc | 0.14 ± 0.02 de | 228.99 ± 8.18 f | 4.75 ± 0.29 d | 66.2 ± 0.61 d |
| W1Se5 | 14.31 ± 0.56 bc | 0.21 ± 0.04 bc | 275.54 ± 1.66 abc | 7.12 ± 0.22 bc | 64.57 ± 0.49 e |
| W1Se10 | 11.96 ± 1.66 d | 0.16 ± 0.01 de | 250.44 ± 4.35 de | 6.54 ± 0.38 c | 64.00 ± 0.61 e |
| W2Se0 | 6.95 ± 1.60 e | 0.08 ± 0.00 fg | 228.32 ± 5.62 f | 3.26 ± 0.88 e | 68.07 ± 0.50 bc |
| W2Se2.5 | 16.44 ± 1.63 ab | 0.21 ± 0.02 bc | 242.00 ± 5.21 ef | 8.50 ± 0.59 b | 70.83 ± 0.49 a |
| W2Se5 | 14.04 ± 1.95 c | 0.18 ± 0.03 cd | 262.28 ± 13.72 cd | 8.06 ± 1.22 b | 68.83 ± 0.21 b |
| W2Se10 | 10.69 ± 1.08 d | 0.17 ± 0.03 cd | 262.77 ± 17.12 cd | 7.54 ± 1.02 bc | 66.30 ± 1.06 d |
| W3Se0 | 11.45 ± 0.53 d | 0.12 ± 0.01 ef | 270.76 ± 8.11 bcd | 4.46 ± 0.32 de | 66.47 ± 0.93 d |
| W3Se2.5 | 18.03 ± 0.65 a | 0.38 ± 0.06 a | 291.57 ± 22.93 a | 13.21 ± 0.26 a | 67.93 ± 0.61 bc |
| W3Se5 | 16.10 ± 0.26 abc | 0.36 ± 0.01 a | 284.17 ± 10.16 ab | 11.87 ± 1.24 a | 67.03 ± 0.25 cd |
| W3Se10 | 16.31 ± 0.39 ab | 0.25 ± 0.01 b | 269.13 ± 7.66 bcd | 11.91 ± 1.40 a | 66.47 ± 0.86 d |
| W | ** | ** | ** | ** | ** |
| Se | ** | ** | ** | ** | ** |
| W × Se | ** | ** | ** | ** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Cui, H.; Li, H.; Qiang, X.; Han, Q.; Liu, H. Foliar Application of Selenium Enhances Drought Tolerance in Tomatoes by Modulating the Antioxidative System and Restoring Photosynthesis. Agronomy 2024, 14, 1184. https://doi.org/10.3390/agronomy14061184
Zhong Y, Cui H, Li H, Qiang X, Han Q, Liu H. Foliar Application of Selenium Enhances Drought Tolerance in Tomatoes by Modulating the Antioxidative System and Restoring Photosynthesis. Agronomy. 2024; 14(6):1184. https://doi.org/10.3390/agronomy14061184
Chicago/Turabian StyleZhong, Yuan, Haixue Cui, Huanhuan Li, Xiaoman Qiang, Qisheng Han, and Hao Liu. 2024. "Foliar Application of Selenium Enhances Drought Tolerance in Tomatoes by Modulating the Antioxidative System and Restoring Photosynthesis" Agronomy 14, no. 6: 1184. https://doi.org/10.3390/agronomy14061184






