Genetic and Genomic Pathways to Improved Wheat (Triticum aestivum L.) Yields: A Review
Abstract
:1. Introduction
1.1. The Challenge of Increasing Global Demand and the Need for Higher Yield
1.2. Environmental Factors Influencing Wheat Yield Losses
2. Biotechnological Approaches in Yield Enhancement
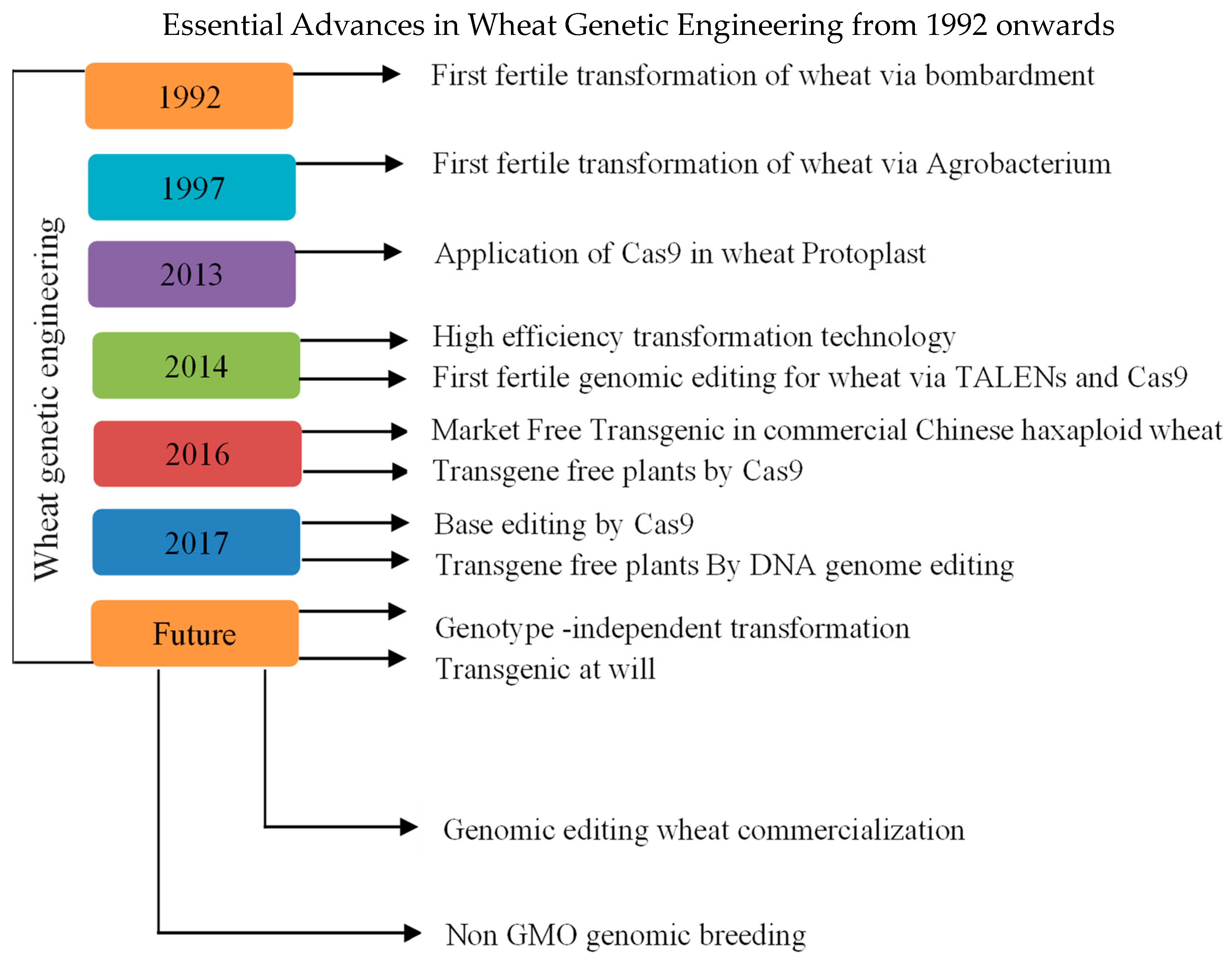
2.1. Genetic Engineering Techniques for Yield Improvement
2.2. Marker-Assisted Breeding (MAB)
2.3. QTLs Mapping
2.4. Next-Generation Sequencing (NGS)
2.5. CRISPR Cas9; Cas13
2.6. Integration of Omics Approaches for Yield Enhancement
3. Molecular Perspectives on Wheat Yield
3.1. Historical Perspective on Wheat Yield Improvements
3.2. Determining Genes Involved in Different Traits for High Yield
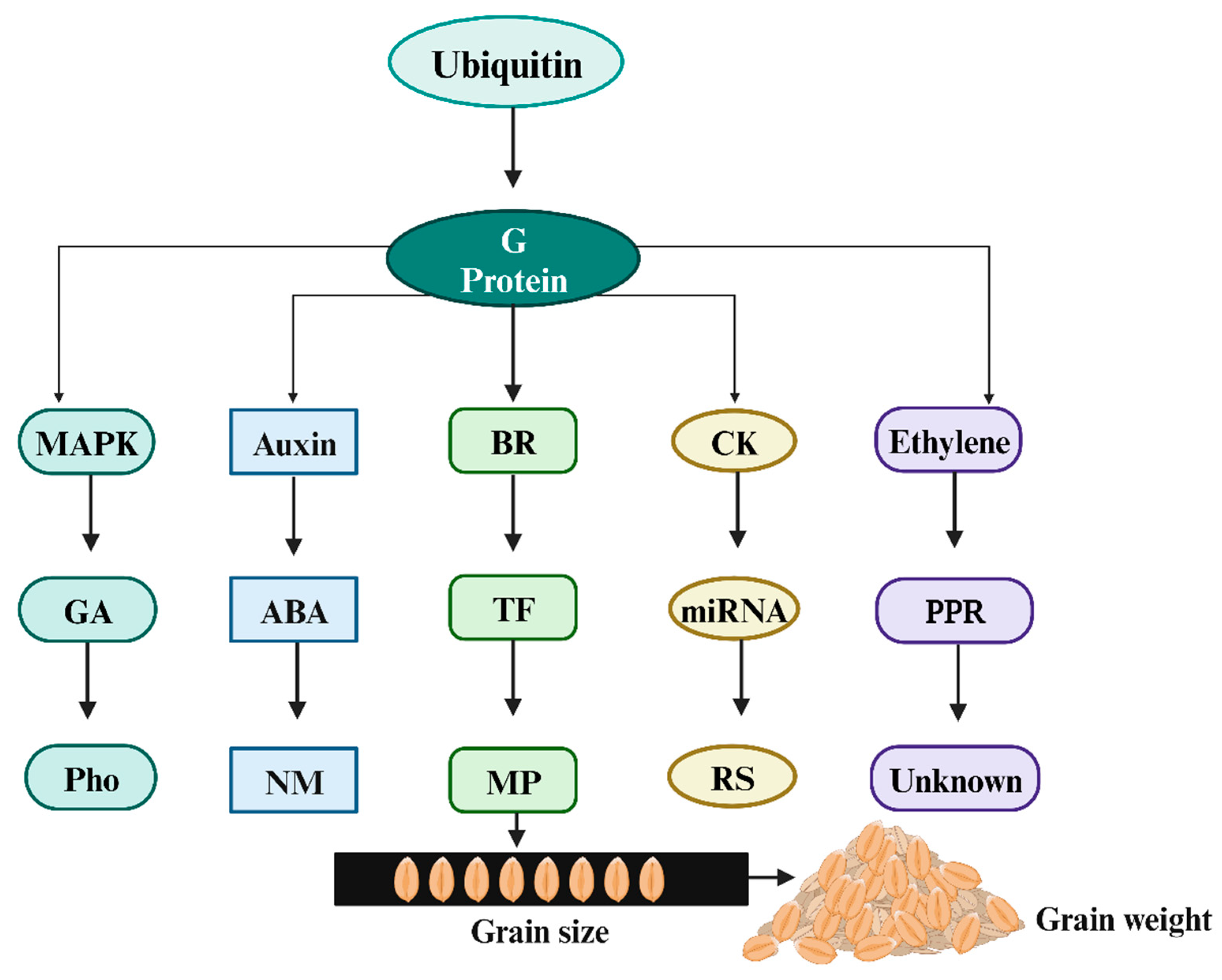
3.3. Molecular Mechanisms behind Wheat Yield Modulation
3.4. Role of Hormone Signaling Genes
3.5. Transcription Factors in Seed Development
3.6. MicroRNAs in Post-Transcriptional Regulation
3.7. Impact of Epigenetic Modifications on Yield
4. Advancements in Wheat Genomics
4.1. Achievements in Wheat Improvement through Genetic and Genomic Pathways
4.2. Genomic Selection and Its Impact on Breeding Programs
4.3. Role of Bioinformatics in Wheat Genomics Research
4.4. Genome Sequencing and Association Mapping
4.5. Functional Genomics and Gene Expression Profiling
5. Future Prospects and Directions for Yield Improvement
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of Food Security and Nutrition in the World 2022; FAO: Rome, Italy, 2022. [Google Scholar]
- FAO. The State of Food Security and Nutrition in the World 2023; FAO: Rome, Italy, 2023. [Google Scholar]
- Ranjbar, Z.; Chizari, M.; Sadighi, H.; Farhadian, H.; Lebailly, P.; Dogot, T.; Rojas, J.A.O.; Parra-Acosta, Y.K.; Azadi, H. Risk Factors in Various Climates of Wheat Production in Western Iran: Experts’ Opinions. Agriculture 2021, 11, 1227. [Google Scholar] [CrossRef]
- Khadka, K.; Earl, H.J.; Raizada, M.N.; Navabi, A. A Physio-Morphological Trait-Based Approach for Breeding Drought Tolerant Wheat. Front. Plant Sci. 2020, 11, 715. [Google Scholar] [CrossRef]
- Yu, K.; Liu, D.; Chen, Y.; Wang, D.; Yang, W.; Yang, W.; Yin, L.; Zhang, C.; Zhao, S.; Sun, J.; et al. Unraveling the Genetic Architecture of Grain Size in Einkorn Wheat through Linkage and Homology Mapping and Transcriptomic Profiling. J. Exp. Bot. 2019, 70, 4671–4688. [Google Scholar] [CrossRef]
- Li, A.; Hao, C.; Wang, Z.; Geng, S.; Jia, M.; Wang, F.; Han, X.; Kong, X.; Yin, L.; Tao, S.; et al. Wheat Breeding History Reveals Synergistic Selection of Pleiotropic Genomic Sites for Plant Architecture and Grain Yield. Mol. Plant 2022, 15, 504–519. [Google Scholar] [CrossRef]
- Alam, I.; Batool, K.; Huang, Y.; Liu, J.; Ge, L. Developing Genetic Engineering Techniques for Control of Seed Size and Yield. Int. J. Mol. Sci. 2022, 23, 13256. [Google Scholar] [CrossRef]
- Hamdan, M.F.; Noor, S.N.M.; Abd-Aziz, N.; Pua, T.-L.; Tan, B.C. Green Revolution to Gene Revolution: Technological Advances in Agriculture to Feed the World. Plants 2022, 11, 1297. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Yu, S.; Sun, M.; Yang, Z.; Gao, Z.-Q. Development of Drought-Tolerant Transgenic Wheat: Achievements and Limitations. Int. J. Mol. Sci. 2019, 20, 3350. [Google Scholar] [CrossRef]
- Albahri, G.; Alyamani, A.A.; Badran, A.; Hijazi, A.; Nasser, M.; Maresca, M.; Baydoun, E. Enhancing Essential Grains Yield for Sustainable Food Security and Bio-Safe Agriculture through Latest Innovative Approaches. Agronomy 2023, 13, 1709. [Google Scholar] [CrossRef]
- Farooq, A.; Farooq, N.; Akbar, H.; Hassan, Z.U.; Gheewala, S.H. A Critical Review of Climate Change Impact at a Global Scale on Cereal Crop Production. Agronomy 2023, 13, 162. [Google Scholar] [CrossRef]
- Dracatos, P.M.; Lück, S.; Douchkov, D.K. Diversifying Resistance Mechanisms in Cereal Crops Using Microphenomics. Plant Phenomics 2023, 5, 0023. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.-H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and Molecular Techniques from Simple Breeding to Speed Breeding in Crop Plants: Recent Advances and Future Outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef] [PubMed]
- Krishna, T.P.A.; Veeramuthu, D.; Maharajan, T.; Soosaimanickam, M. The Era of Plant Breeding: Conventional Breeding to Genomics-assisted Breeding for Crop Improvement. Curr. Genom. 2023, 24, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, L.; Bollina, V.; Soolanayakanahally, R.; Pahari, S.; Elferjani, R.; Kulkarni, M.; Vaid, N.; Risseuw, E.; Cram, D.; Pasha, A.; et al. Multi-Omics Atlas of Combinatorial Abiotic Stress Responses in Wheat. Plant J. 2023, 116, 1118–1135. [Google Scholar] [CrossRef] [PubMed]
- Farhad, M.; Kumar, U.; Tomar, V.; Bhati, P.K.; Krishnan, J.N.; Barek, V.; Brestic, M.; Hossain, A. Heat Stress in Wheat: A Global Challenge to Feed Billions in the Current Era of the Changing Climate. Front. Sustain. Food Syst. 2023, 7, 1203721. [Google Scholar] [CrossRef]
- Bhoite, R.; Han, Y.; Chaitanya, A.K.; Varshney, R.K.; Sharma, D.L. Genomic Approaches to Enhance Adaptive Plasticity to Cope with Soil Constraints Amidst Climate Change in Wheat. Plant Genome 2024, 17, e20358. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Prusty, M.R.; Pandey, M.K.; Singh, P.K.; Bohra, A.; Guo, B.; Varshney, R.K. Application of CRISPR/Cas9-Mediated Gene Editing for Abiotic Stress Management in Crop Plants. Front. Plant Sci. 2023, 14, 1157678. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, F.; Alharbi, S.; Alotaibi, M.; Al Mosallam, M.; Motawei, M.; Alrajhi, A. Wheat Omics: Classical Breeding to New Breeding Technologies. Saudi J. Biol. Sci. 2021, 28, 1433–1444. [Google Scholar] [CrossRef]
- Yang, Y.; Saand, M.A.; Huang, L.; Abdelaal, W.B.; Zhang, J.; Wu, Y.; Li, J.; Sirohi, M.H.; Wang, F. Applications of Multi-Omics Technologies for Crop Improvement. Front. Plant Sci. 2021, 12, 563953. [Google Scholar] [CrossRef]
- Nadolska-Orczyk, A.; Rajchel, I.K.; Orczyk, W.; Gasparis, S. Major Genes Determining Yield-Related Traits in Wheat and Barley. Theor. Appl. Genet. 2017, 130, 1081–1098. [Google Scholar] [CrossRef]
- Sinha, D.; Maurya, A.K.; Abdi, G.; Majeed, M.; Agarwal, R.; Mukherjee, R.; Ganguly, S.; Aziz, R.; Bhatia, M.; Majgaonkar, A.; et al. Integrated Genomic Selection for Accelerating Breeding Programs of Climate-Smart Cereals. Genes 2023, 14, 1484. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, X.; Jin, X.; Peng, J.; Zhang, H.; Wang, Y. CRISPR/Cas Technology Revolutionizes Crop Breeding. Plants 2023, 12, 3119. [Google Scholar] [CrossRef] [PubMed]
- Vagndorf, N.; Kristensen, P.S.; Andersen, J.R.; Jahoor, A.; Orabi, J.; Vagndorf, N.; Kristensen, P.S.; Andersen, J.R.; Jahoor, A.; Orabi, J. Marker-Assisted Breeding in Wheat. Next Gener. Plant Breed. 2018, 1, 3–22. [Google Scholar] [CrossRef]
- Labroo, M.R.; Studer, A.J.; Rutkoski, J.E. Heterosis and Hybrid Crop Breeding: A Multidisciplinary Review. Front. Genet. 2021, 12, 643761. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.P.; Braun, H.J. Wheat Improvement. In Wheat Improvement: Food Security in a Changing Climate; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Vitale, J.; Adam, B.; Vitale, P. Economics of Wheat Breeding Strategies: Focusing on Oklahoma Hard Red Winter Wheat. Agronomy 2020, 10, 238. [Google Scholar] [CrossRef]
- Rathan, N.D.; Krishnappa, G.; Singh, A.-M.; Govindan, V. Mapping QTL for Phenological and Grain-Related Traits in a Mapping Population Derived from High-Zinc-Biofortified Wheat. Plants 2023, 12, 220. [Google Scholar] [CrossRef] [PubMed]
- Lephuthing, M.C.; Khumalo, T.P.; Tolmay, V.L.; Dube, E.; Tsilo, T.J. Genetic Mapping of Quantitative Trait Loci Associated with Plant Height and Yield Component Traits in a Wheat (Triticum aestivum L.) Doubled Haploid Population Derived from Tugela-DN × Elands. Agronomy 2022, 12, 2283. [Google Scholar] [CrossRef]
- Asrat, Z. The Application of Quantitative Trait Loci (QTL) Mapping in Crop Improvement. Int. J. Plant Breed. Genet. 2021, 8, 1–5. [Google Scholar]
- Rani, K.; Kumar, M.; Razzaq, A.; Ajay, B.C.; Kona, P.; Bera, S.K.; Wani, S.H. Recent Advances in Molecular Marker Technology for QTL Mapping in Plants. In QTL Mapping in Crop Improvement; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Zhou, C.; Xiong, H.; Fu, M.; Guo, H.; Zhao, L.; Xie, Y.; Gu, J.; Zhao, S.; Ding, Y.; Li, Y.; et al. Genetic Mapping and Identification of Rht8-B1 That Regulates Plant Height in Wheat. BMC Plant Biol. 2023, 23, 333. [Google Scholar] [CrossRef]
- Ma, F.; Xu, Y.; Wang, R.; Tong, Y.; Zhang, A.; Liu, D.; An, D. Identification of Major QTLs for Yield-Related Traits with Improved Genetic Map in Wheat. Front. Plant Sci. 2023, 14, 1138696. [Google Scholar] [CrossRef]
- Kerimov, N.; Tambets, R.; Hayhurst, J.D.; Rahu, I.; Kolberg, P.; Raudvere, U.; Kuzmin, I.; Chowdhary, A.; Vija, A.; Teras, H.J.; et al. Systematic Visualisation of Molecular QTLs Reveals Variant Mechanisms at GWAS Loci. bioRxiv 2023. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Akpınar, B.A.; Alaux, M.; Algharib, A.M.; Sehgal, D.; Ali, Z.; Aradottir, G.I.; Batley, J.; Bellec, A.; Bentley, A.R.; et al. Capturing Wheat Phenotypes at the Genome Level. Front. Plant Sci. 2022, 13, 851079. [Google Scholar] [CrossRef] [PubMed]
- Zenda, T.; Liu, S.; Dong, A.; Duan, H. Advances in Cereal Crop Genomics for Resilience under Climate Change. Life 2021, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Li, C. Harness the Power of Genomic Selection and the Potential of Germplasm in Crop Breeding for Global Food Security in the Era with Rapid Climate Change. Crop. J. 2020, 8, 688–700. [Google Scholar] [CrossRef]
- Dong, C.; Xi, Y.; Satheesh, V.; Lei, M. Advances in CRISPR/Cas Technologies and Their Application in Plants. Trop. Plants 2023, 2, 2. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas Genome Editing and Precision Plant Breeding in Agriculture. Annu. Rev. Plant Biol. 2019, 70, 667–697. [Google Scholar] [CrossRef]
- van de Wiel, C.C.M.; Schaart, J.G.; Lotz, L.A.P.; Smulders, M.J.M. New Traits in Crops Produced by Genome Editing Techniques Based on Deletions. Plant Biotechnol. Rep. 2017, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Neik, T.X.; Thomas, W.J.W.; Batley, J. CRISPR-Based Genome Editing Tools: An Accelerator in Crop Breeding for a Changing Future. Int. J. Mol. Sci. 2023, 24, 8623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Z.; Unver, T.; Zhang, B. CRISPR/Cas: A Powerful Tool for Gene Function Study and Crop Improvement. J. Adv. Res. 2021, 29, 207–221. [Google Scholar] [CrossRef]
- Gunitseva, N.; Evteeva, M.; Borisova, A.; Patrushev, M.; Subach, F. RNA-Dependent RNA Targeting by CRISPR-Cas Systems: Characterizations and Applications. Int. J. Mol. Sci. 2023, 24, 6894. [Google Scholar] [CrossRef]
- Mahmood, U.; Li, X.; Fan, Y.; Chang, W.; Niu, Y.; Li, J.; Qu, C.; Lu, K. Multi-Omics Revolution to Promote Plant Breeding Efficiency. Front. Plant Sci. 2022, 13, 1062952. [Google Scholar] [CrossRef]
- Sethi, M.; Saini, D.K.; Devi, V.; Kaur, C.; Singh, M.P.; Singh, J.; Pruthi, G.; Kaur, A.; Singh, A.; Chaudhary, D.P. Unravelling the Genetic Framework Associated with Grain Quality and Yield-Related Traits in Maize (Zea mays L.). Front. Genet. 2023, 14, 1248697. [Google Scholar]
- Ko, D.K.; Brandizzi, F. Network-Based Approaches for Understanding Gene Regulation and Function in Plants. Plant J. 2020, 104, 302–317. [Google Scholar] [CrossRef]
- Großkinsky, D.K.; Syaifullah, S.J.; Roitsch, T. Integration of Multi-Omics Techniques and Physiological Phenotyping within a Holistic Phenomics Approach to Study Senescence in Model and Crop Plants. J. Exp. Bot. 2018, 69, 825–844. [Google Scholar] [CrossRef]
- Munaweera, T.I.K.; Jayawardana, N.U.; Rajaratnam, R.; Dissanayake, N. Modern Plant Biotechnology as a Strategy in Addressing Climate Change and Attaining Food Security. Agric. Food Secur. 2022, 11, 26. [Google Scholar] [CrossRef]
- Abdullah-Zawawi, M.-R.; Govender, N.; Harun, S.; Muhammad, N.A.N.; Zainal, Z.; Mohamed-Hussein, Z.-A. Multi-Omics Approaches and Resources for Systems-Level Gene Function Prediction in the Plant Kingdom. Plants 2022, 11, 2614. [Google Scholar] [CrossRef]
- Saha, S.; Bhadana, D.; Arya, R.; Shah, P.K.; Nishtha; Kumar, P. An Overview of Bioinformatics and Computational Genomics in Modern Plant Science. Int. J. Environ. Clim. Chang. 2023, 13, 1530–1544. [Google Scholar] [CrossRef]
- Farrell, R.E. Transcriptomes and Bioinformatics. In RNA Methodologies; Academic Press: Cambridge, MA, USA, 2023. [Google Scholar]
- Dikobe, T.; Masenya, K.; Manganyi, M.C. Molecular Technologies Ending with ‘Omics’: The Driving Force toward Sustainable Plant Production and Protection. F1000Research 2023, 12, 480. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, G. Molecular Prospective on the Wheat Grain Development. Crit. Rev. Biotechnol. 2023, 43, 38–49. [Google Scholar] [CrossRef]
- Hasan, N.; Choudhary, S.; Naaz, N.; Sharma, N.; Laskar, R.A. Recent Advancements in Molecular Marker-Assisted Selection and Applications in Plant Breeding Programmes. J. Genet. Eng. Biotechnol. 2021, 19, 128. [Google Scholar] [CrossRef]
- Fischer, R.A.T. History of Wheat Breeding: A Personal View. In Wheat Improvement: Food Security in a Changing Climate; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Subhasmita, S.; Pradeepkumara, N. Concept of Gene Pool and Distant Hybridization in Vegetable Crops. Int. J. Curr. Microbiol. Appl. Sci. 2022, 11, 148–159. [Google Scholar] [CrossRef]
- Khalid, M.; Afzal, F.; Gul, A.; Amir, R.; Subhani, A.; Ahmed, Z.; Mahmood, Z.; Xia, X.; Rasheed, A.; He, Z. Molecular Characterization of 87 Functional Genes in Wheat Diversity Panel and Their Association with Phenotypes under Well-Watered and Water-Limited Conditions. Front. Plant Sci. 2019, 10, 717. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Yin, C.; Wang, Z.; Wu, H.; Shen, K.; Zhang, Z.; Kang, L.; Xu, S.; Bi, A.; et al. A High-Resolution Genotype-Phenotype Map Identifies the TaSPL17 Controlling Grain Number and Size in Wheat. Genome Biol. 2023, 24, 196. [Google Scholar] [CrossRef]
- Chachar, Z.; Khan, S.U.; Zhang, X.-H.; Leng, P.-F.; Zong, N.; Zhao, J. Characterization of Transgenic Wheat Lines Expressing Maize ABP7 Involved in Kernel Development. J. Integr. Agric. 2023, 22, 389–399. [Google Scholar] [CrossRef]
- Tillett, B.J.; Hale, C.O.; Martin, J.M.; Giroux, M.J. Genes Impacting Grain Weight and Number in Wheat (Triticum aestivum L. ssp. aestivum). Plants 2022, 11, 1772. [Google Scholar] [CrossRef]
- Kuzay, S.; Lin, H.; Li, C.; Chen, S.; Woods, D.P.; Zhang, J.; Lan, T.; von Korff, M.; Dubcovsky, J. WAPO-A1 Is the Causal Gene of the 7AL QTL for Spikelet Number per Spike in Wheat. PLoS Genet. 2022, 18, e1009747. [Google Scholar] [CrossRef]
- Golan, G.; Ayalon, I.; Perry, A.; Zimran, G.; Ade-Ajayi, T.; Mosquna, A.; Distelfeld, A.; Peleg, Z. GNI-A1 Mediates Trade-Off between Grain Number and Grain Weight in Tetraploid Wheat. Theor. Appl. Genet. 2019, 132, 2353–2365. [Google Scholar] [CrossRef]
- Sakuma, S.; Golan, G.; Guo, Z.; Ogawa, T.; Tagiri, A.; Sugimoto, K.; Bernhardt, N.; Brassac, J.; Mascher, M.; Hensel, G.; et al. Unleashing Floret Fertility in Wheat through the Mutation of a Homeobox Gene. Proc. Natl. Acad. Sci. USA 2019, 116, 5182–5187. [Google Scholar] [CrossRef]
- Bustos, D.V.; Hasan, A.K.; Reynolds, M.P.; Calderini, D.F. Combining High Grain Number and Weight through a DH-Population to Improve Grain Yield Potential of Wheat in High-Yielding Environments. Field Crop. Res. 2013, 145, 106–115. [Google Scholar] [CrossRef]
- García, G.A.; Serrago, R.A.; González, F.G.; Slafer, G.A.; Reynolds, M.P.; Miralles, D.J. Wheat Grain Number: Identification of Favourable Physiological Traits in an Elite Doubled-Haploid Population. Field Crop. Res. 2014, 168, 126–134. [Google Scholar] [CrossRef]
- Griffiths, S.; Wingen, L.; Pietragalla, J.; Garcia, G.; Hasan, A.; Miralles, D.; Calderini, D.F.; Ankleshwaria, J.B.; Waite, M.L.; Simmonds, J.; et al. Genetic Dissection of Grain Size and Grain Number Trade-Offs in CIMMYT Wheat Germplasm. PLoS ONE 2015, 10, e0118847. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-J.; Zhang, H.-P.; Cao, J.-J.; Zhu, X.-F.; Wang, S.-X.; Jiang, H.; Wu, Z.Y.; Lu, J.; Chang, C.; Sun, G.-L.; et al. Characterization of an IAA-Glucose Hydrolase Gene TaTGW6 Associated with Grain Weight in Common Wheat (Triticum aestivum L.). Mol. Breed. 2016, 36, 25. [Google Scholar] [CrossRef]
- Hu, M.-J.; Zhang, H.-P.; Liu, K.; Cao, J.-J.; Wang, S.-X.; Jiang, H.; Wu, Z.-Y.; Lu, J.; Zhu, X.F.; Xia, X.-C.; et al. Cloning and Characterization of TaTGW-7A Gene Associated with Grain Weight in Wheat via SLAF-seq-BSA. Front. Plant Sci. 2016, 7, 1902. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Zhang, D.; Zhao, X.; Cao, X.; Dong, L.; Liu, J.; Chen, K.; Zhang, H.; Gao, C.; et al. Analysis of the Functions of TaGW2 Homoeologs in Wheat Grain Weight and Protein Content Traits. Plant J. 2018, 94, 857–866. [Google Scholar] [CrossRef]
- Lv, Q.; Li, L.; Meng, Y.; Sun, H.; Chen, L.; Wang, B.; Li, X. Wheat E3 Ubiquitin Ligase TaGW2-6A Degrades TaAGPS to Affect Seed Size. Plant Sci. 2022, 320, 111274. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, L.; Du, L.-P.; Su, Z.; Wang, J.; Ye, X.; Qi, L.; Zhang, Z. Transcript Suppression of TaGW2 Increased Grain Width and Weight in Bread Wheat. Funct. Integr. Genom. 2014, 14, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, X.; Chen, F.; Cui, D. A Single-Nucleotide Polymorphism of TaGS5 Gene Revealed Its Association with Kernel Weight in Chinese Bread Wheat. Front. Plant Sci. 2015, 6, 1166. [Google Scholar] [CrossRef]
- Ma, L.; Li, T.; Hao, C.; Wang, Y.; Chen, X.; Zhang, X. TaGS5-3A, a Grain Size Gene Selected during Wheat Improvement for Larger Kernel and Yield. Plant Biotechnol. J. 2016, 14, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Jiang, Q.; Hao, C.; Wang, Y.; Zhang, H.; Zhang, X. Global Selection on Sucrose Synthase Haplotypes during a Century of Wheat Breeding. Plant Physiol. 2014, 164, 1918–1929. [Google Scholar] [CrossRef]
- Hou, J.; Liu, Y.; Hao, C.; Li, T.; Liu, H.; Zhang, X. Starch Metabolism in Wheat: Gene Variation and Association Analysis Reveal Additive Effects on Kernel Weight. Front. Plant Sci. 2020, 11, 562008. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kim, J.-Y. Understanding Wheat Starch Metabolism in Properties, Environmental Stress Condition, and Molecular Approaches for Value-Added Utilization. Plants 2021, 10, 2282. [Google Scholar] [CrossRef]
- Kumar, R.; Mukherjee, S.; Ayele, B.T. Molecular Aspects of Sucrose Transport and Its Metabolism to Starch during Seed Development in Wheat: A Comprehensive Review. Biotechnol. Adv. 2018, 36, 954–967. [Google Scholar] [CrossRef]
- Borisjuk, N.; Kishchenko, O.; Eliby, S.; Schramm, C.; Anderson, P.; Jatayev, S.; Kurishbayev, A.; Shavrukov, Y. Genetic Modification for Wheat Improvement: From Transgenesis to Genome Editing. BioMed Res. Int. 2019, 2019, 6216304. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Wang, Y.; Tang, H.; Sui, N.; Zhang, X.; Wang, F. Genetic, Hormonal, and Environmental Control of Tillering in Wheat. Crop. J. 2021, 9, 986–991. [Google Scholar] [CrossRef]
- Castro-Camba, R.; Sánchez, C.; Vidal, N.; Vielba, J.M. Plant Development and Crop Yield: The Role of Gibberellins. Plants 2022, 11, 2650. [Google Scholar] [CrossRef]
- Sosnowski, J.; Truba, M.; Vasileva, V. The Impact of Auxin and Cytokinin on the Growth and Development of Selected Crops. Agriculture 2023, 13, 724. [Google Scholar] [CrossRef]
- Long, Y.; Wang, C.; Liu, C.; Li, H.; Pu, A.; Dong, Z.; Wei, X.; Wan, X. Molecular Mechanisms Controlling Grain Size and Weight and Their Biotechnological Breeding Applications in Maize and Other Cereal Crops. J. Adv. Res. 2023, 19. [Google Scholar] [CrossRef]
- Gasparis, S.; Miłoszewski, M.M. Genetic Basis of Grain Size and Weight in Rice, Wheat, and Barley. Int. J. Mol. Sci. 2023, 24, 16921. [Google Scholar] [CrossRef]
- Chen, K.; Lyskowski, A.; Jaremko, L.; Jaremko, M. Genetic and Molecular Factors Determining Grain Weight in Rice. Front. Plant Sci. 2021, 12, 605799. [Google Scholar] [CrossRef]
- Devate, N.B.; Krishna, H.; Parmeshwarappa, S.K.V.; Manjunath, K.K.; Chauhan, D.; Singh, S.; Singh, J.B.; Kumar, M.; Patil, R.; Khan, H.; et al. Genome-Wide Association Mapping for Component Traits of Drought and Heat Tolerance in Wheat. Front. Plant Sci. 2022, 13, 943033. [Google Scholar] [CrossRef]
- Lu, H.-P.; Wang, J.-J.; Wang, M.-J.; Liu, J.-X. Roles of Plant Hormones in Thermomorphogenesis. Stress Biol. 2021, 1, 20. [Google Scholar] [CrossRef]
- Matilla, A.J. Auxin: Hormonal Signal Required for Seed Development and Dormancy. Plants 2020, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.W.; Lu, J.; Shah, L.; Yang, L.; Chen, C.; Mei, X.D.; Xue, L.; Manzoor, M.A.; Abdullah, M.; Rehman, S.; et al. Expansion and Molecular Characterization of AP2/ERF Gene Family in Wheat (Triticum aestivum L.). Front. Genet. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Daher, H.; Galien, A.; Hugouvieux, V.; Zubieta, C. Structural Basis for Plant MADS Transcription Factor Oligomerization. Comput. Struct. Biotechnol. J. 2019, 17, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.-Y.; Chang, D.C.; Lin, S.-L. The MicroRNA (MiRNA): Overview of the RNA Genes That Modulate Gene Function. Mol. Biotechnol. 2007, 38, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Hu, B.; Zhang, C. microRNAs and Their Roles in Plant Development. Front. Plant Sci. 2022, 13, 824240. [Google Scholar] [CrossRef] [PubMed]
- Mall, A.K.; Misra, V.; Pathak, A.D. Changing Environment and Crop Plant Breeding. In Augmenting Crop Productivity in Stress Environment; Springer: Singapore, 2022; pp. 105–114. [Google Scholar]
- Ďúranová, H.; Šimora, V.; Ďurišová, Ľ.; Olexiková, L.; Kovár, M.; Požgajová, M. Modifications in Ultrastructural Characteristics and Redox Status of Plants under Environmental Stress: A Review. Plants 2023, 12, 1666. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Hu, B.; Jiang, W.; Wang, Y.; Yan, J.; Ma, F.; Guan, Q.; Xu, J. Advances in Plant Epigenome Editing Research and Its Application in Plants. Int. J. Mol. Sci. 2023, 24, 3442. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Khan, S.; Wani, I.A.; Gupta, R.; Verma, S.; Alam, P.; Alaklabi, A. Unravelling the Role of Epigenetic Modifications in Development and Reproduction of Angiosperms: A Critical Appraisal. Front. Genet. 2022, 13, 819941. [Google Scholar] [CrossRef]
- Lucibelli, F.; Valoroso, M.C.; Aceto, S. Plant DNA Methylation: An Epigenetic Mark in Development, Environmental Interactions, and Evolution. Int. J. Mol. Sci. 2022, 23, 8299. [Google Scholar] [CrossRef]
- Husen, A.; Ahmad, A. Genomics, Transcriptomics, Proteomics and Metabolomics of Crop Plants; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Sun, C.; Hu, H.; Cheng, Y.; Yang, X.; Qiao, Q.; Wang, C.; Zhang, L.; Chen, D.; Zhao, S.; Dong, Z.; et al. Genomics-Assisted Breeding: The Next-Generation Wheat Breeding Era. Plant Breed. 2023, 142, 259–268. [Google Scholar] [CrossRef]
- Kang, Y.; Choi, C.; Kim, J.Y.; Min, K.D.; Kim, C. Optimizing Genomic Selection of Agricultural Traits Using K-Wheat Core Collection. Front. Plant Sci. 2023, 14, 1112297. [Google Scholar] [CrossRef] [PubMed]
- Winn, Z.J.; Larkin, D.L.; Lozada, D.N.; DeWitt, N.; Brown-Guedira, G.; Mason, R.E. Multivariate Genomic Selection Models Improve Prediction Accuracy of Agronomic Traits in Soft Red Winter Wheat. Crop. Sci. 2023, 63, 2115–2130. [Google Scholar] [CrossRef]
- Vaghela, U.; Sonagara, M.K.; Pratibha; Yadav, A. A Review on Revolutionary Strategy for Crop Improvement: Genome Editing. Int. J. Environ. Clim. Chang. 2023, 13, 2005–2018. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, M.K.; Tiwari, S.; Solanki, R.S.; Chauhan, S.; Tripathi, N.; Dwivedi, N.; Kandalkar, V.S. The Exploitation of Genetic Variability and Trait Association Analysis for Diverse Quantitative Traits in Bread Wheat (Triticum aestivum L.). Curr. J. Appl. Sci. Technol. 2023, 42, 19–33. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Shen, T.; Wang, X.; Yi, S.; Meng, T.; Sun, J.; Wang, X.; Qu, X.; Chen, S.; et al. A Near-Complete Genome Sequence of Einkorn Wheat Provides Insight into the Evolution of Wheat A Subgenomes. Plant Commun. 2023, 5, 100768. [Google Scholar] [CrossRef] [PubMed]
- Mohideen, H.S.; Gupta, A.; Ghosh, S. Bioinformatics Approach for Whole Transcriptomics-Based Marker Prediction in Agricultural Crops. In Bioinformatics in Agriculture: Next Generation Sequencing Era; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Montesinos-López, A.; Rivera, C.; Pinto, F.; Piñera, F.; Gonzalez, D.; Reynolds, M.; Pérez-Rodríguez, P.; Li, H.; Montesinos-López, O.A.; Crossa, J. Multimodal Deep Learning Methods Enhance Genomic Prediction of Wheat Breeding. G3 Genes Genomes Genet. 2023, 13, jkad045. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.T.; Batool, Z.; Ahmad, C.A.; Khan, H.A.A.; Akhter, A. Application of Modern Biotechnology and Bioinformatics Approaches in Agricultural Sciences; A Systematic Review. World J. Biol. Biotechnol. 2023, 8, 13–17. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Elashtokhy, M.M.A.; Shamseldin, S.A.M.; El-Ballat, E.M.; Zayed, E.M.; Heikal, Y.M. Analysis of Genetic Diversity and Phylogenetic Relationships of Wheat (Triticum aestivum L.) Genotypes Using Phenological, Molecular and DNA Barcoding Markers. Genes 2023, 14, 34. [Google Scholar] [CrossRef]
- Zia, M.A.B.; Yousaf, M.F.; Asim, A.; Naeem, M. An Overview of Genome-Wide Association Mapping Studies in Poaceae Species (Model Crops: Wheat and Rice). Mol. Biol. Rep. 2022, 49, 12077–12090. [Google Scholar] [CrossRef]
- Shepelev, S.S.; Pototskaya, I.V.; Chursin, A.S.; Morgunov, A.I.; Shamanin, V.P. Marker Assisted Selection (MAS) of Spring Bread Wheat to Improve Productivity, Grain Quality, Resistance to Diseases and Drought in the Conditions of Western Siberia. Grain Econ. Russ. 2023, 15, 18–25. [Google Scholar] [CrossRef]
- Montesinos-López, A.; Runcie, D.E.; Ibba, M.I.; Pérez-Rodríguez, P.; Montesinos-López, O.A.; Crespo, L.A.; Bentley, A.R.; Crossa, J. Multi-Trait Genomic-Enabled Prediction Enhances Accuracy in Multi-Year Wheat Breeding Trials. G3 Genes Genomes Genet. 2021, 11, jkab27. [Google Scholar] [CrossRef]
- Altaf, A.; Shah, A.Z.; Gull, S.; Hussain, S.; Faheem, M.; Zada, A.; Saeed, A.; Al Amin Miah, A.; Zhu, M.; Zhu, X. Progress in Modern Crop Science Research in Wheat Biology. J. Glob. Innov. Agric. Sci. 2022, 10, 43–49. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, J.; Yang, G.; Wan, Y.; Li, Y. Harnessing Knowledge from Plant Functional Genomics and Multi-Omics for Genetic Improvement. Int. J. Mol. Sci. 2023, 24, 10347. [Google Scholar] [CrossRef]
- Xiong, H.; Guo, H.; Fu, M.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Ding, Y.; Du, Q.; Zhang, J.; et al. A Large-Scale Whole-Exome Sequencing Mutant Resource for Functional Genomics in Wheat. Plant Biotechnol. J. 2023, 21, 2047–2056. [Google Scholar] [CrossRef]
- Wei, J.; Fang, Y.; Jiang, H.; Wu, X.-T.; Zuo, J.-H.; Xia, X.-C.; Li, J.-Q.; Stich, B.; Cao, H.; Liu, Y.-X. Combining QTL Mapping and Gene Co-Expression Network Analysis for Prediction of Candidate Genes and Molecular Network Related to Yield in Wheat. BMC Plant Biol. 2022, 22, 228. [Google Scholar] [CrossRef]
- Goyal, A.; Lakra, N.; Soni, A.; Kumari, A.; Annu; Manorma; Meenakshi; Reena; Munjal, R. Functional Genomics Approaches for Combating the Abiotic Stresses in Wheat. In Abiotic Stresses in Wheat: Unfolding the Challenges; Academic Press: Cambridge, MA, USA, 2023. [Google Scholar]

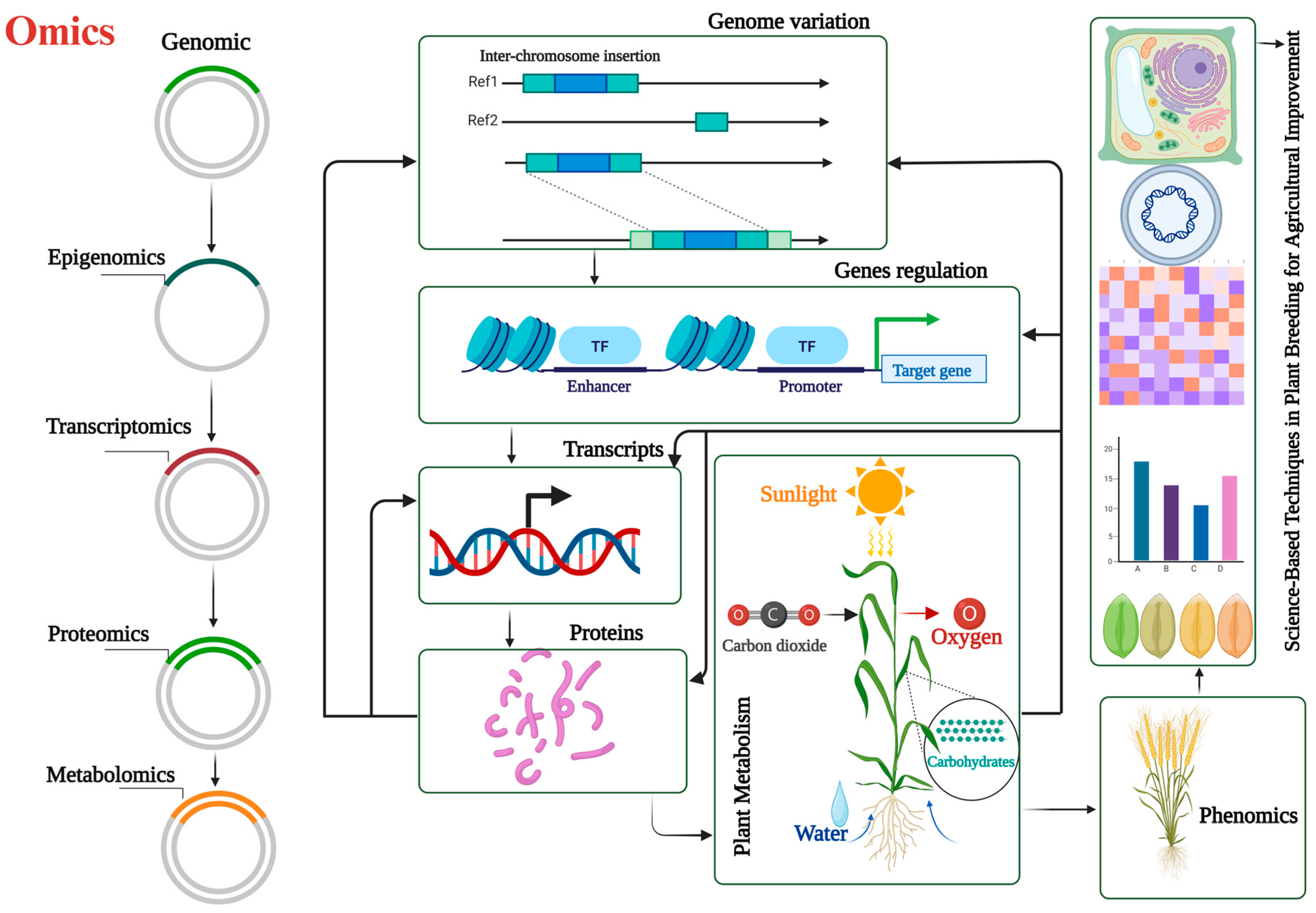

| Omics Type | Key Findings | Contribution to Yield Enhancement | References |
|---|---|---|---|
| Genomics and Transcriptomics | Identification of genes linked to grain size and biomass accumulation. | Enabled the development of wheat varieties with optimized grain size and biomass traits. | [49] |
| Transcriptomics | Discovery of genetic markers for grain number and size. | Facilitated marker-assisted selection for traits directly influencing yield. | [46] |
| Proteomics | Analysis of protein expression related to nutrient utilization. | Improved understanding of nutrient metabolism aiding in breeding for better nutrient efficiency. | [47] |
| Metabolomics and Environmental Genomics | Identified metabolic pathways and gene–environment interactions affecting yield under stress conditions. | Supported the breeding of wheat varieties tailored to specific environmental conditions for enhanced yield stability. | [20] |
| Metabolomics | Profiling of metabolites in high-yielding lines under various conditions. | Provided targets for genetic manipulation to improve metabolic pathways crucial for yield. | [49] |
| Study | Gene | Function of Gene | Primer Sequence (F/R) | Reference |
|---|---|---|---|---|
| TaSPL17 controlling grain number and size in wheat. | TaSPL17 | Grain Number & Size | F: TACGTTACCCTAAGTCTGCGC R: GAGCCCTTCCTTCCCATACC | [59] |
| ABP7 can be utilized in wheat breeding for grain yield improvement. | ABP7 | Grain yield | F: GGTGGGTACCAAGACCTGTGGCAAAC R: TGCGGGACTCTAATCATAAAAACC | [60] |
| These genes that impact grain weight and number and the most beneficial alleles of those genes with respect to increasing the yield. | TaGNI, TaCKX6, TaGS5, TaDA1, WAPO1, and TaRht1 | Grain Weight and Number in Wheat | F: ACAAAATAGGCGCTATAGCTGCTC R: CGGGACAGATGATTTCTAGAGGTT | [61] https://www.mdpi.com/article/10.3390/plants11131772/s1 (accessed on 24 January 2024) |
| TGW7A can be used for improvement of TGW in breeding programs. | TaTGW-7A | Grain Weight | F: AATGATACGGCGACCACCGA R: CAAGCAGAAGACGGCATACG | [68] |
| This gene increase, thousand-grain weight, and grain size in wheat. | TaGW2 | Grain Size | F: AGA GCA ATT TGT AAG TCT TAT TCC R: GCT TCA ATG ACT TTC TGT TCT TCC | [72] |
| TaGS5 gene revealed its association with kernel weight in Chinese bread wheat. | TaGS5 | Grain Weight | F: CAAGCCACTCACTCTCACAT R: GATCAGCGCTATCCCTTCTG | [73] |
| TaGS5-3A is a positive regulator of grain size in wheat. | TaGS5-3A | Larger grain size | F: TGTCAATGGGATGTTGCCTG R: TCATCGGTGTGTAGGAAGCTG | [74] |
| Study of these genes shows that the endosperm starch synthesis pathway is a major target of indirect selection in global wheat breeding for higher yield. | TaSus2-2A, TaSus2-2B, TaSus1-7A, and TaSus1-7B | Thousand-grain weight (TGW) | F: ATGGCTGCCAAGCTGACTCG R: CACACCGGTCAGGGTCATCA | [75] |
| Achievement | Genetic and Genomic Pathways | Description | Impact | References |
|---|---|---|---|---|
| Enhanced Yield | Genomic Selection, Marker-Assisted Selection | Development of varieties with higher yields through genomic selection and marker-assisted selection. | Increases agricultural productivity and efficiency. | [20] |
| Disease Resistance | Genetic Mapping, CRISPR/Cas9 | Introduction of disease resistance traits using genetic mapping and CRISPR/Cas9. | Reduces crop losses and chemical pesticide use. | [21] |
| Improved Grain Quality | Gene Editing, Genomic Analysis | Modification of genes related to grain size, gluten content, and nutrition. | Meets higher food quality standards and safety. | [98] |
| Stress Tolerance | Genetic Mapping, Omics Technologies | Breeding of strains that tolerate abiotic stresses such as drought, salinity, and extreme temperatures. | Enhances resilience to climate change. | [15] |
| Resource Use Efficiency | Genomic Selection, Physiological Genomics | Development of varieties that use water and nutrients more efficiently. | Improves sustainability in resource-limited settings. | [79] |
| Tailored Wheat Varieties | Genomic Information, Association Mapping | Custom development of varieties suited to specific climates and soil types using genomic information. | Enhances local farming success and sustainability. | International Wheat Genome Sequencing Consortium (IWGSC). https://www.wheatgenome.org/ (accessed on 24 January 2024) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chachar, Z.; Fan, L.; Chachar, S.; Ahmed, N.; Narejo, M.-u.-N.; Ahmed, N.; Lai, R.; Qi, Y. Genetic and Genomic Pathways to Improved Wheat (Triticum aestivum L.) Yields: A Review. Agronomy 2024, 14, 1201. https://doi.org/10.3390/agronomy14061201
Chachar Z, Fan L, Chachar S, Ahmed N, Narejo M-u-N, Ahmed N, Lai R, Qi Y. Genetic and Genomic Pathways to Improved Wheat (Triticum aestivum L.) Yields: A Review. Agronomy. 2024; 14(6):1201. https://doi.org/10.3390/agronomy14061201
Chicago/Turabian StyleChachar, Zaid, Lina Fan, Sadaruddin Chachar, Nazir Ahmed, Mehar-un-Nisa Narejo, Naseer Ahmed, Ruiqiang Lai, and Yongwen Qi. 2024. "Genetic and Genomic Pathways to Improved Wheat (Triticum aestivum L.) Yields: A Review" Agronomy 14, no. 6: 1201. https://doi.org/10.3390/agronomy14061201
APA StyleChachar, Z., Fan, L., Chachar, S., Ahmed, N., Narejo, M.-u.-N., Ahmed, N., Lai, R., & Qi, Y. (2024). Genetic and Genomic Pathways to Improved Wheat (Triticum aestivum L.) Yields: A Review. Agronomy, 14(6), 1201. https://doi.org/10.3390/agronomy14061201








