Transcriptomic Analysis of Sodium-Silicate-Induced Resistance against Rhizoctonia solani AG-3 in Potato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Potato and Pathogen
2.2. Potato Inoculation
2.3. RNA Sequencing and Library Construction
2.4. Sequence Alignment
2.5. Identification and Functional Enrichment Analysis of Differentially Expressed Genes
2.6. Gene Ontology and KEGG Pathways
2.7. Real-Time qPCR Analysis
3. Results
3.1. Prediction of Novel Transcripts
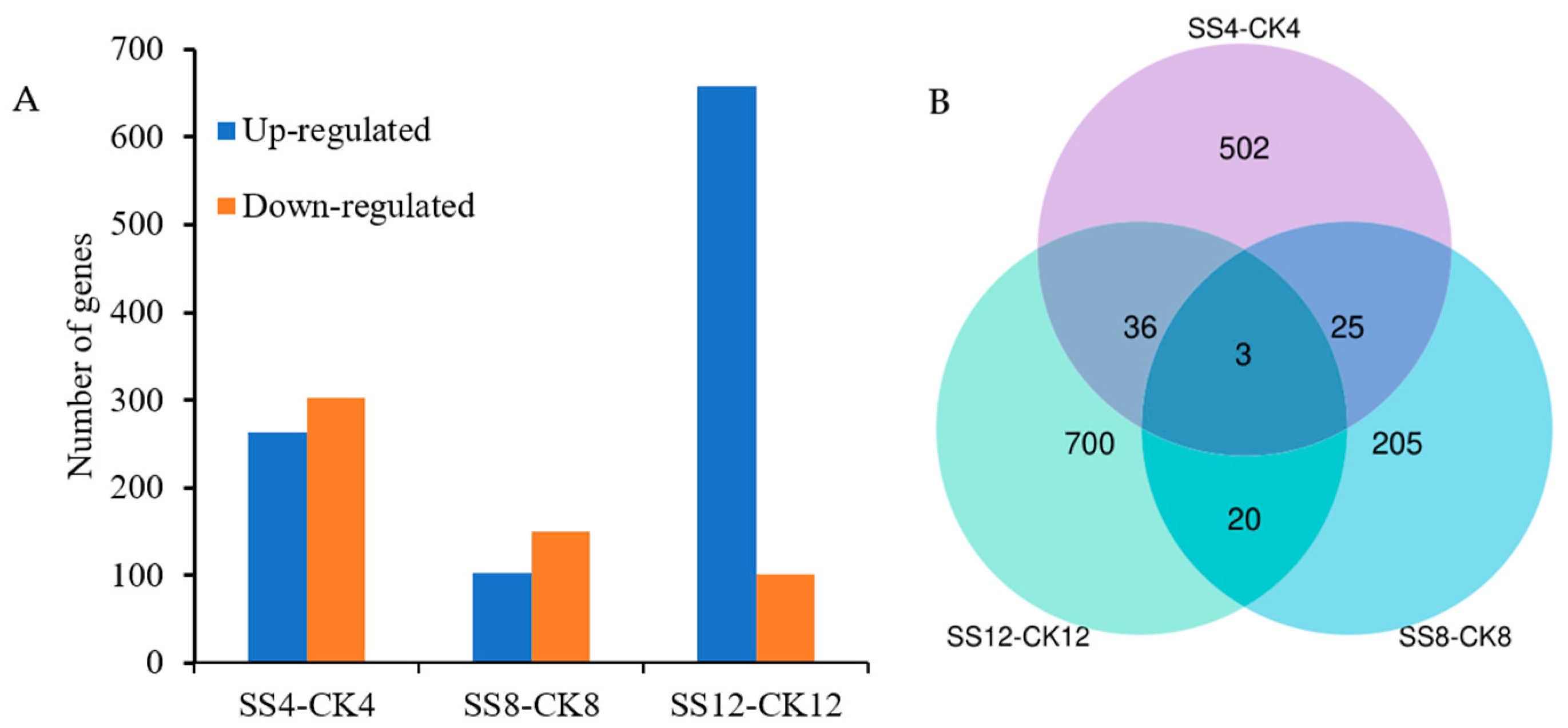
3.2. Global Gene Expression Profiling and Differentially Expressed Genes
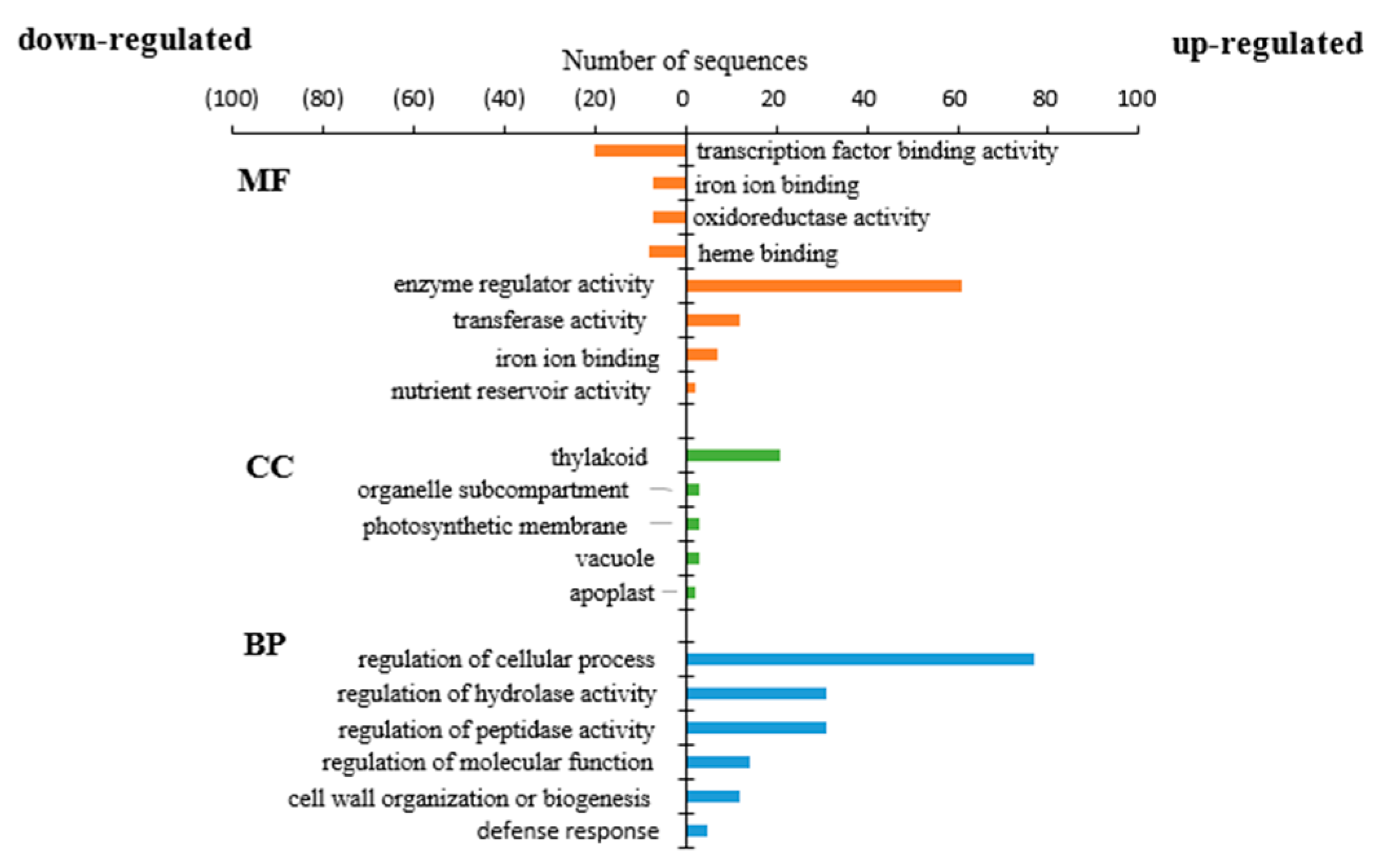
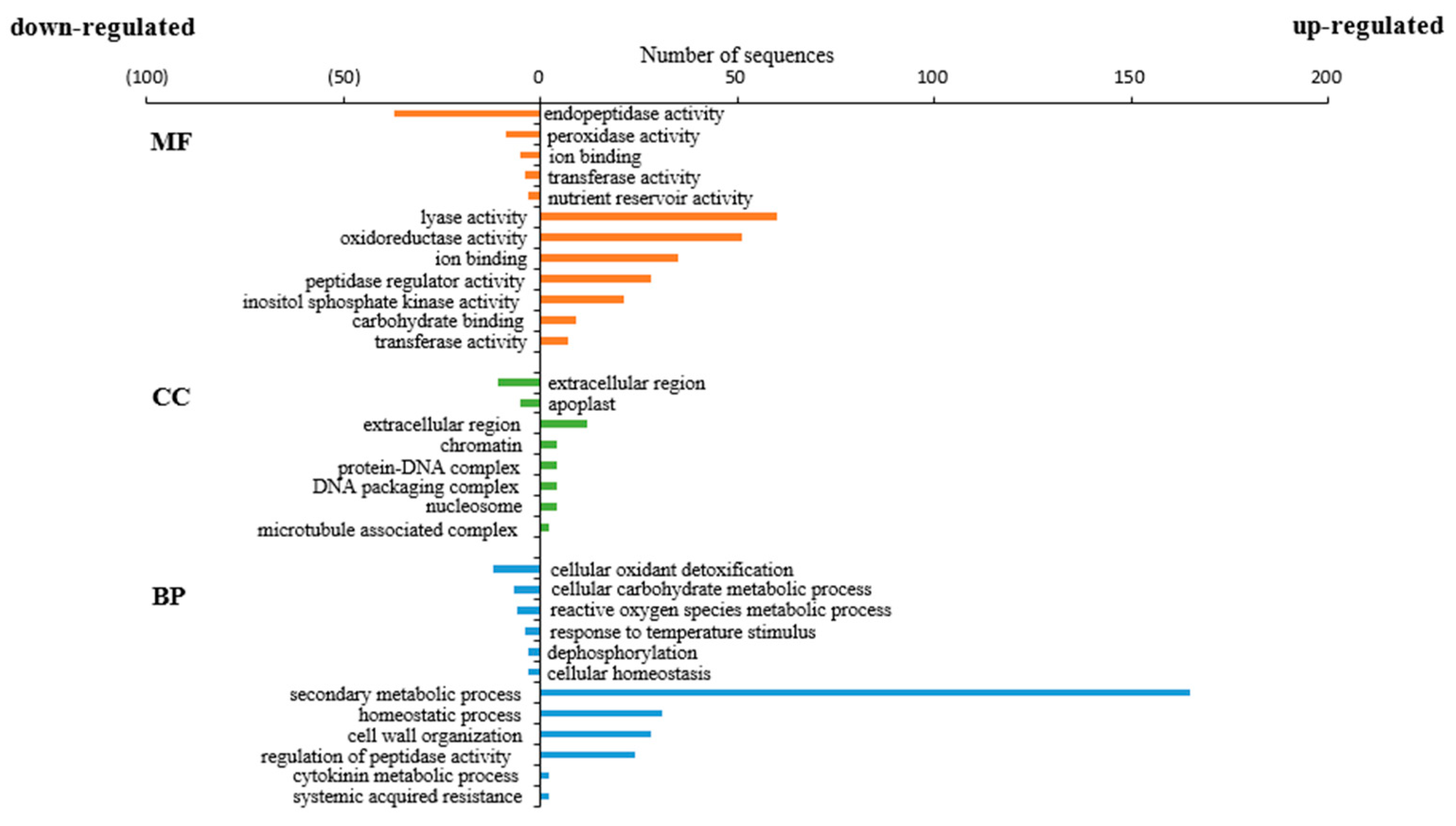
3.3. Gene Ontology and Kyoto Encyclopedia Analysis of Differentially Expressed Genes
3.4. Differentially Expressed Genes Involved in the Plant Hormone Signal Transduction Pathway
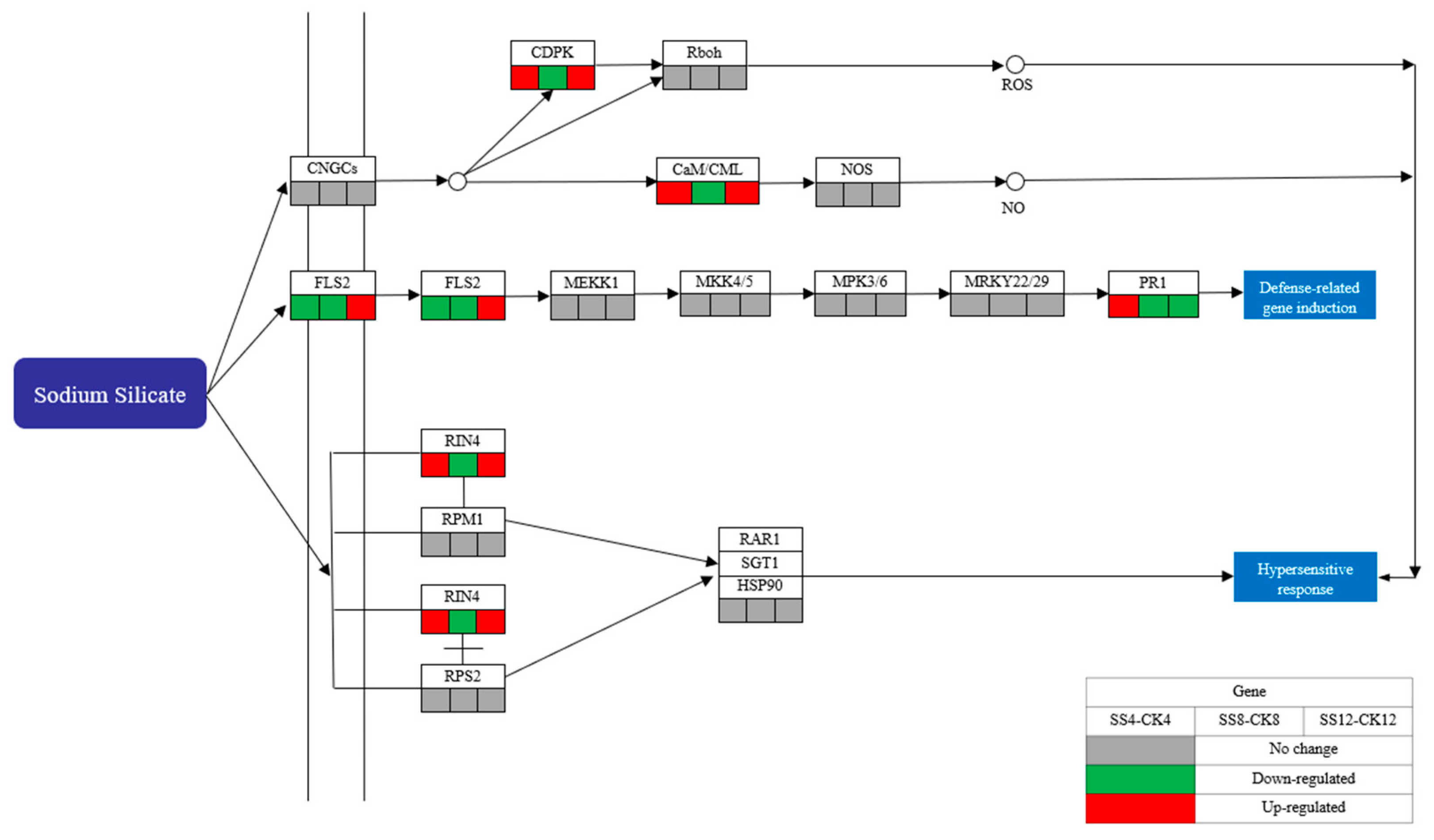
3.5. Differentially Expressed Genes Involved in the Plant–Pathogen Interaction Pathway
3.6. Differentially Expressed Genes Involved in the Phenylpropanoid Biosynthesis Pathway
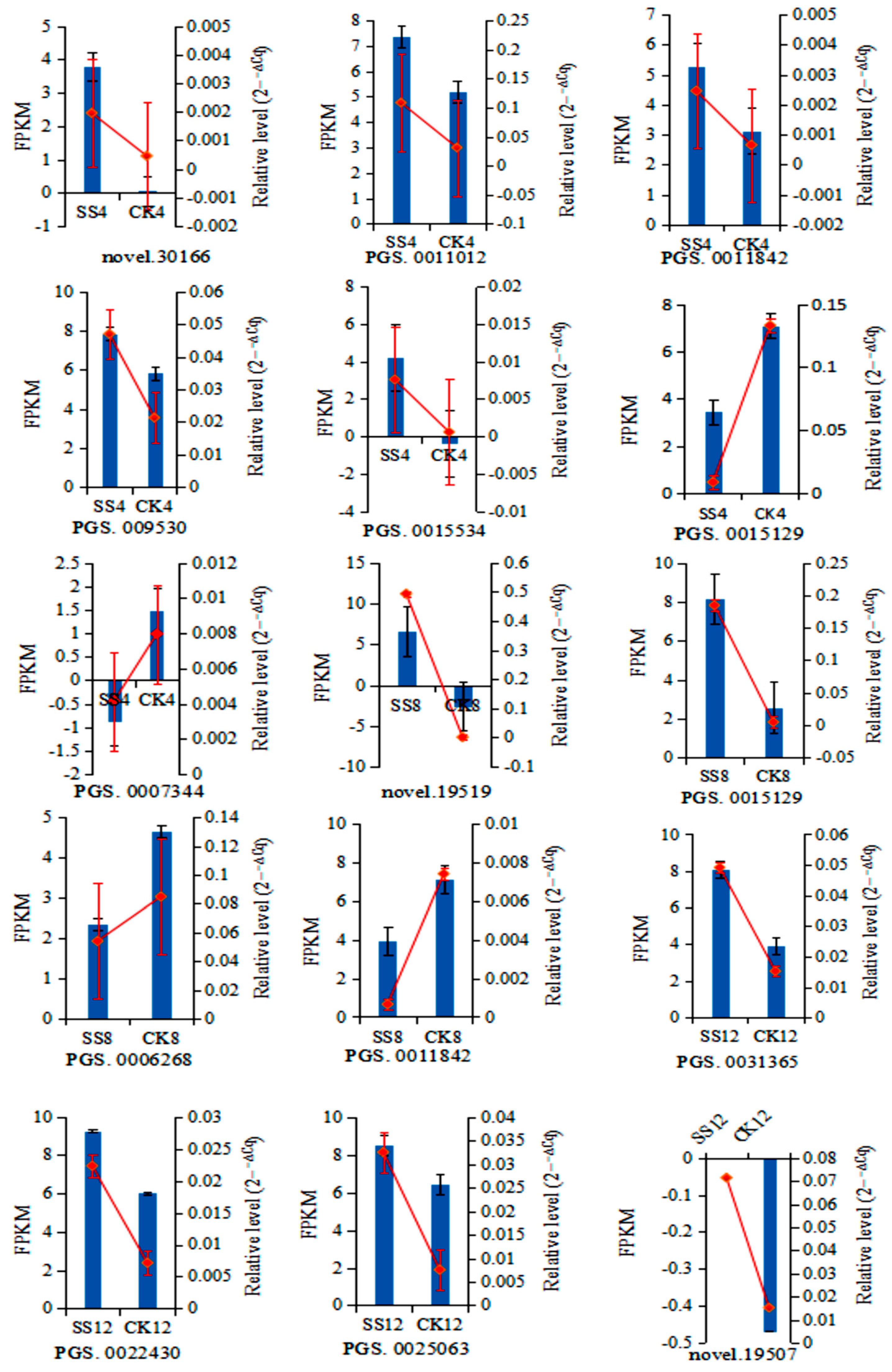
3.7. Validation of the Gene Expression of Differentially Expressed Genes by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, M.R.; Parmar, A.; Niedbała, G.; Wojciechowski, T.; Abou El-Yazied, A.; El-Gawad, H.G.A.; Nahhas, N.E.; Ibrahim, M.F.M.; El-Mogy, M.M. Improved shelf-life and consumer acceptance of fresh-cut and fried potato strips by an edible coating of garden cress seed mucilage. Foods 2021, 10, 1536. [Google Scholar] [CrossRef]
- Tian, J.; Chen, J.; Ye, X.; Chen, S. Health benefits of the potato affected by domestic cooking: A review-ScienceDirect. Food Chem. 2016, 202, 165–175. [Google Scholar] [CrossRef]
- Banville, G.J. Yield losses and damage to potato plants caused by Rhizoctonia solani Kuhn. Am. Potato J. 1989, 66, 821–834. [Google Scholar] [CrossRef]
- Johnson, D.A.; Cummings, T.F. Effect of extended crop rotations on incidence of black dot, silver scurf, and Verticillium wilt of potato. Plant Dis. 2015, 99, 257–262. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, C.; Guo, Z.; Wu, X. Anastomosis group and pathogenicity of Rhizoctonia solani associated with stem canker and black scurf of potato in China. Eur. J. Plant Pathol. 2015, 143, 99–111. [Google Scholar] [CrossRef]
- Ajayi-Oyetunde, O.O.; Bradley, C.A. Rhizoctonia solani: Taxonomy, population biology and management of Rhizoctonia seedling disease of soybean. Plant Pathol. 2018, 67, 3–17. [Google Scholar] [CrossRef]
- Tsror, L. Biology, epidemiology and management of Rhizoctonia solani on potato. J. Phytopathol. 2010, 158, 649–658. [Google Scholar] [CrossRef]
- Balali, G.R.; Neate, S.M.; Kasalkheh, A.M.; Stodart, B.J.; Melanson, D.L.; Scott, E.S. Intraspecific variation of Rhizoctonia solani AG 3 isolates recovered from potato fields in Central Iran and South Australia. Mycopathologia 2007, 163, 105–115. [Google Scholar] [CrossRef]
- Yang, L.; Wang, D.; Xu, Y.; Zhao, H.; Wang, L.; Cao, X.; Chen, Y.; Chen, Q. A new resistance gene against potato late blight originating from Solanum pinnatisectum located on potato chromosome 7. Front. Plant Sci. 2017, 8, 1729. [Google Scholar] [CrossRef]
- Carling, D.E.; Leiner, R.H.; Westphale, P.C. Symptoms, signs and yield reduction associated with Rhizoctonia disease of potato induced by tuberborne inoculum of Rhizoctonia solani AG-3. Am. Potato J. 1989, 66, 693–701. [Google Scholar] [CrossRef]
- Read, P.J.; Hide, G.A.; Firmager, J.P.; Hall, S.M. Growth and yield of potatoes as affected by severity of stem canker (Rhizoctonia solani). Potato Res. 1989, 32, 9–15. [Google Scholar] [CrossRef]
- Bohinc, T.; Vuajnk, F.; Trdan, S. The efficacy of environmentally acceptable products for the control of major potato pests and diseases. Zemdirbyste-Agric. 2019, 106, 135–142. [Google Scholar] [CrossRef]
- Pierre, M.; Mélanie, W.; Clarke, R.H. Pesticide acute toxicity is a better correlate of U.S. grassland bird declines than agricultural intensification. PLoS ONE 2013, 8, e57457. [Google Scholar] [CrossRef]
- Sabes, P.L.P.; Lon, M.M.; Peter, M.A.; Maruyama, J.; Koyama, S.; Watanabe, T.; Koizumi, S. Effect of increased silicon content of paddy rice on sheath blight development through carbonized rice husk application. Jpn. Agric. Res. Q. 2020, 54, 145–151. [Google Scholar] [CrossRef]
- Saludares, G.G.; Vera Cruz, C.; Alovera, R.; Cabahug, L. Application of calcium silicate for the management of sheath blight (Rhizoctonia solani) in rice cultivars. Philipp. J. Crop Sci. 2011, 36, 101–102. [Google Scholar]
- Mandlik, R.; Thakral, V.; Raturi, G.; Shinde, S.; Nikolić, M.; Tripathi, D.K.; Sonah, H.; Deshmukh, R. Significance of silicon uptake, transport, and deposition in plants. J. Exp. Bot. 2020, 71, 6703–6718. [Google Scholar] [CrossRef]
- Zhang, C.C.; Wang, L.J.; Zhang, W.X.; Zhang, F.S. Do lignification and silicification of the cell wall precede silicon deposition in the silica cell of the rice (Oryza sativa L.) leaf epidermis? Plant Soil 2013, 372, 137–149. [Google Scholar] [CrossRef]
- Bozkurt, T.O.; Schornack, S.; Banfield, M.J.; Kamoun, S. Oomycetes, effectors, and all that jazz. Curr. Opin. Plant Biol. 2012, 15, 483–492. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y. Phytophthora sojae effectors orchestrate warfare with host immunity. Curr. Opin. Microbiol. 2018, 46, 7–13. [Google Scholar] [CrossRef]
- Thorsten, N.; Volker, L. Non-host resistance in plants: New insights into an old phenomenon. Mol. Plant Pathol. 2005, 6, 335–345. [Google Scholar] [CrossRef]
- Rasoolizadeh, A.; Santhanam, P.; Labbé, C.; Shivaraj, S.M.; Germain, H.; Bélanger, R.R. Silicon influences the localization and expression of Phytophthora sojae effectors in interaction with soybean. J. Exp. Bot. 2020, 71, 6844–6855. [Google Scholar] [CrossRef]
- Zheng, A.P.; Lin, R.M.; Zhang, D.H.; Qin, P.G.; Xu, L.Z.; Ai, P.; Ding, L.; Wang, Y.R.; Chen, Y.; Liu, Y.; et al. The genome and pathogenic mechanisms of the rice sheath blight pathogen. Nat. Commun. 2013, 4, 1424. [Google Scholar] [CrossRef]
- Fawe, A.; Abou-Zaid, M.; Menzies, J.G.; Bélanger, R.R. Silicon-mediated accumulation of flavonoid phytoalexins in cucumber. Phytopathology 1998, 88, 396–401. [Google Scholar] [CrossRef]
- Samuels, A.; Glass, A.; Ehret, D.L.; Menzies, J.G. Mobility and deposition of silicon in cucumber plants. Plant Cell Environ. 1991, 14, 485–492. [Google Scholar] [CrossRef]
- Cherif, M.; Belanger, R.R. Use of potassium silicate amendments in recirculating nutrient solutions to suppress Pythium ultimum on long English cucumber. Plant Dis. 1992, 6, 1008–1011. [Google Scholar] [CrossRef]
- Bélanger, R.R.; Benhamou, N.; Menzies, J.G. Cytological evidence of an active role of silicon in wheat resistance to powdery mildew (Blumeria graminis f. sp. tritici). Phytopathology 2003, 93, 402–412. [Google Scholar]
- Rodrigues, F.Á.; McNally, D.J.; Datnoff, L.E.; Jones, J.B.; Labbé, C.; Benhamou, N.; Menzies, J.G.; Bélanger, R.R. Silicon enhances the accumulation of diterpenoid phytoalexins in rice: A potential mechanism for blast resistance. Phytopathology 2004, 94, 177–183. [Google Scholar] [CrossRef]
- Cai, K.Z.; Gao, D.; Luo, S.M.; Zeng, R.S.; Yang, J.Y.; Zhu, X.Y. Physiological and cytological mechanisms of silicon-induced resistance in rice against blast disease. Physiol. Plant. 2008, 134, 324–333. [Google Scholar] [CrossRef]
- Resende, R.S.; Rodrigues, F.Á.; Ferraz, H.G.M.; Moreira, W.R.; Oliveira, J.R.; Mariano, R.L.R. Silicon reduces bacterial speck development on tomato leaves. Trop. Plant Pathol. 2013, 38, 436–442. [Google Scholar]
- Dannon, E.A.; Wydra, K. Interaction between silicon amendment, bacterial wilt development and phenotype of Ralstonia solanacearum in tomato genotypes. Physiol. Mol. Plant Pathol. 2004, 64, 233–243. [Google Scholar] [CrossRef]
- David, D.; Weerahewa, H.L.D. Silicon Suppresses Anthracnose Diseases in Tomato (Lycopersicon esculentum) by Enhancing Disease Resistance. Annu. Acad. Sess. 2012, 210–213. [Google Scholar]
- Ghareeb, H.; Zoltán Bozsó, P.G.; Repenning, C.; Stahl, F.; Wydra, K. Transcriptome of silicon-induced resist Ralstonia solanacearum in the silicon non-accumulator tomato implicates priming effect. Physiol. Mol. Plant Pathol. 2011, 75, 83–89. [Google Scholar] [CrossRef]
- Heine, G.; Tikum, G.; Horst, W.J. The effect of silicon on the infection by and spread of Pythium aphanidermatum in single roots of tomato and bitter gourd. J. Exp. Bot. 2007, 58, 569–577. [Google Scholar] [CrossRef]
- Cheng, H.H.; Roberts, P.D.; Datnoff, L.E. Silicon suppresses Fusarium crown and root rot of tomato. J. Phytopathol. 2011, 159, 546–554. [Google Scholar] [CrossRef]
- Huo, H.L. Sodium Silicate Increases the Resistance of Potato and Its Physiological and Biochemical Mechanism on Stem Canker and Black Scurf. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2018. [Google Scholar]
- Zhang, X.Y.; Huo, H.L.; Zhang, D.M.; Xing, X.; Li, D.Z.; Wang, F.W. Effects of sodium silicate on resistance to potato stem canker and its resistance-related substances. J. Plant Prot. 2020, 47, 1287–1296. [Google Scholar]
- Vulavala, V.K.R.; Elbaum, R.; Yermiyahu, U.; Fogelman, E.; Kumar, A.; Ginzberg, I. Silicon fertilization of potato: Expression of putative transporters and tuber skin quality. Planta 2016, 243, 217–229. [Google Scholar] [CrossRef]
- Gao, X.Q.; Cox, K.L.; He, P. Functions of calcium-dependent protein kinases in plant innate immunity. Plants 2014, 3, 160–176. [Google Scholar] [CrossRef]
- Lee, H.; Chah, O.K.; Sheen, J. Stem cell-triggered immunity via CLV3p-FLS2 signaling. Nature 2011, 473, 376–379. [Google Scholar] [CrossRef]
- Jones, J.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Taheri, P.; Tarighi, S. Riboflavin induces resistance in rice against Rhizoctonia solani via jasmonate-mediated priming of phenylpropanoid pathway. J. Plant Physiol. 2010, 167, 201–208. [Google Scholar] [CrossRef]
- Mengiste, T. Plant Immunity to Necrotrophs. Annu. Rev. Phytopathol. 2012, 50, 267–294. [Google Scholar] [CrossRef]
- Hayasaka, T.; Fujii, H.; Ishiguro, K. The role of silicon in preventing appressorial penetration by the rice blast fungus. Phytopathology 2008, 98, 1038–1044. [Google Scholar] [CrossRef]
- Zhang, G.L.; Dai, Q.G.; Zhang, H.C. Silicon application enhances resistance to sheath blight (Rhizoctonia solani) in rice. J. Plant Physiol. Mol. Biol. 2006, 32, 600–606. [Google Scholar]
- Monaghan, J.; Zipfel, C. Plant pattern recognition receptor complexes at the plasma membrane. Curr. Opin. Plant Biol. 2012, 15, 348–357. [Google Scholar] [CrossRef]
- Yuan, M.H.; Jiang, Z.Y.; Bi, G.Z.; Nomura, K.; Liu, M.H.; Wang, Y.P.; Cai, B.Y.; Zhou, J.M.; He, S.Y.; Xin, X.F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Johnson, J.M.; Reichelt, M.; Vadassery, J.; Gershenzon, J.; Oelmüller, R. An Arabidopsis mutant impaired in intracellular calcium elevation is sensitive to biotic and abiotic stress. BMC Plant Biol. 2014, 14, 162. Available online: http://www.biomedcentral.com/1471-2229/14/162 (accessed on 11 June 2014).
- Pane, C.; Caputo, M.; Francese, G.; Manganiello, G.; Scalzo, R.L.; Mennella, G.; Zaccardelli, M. Managing Rhizoctonia damping-off of rocket (Eruca sativa) seedlings by drench application of bioactive potato leaf phytochemical extracts. Biology 2020, 9, 270. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Z.J.; Lei, C.; Zheng, C.F.; Wang, J.; Shao, S.J.; Li, X.; Xia, X.J.; Cai, X.Z.; Zhou, J.; et al. A plant phytosulfokine peptide initiates auxin-dependent immunity through cytosolic Ca2+ signaling in tomato. Plant Cell. 2018, 30, 652–667. [Google Scholar] [CrossRef]
- Hong, R.; Gray, W.M. SAUR Proteins as effectors of hormonal and environmental signals in plant growth. Mol. Plant 2015, 8, 1153–1164. [Google Scholar] [CrossRef]
- Domingo, C.; Andrés, F.; Tharreau, D.; Iglesias, D.J.; Talón, M. Constitutive expression of OsGH3.1 reduces auxin content and enhances defense response and resistance to a fungal pathogen in rice. Mol. Plant-Microbe Interact. 2009, 22, 201–210. [Google Scholar] [CrossRef]
- Zrenner, R.; Verwaaijen, B.; Genzel, F.; Flemer, B.; Grosch, R. Transcriptional changes in potato sprouts upon interaction with Rhizoctonia solani indicate pathogen-induced interference in the defence pathways of potato. Int. J. Mol. Sci. 2021, 22, 3094. [Google Scholar] [CrossRef]
- Yang, D.L.; Yang, Y.; He, Z. Roles of plant hormones and their interplay in rice immunity. Mol. Plant. 2013, 6, 675–685. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–207. [Google Scholar] [CrossRef]
- Velivelli, S.L.; Lojan, P.; Cranenbrouck, S.; Boulois, H.D.; Suarez, J.P.; Declerck, S.; Franco, J.; Prestwich, B.D. The induction of Ethylene response factor 3 (ERF3) in potato as a result of co-inoculation with Pseudomonas sp. R41805 and Rhizophagus irregularis MUCL 41833- a possible role in plant defense. Plant Signal. Behav. 2015, 10, e988076. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Du, M.M.; Deng, L.; Shen, J.F.; Fang, M.M.; Chen, Q.; Lu, Y.H.; Wang, Q.M.; Li, C.Y.; Zhai, Q.Z. MYC2 regulates the termination of jasmonate signaling via an autoregulatory negative feedback loop. Plant Cell. 2019, 31, 106–127. [Google Scholar] [CrossRef]
- Halder, V.; Suliman, M.N.S.; Kaschani, F.; Kaiser, M. Plant chemical genetics reveals colistin sulphate as a SA and NPR1-independent PR1 inducer functioning via a p38-like kinase pathway. Sci. Rep. 2019, 9, 11196. [Google Scholar] [CrossRef]
- Infantes, L.; Moreno, M.R.; Mozo, M.D.; Benavente, J.L.; Cuesta, J.O.; Coego, A.; Juste, J.L.; Rodriguez, P.L.; Albert, A. Structure-based modulation of the ligand sensitivity of a tomato dimeric abscisic acid receptor through a Glu to Asp mutation in the latch loop. Front. Plant Sci. 2022, 13, 884029. [Google Scholar] [CrossRef]
- Xu, W.; Purugganan, M.M.; Polisensky, D.H.; Antosiewicz, D.M.; Fry, S.C.; Braam, J. Arabidopsis TCH4, regulated by hormones and the environment, encodes a xyloglucan endotransglycosylase. Plant Cell. 1995, 7, 1555–1567. [Google Scholar]
- Iliev, E.A.; Xu, W.; Polisensky, D.H.; Oh, M.H.; Torisky, R.S.; Clouse, S.D.; Braam, J. Transcriptional and posttranscriptional regulation of arabidopsis TCH4 expression by diverse stimuli. Roles of cis regions and brassinosteroids. Plant Physiol. 2002, 130, 770–783. [Google Scholar] [CrossRef]
- Zhang, J.F.; Chen, L.; Fu, C.L.; Wang, L.X.; Liu, H.N.; Cheng, Y.Z.; Li, S.C.; Deng, Q.M.; Wang, S.Q.; Zhu, J.; et al. Comparative transcriptome analyses of gene expression changes triggered by Rhizoctonia solani AG1 IA infection in resistant and susceptible rice varieties. Front. Plant Sci. 2017, 8, 1422. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, S.T.; Wang, H.Y.; Wu, J.H.; Luo, Y.M.; Wang, Q.; Wang, F.X.; Xia, G.X. iTRAQ-based proteomic analysis of defence responses triggered by the necrotrophic pathogen Rhizoctonia solani in cotton. J. Proteom. 2016, 152, 226–235. [Google Scholar] [CrossRef]
- Taheri, P.; Tarighi, S. A survey on basal resistance and riboflavin-induced defense responses of sugar beet against Rhizoctonia solani. J. Plant Physiol. 2011, 168, 1114–1122. [Google Scholar] [CrossRef]
- Li, Q.; Qin, X.J.; Qi, J.J.; Dou, W.F.; Dunand, C.; Chen, S.C.; He, Y.R. CsPrx25, a class III peroxidase in Citrus sinensis, confers resistance to citrus bacterial canker through the maintenance of ROS homeostasis and cell wall lignification. Hortic. Res. 2020, 7, 192. [Google Scholar] [CrossRef]
- Jonas, V.B.; David, D.V.; Monica, H. Towards establishing broad-spectrum disease resistance in plants: Silicon leads the way. J. Exp. Bot. 2013, 64, 1281–1293. [Google Scholar] [CrossRef]
- Gehring, C.; Turek, I.S. Cyclic nucleotide monophosphates and their cyclases in plant signaling. Front. Plant Sci. 2017, 8, 1704. [Google Scholar] [CrossRef]
- Isner, J.C.; Maathuis, F.J.M. cGMP signalling in plants: From enigma to main stream. Funct. Plant Biol. 2018, 45, 93–101. [Google Scholar] [CrossRef]



| Gene ID | KEGG Entry | KO ID | Times Identified as a DEG a | Gene Name (Predicted) | KEGG Definition | Gene Description |
|---|---|---|---|---|---|---|
| PGSC0003DMG400003155 | 102581073 | K01904 | 12 | 4CL3 | 4-coumarate-CoA ligase | 4-coumarate:CoA ligase |
| PGSC0003DMG400007178 | 102578747 | K09754 | 12 | CYP450 | 5-O-(4-coumaroyl)-D-quinate 3′-monooxygenase | P-coumaroyl quinate/shikimate 3′-hydroxylase |
| PGSC0003DMG400010465 | 102605677 | K00430 | 8 | peroxidase 21 | peroxidase | Peroxidase |
| PGSC0003DMG400023458 | 102582618 | K10775 | 12 | PAL | phenylalanine ammonia-lyase | Phenylalanine ammonia-lyase |
| PGSC0003DMG400024967 | 102592844 | K00430 | 4 | peroxidase 51 | peroxidase | Peroxidase |
| PGSC0003DMG400031457 | 102596891 | K10775 | 4 | PAL | phenylalanine ammonia-lyase | Phenylalanine ammonia-lyase 1 |
| PGSC0003DMG401021549 | 102596343 | K10775 | 12 | PAL | phenylalanine ammonia-lyase | Phenylalanine ammonia-lyase |
| PGSC0003DMG402014734 | 102590830 | K09754 | 12 | CYP450 | 5-O-(4-coumaroyl)-D-quinate 3′-monooxygenase | P-coumaroyl quinate/shikimate 3′-hydroxylase |
| PGSC0003DMG402025083 | 102585990 | K00430 | 12 | peroxidase 12 | peroxidase | Peroxidase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Hao, J.; Zhang, D.; Huo, H.; Li, L.; Xiu, Z.; Yang, C.; Zhang, X. Transcriptomic Analysis of Sodium-Silicate-Induced Resistance against Rhizoctonia solani AG-3 in Potato. Agronomy 2024, 14, 1207. https://doi.org/10.3390/agronomy14061207
Feng Y, Hao J, Zhang D, Huo H, Li L, Xiu Z, Yang C, Zhang X. Transcriptomic Analysis of Sodium-Silicate-Induced Resistance against Rhizoctonia solani AG-3 in Potato. Agronomy. 2024; 14(6):1207. https://doi.org/10.3390/agronomy14061207
Chicago/Turabian StyleFeng, Yayan, Jianjun Hao, Dongmei Zhang, Hongli Huo, Lele Li, Zhijun Xiu, Chunfang Yang, and Xiaoyu Zhang. 2024. "Transcriptomic Analysis of Sodium-Silicate-Induced Resistance against Rhizoctonia solani AG-3 in Potato" Agronomy 14, no. 6: 1207. https://doi.org/10.3390/agronomy14061207
APA StyleFeng, Y., Hao, J., Zhang, D., Huo, H., Li, L., Xiu, Z., Yang, C., & Zhang, X. (2024). Transcriptomic Analysis of Sodium-Silicate-Induced Resistance against Rhizoctonia solani AG-3 in Potato. Agronomy, 14(6), 1207. https://doi.org/10.3390/agronomy14061207






