Effects of Ni and Cu Stresses on Morphological and Physiological Characteristics of Euphorbia marginata Pursh Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Conditions and Ni and Cu Treatment
2.2. Measurement Items and Methods
2.2.1. Growth Indicators
2.2.2. Measurement of Physiological Indicators
2.2.3. Determination of Ni and Cu Content under Different Treatments
2.3. Statistical Analysis
3. Results
3.1. Effect of Ni and Cu Stresses on Morphology Traits
3.2. Effect of Ni and Cu Stresses on Root Morphology Traits
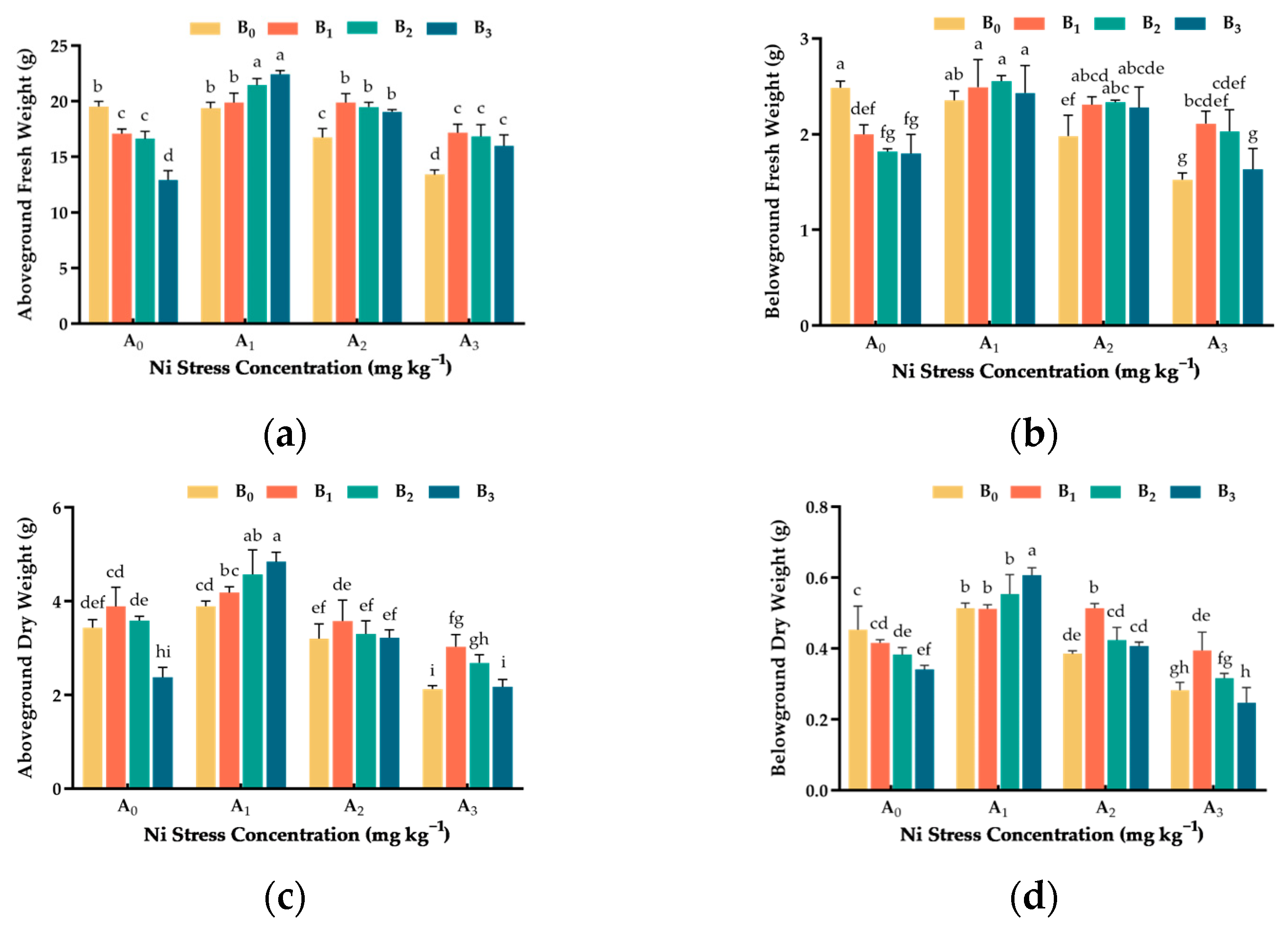
3.3. Effect of Ni and Cu Stresses on Biomass
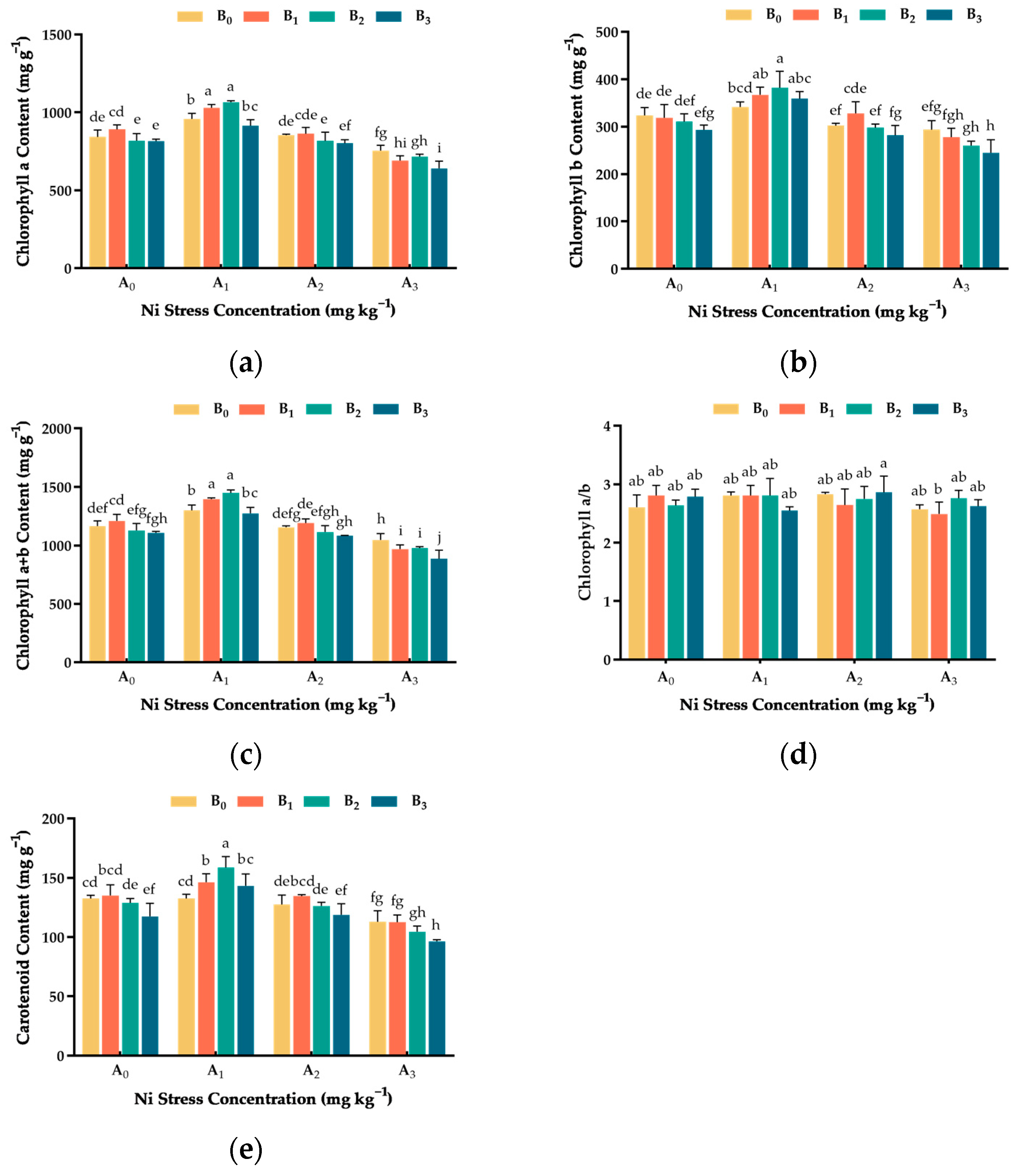
3.4. Effect of Ni and Cu Stresses on Photosynthetic Pigment Content
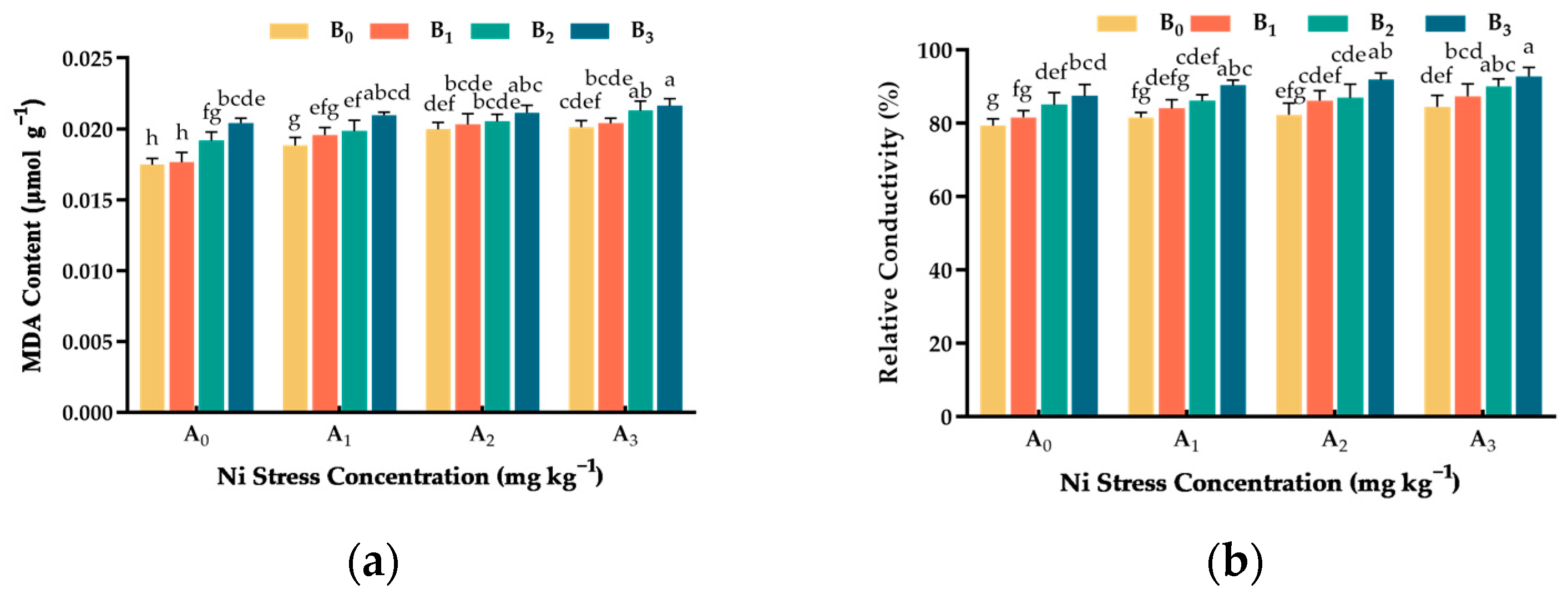
3.5. Effect of Ni and Cu Stresses on Antioxidant Activity
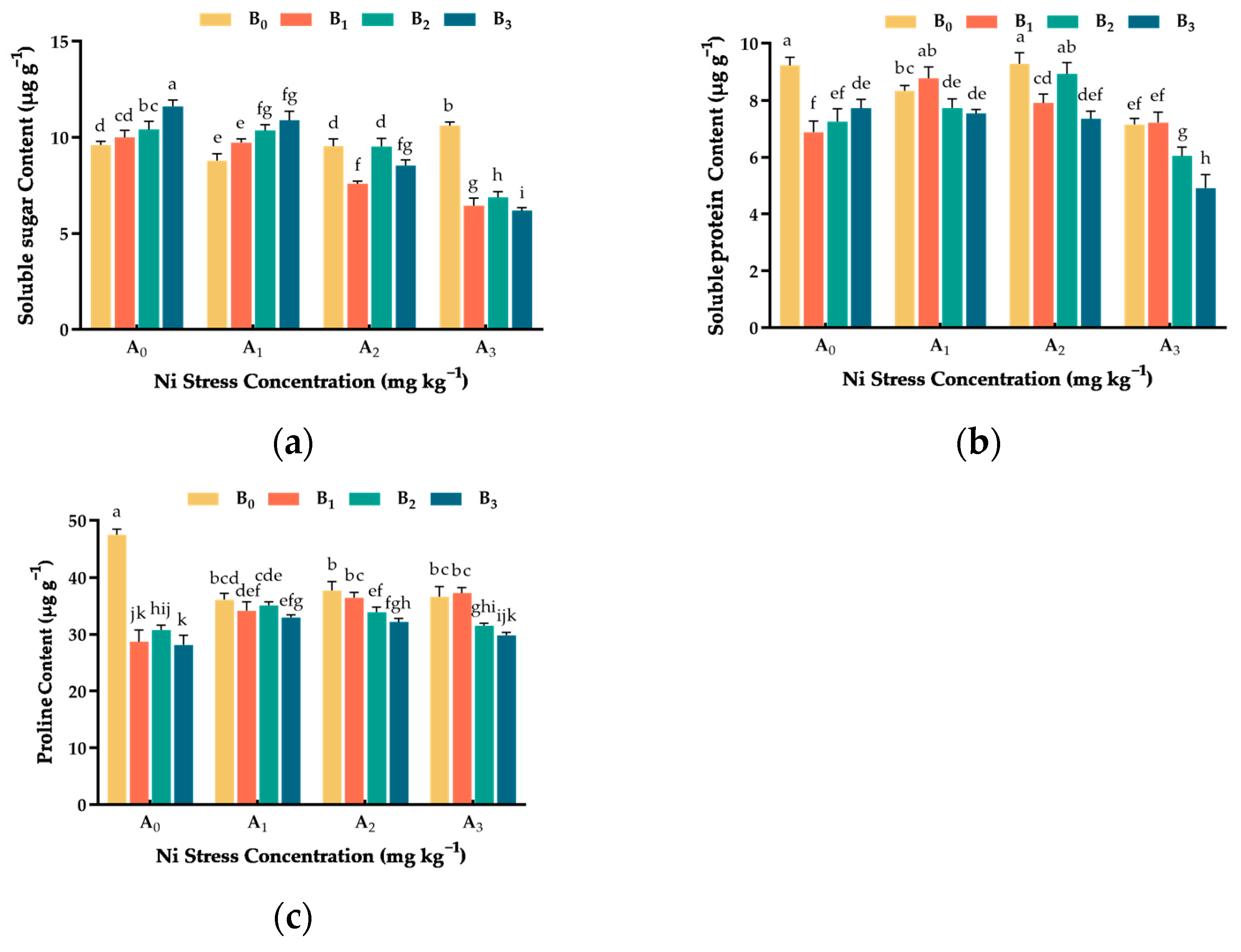
3.6. Effect of Ni and Cu Stresses on Osmoregulatory Substance Content
3.7. Effect of Ni and Cu Stresses on Cell Membrane Permeability
3.8. Ni and Cu Uptake and Transport in Different Treatments
4. Discussion
4.1. Ni and Cu Effect Morphology Traits of Seedings
4.2. Ni and Cu Effect Root Morphology Traits of Seedings
4.3. Ni and Cu Effect Biomass of Seedings
4.4. Ni and Cu Effect Photosynthetic Pigment Content of Seedings
4.5. Ni and Cu Effect Antioxidant Activity of Seedings
4.6. Ni and Cu Effect Osmoregulatory Substance Content of Seedings
4.7. Ni and Cu Effect Cell Membrane Permeability of Seedings
4.8. Ni and Cu Accumulation and Transport
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, S. Heavy metal pollution in China: Origin, pattern and control. Environ. Sci. Pollut. Res. 2003, 10, 192–198. [Google Scholar] [CrossRef]
- RoyChowdhury, A.; Datta, R.; Sarkar, D. Heavy metal pollution and remediation. In Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 359–373. [Google Scholar]
- Bradl, H. Sources and origins of heavy metals. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 6, pp. 1–27. [Google Scholar]
- Yang, J.; Sun, Y.; Wang, Z.; Gong, J.; Gao, J.; Tang, S.; Ma, S.; Duan, Z. Heavy metal pollution in agricultural soils of a typical volcanic area: Risk assessment and source appointment. Chemosphere 2022, 304, 135340. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512, 143–153. [Google Scholar] [CrossRef]
- Hutchinson, T.; Whitby, L. Heavy-metal pollution in the Sudbury mining and smelting region of Canada, I. Soil and vegetation contamination by nickel, copper, and other metals. Environ. Conserv. 1974, 1, 123–132. [Google Scholar] [CrossRef]
- Likuku, A.S.; Mmolawa, K.B.; Gaboutloeloe, G.K. Assessment of heavy metal enrichment and degree of contamination around the copper-nickel mine in the Selebi Phikwe Region, Eastern Botswana. Environ. Ecol. Res. 2013, 1, 32–40. [Google Scholar] [CrossRef]
- Kozlov, M.; Haukioja, E.; Bakhtiarov, A.; Stroganov, D.; Zimina, S. Root versus canopy uptake of heavy metals by birch in an industrially polluted area: Contrasting behaviour of nickel and copper. Environ. Pollut. 2000, 107, 413–420. [Google Scholar] [CrossRef]
- Martín, J.R.; De Arana, C.; Ramos-Miras, J.J.; Gil, C.; Boluda, R. Impact of 70 years urban growth associated with heavy metal pollution. Environ. Pollut. 2015, 196, 156–163. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Q.; Deng, M.; Japenga, J.; Li, T.; Yang, X.; He, Z. Heavy metal pollution and health risk assessment of agricultural soils in a typical peri-urban area in southeast China. J. Environ. Manag. 2018, 207, 159–168. [Google Scholar] [CrossRef]
- Rai, L.; Gaur, J.; Kumar, H.D. Phycology and heavy-metal pollution. Biol. Rev. 1981, 56, 99–151. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Aamer, M.; Nawaz, M.; Ali, A.; Khan, M.A.U.; Khan, T.A. Nickel toxicity in plants: Reasons, toxic effects, tolerance mechanisms, and remediation possibilities—A review. Environ. Sci. Pollut. Res. Int. 2019, 26, 12673–12688. [Google Scholar] [CrossRef]
- Barón, M.; Arellano, J.B.; Gorgé, J.L. Copper and photosystem II: A controversial relationship. Physiol. Plant. 1995, 94, 174–180. [Google Scholar] [CrossRef]
- Droppa, M.; Horváth, G. The role of copper in photosynthesis. Crit. Rev. Plant Sci. 1990, 9, 111–123. [Google Scholar] [CrossRef]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of lead and copper on photosynthetic apparatus in citrus (Citrus aurantium L.) plants. The role of antioxidants in oxidative damage as a response to heavy metal stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef]
- Ahmad, M.S.A.; Ashraf, M. Essential roles and hazardous effects of nickel in plants. Rev. Environ. Contam. Toxicol. 2011, 214, 125–167. [Google Scholar]
- Mir, A.R.; Pichtel, J.; Hayat, S. Copper: Uptake, toxicity and tolerance in plants and management of Cu-contaminated soil. BioMetals 2021, 34, 737–759. [Google Scholar] [CrossRef]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar heavy metal uptake, toxicity and detoxification in plants: A comparison of foliar and root metal uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef]
- Owens, L.D. Toxins in Plant Disease: Structure and Mode of Action: Toxins may cause disease symptoms by inhibiting enzymes or changing the permeability of membranes. Science 1969, 165, 18–25. [Google Scholar] [CrossRef]
- Lange, B.; van Der Ent, A.; Baker, A.J.M.; Echevarria, G.; Mahy, G.; Malaisse, F.; Meerts, P.; Pourret, O.; Verbruggen, N.; Faucon, M. Copper and cobalt accumulation in plants: A critical assessment of the current state of knowledge. New Phytol. 2017, 213, 537–551. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Li, L.; Yi, H. Effect of sulfur dioxide on ROS production, gene expression and antioxidant enzyme activity in Arabidopsis plants. Plant Physiol. Biochem. 2012, 58, 46–53. [Google Scholar] [CrossRef]
- Bian, S.; Jiang, Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci. Hortic. 2009, 120, 264–270. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Sun, J.; Nawaz, M.A.; Li, G.; Hasanuzzaman, M.; Liu, J. Heavy metal and metalloid toxicity in horticultural plants: Tolerance mechanism and remediation strategies. Chemosphere 2022, 303, 135196. [Google Scholar] [CrossRef]
- Aila; Saradhi, P.P. Proline accumulation under heavy metal stress. J. Plant Physiol. 1991, 138, 554–558. [Google Scholar] [CrossRef]
- Guo, T.R.; Zhang, G.P.; Zhang, Y.H. Physiological changes in barley plants under combined toxicity of aluminum, copper and cadmium. Colloids Surf. B Biointerfaces 2007, 57, 182–188. [Google Scholar] [CrossRef]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Shahzad, B.; Ashraf, U.; Fahad, S.; Hassan, W.; Jan, S.; Khan, I.; Saleem, M.F.; et al. Osmoregulation and antioxidant production in maize under combined cadmium and arsenic stress. Environ. Sci. Pollut. Res. 2016, 23, 11864–11875. [Google Scholar] [CrossRef]
- Du, B.; Haensch, R.; Alfarraj, S.; Rennenberg, H. Strategies of plants to overcome abiotic and biotic stresses. Biol. Rev. 2024. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Li, X. Technical solutions for the safe utilization of heavy metal-contaminated farmland in China: A critical review. Land Degrad. Dev. 2019, 30, 1773–1784. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Cost–benefit calculation of phytoremediation technology for heavy-metal-contaminated soil. Sci. Total Environ. 2016, 563, 796–802. [Google Scholar] [CrossRef]
- Kutrowska, A.; Małecka, A.; Piechalak, A.; Masiakowski, W.; Hanć, A.; Barałkiewicz, D.; Andrzejewska, B.; Zbierska, J.; Tomaszewska, B. Effects of binary metal combinations on zinc, copper, cadmium and lead uptake and distribution in Brassica juncea. J. Trace Elements Med. Biol. 2017, 44, 32–39. [Google Scholar] [CrossRef]
- Lanier, C.; Bernard, F.; Dumez, S.; Leclercq-Dransart, J.; Lemiere, S.; Vandenbulcke, F.; Nesslany, F.; Platel, A.; Devred, I.; Hayet, A.; et al. Combined toxic effects and DNA damage to two plant species exposed to binary metal mixtures (Cd/Pb). Ecotoxicol. Environ. Saf. 2019, 167, 278–287. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Carranza-Álvarez, C.; Alfaro-De la Torre, M.C.; Chávez-Guerrero, L.; García-De la Cruz, R.F. Removal and accumulation of cadmium and lead by Typha latifolia exposed to single and mixed metal solutions. Arch. Environ. Contam. Toxicol. 2009, 57, 688–696. [Google Scholar] [CrossRef]
- Arvola, L. Spectrophotometric determination of chlorophyll a and phaeopigments in ethanol extractions. Ann. Bot. Fenn. 1981, 18, 221–227. [Google Scholar]
- Chance, B.; Maehly, A. [136] Assay of catalases and peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Loewus, F.A. Improvement in anthrone method for determination of carbohydrates. Anal. Chem. 1952, 24, 219. [Google Scholar] [CrossRef]
- Neuhoff, V.; Arold, N.; Taube, D.; Ehrhardt, W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis 1988, 9, 255–262. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Vieira, R.D.; Scappa Neto, A.; Bittencourt, S.R.M.d.; Panobianco, M. Electrical conductivity of the seed soaking solution and soybean seedling emergence. Sci. Agricola 2004, 61, 164–168. [Google Scholar] [CrossRef]
- Millour, S.; Noel, L.; Kadar, A.; Chekri, R.; Vastel, C.; Guerin, T. Simultaneous analysis of 21 elements in foodstuffs by ICP-MS after closed-vessel microwave digestion: Method validation. J. Food Compos. Anal. 2011, 24, 111–120. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Die and let live: Leaf senescence contributes to plant survival under drought stress. Funct. Plant Biol. 2004, 31, 203–216. [Google Scholar] [CrossRef]
- Telewski, F.W. A unified hypothesis of mechanoperception in plants. Am. J. Bot. 2006, 93, 1466–1476. [Google Scholar] [CrossRef]
- Hossain, M.S.; Abdelrahman, M.; Tran, C.D.; Nguyen, K.H.; Chu, H.D.; Watanabe, Y.; Hasanuzzaman, M.; Mohsin, S.M.; Fujita, M.; Tran, L.-S.P. Insights into acetate-mediated copper homeostasis and antioxidant defense in lentil under excessive copper stress. Environ. Pollut. 2020, 258, 113544. [Google Scholar] [CrossRef]
- Reckova, S.; Tuma, J.; Dobrev, P.; Vankova, R. Influence of copper on hormone content and selected morphological, physiological and biochemical parameters of hydroponically grown Zea mays plants. Plant Growth Regul. 2019, 89, 191–201. [Google Scholar] [CrossRef]
- Sofo, A.; Khan, N.A.; D’Ippolito, I.; Reyes, F. Subtoxic levels of some heavy metals cause differential root-shoot structure, morphology and auxins levels in Arabidopsis thaliana. Plant Physiol. Biochem. 2022, 173, 68–75. [Google Scholar] [CrossRef]
- Benáková, M.; Ahmadi, H.; Dučaiová, Z.; Tylová, E.; Clemens, S.; Tůma, J. Effects of Cd and Zn on physiological and anatomical properties of hydroponically grown Brassica napus plants. Sci. Pollut. Res. 2017, 24, 20705–20716. [Google Scholar] [CrossRef]
- Nazir, F.; Hussain, A.; Fariduddin, Q. Interactive role of epibrassinolide and hydrogen peroxide in regulating stomatal physiology, root morphology, photosynthetic and growth traits in Solanum lycopersicum L. under nickel stress. Environ. Exp. Bot. 2019, 162, 479–495. [Google Scholar] [CrossRef]
- Cambrollé, J.; García, J.; Ocete, R.; Figueroa, M.; Cantos, M. Growth and photosynthetic responses to copper in wild grapevine. Chemosphere 2013, 93, 294–301. [Google Scholar] [CrossRef]
- Subba, P.; Mukhopadhyay, M.; Mahato, S.K.; Bhutia, K.D.; Mondal, T.K.; Ghosh, S.K. Zinc stress induces physiological, ultra-structural and biochemical changes in mandarin orange (Citrus reticulata Blanco) seedlings. Physiol. Mol. Biol. Plants 2014, 20, 461–473. [Google Scholar] [CrossRef]
- Rizvi, A.; Khan, M.S. Biotoxic impact of heavy metals on growth, oxidative stress and morphological changes in root structure of wheat (Triticum aestivum L.) and stress alleviation by Pseudomonas aeruginosa strain CPSB1. Chemosphere 2017, 185, 942–952. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Furini, A. An overview of heavy metal challenge in plants: From roots to shoots. Metallomics 2013, 5, 1117–1132. [Google Scholar] [CrossRef]
- AbdElgawad, H.; Zinta, G.; Hamed, B.A.; Selim, S.; Beemster, G.; Hozzein, W.N.; Wadaan, M.A.; Asard, H.; Abuelsoud, W. Maize roots and shoots show distinct profiles of oxidative stress and antioxidant defense under heavy metal toxicity. Environ. Pollut. 2020, 258, 113705. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Peco, J.; Higueras, P.; Campos, J.; Olmedilla, A.; Romero-Puertas, M.C.; Sandalio, L. Deciphering lead tolerance mechanisms in a population of the plant species Biscutella auriculata L. from a mining area: Accumulation strategies and antioxidant defenses. Chemosphere 2020, 261, 127721. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeant, K.; Hausman, J.-F.; Lutts, S.; Cai, G.; Guerriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Chen, J.; Shafi, M.; Li, S.; Wang, Y.; Wu, J.; Ye, Z.; Peng, D.; Yan, W.; Liu, D. Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens). Sci. Rep. 2015, 5, 13554. [Google Scholar] [CrossRef]
- Ghazaryan, K.; Movsesyan, H.; Ghazaryan, N.; Watts, B.A. Copper phytoremediation potential of wild plant species growing in the mine polluted areas of Armenia. Environ. Pollut. 2019, 249, 491–501. [Google Scholar] [CrossRef]
- Sebelik, V.; Kuznetsova, V.; Lokstein, H.; Polivka, T. Transient absorption of chlorophylls and carotenoids after two-photon excitation of LHCII. J. Phys. Chem. Lett. 2021, 12, 3176–3181. [Google Scholar] [CrossRef]
- Houri, T.; Khairallah, Y.; Al Zahab, A.; Osta, B.; Romanos, D.; Haddad, G. Heavy metals accumulation effects on the photosynthetic performance of geophytes in Mediterranean reserve. J. King Saud Univ. Sci. 2020, 32, 874–880. [Google Scholar] [CrossRef]
- Papenbrock, J.; Pfündel, E.; Mock, H.P.; Grimm, B. Decreased and increased expression of the subunit CHL I diminishes Mg chelatase activity and reduces chlorophyll synthesis in transgenic tobacco plants. Plant J. 2000, 22, 155–164. [Google Scholar] [CrossRef]
- Michael, P.I.; Krishnaswamy, M. The effect of zinc stress combined with high irradiance stress on membrane damage and antioxidative response in bean seedlings. Environ. Exp. Bot. 2011, 74, 171–177. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Varun, M.; Paul, M.S. Volume 3. Phytoremediation: Uptake and role of metal transporters in some members of Brassicaceae. In Phytoremediation; Springer: Cham, Switzerland, 2016; pp. 453–468. [Google Scholar]
- Sun, Y.; Zhou, Q.; Diao, C. Effects of cadmium and arsenic on growth and metal accumulation of Cd-hyperaccumulator Solanum nigrum L. Bioresour. Technol. 2008, 99, 1103–1110. [Google Scholar] [CrossRef]
- Zhao, H.; Guan, J.; Liang, Q.; Zhang, X.; Hu, H.; Zhang, J. Effects of cadmium stress on growth and physiological characteristics of sassafras seedlings. Sci. Rep. 2021, 11, 9913. [Google Scholar] [CrossRef]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Din, M.; Ullah, A.; Sabagh, A.E.; Hossain, A.; Llanes, A.; Liu, L. Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ. Sci. Pollut. Res. 2020, 27, 5211–5221. [Google Scholar] [CrossRef]
- Hussain, I.; Afzal, S.; Ashraf, M.A.; Rasheed, R.; Saleem, M.H.; Alatawi, A.; Ameen, F.; Fahad, S. Effect of metals or trace elements on wheat growth and its remediation in contaminated soil. J. Plant Growth Regul. 2023, 42, 2258–2282. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.-G. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 2010, 18, 309–319. [Google Scholar] [CrossRef]
- Saud, S.; Wang, L. Mechanism of cotton resistance to abiotic stress, and recent research advances in the osmoregulation related genes. Front. Plant Sci. 2022, 13, 972635. [Google Scholar] [CrossRef]
- Al Mahmud, J.; Hasanuzzaman, M.; Nahar, K.; Bhuyan, M.B.; Fujita, M. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: Coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol. Environ. Saf. 2018, 147, 990–1001. [Google Scholar] [CrossRef]
- Deng, F.; Yamaji, N.; Xia, J.; Ma, J.F. A member of the heavy metal P-type ATPase OsHMA5 is involved in xylem loading of copper in rice. Plant Physiol. 2013, 163, 1353–1362. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.; Chen, L. Growth and physiological responses of Pennisetum sp. to cadmium stress under three different soils. Environ. Sci. Pollut. Res. 2021, 28, 14867–14881. [Google Scholar] [CrossRef]
- Haque, A.M.; Tasnim, J.; El-Shehawi, A.M.; Rahman, M.A.; Parvez, M.S.; Ahmed, M.B.; Kabir, A.H. The Cd-induced morphological and photosynthetic disruption is related to the reduced Fe status and increased oxidative injuries in sugar beet. Plant Physiol Biochem. 2021, 166, 448–458. [Google Scholar] [CrossRef]
- Shahzad, K.; Ali, A.; Ghani, A.; Nadeem, M.; Khalid, T.; Nawaz, S.; Jamil, M.; Anwar, T. Exogenous application of proline and glycine betaine mitigates nickel toxicity in mung bean plants by up-regulating growth, physiological and yield attributes. Pak. J. Bot. 2023, 55, 27–32. [Google Scholar] [CrossRef]
- Georgiadou, E.C.; Kowalska, E.; Patla, K.; Kulbat, K.; Smolińska, B.; Leszczyńska, J.; Fotopoulos, V.J.F.i.P.S. Influence of heavy metals (Ni, Cu, and Zn) on nitro-oxidative stress responses, proteome regulation and allergen production in basil (Ocimum basilicum L.) plants. Front. Plant Sci. 2018, 9, 374129. [Google Scholar] [CrossRef]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D.; et al. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar] [CrossRef]
- Spiteller, G. The relationship between changes in the cell wall, lipid peroxidation, proliferation, senescence and cell death. Physiol. Plant. 2003, 119, 5–18. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.-J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef]
- Gajewska, E.; SkŁodowska, M. Differential effect of equal copper, cadmium and nickel concentration on biochemical reactions in wheat seedlings. Ecotoxicol. Environ. Saf. 2010, 73, 996–1003. [Google Scholar] [CrossRef]
- Seregin, I.; Kozhevnikova, A.D. Physiological role of nickel and its toxic effects on higher plants. Russ. J. Plant Physiol. 2006, 53, 257–277. [Google Scholar] [CrossRef]
- Dresler, S.; Hanaka, A.; Bednarek, W.; Maksymiec, W. Accumulation of low-molecular-weight organic acids in roots and leaf segments of Zea mays plants treated with cadmium and copper. Acta Physiol. Plant. 2014, 36, 1565–1575. [Google Scholar] [CrossRef]
- Song, J.; Zhao, F.-J.; Luo, Y.-M.; McGrath, S.P.; Zhang, H. Copper uptake by Elsholtzia splendens and Silene vulgaris and assessment of copper phytoavailability in contaminated soils. Environ. Pollut. 2004, 128, 307–315. [Google Scholar] [CrossRef]



| CuSO4·5H2O Treatment | 0 mg·kg−1 | 300 mg·kg−1 | 600 mg·kg−1 | 900 mg·kg−1 | |
|---|---|---|---|---|---|
| NiCl2·6H2O Treatment | |||||
| 0 mg·kg−1 | A0B0 | B1 | B2 | B3 | |
| 100 mg·kg−1 | A1 | A1B1 | A1B2 | A1B3 | |
| 300 mg·kg−1 | A2 | A2B1 | A2B2 | A2B3 | |
| 500 mg·kg−1 | A3 | A3B1 | A3B2 | A3B3 | |
| Treatment | Plant Height (cm) | Stem Diameter (cm) | Leaves Length (cm) | Leaves Width (cm) |
|---|---|---|---|---|
| A0B0 | 51.25 ± 0.77 g | 0.49 ± 0.02 ab | 7.87 ± 0.19 d | 4.13 ± 0.02 abcde |
| A1 | 52.67 ± 1.39 ef | 0.49 ± 0.04 ab | 8.17 ± 0.06 c | 4.59 ± 0.05 a |
| A2 | 51.90 ± 0.36 fg | 0.47 ± 0.03 ab | 7.65 ± 0.02 e | 4.21 ± 0.04 abcde |
| A3 | 48.86 ± 0.79 i | 0.44 ± 0.10 b | 7.25 ± 0.08 f | 4.07 ± 0.11 abcde |
| B1 | 53.08 ± 0.81 ef | 0.50 ± 0.04 ab | 7.90 ± 0.04 d | 4.19 ± 0.03 abcde |
| B2 | 53.32 ± 0.63 de | 0.51 ± 0.05 ab | 7.65 ± 0.07 e | 4.09 ± 0.08 abcde |
| B3 | 51.07 ± 0.65 g | 0.48 ± 0.01 ab | 7.33 ± 0.12 f | 3.82 ± 0.19 e |
| A1B1 | 55.31 ± 1.24 bc | 0.51 ± 0.12 ab | 8.36 ± 0.10 ab | 4.39 ± 0.74 abcd |
| A1B2 | 56.09 ± 0.81 b | 0.51 ± 0.08 ab | 8.38 ± 0.05 ab | 4.41 ± 0.11 abc |
| A1B3 | 59.00 ± 0.36 a | 0.56 ± 0.03 a | 8.51 ± 0.07 a | 4.56 ± 0.13 ab |
| A2B1 | 55.60 ± 0.39 bc | 0.49 ± 0.06 ab | 8.23 ± 0.20 bc | 4.32 ± 0.03 abcde |
| A2B2 | 54.47 ± 0.33 cd | 0.49 ± 0.01 ab | 8.16 ± 0.15 c | 4.16 ± 0.05 abcde |
| A2B3 | 51.32 ± 0.51 g | 0.49 ± 0.05 ab | 7.94 ± 0.08 d | 4.04 ± 0.09 bcde |
| A3B1 | 50.54 ± 0.53 gh | 0.48 ± 0.02 ab | 7.92 ± 0.14 d | 3.93 ± 0.71 cde |
| A3B2 | 49.41 ± 1.12 hi | 0.47 ± 0.08 ab | 7.89 ± 0.07 d | 3.98 ± 0.04 cde |
| A3B3 | 48.81 ± 0.29 i | 0.46 ± 0.02 ab | 7.67 ± 0.03 e | 3.88 ± 0.01 de |
| Treatment | Root Length (cm) | Root Surface Area (cm2) | Root Volume (cm3) |
|---|---|---|---|
| A0B0 | 301.84 ± 6.24 c | 55.06 ± 0.47 g | 0.80 ± 0.01 abcd |
| A1 | 339.58 ± 15.18 b | 65.66 ± 0.28 c | 1.01 ± 0.10 ab |
| A2 | 273.75 ± 5.71 de | 54.84 ± 0.95 g | 0.90 ± 0.25 abcd |
| A3 | 265.79 ± 12.30 def | 49.57 ± 0.04 i | 0.70 ± 0.21 d |
| B1 | 315.04 ± 4.84 c | 61.45 ± 1.06 e | 0.97 ± 0.23 abc |
| B2 | 299.37 ± 11.97 c | 56.00 ± 0.50 fg | 0.73 ± 0.19 cd |
| B3 | 232.15 ± 13.63 hi | 47.50 ± 0.08 j | 0.68 ± 0.05 d |
| A1B1 | 365.39 ± 8.23 a | 69.06 ± 0.11 b | 1.04 ± 0.10 a |
| A1B2 | 356.31 ± 10.34 ab | 70.58 ± 0.54 a | 1.04 ± 0.15 a |
| A1B3 | 263.73 ± 1.48 ef | 68.30 ± 1.78 b | 1.03 ± 0.03 ab |
| A2B1 | 345.45 ± 17.78 b | 64.26 ± 1.42 d | 1.00 ± 0.05 ab |
| A2B2 | 282.41 ± 9.52 d | 56.89 ± 0.38 f | 0.97 ± 0.15 abc |
| A2B3 | 250.66 ± 3.14 fg | 55.93 ± 0.61 fg | 0.97 ± 0.04 abc |
| A3B1 | 237.97 ± 10.02 gh | 51.68 ± 0.44 h | 0.92 ± 0.17 abcd |
| A3B2 | 220.44 ± 2.85 i | 44.54 ± 0.23 k | 0.76 ± 0.09 bcd |
| A3B3 | 184.64 ± 6.60 j | 39.61 ± 1.22 l | 0.66 ± 0.12 d |
| Treatment | Ni2+ Content in Roots (mg kg−1) | Ni2+ Content in Stems (mg kg−1) | Ni2+ Content in Leaves (mg kg−1) | BCF | Translocation Factor |
|---|---|---|---|---|---|
| A0B0 | 7.23 ± 0.33 g | 1.85 ± 0.35 ef | 3.66 ± 0.61 h | 0.38 ± 0.03 d | 0.77 ± 0.16 e |
| A1 | 8.55 ± 0.19 f | 2.69 ± 0.35 bcd | 7.94 ± 0.91 f | 0.44 ± 0.01 c | 1.24 ± 0.04 b |
| A2 | 11.94 ± 0.54 cd | 3.94 ± 0.12 a | 14.76 ± 0.76 b | 0.51 ± 0.01 b | 1.57 ± 0.13 a |
| A3 | 23.42 ± 0.60 a | 2.85 ± 0.17 bc | 16.86 ± 0.87 a | 0.56 ± 0.00 a | 0.84 ± 0.05 de |
| A1B1 | 10.56 ± 0.57 e | 1.39 ± 0.42 f | 4.36 ± 0.36 gh | 0.39 ± 0.03 d | 0.55 ± 0.04 f |
| A1B2 | 13.16 ± 0.70 b | 1.52 ± 0.45 f | 4.44 ± 0.25 gh | 0.47 ± 0.01 bc | 0.45 ± 0.01 f |
| A1B3 | 12.79 ± 0.20 b | 1.53 ± 0.41 f | 4.78 ± 0.70 g | 0.48 ± 0.02 bc | 0.49 ± 0.03 f |
| A2B1 | 11.38 ± 0.44 d | 2.15 ± 0.25 de | 7.81 ± 0.34 f | 0.44 ± 0.02 c | 0.88 ± 0.03 de |
| A2B2 | 12.94 ± 0.2 b | 2.66 ± 0.23 bcd | 9.48 ± 0.47 cd | 0.57 ± 0.02 a | 0.94 ± 0.07 cd |
| A2B3 | 12.34 ± 0.35 bc | 2.15 ± 0.19 de | 8.54 ± 0.49 def | 0.50 ± 0.03 b | 0.86 ± 0.03 de |
| A3B1 | 12.36 ± 0.44 bc | 2.45 ± 0.40 cd | 8.28 ± 0.24 ef | 0.38 ± 0.02 d | 0.87 ± 0.02 de |
| A3B2 | 12.83 ± 0.11 b | 3.79 ± 0.25 a | 9.20 ± 0.32 cde | 0.45 ± 0.02 c | 1.01 ± 0.05 c |
| A3B3 | 12.54 ± 0.57 bc | 3.04 ± 0.30 b | 9.89 ± 0.22 c | 0.44 ± 0.02 c | 1.03 ± 0.04 c |
| Treatment | Cu2+ Content in Roots (mg kg−1) | Cu 2+ Content in Stems (mg kg−1) | Cu 2+ Content in Leaves (mg kg−1) | BCF | Translocation Factor |
|---|---|---|---|---|---|
| A0B0 | 7.93 ± 0.33 j | 3.85 ± 0.35 g | 5.96 ± 0.52 f | 0.55 ±0.01 h | 1.24 ± 0.05 d |
| B1 | 15.54 ± 0.80 h | 6.26 ± 1.79 def | 13.43 ± 0.91 de | 0.58 ± 0.03 gh | 1.27 ± 0.22 d |
| B2 | 19.89 ± 0.89 g | 9.52 ± 1.02 ab | 26.34 ± 1.62 b | 0.65 ± 0.01 f | 1.81 ± 0.12 b |
| B3 | 23.87 ± 1.93 f | 5.88 ± 1.86 ef | 49.36 ± 0.62 a | 0.72 ± 0.02 e | 2.32 ± 0.14 a |
| A1B1 | 14.09 ± 0.21 hi | 5.99 ± 0.97 ef | 14.43 ± 0.58 d | 0.63 ± 0.04 fg | 1.45 ± 0.09 c |
| A1B2 | 24.16 ± 0.82 ef | 5.40 ± 0.49 fg | 12.26 ± 0.30 e | 0.77 ± 0.01 de | 0.73 ± 0.06 f |
| A1B3 | 34.74 ± 0.38 a | 7.61 ± 0.25 cde | 12.27 ± 0.44 e | 0.84 ± 0.02 c | 0.57 ± 0.01 g |
| A2B1 | 12.65 ± 0.58 i | 7.80 ± 1.16 bcd | 16.96 ± 0.85 c | 0.72 ± 0.06 e | 1.95 ± 0.07 b |
| A2B2 | 26.23 ± 0.41 cd | 8.04 ± 0.25 bc | 17.60 ± 0.54 c | 1.15 ± 0.04 b | 0.98 ± 0.02 ef |
| A2B3 | 30.37 ± 1.22 b | 7.91 ± 0.77 bcd | 17.55 ± 0.45 c | 0.89 ± 0.04 c | 0.84 ± 0.04 fg |
| A3B1 | 14.06 ± 0.23 hi | 8.40 ± 0.35 bc | 18.20 ± 1.03 c | 0.83 ± 0.01 cd | 1.89 ± 0.07 b |
| A3B2 | 26.99 ± 0.84 c | 10.55 ± 0.61 a | 18.32 ± 1.27 c | 1.36 ± 0.09 a | 1.07 ± 0.04 e |
| A3B3 | 25.47 ± 0.67 de | 7.61 ± 0.37 cde | 17.23 ± 0.25 c | 0.73 ± 0.01 e | 0.98 ± 0.05 ef |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; An, Y.; Qu, T.; Jin, T.; Zhao, L.; Guo, H.; Wang, W.; Zhao, C. Effects of Ni and Cu Stresses on Morphological and Physiological Characteristics of Euphorbia marginata Pursh Seedlings. Agronomy 2024, 14, 1223. https://doi.org/10.3390/agronomy14061223
Zhou X, An Y, Qu T, Jin T, Zhao L, Guo H, Wang W, Zhao C. Effects of Ni and Cu Stresses on Morphological and Physiological Characteristics of Euphorbia marginata Pursh Seedlings. Agronomy. 2024; 14(6):1223. https://doi.org/10.3390/agronomy14061223
Chicago/Turabian StyleZhou, Xudan, Yue An, Tongbao Qu, Tian Jin, Lei Zhao, Hongliang Guo, Wei Wang, and Chunli Zhao. 2024. "Effects of Ni and Cu Stresses on Morphological and Physiological Characteristics of Euphorbia marginata Pursh Seedlings" Agronomy 14, no. 6: 1223. https://doi.org/10.3390/agronomy14061223
APA StyleZhou, X., An, Y., Qu, T., Jin, T., Zhao, L., Guo, H., Wang, W., & Zhao, C. (2024). Effects of Ni and Cu Stresses on Morphological and Physiological Characteristics of Euphorbia marginata Pursh Seedlings. Agronomy, 14(6), 1223. https://doi.org/10.3390/agronomy14061223






