Purpureocillium lilacinum as an Agent of Nematode Control and Plant Growth-Promoting Fungi
Abstract
1. Introduction
2. Nematode Control
3. Plant Growth
4. Control of Other Pathogens
5. Conclusions
6. Futures and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, C.J.; Wang, L.L.; Li, Q.; Shang, Q.M. Bacterial communities in the rhizosphere, phyllosphere and endosphere of tomato plants. PLoS ONE 2019, 14, e0223847. [Google Scholar] [CrossRef] [PubMed]
- Pathania, P.; Bhatia, R.; Khatri, M. Cross-competence and affectivity of maize rhizosphere bacteria Bacillus sp. MT7 in tomato rhizosphere. Sci. Hortic. 2020, 272, 109480. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Manici, L.M.; Kelderer, M.; Caputo, F.; Mazzola, M. Auxin-mediated relationships between apple plants and root inhabiting fungi: Impact on root pathogens and potentialities of growth-promoting populations. Plant Pathol. 2015, 64, 843–851. [Google Scholar] [CrossRef]
- Attia, M.S.; Abdelaziz, A.M.; Al-Askar, A.A.; Arishi, A.A.; Abdelhakim, A.M.; Hashem, A.H. Plant growth-promoting fungi as biocontrol tool against Fusarium wilt disease of tomato plant. J. Fungi 2022, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Turbat, A.; Rakk, D.; Vigneshwari, A.; Kocsubé, S.; Thu, H.; Szepesi, Á.; Bakacsy, L.; Škrbić, B.D.; Jigjiddorj, E.A.; Vágvölgyi, C.; et al. Characterization of the plant growth-promoting activities of endophytic fungi isolated from Sophora flavescens. Microorganisms 2020, 8, 683. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; Del Rio, T.G.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.K.; McInroy, J.A.; Kloepper, J.W. The interactions of rhizodeposits with plant growth-promoting rhizobacteria in the rhizosphere: A Review. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Shamseldin, A. Future outlook of transferring biological nitrogen fixation (BNF) to cereals and challenges to retard achieving this dream. Curr. Microbiol. 2022, 79, 171. [Google Scholar] [CrossRef]
- Jensen, E.S.; Carlsson, G.; Hauggaard-Nielsen, H. Intercropping of grain legumes and cereals improves the use of soil and resources and reduces the requirement for synthetic fertilizer n: A global-scale analysis. Agron. Sustain. Dev. 2020, 40, 5. [Google Scholar] [CrossRef]
- Krey, T.; Vassilev, N.; Baum, C.; Eichler-Löbermann, B. Effects of long-term phosphorus application and plant-growth promoting rhizobacteria on maize phosphorus nutrition under field conditions. Eur. J. Soil Biol. 2013, 55, 124–130. [Google Scholar] [CrossRef]
- Kijpornyongpan, T.; Aime, M.C. Comparative transcriptomics reveal different mechanisms for hyphal growth across four plant-associated dimorphic fungi. Fungal Genet. Biol. 2021, 152, 103565. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hua, J.; Li, H.; Zhang, J.; Luo, S. Inoculation with carbofuran-degrading rhizobacteria promotes maize growth through production of IAA and regulation of the release of plant-specialized metabolites. Chemosphere 2022, 307, 136027. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Engelberth, J.; Alborn, H.T.; O’Donnell, P.; Sammons, M.; Toshima, H.; Tumlinson, J.H. Simultaneous analysis of phytohormones, phytotoxins, and volatile organic compounds in plants. Proc. Natl. Acad. Sci. USA 2003, 100, 10552–10557. [Google Scholar] [CrossRef] [PubMed]
- Costacurta, A.; Vanderleyden, J. Synthesis of phytohormones by plant-associated bacteria. Crit. Rev. Microbiol. 1995, 21, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liang, J.; Björn, L.O.; Li, J.; Shu, W.; Wang, Y. Phosphorus-arsenic interaction in the ‘soil-plant-microbe’ system and its influence on arsenic pollution. Sci. Total Environ. 2022, 802, 149796. [Google Scholar] [CrossRef]
- Kour, D.; Rana, K.L.; Kaur, T.; Yadav, N.; Yadav, A.N.; Kumar, M.; Kumar, V.; Dhaliwal, H.S.; Saxena, A.K. Biodiversity, current developments and potential biotechnological applications of phosphorus-solubilizing and -mobilizing microbes: A Review. Pedosphere 2021, 31, 43–75. [Google Scholar] [CrossRef]
- Barbosa, R.B.G.; Borges, A.C.; de Araújo, H.H.; Vergütz, L.; Rosa, A.P. Effects of the addition of phytohormone and plant growth-promoting bacteria on the health and development of polygonum hydropiperoides cultivated in constructed wetlands treating chromium-contaminated wastewater. Ecol. Eng. 2023, 190, 106931. [Google Scholar] [CrossRef]
- Mahmud, K.; Missaoui, A.; Lee, K.; Ghimire, B.; Presley, H.W.; Makaju, S. Rhizosphere microbiome manipulation for sustainable crop production. Curr. Plant Biol. 2021, 27, 100210. [Google Scholar] [CrossRef]
- Raghavendra, M.P.; Nayaka, S.C.; Nuthan, B.R. Role of rhizosphere microflora in potassium solubilization. In Potassium solubilizing Microorganisms for Sustainable Agriculture; Springer Nature: Berlin/Heidelberg, Germany, 2016; pp. 43–59. [Google Scholar] [CrossRef]
- Díaz-Urbano, M.; Goicoechea, N.; Velasco, P.; Poveda, J. Development of agricultural bio-inoculants based on mycorrhizal fungi and endophytic filamentous fungi: Co-inoculants for improve plant-physiological responses in sustainable agriculture. Biol. Control 2023, 182, 105223. [Google Scholar] [CrossRef]
- Giri, B.; Mukerji, K.G. Mycorrhizal inoculant alleviates salt stress in Sesbania aegyptiaca and Sesbania grandiflora under field conditions: Evidence for reduced sodium and improved magnesium uptake. Mycorrhiza 2004, 14, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Efthymiou, A.; Jensen, B.; Jakobsen, I. The roles of mycorrhiza and Penicillium inoculants in phosphorus uptake by biochar-amended wheat. Soil Biol. Biochem. 2018, 127, 168–177. [Google Scholar] [CrossRef]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-mediated stress resistance in plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.; Koskella, B. Nutrient- and dose-dependent microbiome-mediated protection against a plant pathogen. Curr. Biol. 2018, 28, 2487–2492.e3. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.H.; Baki, M.Z.I.; Ahmed, B.; Mostofa, M.G. Current, faltering, and future strategies for advancing microbiome-assisted sustainable agriculture and environmental resilience. New Crop. 2024, 1, 100013. [Google Scholar] [CrossRef]
- Chadha, N.; Mishra, M.; Rajpal, K.; Bajaj, R.; Choudhary, D.K.; Varma, A. An ecological role of fungal endophytes to ameliorate plants under biotic stress. Arch. Microbiol. 2015, 197, 869–881. [Google Scholar] [CrossRef]
- Anjum, R.; Afzal, M.; Baber, R.; Khan, M.A.J.; Kanwal, W.; Sajid, W.; Raheel, A. Endophytes: As potential biocontrol agent—Review and future prospects. J. Agric. Sci. 2019, 11, 113. [Google Scholar] [CrossRef]
- Baron, N.C.; de Souza Pollo, A.; Rigobelo, E.C. Purpureocillium lilacinum and Metarhizium marquandii as plant growth-promoting fungi. PeerJ 2020, 8, e9005. [Google Scholar] [CrossRef] [PubMed]
- Luangsa-Ard, J.; Houbraken, J.; van Doorn, T.; Hong, S.B.; Borman, A.M.; Hywel-Jones, N.L.; Samson, R.A. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol. Lett. 2011, 321, 141–149. [Google Scholar] [CrossRef]
- Tariq, M.; Khan, A.; Asif, M.; Khan, F.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Biological control: A sustainable and practical approach for plant disease management. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2020, 70, 507–524. [Google Scholar] [CrossRef]
- Alves, G.S.; Bertini, S.C.B.; Barbosa, B.B.; Pimentel, J.P.; Ribeiro Junior, V.A.; de Oliveira Mendes, G.; Azevedo, L.C.B. Fungal endophytes inoculation improves soil nutrient availability, arbuscular mycorrhizal colonization and common bean growth. Rhizosphere 2021, 18, 100330. [Google Scholar] [CrossRef]
- Lan, X.; Zhang, J.; Zong, Z.; Ma, Q.; Wang, Y. Evaluation of the biocontrol potential of Purpureocillium lilacinum qlp12 against Verticillium dahliae in eggplant. BioMed Res. Int. 2017, 2017, 4101357. [Google Scholar] [CrossRef] [PubMed]
- Girardi, N.S.; Sosa, A.L.; Etcheverry, M.G.; Passone, M.A. In vitro characterization bioassays of the nematophagous fungus Purpureocillium lilacinum: Evaluation on growth, extracellular enzymes, mycotoxins and survival in the surrounding agroecosystem of tomato. Fungal Biol. 2022, 126, 300–307. [Google Scholar] [CrossRef]
- Sharon, E.; Chet, I.; Viterbo, A.; Bar-Eyal, M.; Nagan, H.; Samuels, G.J.; Spiegel, Y. Parasitism of Trichoderma on Meloidogyne javanica and role of the gelatinous matrix. Eur. J. Plant Pathol. 2007, 118, 247–258. [Google Scholar] [CrossRef]
- Khan, M.; Tanaka, K. Purpureocillium lilacinum for plant growth promotion and biocontrol against root-knot nematodes infecting eggplant. PLoS ONE 2023, 18, e0283550. [Google Scholar] [CrossRef] [PubMed]
- Kepenekci, I.; Hazir, S.; Oksal, E.; Lewis, E.E. Application methods of Steinernema feltiae, Xenorhabdus bovienii and Purpureocillium lilacinum to control root-knot nematodes in greenhouse tomato systems. Crop Prot. 2018, 108, 31–38. [Google Scholar] [CrossRef]
- Lopez, D.C.; Sword, G.A. The endophytic fungal entomopathogens Beauveria bassiana and Purpureocillium lilacinum enhance the growth of cultivated cotton (Gossypium hirsutum) and negatively affect survival of the cotton bollworm (Helicoverpa zea). Biol. Control 2015, 89, 53–60. [Google Scholar] [CrossRef]
- Kiriga, A.W.; Haukeland, S.; Kariuki, G.M.; Coyne, D.L.; Beek, N.V. Effect of Trichoderma spp. and Purpureocillium lilacinum on Meloidogyne javanica in commercial pineapple production in Kenya. Biol. Control 2018, 119, 27–32. [Google Scholar] [CrossRef]
- Zhang, J.; Song, J.; Wei, J.; Qi, S.; Li, J.; Jin, Y.; Luan, X.; Li, P.; Yan, J. Effect of Purpureocillium lilacinum on inter-root soil microbial community and metabolism of tobacco. Ann. Microbiol. 2023, 73, 30. [Google Scholar] [CrossRef]
- Topalović, O.; Hussain, M.; Heuer, H. Plants and associated soil microbiota cooperatively suppress plant-parasitic nematodes. Front. Microbiol. 2020, 11, 491484. [Google Scholar] [CrossRef]
- Silva, J.C.P.; Nunes, T.C.S.; Guimarães, R.A.; Pylro, V.S.; Costa, L.S.A.S.; Zaia, R.; Campos, V.P.; Medeiros, F.H.V. Organic practices intensify the microbiome assembly and suppress root-knot nematodes. J. Pest Sci. 2022, 95, 709–721. [Google Scholar] [CrossRef]
- Wille, C.N.; Gomes, C.B.; Minotto, E.; Nascimento, J.S. Potential of aqueous extracts of basidiomycetes to control root-knot nematodes on lettuce. Hortic. Bras. 2019, 37, 54–59. [Google Scholar] [CrossRef]
- Abiola, O.A.; Abiola, O.A. Vegetable production and nematodes infestation: Impacts on small-scale farming communities of South Africa. World J. Adv. Res. Rev. 2020, 7, 168–177. [Google Scholar] [CrossRef]
- Nagachandrabose, S. Management of potato cyst nematodes using liquid bioformulations of Pseudomonas fluorescens, Purpureocillium lilacinum and Trichoderma viride. Potato Res. 2020, 63, 479–496. [Google Scholar] [CrossRef]
- Van Damme, V.; Hoedekie, A.; Nemat, N.V. undefined long-term efficacy of Pochonia chlamydosporia for management of Meloidogyne javanica in glasshouse crops. Nematology 2005, 7, 727–736. [Google Scholar]
- Hu, W.; Samac, D.A.; Liu, X.; Chen, S. Microbial communities in the cysts of soybean cyst nematode affected by tillage and biocide in a suppressive soil. Appl. Soil Ecol. 2017, 119, 396–406. [Google Scholar] [CrossRef]
- Chen, W.; Hu, Q. Secondary Metabolites of Purpureocillium lilacinum. Molecules 2022, 27, 18. [Google Scholar] [CrossRef]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Front. Microbiol. 2020, 11, 530260. [Google Scholar] [CrossRef]
- Baron, N.C.; Rigobelo, E.C.; Zied, D.C. Filamentous fungi in biological control: Current status and future perspectives. Chil. J. Agric. Res. 2019, 79, 307–315. [Google Scholar] [CrossRef]
- Shah, P.A.; Pell, J.K. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Ayilara, M.S.; Akinola, S.A.; Babalola, O.O. Biocontrol mechanisms of endophytic fungi. Egypt. J. Biol. Pest Control 2022, 32, 46. [Google Scholar] [CrossRef]
- Latz, M.A.C.; Jensen, B.; Collinge, D.B.; Jørgensen, H.J.L. Endophytic fungi as biocontrol agents: Elucidating mechanisms in disease suppression. Plant Ecol. Divers. 2018, 11, 555–567. [Google Scholar] [CrossRef]
- Giné, A.; Sorribas, F.J. Effect of Plant Resistance and BioAct WG (Purpureocillium lilacinum Strain 251) on Meloidogyne incognita in a tomato–cucumber rotation in a greenhouse. Pest Manag. Sci. 2017, 73, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.R.; Ownley, B.H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control 2018, 116, 36–45. [Google Scholar] [CrossRef]
- Johnston-Fennell, L.; Tooker, J.; Nault, B.A.; Wickings, K. Preventative pest management in field crops influences the biological control potential of epigeal arthropods and soil-borne entomopathogenic fungi. Field Crop. Res. 2021, 272, 108265. [Google Scholar] [CrossRef]
- Dahlin, P.; Eder, R.; Consoli, E.; Krauss, J.; Kiewnick, S. Integrated control of Meloidogyne incognita in tomatoes using fluopyram and Purpureocillium lilacinum strain 251. Crop Prot. 2019, 124, 104874. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Sabir, N.; El-Sheikh, M.A.; Alyemeni, M. Impact assessment of Karanja deoiled cake and sundried biogas slurry as a mixed substrate on the nematicidal potential of Purpureocillium lilacinum. J. King Saud Univ. Sci. 2021, 33, 101399. [Google Scholar] [CrossRef]
- de Paula, L.L.; Campos, V.P.; Terra, W.C.; de Brum, D.; Jacobs, D.C.; Xuan Bui, H.; Desaeger, J. The combination of Bacillus amyloliquefaciens and Purpureocillium lilacinum in the control of Meloidogyne enterolobii. Biol. Control 2024, 189, 105438. [Google Scholar] [CrossRef]
- Hu, J.; Hou, S.; Li, M.; Wang, J.; Wu, F.; Lin, X. The better suppression of pepper phytophthora blight by arbuscular mycorrhizal (am) fungus than Purpureocillium lilacinum alone or combined with AM fungus. J. Soils Sediments 2020, 20, 793–800. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, H.; Zhou, W.; Yuan, Z.; Hao, J.; Liu, X.; Han, L. Effects of indole derivatives from Purpureocillium lilacinum in controlling tobacco mosaic virus. Pestic. Biochem. Physiol. 2022, 183, 105077. [Google Scholar] [CrossRef]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Gutiérrez Martínez, P.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Zhang, H.; Serwah Boateng, N.A.; Ngolong Ngea, G.L.; Shi, Y.; Lin, H.; Yang, Q.; Wang, K.; Zhang, X.; Zhao, L.; Droby, S. Unravelling the fruit microbiome: The key for developing effective biological control strategies for postharvest diseases. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4906–4930. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, X. Effect of harpin on control of postharvest decay and resistant responses of tomato fruit. Postharvest Biol. Technol. 2016, 112, 241–246. [Google Scholar] [CrossRef]
- Erasmus, A.; Lennox, C.L.; Korsten, L.; Lesar, K.; Fourie, P.H. Imazalil resistance in Penicillium digitatum and P. italicum causing citrus postharvest green and blue mould: Impact and options. Postharvest Biol. Technol. 2015, 107, 66–76. [Google Scholar] [CrossRef]
- Ray, R.C.; Ravi, V. post harvest spoilage of sweetpotato in tropics and control measures. Crit. Rev. Food Sci. Nutr. 2005, 45, 623–644. [Google Scholar] [CrossRef] [PubMed]
- Talibi, I.; Boubaker, H.; Boudyach, E.H.; Ait Ben Aoumar, A. Alternative methods for the control of postharvest citrus diseases. J. Appl. Microbiol. 2014, 117, 1–17. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Taher, M.A.; Abd El-Aziz, M.H.; Mohamed, S.Y. Action mechanisms and biocontrol of Purpureocillium lilacinum against green mould caused by Penicillium digitatum in orange fruit. J. Appl. Microbiol. 2021, 131, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Salazar, R.; Sánchez-García, I.; Chan-Cupul, W.; Ruiz-Sánchez, E.; Hernández-Ortega, H.A.; Pineda-Lucatero, J.; Figueroa-Chávez, D. Plant growth, foliar nutritional content and fruit yield of capsicum chinense biofertilized with Purpureocillium lilacinum under greenhouse conditions. Sci. Hortic. 2020, 261, 108950. [Google Scholar] [CrossRef]
- Khan, J.A.; Siddiq, R.; Arshad, H.M.I.; Anwar, H.S.; Saleem, K.; Jamil, F.F.; Pakistan, F.; Saleem, K. Chemical control of bacterial leaf blight of rice caused by Xanthomonas oryzae pv oryzae. Pak. J. Phytopathol. 2012, 24, 97–100. [Google Scholar]
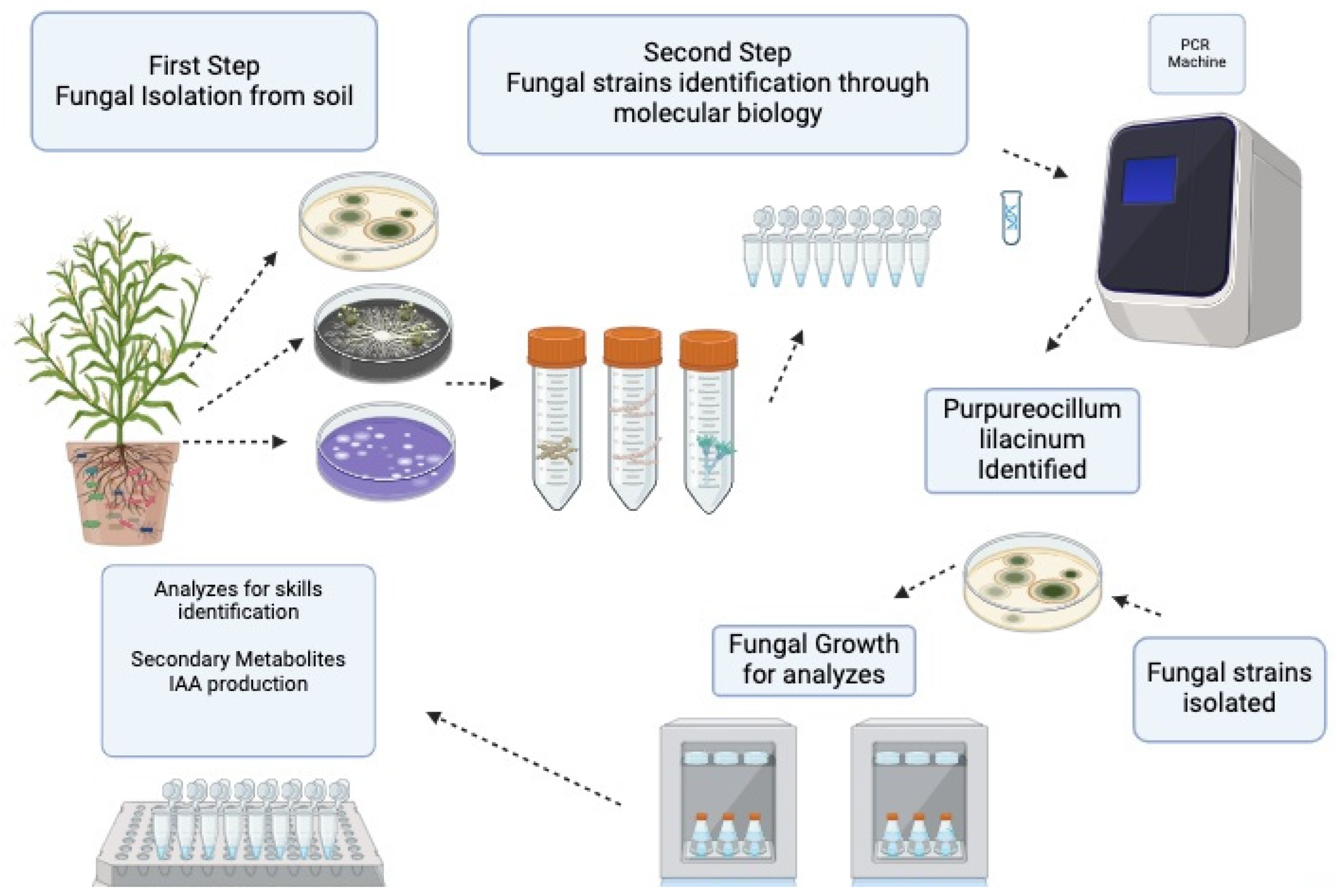
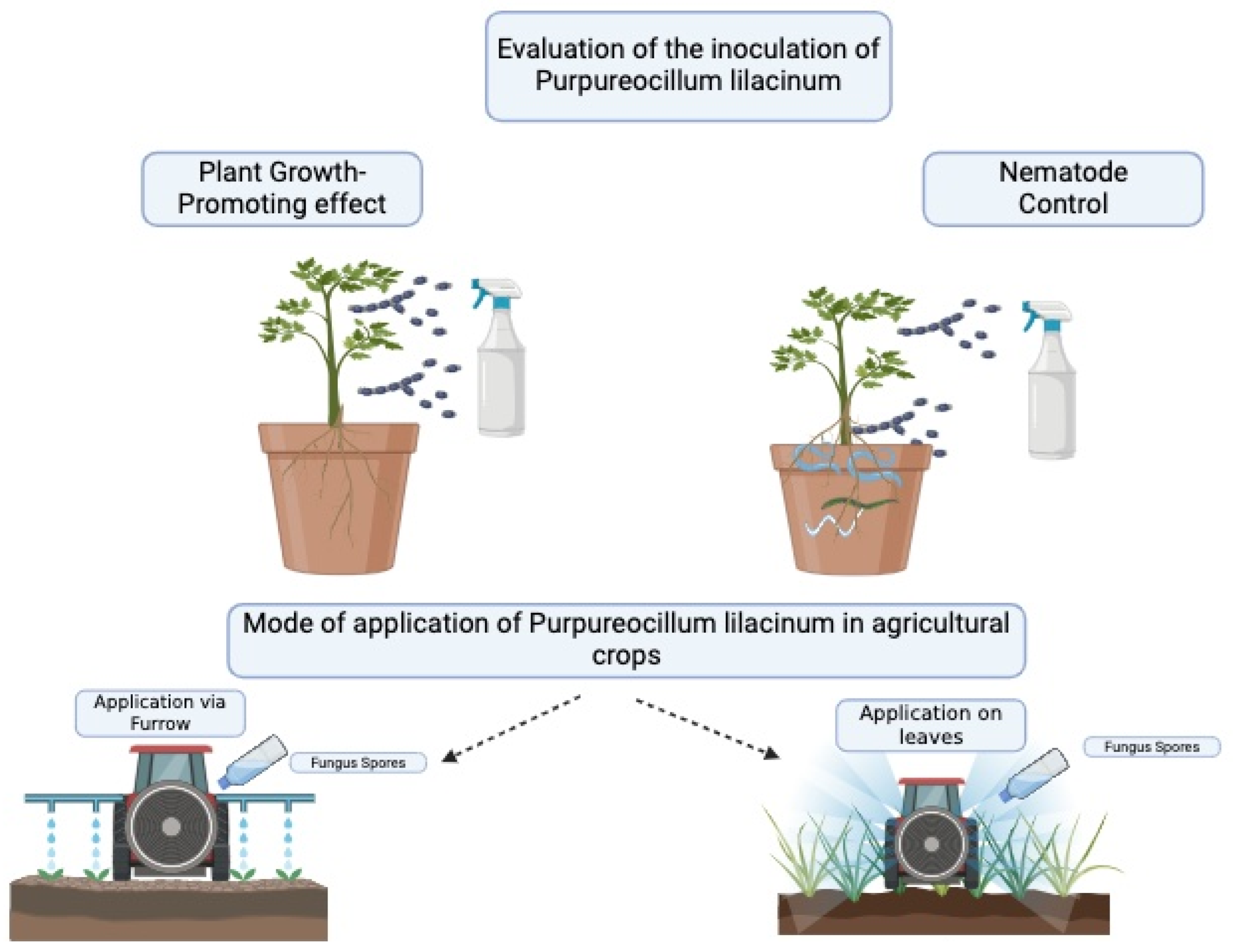
| Effect | Results | References |
|---|---|---|
| Plant Growth | In soybean, stimulated root growth and nutrient absorption | [19] |
| Plant Growth | Increased the availability of P and N and promoted the growth of maize, beans and soybean | [30] |
| Plant Growth | Promoted significant increases in plant dry biomass in cotton crop | [33] |
| Plant Growth | Improved soil nutrient availability in common bean growth | [9] |
| Nematode Control | Suppressed nematode Meloidogyne incognita population in a tomato–cucumber rotation in a greenhouse | [34] |
| Nematode Control | The fungus was effectively applied as a biocontrol agent of phytoparasitic nematodes in tomatoes under variable agroecological conditions | [35] |
| Nematode Control | The fungus showed maximum egg mass inhibition of Meloidogyne incognita | [36] |
| Nematode Control | The fungus controlled root-knot nematodes infecting eggplant | [37] |
| Nematode Control | Control of root-knot nematodes in tomatoes | [38] |
| Nematode Control | Potential control against cotton aphids | [39] |
| Nematode Control | Control of Meloidogyne javanica in commercial pineapple | [40] |
| Nematode Control | Controlled the insect Thrips palmi in orchid farms | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigobelo, E.C.; Nicodemo, D.; Babalola, O.O.; Desoignies, N. Purpureocillium lilacinum as an Agent of Nematode Control and Plant Growth-Promoting Fungi. Agronomy 2024, 14, 1225. https://doi.org/10.3390/agronomy14061225
Rigobelo EC, Nicodemo D, Babalola OO, Desoignies N. Purpureocillium lilacinum as an Agent of Nematode Control and Plant Growth-Promoting Fungi. Agronomy. 2024; 14(6):1225. https://doi.org/10.3390/agronomy14061225
Chicago/Turabian StyleRigobelo, Everlon Cid, Daniel Nicodemo, Olubukola Oluranti Babalola, and Nicolas Desoignies. 2024. "Purpureocillium lilacinum as an Agent of Nematode Control and Plant Growth-Promoting Fungi" Agronomy 14, no. 6: 1225. https://doi.org/10.3390/agronomy14061225
APA StyleRigobelo, E. C., Nicodemo, D., Babalola, O. O., & Desoignies, N. (2024). Purpureocillium lilacinum as an Agent of Nematode Control and Plant Growth-Promoting Fungi. Agronomy, 14(6), 1225. https://doi.org/10.3390/agronomy14061225








