Foliar Application of Wood Distillate Protects Basil Plants against Ozone Damage by Preserving Membrane Integrity and Triggering Antioxidant Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
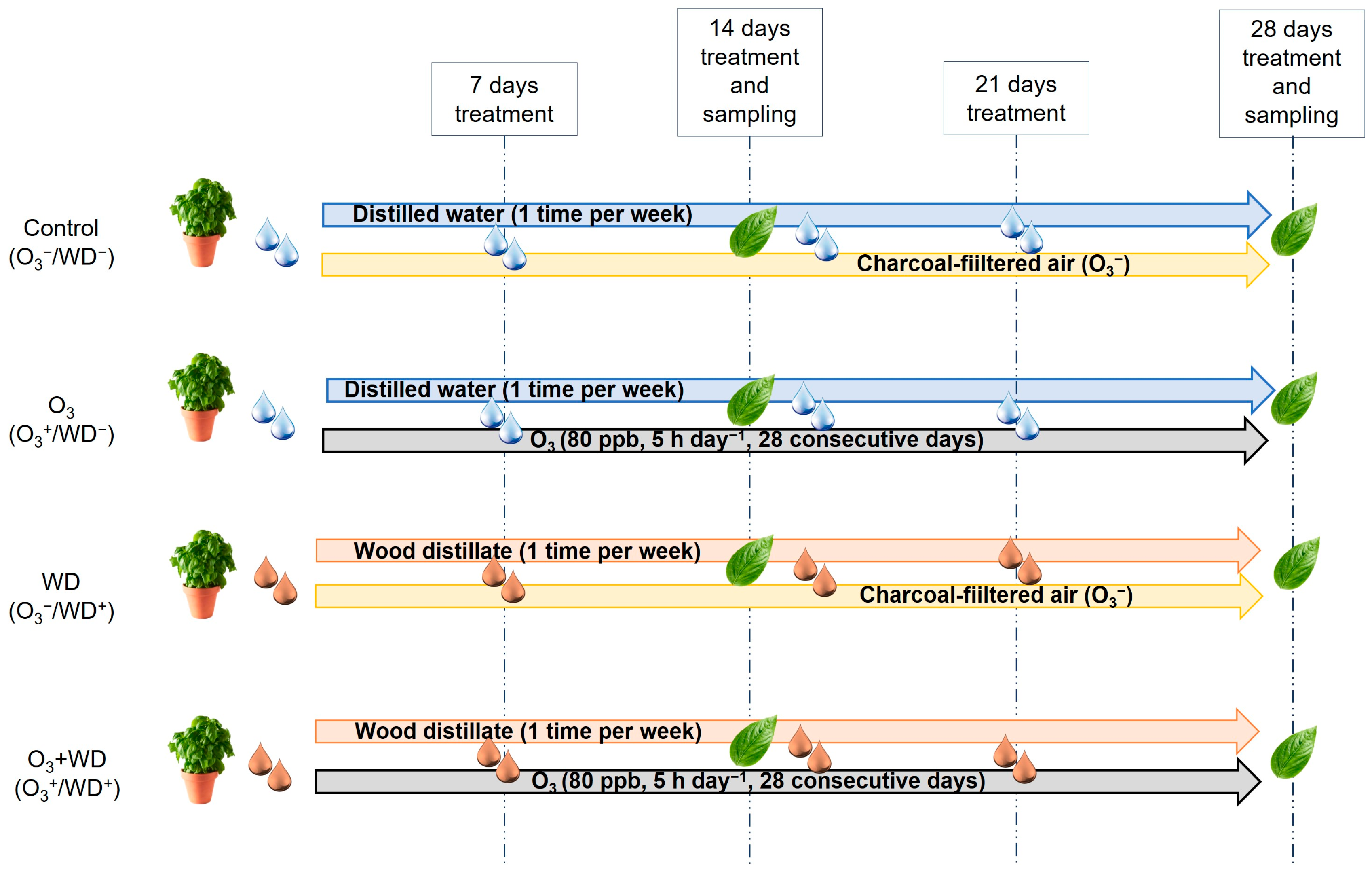
2.1. Plant Material and Experimental Design
2.2. Visible Injury and Leaf Biometric Traits
2.3. Photosynthetic Pigments Determination
2.4. Hydrogen Peroxide and Malondialdehyde Determination
2.5. Endogenous Phytohormones and Antioxidants Determination
2.6. Statistical Analysis
3. Results
3.1. Visible Injury and Leaf Biometric Traits
3.2. Photosynthetic Pigments Content
3.3. Hydrogen Peroxide and Malondialdehyde Content
3.4. Phytohormones and Antioxidants Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meschini, R.; Paoletti, E.; Hoshika, Y.; Sideri-Manoka, Z.-A.; Dell’Orso, A.; Magni, E.; Kuzminsky, E. Comet assay as an early predictor tool to detect ozone enhanced sensitivity of vegetation in a free-air controlled long-term exposure. Plant Stress 2023, 10, 100236. [Google Scholar] [CrossRef]
- Duan, J.; Tan, J.; Yang, L.; Wu, S.; Hao, J. Concentration, sources and ozone formation potential of volatile organic compounds (VOCs) during ozone episode in Beijing. Atmos. Res. 2008, 88, 25–35. [Google Scholar] [CrossRef]
- Zhang, K.; Li, L.; Huang, L.; Wang, Y.; Huo, J.; Duan, Y.; Wang, Y.; Fu, Q. The impact of volatile organic compounds on ozone formation in the suburban area of Shanghai. Atmos. Environ. 2020, 232, 117511. [Google Scholar] [CrossRef]
- Gupta, S.K.; Da, Y.; Zhang, Y.-B.; Pandey, V.; Zhang, J.-L. Tropospheric ozone is a catastrophe, and ethylenediurea (EDU) is a phytoprotectant, recent reports on climate change scenario: A review. Atmos. Pollut. Res. 2023, 14, 101907. [Google Scholar] [CrossRef]
- Zhang, L.; Hoshika, Y.; Carrari, E.; Burkey, K.O.; Paoletti, E. Protecting the photosynthetic performance of snap bean under free air ozone exposure. J. Environ. Sci. 2018, 66, 31–40. [Google Scholar] [CrossRef]
- Nowroz, F.; Hasanuzzaman, M.; Siddika, A.; Parvin, K.; Caparros, P.G.; Nahar, K.; Prasad, P.V. Elevated tropospheric ozone and crop production: Potential negative effects and plant defense mechanisms. Front. Plant Sci. 2024, 14, 1244515. [Google Scholar] [CrossRef] [PubMed]
- Poornima, R.; Dhevagi, P.; Ramya, A.; Agathokleous, E.; Sahasa, R.G.K.; Ramakrishnan, S. Protectants to ameliorate ozone-induced damage in crops–A possible solution for sustainable agriculture. Crop Prot. 2023, 170, 106267. [Google Scholar] [CrossRef]
- Pisuttu, C.; Risoli, S.; Cotrozzi, L.; Nali, C.; Pellegrini, E.; Hoshika, Y.; Moura, B.B.; Paoletti, E. Untangling the role of leaf age specific osmoprotectant and antioxidant responses of two poplar clones under increasing ozone concentrations. Plant Physiol. Biochem. 2024, 208, 108450. [Google Scholar] [CrossRef]
- Singh, A.A.; Fatima, A.; Mishra, A.K.; Chaudhary, N.; Mukherjee, A.; Agrawal, M.; Agrawal, S.B. Assessment of ozone toxicity among 14 Indian wheat cultivars under field conditions: Growth and productivity. Environ. Monit. Assess. 2018, 190, 190. [Google Scholar] [CrossRef]
- Saitanis, C.J.; Lekkas, D.V.; Agathokleous, E.; Flouri, F. Screening agrochemicals as potential protectants of plants against ozone phytotoxicity. Environ. Pollut. 2015, 197, 247–255. [Google Scholar] [CrossRef]
- Godínez-Mendoza, P.; Pico-Chávez, A.K.; Ferrusquía-Jimenez, N.I.; Carbajal-Valenzuela, I.R.; Villagómez-Aranda, A.L.; Torres-Pacheco, I.; Guevara-González, R.G. Plant hormesis: Revising of the concepts of biostimulation, elicitation and their application in a sustainable agricultural production. Sci. Total Environ. 2023, 894, 164883. [Google Scholar] [CrossRef] [PubMed]
- Saitanis, C.J.; Agathokleous, E. Exogenous application of chemicals for protecting plants against ambient ozone pollution: What should come next? Curr. Opin. Environ. Sci. Health 2021, 19, 100215. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.; Abbey, L.; Gunupuru, L.R. Production, prospects and potential application of pyroligneous acid in agriculture. J. Anal. Appl. Pyrolysis 2018, 135, 152–159. [Google Scholar] [CrossRef]
- Akley, E.K.; Ampim, P.A.; Obeng, E.; Sanyare, S.; Yevu, M.; Owusu Danquah, E.; Seidu, A.F. Wood vinegar promotes soil health and the productivity of cowpea. Agronomy 2023, 13, 2497. [Google Scholar] [CrossRef]
- Celletti, S.; Fedeli, R.; Ghorbani, M.; Aseka, J.M.; Loppi, S. Exploring sustainable alternatives: Wood distillate alleviates the impact of bioplastic in basil plants. Sci. Total Environ. 2023, 900, 166484. [Google Scholar] [CrossRef] [PubMed]
- Mohd Amnan, M.A.; Teo, W.F.A.; Aizat, W.M.; Khaidizar, F.D.; Tan, B.C. Foliar application of oil palm wood vinegar enhances Pandanus amaryllifolius tolerance under drought stress. Plants 2023, 12, 785. [Google Scholar] [CrossRef]
- Ofoe, R.; Qin, D.; Gunupuru, L.R.; Thomas, R.H.; Abbey, L. Effect of pyroligneous acid on the productivity and nutritional quality of greenhouse tomato. Plants 2022, 11, 1650. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, R.; Fiaschi, T.; Angiolini, C.; Maccherini, S.; Loppi, S.; Fanfarillo, E. Dose-dependent and species-specific effects of wood distillate addition on the germination performance of threatened arable plants. Plants 2023, 12, 3028. [Google Scholar] [CrossRef] [PubMed]
- Hagner, M.; Pasanen, T.; Lindqvist, B. Effects of birch tar oils on soil organisms and plants. Agric. Food Sci. 2010, 19, 13–23. [Google Scholar] [CrossRef]
- Hagner, M.; Penttinen, O.P.; Pasanen, T.; Tiilikkala, K.; Setälä, H. Acute toxicity of birch tar oil on aquatic organisms. Agric. Food. Sci. 2010, 19, 24–33. [Google Scholar] [CrossRef]
- Filippelli, A.; Ciccone, V.; Loppi, S.; Morbidelli, L. Characterization of the safety profile of sweet chestnut wood distillate employed in agriculture. Safety 2021, 7, 79. [Google Scholar] [CrossRef]
- Fedeli, R.; Marotta, L.; Frattaruolo, L.; Panti, A.; Carullo, G.; Fusi, F.; Loppi, S. Nutritionally enriched tomatoes (Solanum lycopersicum L.) grown with wood distillate: Chemical and biological characterization for quality assessment. J. Food Sci. 2023, 88, 5324–5338. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; H’ng, P.S.; Lee, A.N.; Sajap, A.S.; Tey, B.T.; Salmiah, U. Production of pyroligneous acid from lignocellulosic biomass and their effectiveness against biological attacks. Appl. Sci. 2010, 10, 2440–2446. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Song, Q.; Wang, S.; Wang, Y.; Ge, Y. Root proteomics reveals the effects of wood vinegar on wheat growth and subsequent tolerance to drought stress. Int. J. Mol. Sci. 2019, 20, 943. [Google Scholar] [CrossRef] [PubMed]
- Vannini, A.; Fedeli, R.; Guarnieri, M.; Loppi, S. Foliar application of wood distillate alleviates ozone-induced damage in lettuce (Lactuca sativa L.). Toxics 2022, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Barickman, T.C.; Olorunwa, O.J.; Sehgak, A.; Walne, C.H.; Reddy, K.R.; Gao, W. Yield, physiological performance, and phytochemistry of basil (Ocimum basilicum L.) under temperature stress and elevated CO2 concentrations. Plants 2021, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, B.; Šatović, Z.; Nimac, A.; Vidak, M.; Gunjača, J.; Politeo, O.; Carović-Stanko, K. Application of phenotyping methods in detection of drought and salinity stress in basil (Ocimum basilicum L.). Front. Plant Sci. 2021, 12, 629441. [Google Scholar] [CrossRef]
- Oloumi, H.; Zamani, A.; Ghotbzadeh, S.; Mozaffari, H. Zinc and copper toxicity in basil (Ocimum basilicum L.) seedlings: Role of melatonin in mitigating stress. Plant Stress 2024, 11, 100365. [Google Scholar] [CrossRef]
- Marchica, A.; Ascrizzi, R.; Flamini, G.; Cotrozzi, L.; Tonelli, M.; Lorenzini, G.; Nali, C.; Pellegrini, E. Ozone as eustress for enhancing secondary metabolites and bioactive properties in Salvia officinalis. Ind. Crops Prod. 2021, 170, 113730. [Google Scholar] [CrossRef]
- Vougeleka, V.; Risoli, S.; Saitanis, C.; Agathokleous, E.; Ntatsi, G.; Lorenzini, G.; Nali, C.; Pellegrini, E.; Pisuttu, C. Exogenous application of melatonin protects bean and tobacco plants against ozone damage by improving antioxidant enzyme activities, enhancing photosynthetic performance, and preventing membrane damage. Environ. Pollut. 2024, 343, 123180. [Google Scholar] [CrossRef]
- Shin, R.; Berg, R.H.; Schachtman, D.P. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 2005, 46, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Landi, M. Commentary to: “Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds” by Hodges et al., Planta (1999) 207: 604–611. Planta 2017, 245, 1067. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-H.; Wang, Z.-L.; Shi, B.-L.; Wei, D.; Chen, J.-X.; Wang, S.-L.; Gao, B.-J. Simultaneous determination of salicylic acid, jasmonic acid, methyl salicylate, and methyl jasmonate from Ulmus pumila leaves by GC-MS. Int. J. Anal. Chem. 2015, 2015, 698630. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Montenegro, L.; Lopez-Fernandez, M.; Gimenez, E. Wordlwide research on the ozone influences in plants. Agronomy 2021, 11, 1504. [Google Scholar] [CrossRef]
- Ansari, N.; Yadav, D.S.; Singh, P.; Agrawal, M.; Agrawal, S.B. Ozone exposure response on physiological and biochemical parameters vis-à-vis secondary metabolites in a traditional medicinal plant Sida cordifolia L. Ind. Crops Prod. 2023, 194, 116267. [Google Scholar] [CrossRef]
- Calatayud, A.; Barreno, E. Response to ozone in two lettuce varieties on chlorophyll a fluorescence, photosynthetic pigments and lipid peroxidation. Plant Physiol. Biochem. 2004, 42, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Pintó-Marijuan, M.; Munné-Bosch, S. Photo-oxidative stress markers as a measure of abiotic stress-induced leaf senescence: Advantages and limitations. J. Exp. Bot. 2014, 65, 3845–3857. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ullak, F.; Xhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Czarnocka, W.; Karpińsky, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Yu, Q.; Liu, Y.P.; Qin, G.M.; Li, J.H.; Xu, C.Y.; He, W.J. Response of plant functional traits and leaf economics spectrum to urban thermal environment. J. Beijing For. Univ. 2018, 40, 72–81. [Google Scholar]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic acid: A key regulator of redox signalling and plant immunity. Plant Physiol. Biochem. 2021, 168, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Sun, Y.; Yan, H.; Li, C.; Ge, F. O3-induced leaf senescence in tomato plants is ethylene signaling-dependent and enhances the population abundance of Bemisia tabaci. Front. Plant Sci. 2018, 9, 764. [Google Scholar] [CrossRef] [PubMed]
- González-Villagra, J.; Cohen, J.D.; Reyes-Diaz, M.M. Abscisic acid (ABA) is involved in phenolic compounds biosynthesis mainly anthocyanins, in leaves of Aristotelia chilensis plants (Mol.) subjected to drought stress. Physiol. Plant. 2018, 165, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.A.; Singh, S.; Agrawal, M.; Agrawal, S.B. Assessment of ethylene diurea-induced protection in plants against ozone phytotoxicity. Rev. Environ. Contam. Toxicol. 2015, 233, 129–184. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Tang, S.; Mei, F.; Peng, X.; Li, J.; Li, X.; Yan, X.; Zeng, X.; Liu, F.; Wu, Y.; et al. BnSIP1-1, a trihelix family gene, mediates abiotic stress tolerance and ABA signaling in Brassica napus. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; Hussain, S.; Jalal, F.; Khan, M.A.; Imtiaz, M.; Said, F.; Ismail, M.; Khan, S.; Ali, H.M.; Hatamleh, A.A.; et al. Salicylic acid-mitigates abiotic stress tolerance via altering defense mechanisms in Brassica napus (L.). Front. Plant Sci. 2023, 14, 1187260. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Calabrese, E.J. Hormesis: The dose response for the 21st century: The future has arrived. Toxicology 2019, 425, 152249. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Yu, Z.M.; Wu, W.Q.; Wu, Q.L. Preliminary study of application effect of bamboo vinegar on vegetable growth. For. Stud. China 2006, 8, 43–47. [Google Scholar] [CrossRef]
- Chalermsan, Y.; Peerapan, S. Wood vinegar: By-product from rural charcoal kiln and its role in plant protection. Asian J. Food Agro-Ind. 2009, 2, 189–195. [Google Scholar]
- Zhu, K.; Gu, S.; Liu, J.; Luo, T.; Khan, Z.; Zhang, K.; Hu, L. Wood vinegar as a complex growth regulator promotes the growth, yield, and quality of rapeseed. Agronomy 2021, 11, 510. [Google Scholar] [CrossRef]
- Berahim, Z.; Panhwar, Q.A.; Ismail, M.R.; Saud, H.M.; Monjurul, M.; Mondal, A.; Naher, U.A.; Islam, M.R. Rice yield improvement by foliar application of phytohormone. J. Food Agric. Environ. 2014, 12, 399–404. [Google Scholar]
- Benzon, H.R.L.; Lee, S.C. Pyroligneous acids enhance phytoremediation of heavy metal-contaminated soils using mustard. Commun. Soil Sci. Plant Anal. 2017, 48, 2061–2073. [Google Scholar] [CrossRef]
- Theerakulpisut, P.; Kanawapee, N.; Panwong, B. Seed priming alleviated salt stress effects on rice seedlings by improving Na+/K+and maintaining membrane integrity. Int. J. Plant Biol. 2016, 7, 6402. [Google Scholar] [CrossRef]

 ) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns
) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns  ) and untreated or treated with wood distillate (WD; white
) and untreated or treated with wood distillate (WD; white  and grey
and grey  pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 8). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a–c) indicate significant differences among means (p ≤ 0.05).
pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 8). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a–c) indicate significant differences among means (p ≤ 0.05).
 ) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns
) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns  ) and untreated or treated with wood distillate (WD; white
) and untreated or treated with wood distillate (WD; white  and grey
and grey  pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 8). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a–c) indicate significant differences among means (p ≤ 0.05).
pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 8). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a–c) indicate significant differences among means (p ≤ 0.05).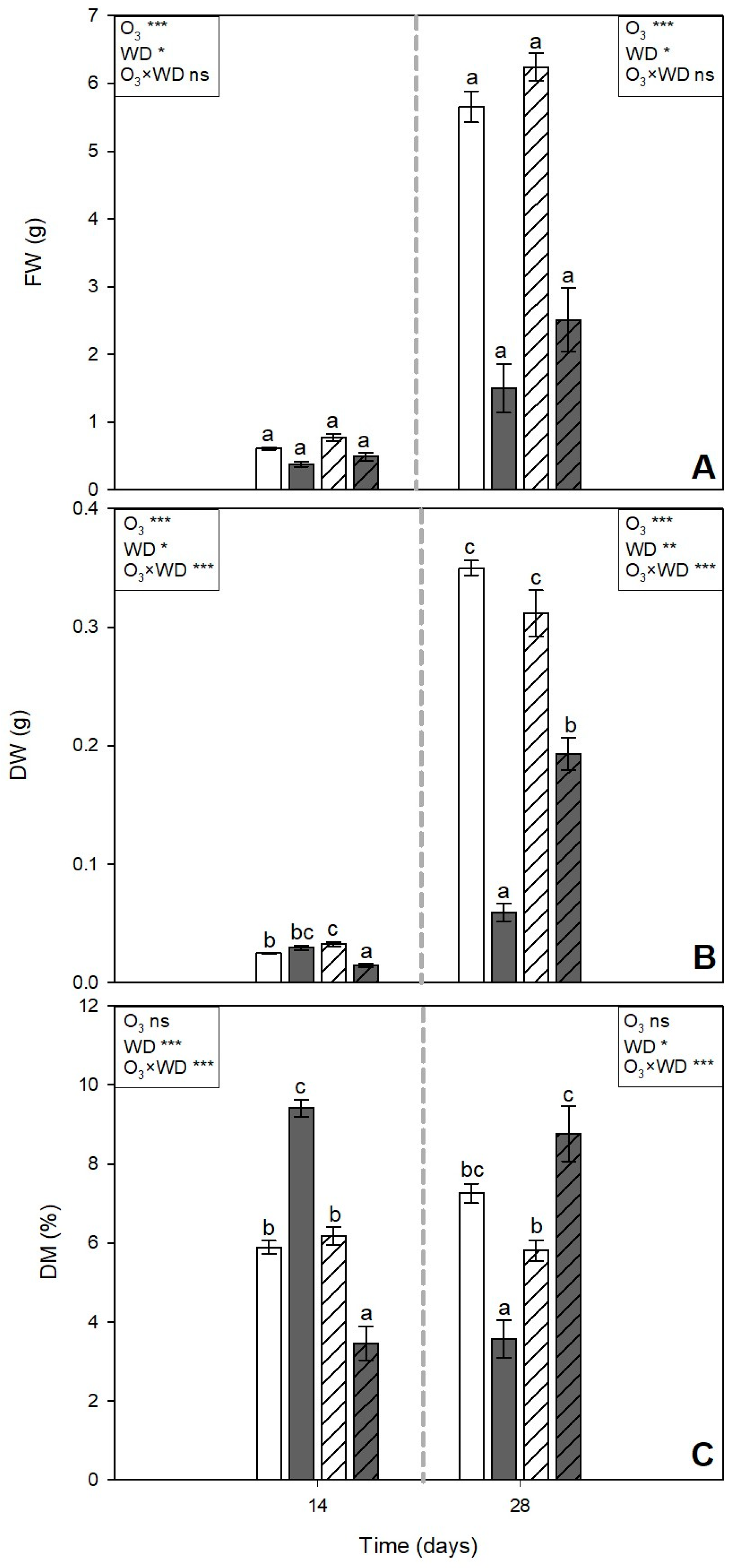
 ) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns
) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns  ) and untreated or treated with wood distillate (WD; white
) and untreated or treated with wood distillate (WD; white  and grey
and grey  pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 4). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a,b) indicate significant differences among means (p ≤ 0.05).
pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 4). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a,b) indicate significant differences among means (p ≤ 0.05).
 ) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns
) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns  ) and untreated or treated with wood distillate (WD; white
) and untreated or treated with wood distillate (WD; white  and grey
and grey  pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 4). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a,b) indicate significant differences among means (p ≤ 0.05).
pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 4). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a,b) indicate significant differences among means (p ≤ 0.05).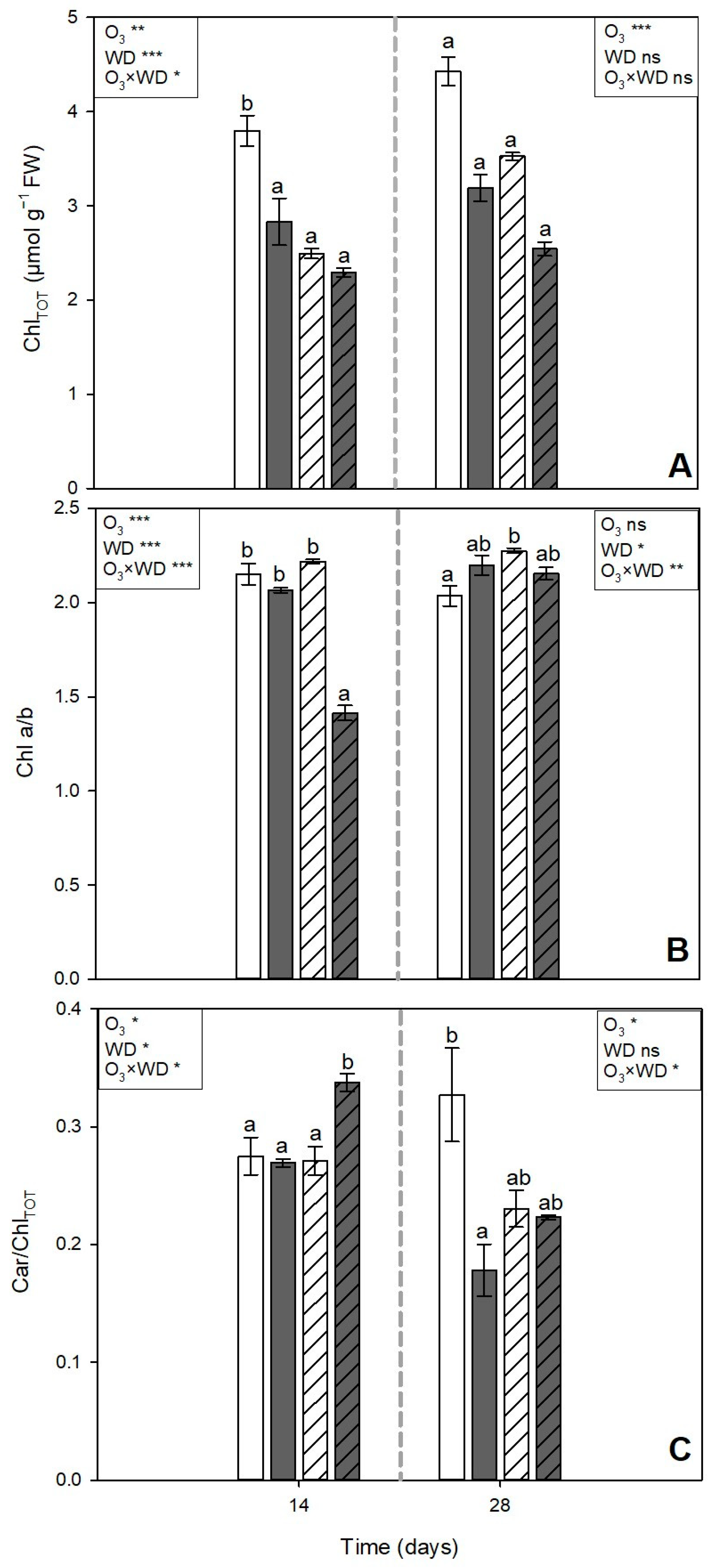
 ) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns
) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns  ) and untreated or treated with wood distillate (WD; white
) and untreated or treated with wood distillate (WD; white  and grey
and grey  pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 4). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a–c) indicate significant differences among means (p ≤ 0.05).
pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 4). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a–c) indicate significant differences among means (p ≤ 0.05).
 ) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns
) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns  ) and untreated or treated with wood distillate (WD; white
) and untreated or treated with wood distillate (WD; white  and grey
and grey  pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 4). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a–c) indicate significant differences among means (p ≤ 0.05).
pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 4). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a–c) indicate significant differences among means (p ≤ 0.05).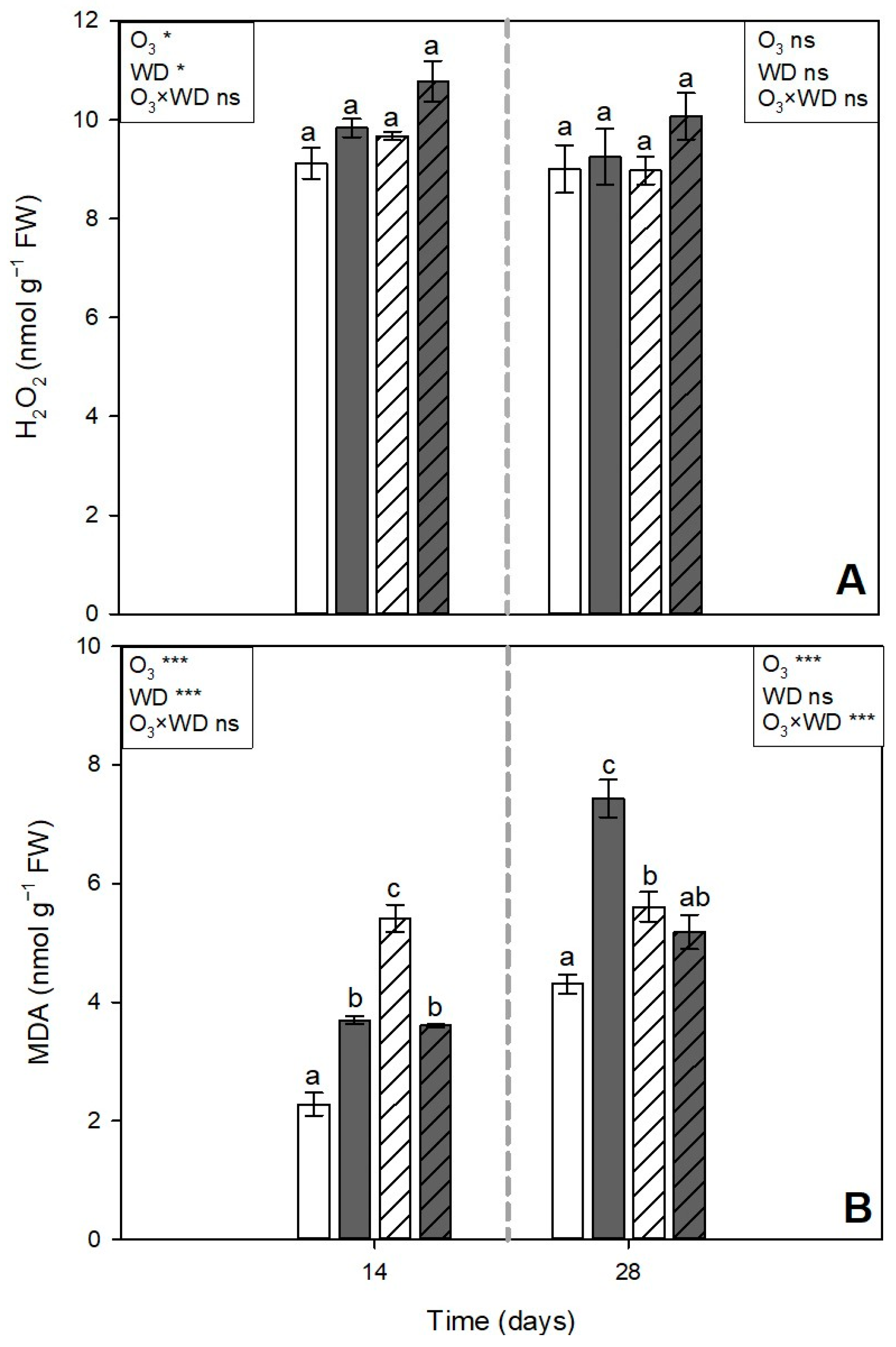
 ) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns
) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns  ) and untreated or treated with wood distillate (WD; white
) and untreated or treated with wood distillate (WD; white  and grey
and grey  pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 4). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a–d) indicate significant differences among means (p ≤ 0.05).
pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 4). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a–d) indicate significant differences among means (p ≤ 0.05).
 ) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns
) or to 80 ppb of ozone (O3, 5 h day−1) for 28 consecutive days (grey and solid filled columns  ) and untreated or treated with wood distillate (WD; white
) and untreated or treated with wood distillate (WD; white  and grey
and grey  pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 4). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a–d) indicate significant differences among means (p ≤ 0.05).
pattern filled columns, respectively). Data are shown as mean ± standard error (number of leaf samples = 4). For each time (14 and 28 days from the beginning of exposure, in each figure on the left and right, respectively), ANOVA: *** p ≤ 0.001; ** p ≤ 0.01; * p ≤ 0.05; ns denotes not significant. According to Tukey’s post hoc test, different letters inside each time window (a–d) indicate significant differences among means (p ≤ 0.05).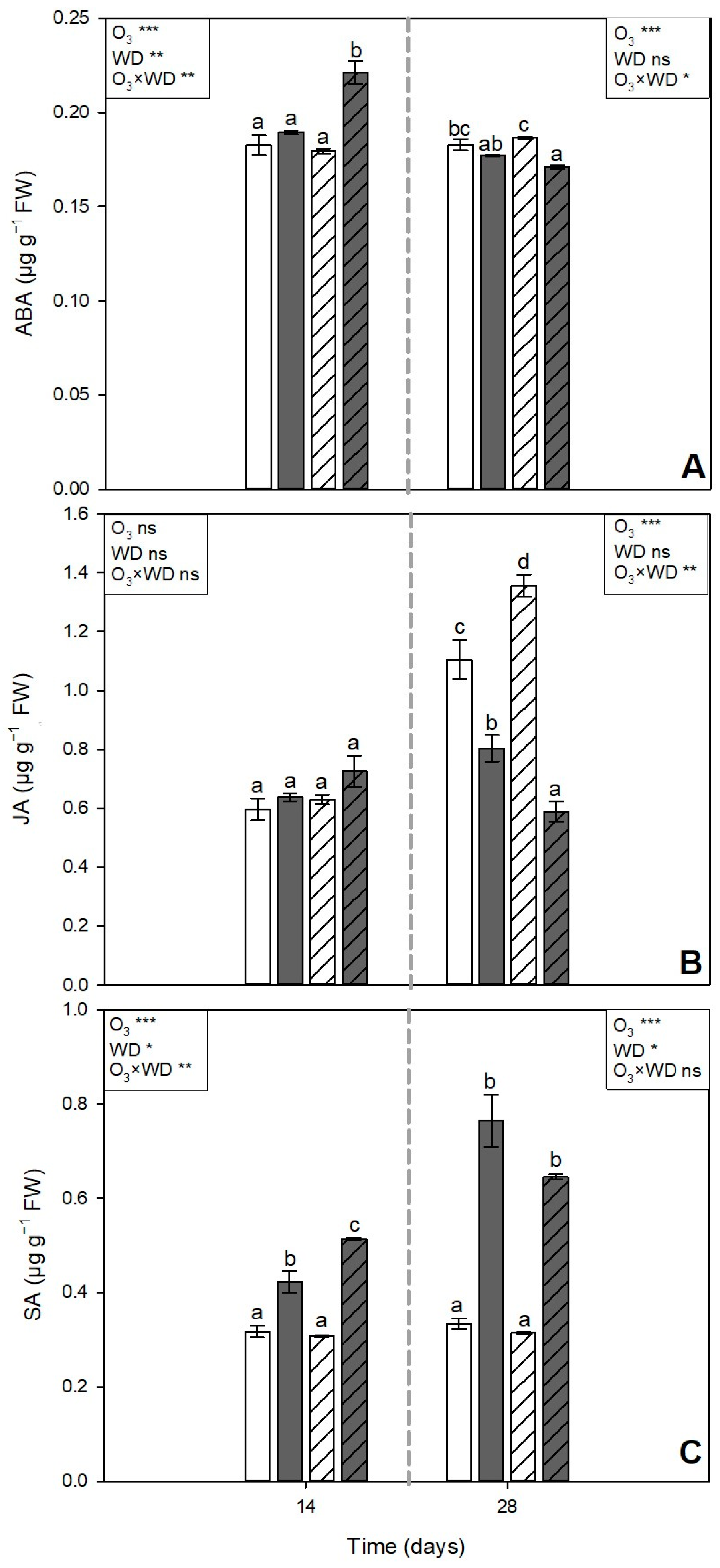
| pH | 5.30 ± 0.03 |
| EC (mS cm−1) | 1.12 ± 0.01 |
| CEC (meq 100 g−1 DW) | 56.89 ± 2.67 |
| Porosity (%) | 92 |
| Moisture content (%) | 43 |
| Ca (mg kg−1 DW) | 23,159 ± 296 |
| Mg (mg kg−1 DW) | 2846 ± 22 |
| Na (mg kg−1 DW) | 1379 ± 19 |
| K (mg kg−1 DW) | 1198 ± 17 |
| P (mg kg−1 DW) | 614 ± 14 |
| S (mg kg−1 DW) | 1410 ± 141 |
| Fe (mg kg−1 DW) | 1097 ± 10 |
| Mn (mg kg−1 DW) | 31 ± 1 |
| Cu (mg kg−1 DW) | 23 ± 1 |
| Zn (mg kg−1 DW) | 38 ± 1 |
| Mo (mg kg−1 DW) | 0.89 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, G.; Fedeli, R.; Mariotti, L.; Pisuttu, C.; Nali, C.; Pellegrini, E.; Loppi, S. Foliar Application of Wood Distillate Protects Basil Plants against Ozone Damage by Preserving Membrane Integrity and Triggering Antioxidant Mechanisms. Agronomy 2024, 14, 1233. https://doi.org/10.3390/agronomy14061233
Bianchi G, Fedeli R, Mariotti L, Pisuttu C, Nali C, Pellegrini E, Loppi S. Foliar Application of Wood Distillate Protects Basil Plants against Ozone Damage by Preserving Membrane Integrity and Triggering Antioxidant Mechanisms. Agronomy. 2024; 14(6):1233. https://doi.org/10.3390/agronomy14061233
Chicago/Turabian StyleBianchi, Gemma, Riccardo Fedeli, Lorenzo Mariotti, Claudia Pisuttu, Cristina Nali, Elisa Pellegrini, and Stefano Loppi. 2024. "Foliar Application of Wood Distillate Protects Basil Plants against Ozone Damage by Preserving Membrane Integrity and Triggering Antioxidant Mechanisms" Agronomy 14, no. 6: 1233. https://doi.org/10.3390/agronomy14061233








