Effects of GmERF5-Responsive Effector on Soybean Symbiotic Nodulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
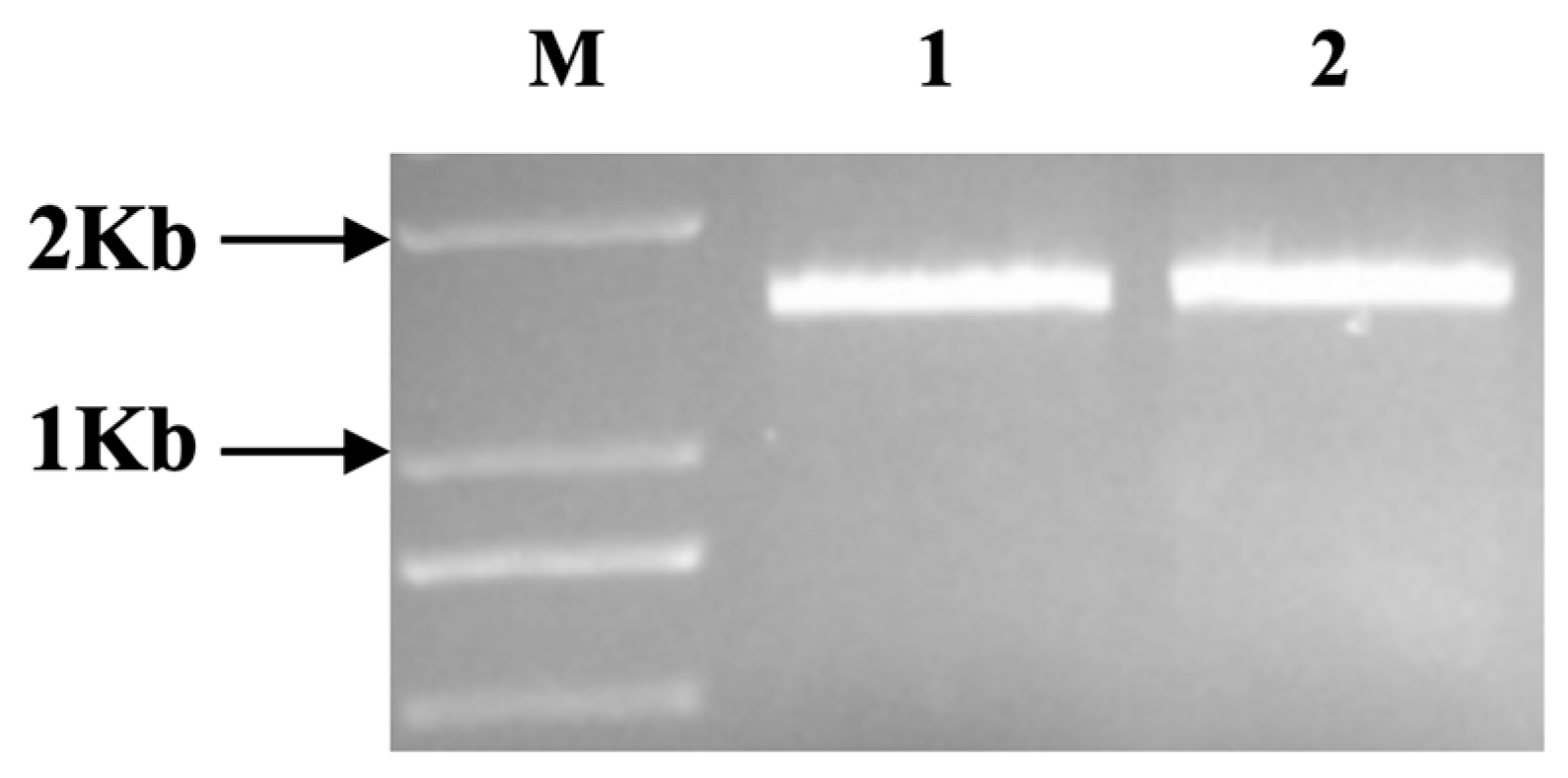
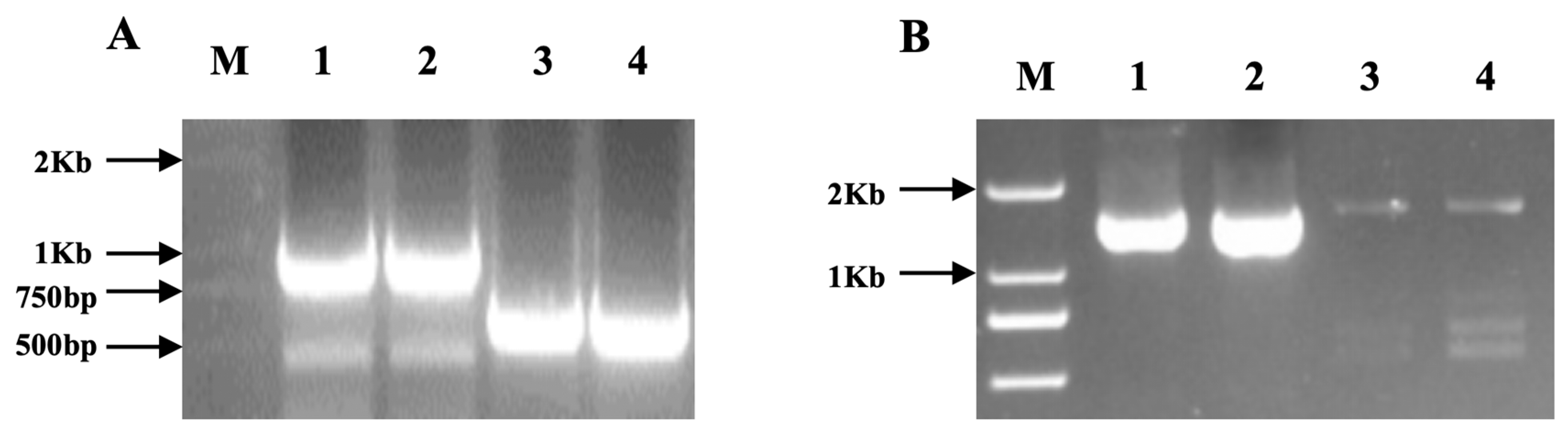
2.2. Type III Effector NopC Gene Cloning and Mutation
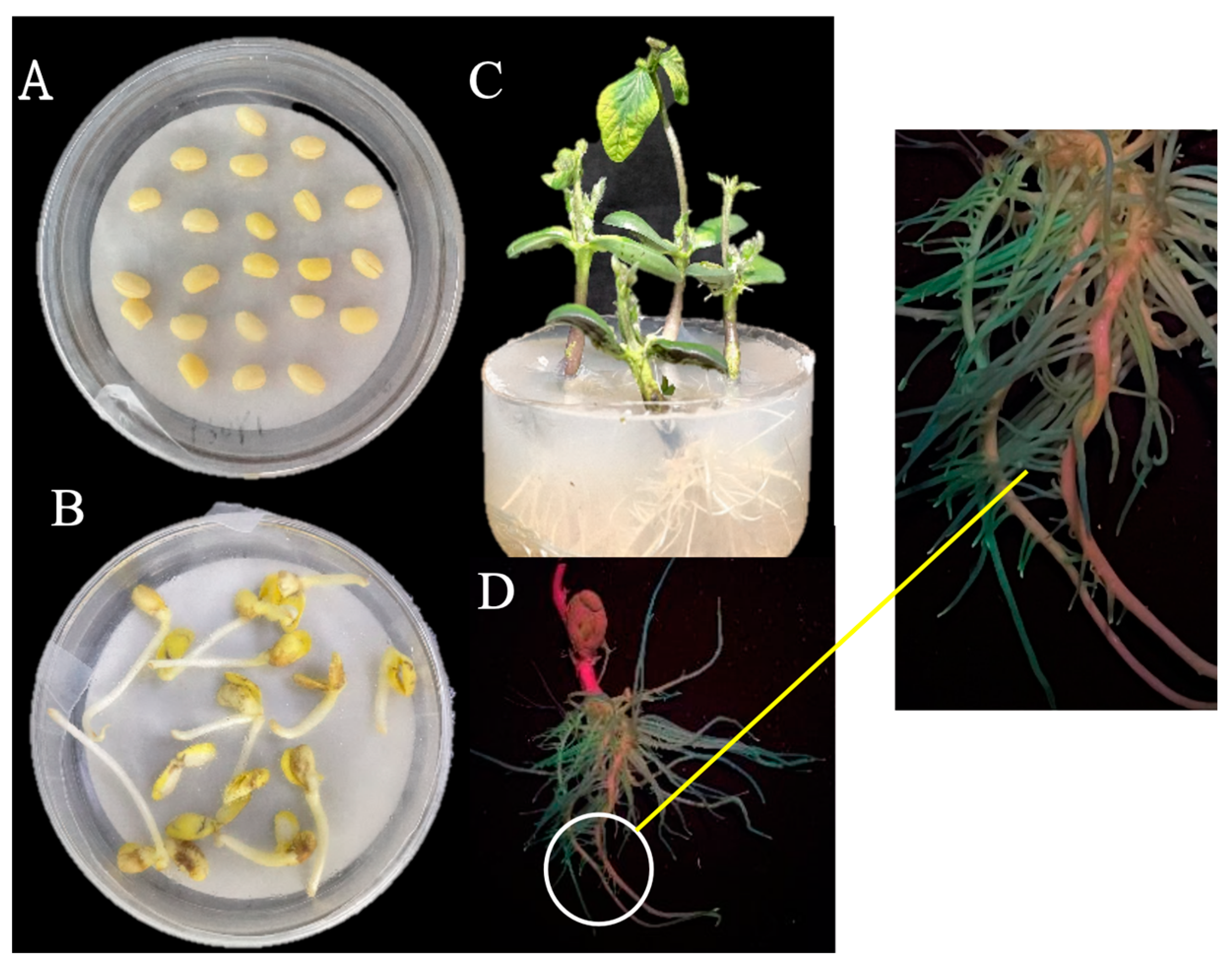
2.3. Soybean Nodulation Experiment
2.4. mRNA-Seq (mRNA Sequencing) Analysis
2.5. RNA Extraction and qRT-PCR Analysis
2.6. Transcriptional Activity Analysis Assay
2.7. Nodules Identification Method
2.8. Phenotypic Statistics and Data Analysis
3. Results
3.1. Construction of the NopC Mutant
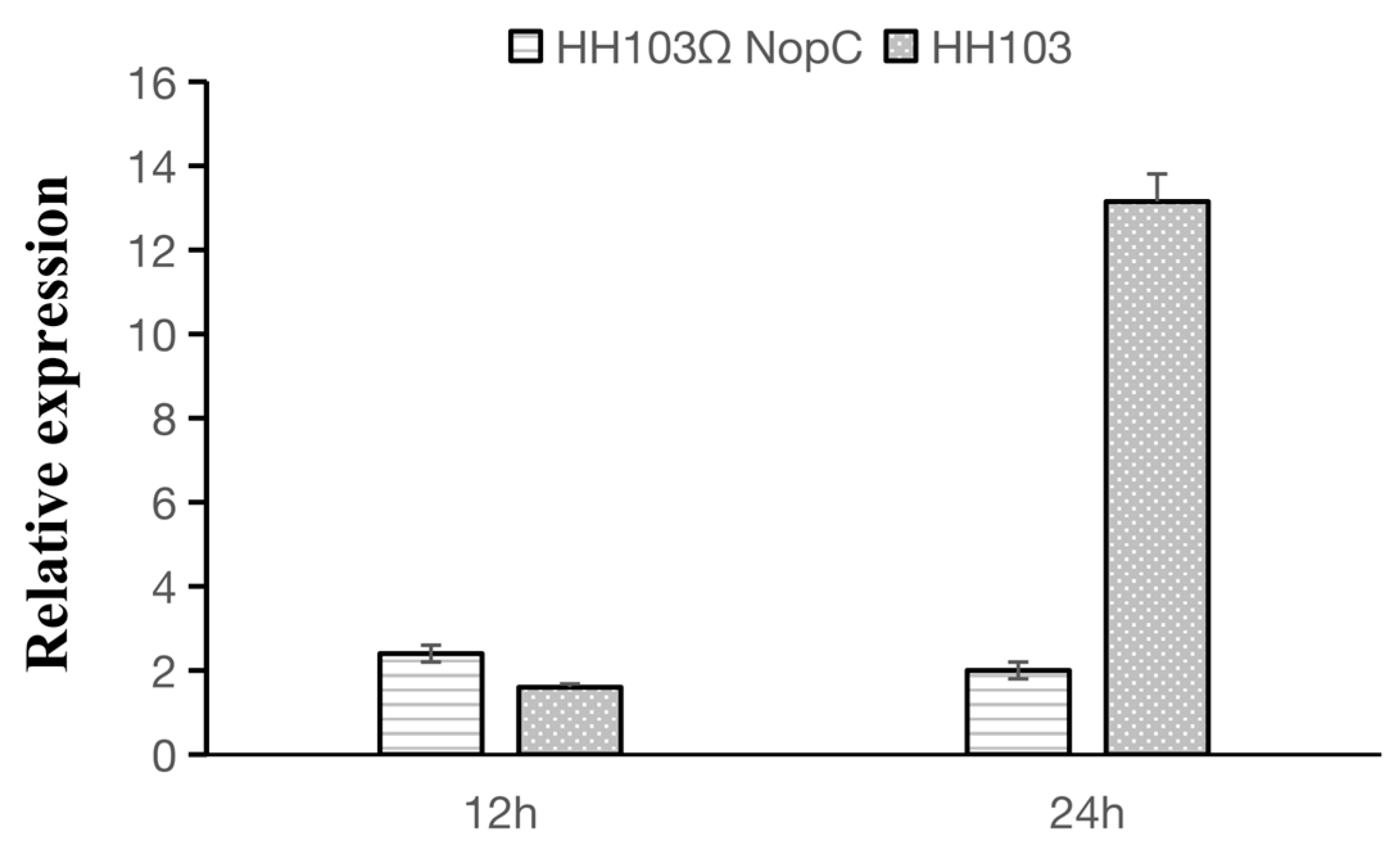
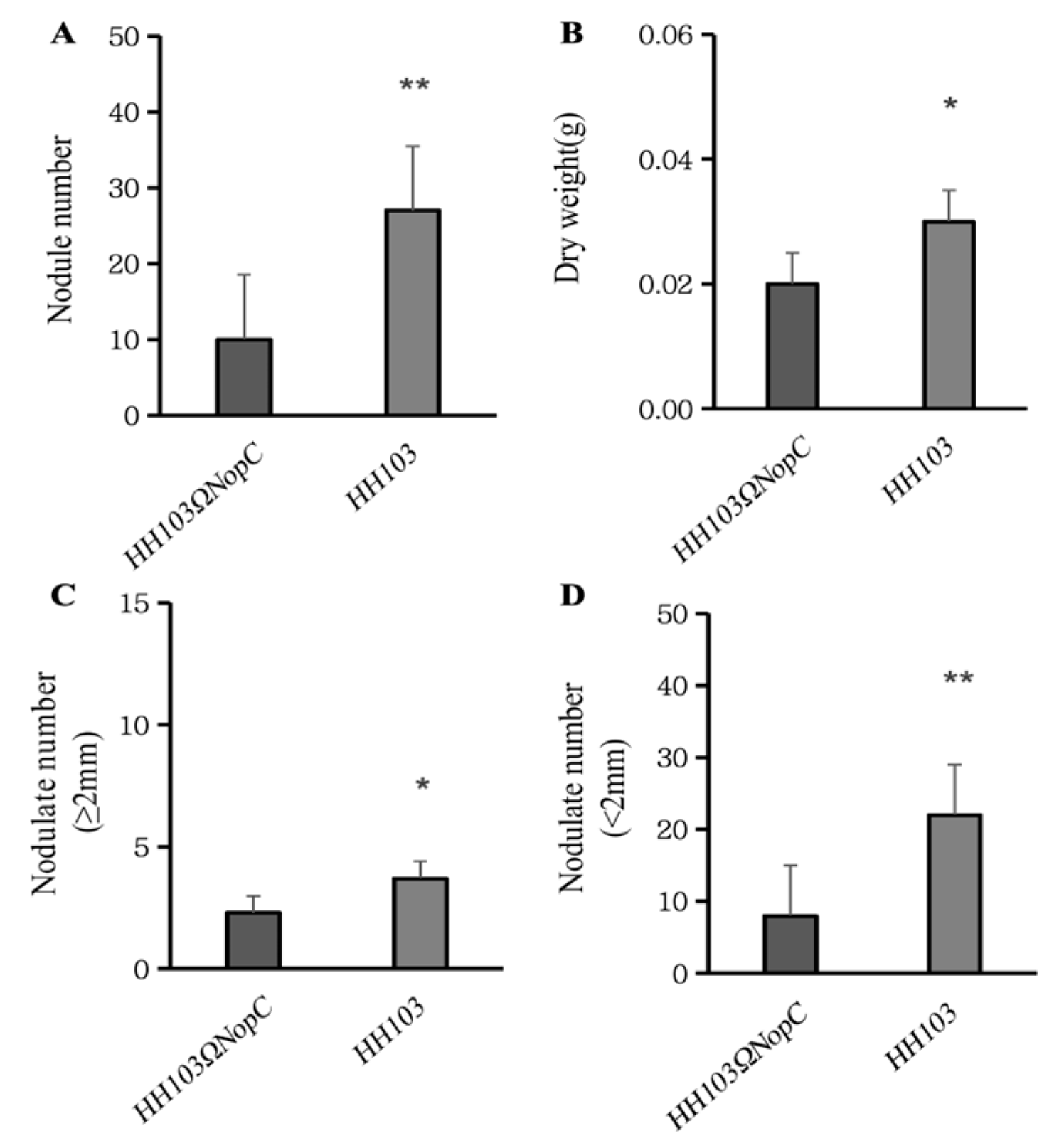
3.2. Characterization of the Effect on Nodulation of NopC
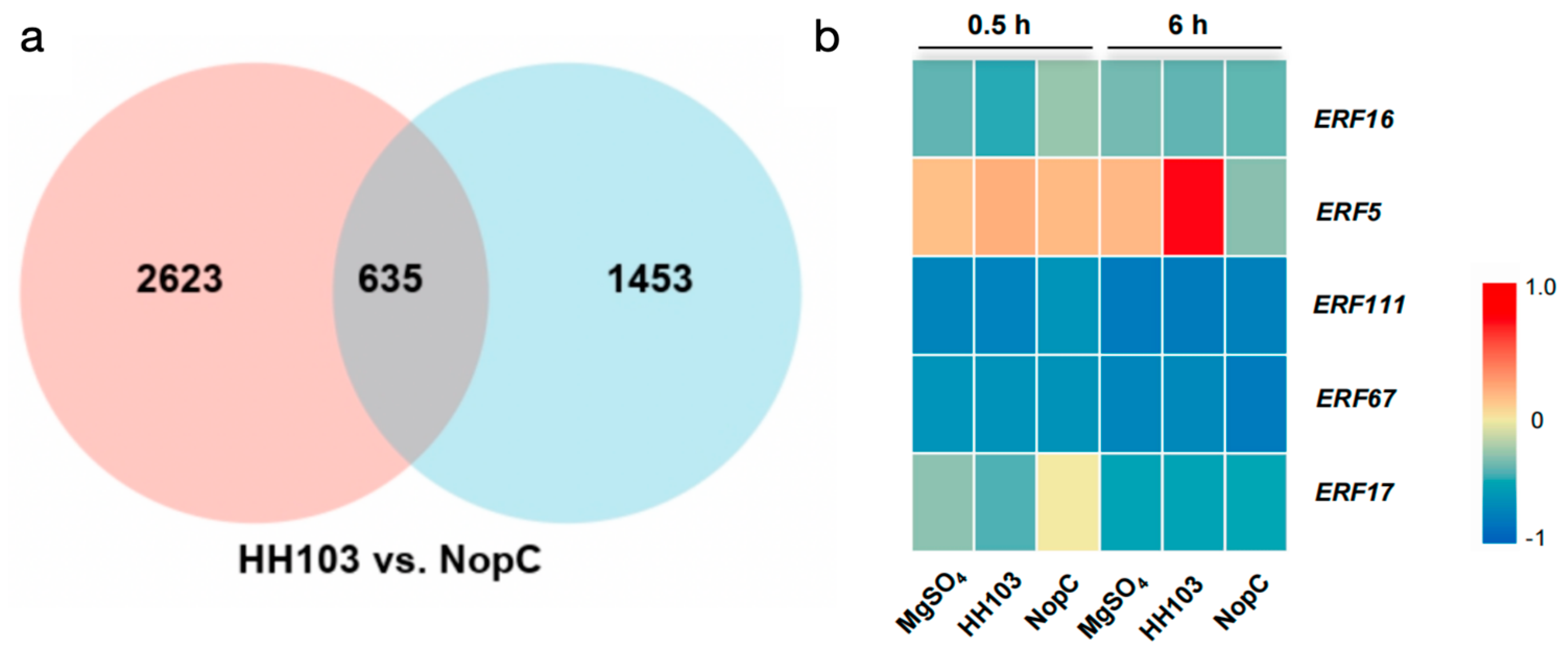
3.3. Analysis of GmERF5 Response Effector Regulation
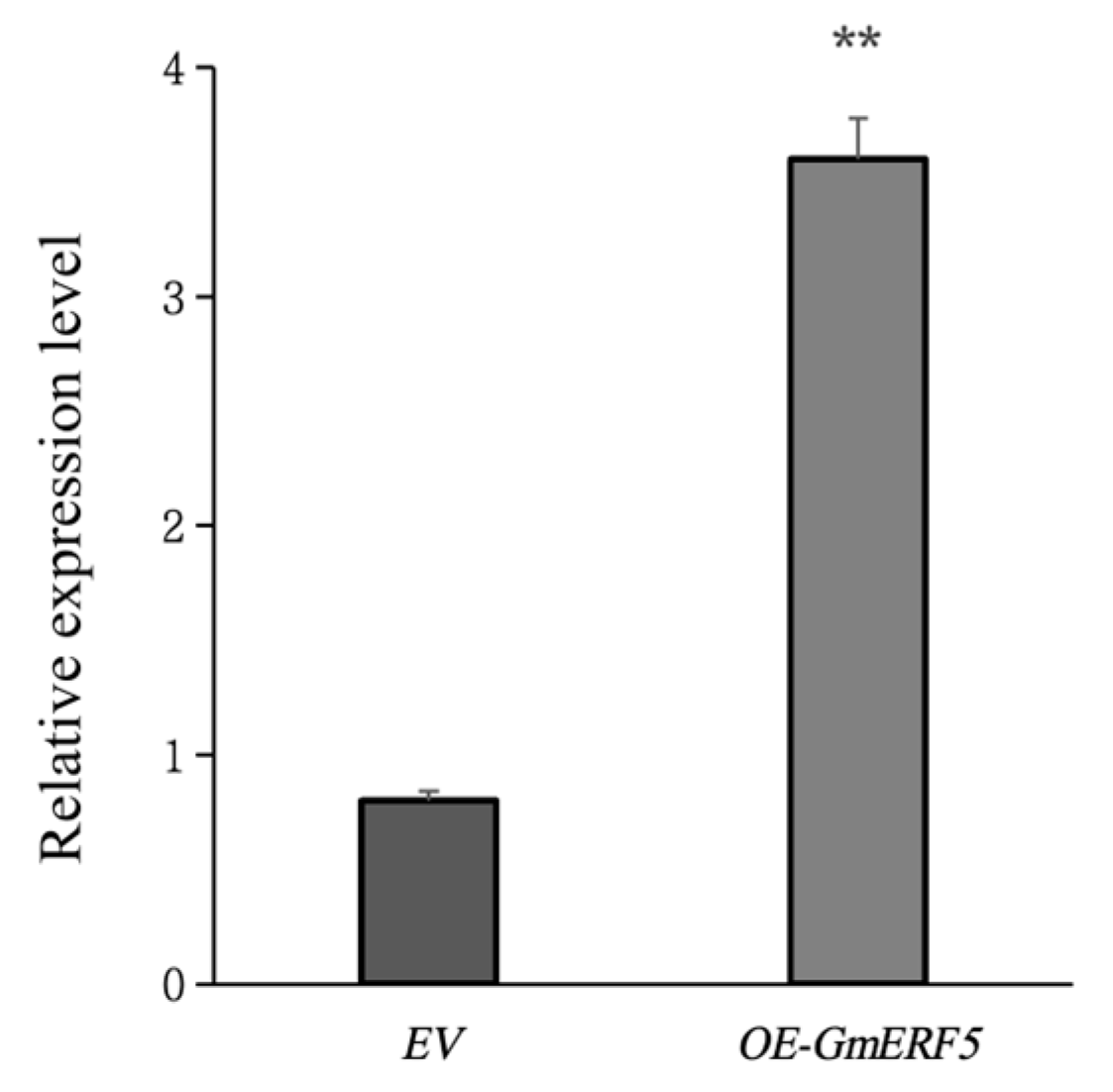
3.4. Functional Analysis of Plant Candidate Genes
GmERF5 Gene Functional Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.; Han, W.; Zhang, W.; Christie, P.; Goulding, K.; Vitousek, P.; Zhang, F. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Schroder, J.L.; Zhang, H.; Girma, K.; Raun, W.R.; Penn, C.J.; Payton, M.E. Soil acidification from long-term use of nitrogen fertilizers on winter wheat. Soil Sci. Soc. Am. J. 2011, 75, 957–964. [Google Scholar] [CrossRef]
- Kim, W.-S.; Krishnan, H.B. A nopA deletion mutant of Sinorhizobium fredii USDA257, a soybean symbiont, is impaired in nodulation. Curr. Microbiol. 2014, 68, 239–246. [Google Scholar] [CrossRef]
- Krishnan, H.B. NolX of Sinorhizobium fredii USDA257, a type III-secreted protein involved in host range determination, is localized in the infection threads of cowpea (Vigna unguiculata [L.] Walp) and soybean (Glycine max [L.] Merr.) nodules. J. Bacteriol. 2002, 184, 831–839. [Google Scholar] [CrossRef]
- Zehner, S.; Schober, G.; Wenzel, M.; Lang, K.; Göttfert, M. Expression of the Bradyrhizobium japonicum type III secretion system in legume nodules and analysis of the associated tts box promoter. Mol. Plant-Microbe Interact. 2008, 21, 1087–1093. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.-J.; Lu, H.-B.; Xie, Z.-P.; Staehelin, C. Functional analysis of the type 3 effector nodulation outer protein L (NopL) from Rhizobium sp. NGR234: Symbiotic effects, phosphorylation, and interference with mitogen-activated protein kinase signaling. J. Biol. Chem. 2011, 286, 32178–32187. [Google Scholar] [CrossRef]
- Balatti, P.A.; Kovács, L.G.; Krishnan, H.B.; Pueppke, S.G. Rhizobium sp. NGR234 contains a functional copy of the soybean cultivar specificity locus, nolXWBTUV. MPMI-Mol. Plant Microbe Interact. 1995, 8, 693–699. [Google Scholar] [CrossRef]
- Perret, X.; Freiberg, C.; Rosenthal, A.; Broughton, W.J.; Fellay, R. High-resolution transcriptional analysis of the symbiotic plasmid of Rhizobium sp. NGR234. Mol. Microbiol. 1999, 32, 415–425. [Google Scholar] [CrossRef]
- Gourion, B.; Berrabah, F.; Ratet, P.; Stacey, G. Rhizobium–legume symbioses: The crucial role of plant immunity. Trends Plant Sci. 2015, 20, 186–194. [Google Scholar] [CrossRef]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-triggered immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef]
- Feng, F.; Zhou, J.-M. Plant–bacterial pathogen interactions mediated by type III effectors. Curr. Opin. Plant Biol. 2012, 15, 469–476. [Google Scholar] [CrossRef]
- Macho, A.P.; Zipfel, C. Targeting of plant pattern recognition receptor-triggered immunity by bacterial type-III secretion system effectors. Curr. Opin. Microbiol. 2015, 23, 14–22. [Google Scholar] [CrossRef]
- Gazi, A.D.; Sarris, P.F.; Fadouloglou, V.E.; Charova, S.N.; Mathioudakis, N.; Panopoulos, N.J.; Kokkinidis, M. Phylogenetic analysis of a gene cluster encoding an additional, rhizobial-like type III secretion system that is narrowly distributed among Pseudomonas syringae strains. BMC Microbiol. 2012, 12, 188. [Google Scholar] [CrossRef]
- Tampakaki, A.P. Commonalities and differences of T3SSs in rhizobia and plant pathogenic bacteria. Front. Plant Sci. 2014, 5, 80600. [Google Scholar] [CrossRef]
- Jimenez-Guerrero, I.; Perez-Montano, F.; Medina, C.; Ollero, F.J.; López-Baena, F.J. NopC is a Rhizobium-specific type 3 secretion system effector secreted by Sinorhizobium (Ensifer) fredii HH103. PLoS ONE 2015, 10, e0142866. [Google Scholar] [CrossRef]
- Bao, H.; yanan, w.; Li, H.; Wang, Q.; Lei, Y.; Ye, Y.; Zhu, H.; Stacey, G.; Xu, S.; Cao, Y. The Rhizobial effector NopT targets Nod factor receptors to regulate symbiosis in Lotus japonicus. bioRxiv 2024. [Google Scholar] [CrossRef]
- Fujimoto, S.Y.; Ohta, M.; Usui, A.; Shinshi, H.; Ohme-Takagi, M. Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box–mediated gene expression. Plant Cell 2000, 12, 393–404. [Google Scholar]
- Gu, Y.-Q.; Yang, C.; Thara, V.K.; Zhou, J.; Martin, G.B. Pti4 is induced by ethylene and salicylic acid, and its product is phosphorylated by the Pto kinase. Plant Cell 2000, 12, 771–785. [Google Scholar] [CrossRef]
- Grant, M.R.; Jones, J.D. Hormone (dis) harmony moulds plant health and disease. Science 2009, 324, 750–752. [Google Scholar] [CrossRef]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Verhage, A.; van Wees, S.C.; Pieterse, C.M. Plant immunity: It’s the hormones talking, but what do they say? Plant Physiol. 2010, 154, 536–540. [Google Scholar] [CrossRef]
- Koornneef, A.; Pieterse, C.M. Cross talk in defense signaling. Plant Physiol. 2008, 146, 839–844. [Google Scholar] [CrossRef]
- Yu, Z.; Qu, X.; Lv, B.; Li, X.; Sui, J.; Yu, Q.; Ding, Z. MAC3A and MAC3B mediate degradation of the transcription factor ERF13 and thus promote lateral root emergence. Plant Cell 2024, koae047. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Liu, C.; Ma, C.; Li, C.; Zhang, Y.; Qi, Z.; Zhu, R.; Shi, Y.; Zou, J. Identification of soybean genes whose expression is affected by the Ensifer fredii HH103 effector protein NopP. Int. J. Mol. Sci. 2018, 19, 3438. [Google Scholar] [CrossRef]
- Ni, H.; Peng, Y.; Wang, J.; Wang, J.; Yuan, Y.; Fu, T.; Zhu, Z.; Zhang, J.; Pan, X.; Cui, Z. Mapping of Quantitative Trait Loci Underlying Nodule Traits in Soybean (Glycine max (L.) Merr.) and Identification of Genes Whose Expression Is Affected by the Sinorhizobium fredii HH103 Effector Proteins NopL and NopT. Agronomy 2022, 12, 946. [Google Scholar] [CrossRef]
- Xin, D.W.; Liao, S.; Xie, Z.P.; Hann, D.R.; Steinle, L.; Boller, T.; Staehelin, C. Functional analysis of NopM, a novel E3 ubiquitin ligase (NEL) domain effector of Rhizobium sp. strain NGR234. PLoS Pathog. 2012, 8, e1002707. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Chen, L.; Fu, Y.; Li, C.; Qi, Z.; Zou, J.; Zhu, R.; Li, S.; Wei, W. Mining for genes encoding proteins associated with NopL of Sinorhizobium fredii HH103 using quantitative trait loci in soybean (Glycine max Merr.) recombinant inbred lines. Plant Soil 2018, 431, 245–255. [Google Scholar] [CrossRef]
- Staehelin, C.; Krishnan, H.B. Nodulation outer proteins: Double-edged swords of symbiotic rhizobia. Biochem. J. 2015, 470, 263–274. [Google Scholar] [CrossRef]
- Bellato, C.; Krishnan, H.; Cubo, T.; Temprano, F.; Pueppke, S. The soybean cultivar specificity gene noIX is present, expressed in a nodD-dependent manner, and of symbiotic significance in cultivar-nonspecific strains of Rhizobium (Sinorhizobium) fredii. Microbiology 1997, 143, 1381–1388. [Google Scholar] [CrossRef]
- Marie, C.; Deakin, W.J.; Viprey, V.; Kopciñska, J.; Golinowski, W.; Krishnan, H.B.; Perret, X.; Broughton, W.J. Characterization of Nops, nodulation outer proteins, secreted via the type III secretion system of NGR234. Mol. Plant-Microbe Interact. 2003, 16, 743–751. [Google Scholar] [CrossRef]
- Thomas, N.A.; Ma, I.; Prasad, M.E.; Rafuse, C. Expanded roles for multicargo and class 1B effector chaperones in type III secretion. J. Bacteriol. 2012, 194, 3767–3773. [Google Scholar] [CrossRef]
- Kawaharada, Y.; James, E.K.; Kelly, S.; Sandal, N.; Stougaard, J. The ethylene responsive factor required for nodulation 1 (ERN1) transcription factor is required for infection-thread formation in Lotus japonicus. Mol. Plant-Microbe Interact. 2017, 30, 194–204. [Google Scholar] [CrossRef]



| Primer | Sequences (5’→3’) | Target Segment Length |
|---|---|---|
| NopC-F | gggtttgttgaagtggatg | 1461 |
| NopC-R | cgccgccttcttggttg | |
| NopC-SY-R | tcagggtcatcagaattctcatcatcacatcagctactcctgcct | 1488 |
| NopC-XY-F | tgatgatgagaattctgatgaccctgagtcggagtgattggaagtggag |
| Primer | Homologous Primer Sequence (5′→3′) | Target Segment Length |
|---|---|---|
| GmERF5-TY-F | GATCTTCTAGAGATATCACTAGATGGCAAACGCTCTTGAAGT | 828 bp |
| GmERF5-TY-R | GCAATGCATATTAATGTCGACACAACCATAAGCTGTGGAA |
| Strains | Nodulate Number (nos.) | Dry Weight (g) | Number of Large Nodules (≥2 mm) | Number of Small Nodules (<2 mm) |
|---|---|---|---|---|
| HH103Ω NopC | 9.69 ± 1.759 | 0.02 ± 0.003 | 2.31 ± 0.624 | 7.38 ± 0.1470 |
| HH103 | 26.20 ± 4.957 | 0.03 ± 0.005 | 3.87 ± 1.046 | 22.07 ± 4.794 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Jin, Y.; Tang, W.; Li, X.; Liu, C.; Wang, J.; Wu, X.; Chen, Q.; Luan, F.; Xin, D. Effects of GmERF5-Responsive Effector on Soybean Symbiotic Nodulation. Agronomy 2024, 14, 1239. https://doi.org/10.3390/agronomy14061239
Li C, Jin Y, Tang W, Li X, Liu C, Wang J, Wu X, Chen Q, Luan F, Xin D. Effects of GmERF5-Responsive Effector on Soybean Symbiotic Nodulation. Agronomy. 2024; 14(6):1239. https://doi.org/10.3390/agronomy14061239
Chicago/Turabian StyleLi, Candong, Yuxin Jin, Weinan Tang, Xuemei Li, Chunyan Liu, Jinhui Wang, Xiaoxia Wu, Qingshan Chen, Feishi Luan, and Dawei Xin. 2024. "Effects of GmERF5-Responsive Effector on Soybean Symbiotic Nodulation" Agronomy 14, no. 6: 1239. https://doi.org/10.3390/agronomy14061239





