15N Isotope Labeled Tracking of the Nitrogen Utilization of Apple under Water and Nitrogen Coupling Application in Arid and Semiarid Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Location and Growing Conditions
2.2. Experimental Design
2.3. Determination Items and Methods
2.3.1. Leaf Blade Growth
2.3.2. Photosynthetic Characteristics
2.3.3. Leaf Chlorophyll
2.3.4. Determination of Mineral Elements and 15N in Leaves
2.3.5. Nitrogen Use Efficiency
2.3.6. Data Statistical Analysis
3. Results and Analysis
3.1. Effects of Different Water and Nitrogen Treatments on the Growth of Apple Leaves
3.2. Effects of Different Water and Nitrogen Treatments on Photosynthetic Efficiency of Apple Leaves
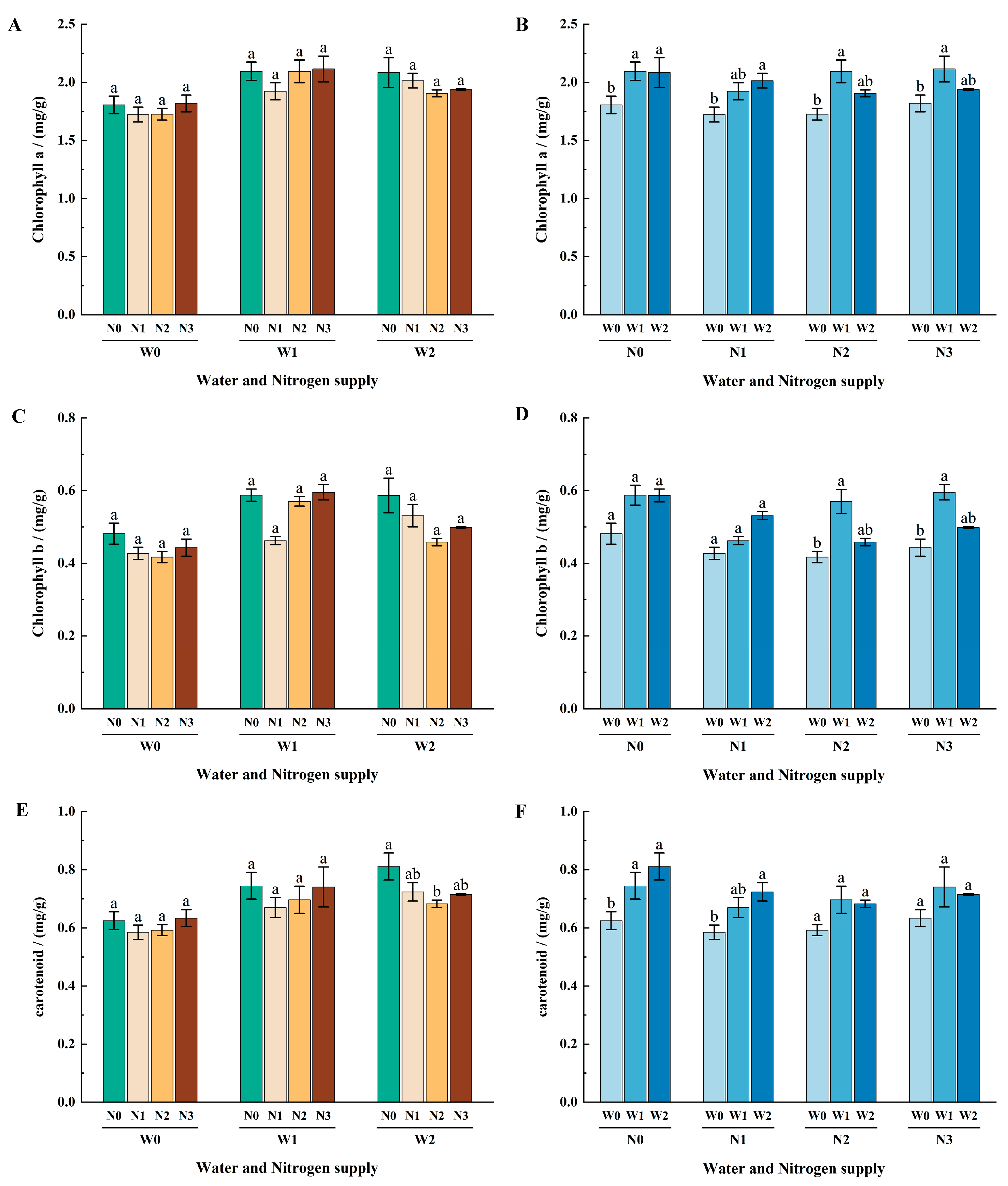
3.3. Effects of Different Water and Nitrogen Treatments on Chlorophyll Content in Apple Leaves
3.4. Effects of Different Water and Nitrogen Treatments on Mineral Elements in Apple Leaves
3.5. Correlation Analysis of Functional Structure, Functional Traits and Nitrogen Use Efficiency of Apple Leaves under Different Water and Nitrogen Treatments
3.6. Effects of Different Water and Nitrogen Treatments on the Absorption and Utilization of 15N-Urea in Apple Tree Tissues and Organs
3.6.1. Ndff% of Different Organs of Apple Plant under Different Water and Nitrogen Treatments
3.6.2. Effects of Different Water and Nitrogen Treatments on TN Content, 15N Uptake and 15N Utilization Rate of Plants
3.7. Economic Analysis of Irrigation Nitrogen Coupling
4. Discussion
4.1. Effects of Different Water and Nitrogen Treatments on the Growth of Apple Leaves
4.2. Effects of Different Water and Nitrogen Treatments on Functional Traits and Nitrogen Use Efficiency of Apple Leaves
4.3. Effects of Different Water and Nitrogen Treatments on 15N (Ndff%) and Nitrogen Use Efficiency in Different Organs of Apple Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, X.; Fahad, S.; Zhao, P.; Yan, B.; Liu, T. The Trilogy of the Chinese Apple Futures Market: Price Discovery, Risk-Hedging and Cointegration. Sustainability 2022, 14, 12864. [Google Scholar] [CrossRef]
- Fan, K.; Fu, L.; Liu, H.; Qu, J.; Zhang, G.; Zhang, S.; Qiao, K. Reduced Sensitivity to Tebuconazole in Botryosphaeria dothidea Isolates Collected from Major Apple Production Areas of China. Plant Dis. 2022, 106, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, T. Spatiotemporal evolution and suitability of apple production in China from climate change and land use transfer perspectives. Food Energy Secur. 2022, 11, e386. [Google Scholar] [CrossRef]
- Dai, Z.; Fei, L.; Zeng, J.; Huang, D.; Liu, T. Optimization of water and nitrogen management for surge-root irrigated apple trees in the Loess Plateau of China. J. Integr. Agric. 2021, 20, 260–273. [Google Scholar] [CrossRef]
- Atkinson, J.C.; Taylor, L.; Taylor, M.J. The influence of temperature and water supply on apple fruit growth and the development of orchard-grown trees. J. Hortic. Sci. 2015, 70, 691–703. [Google Scholar]
- Giuliani, N.; Aguzzoni, A.; Penna, D.; Tagliavini, M. Estimating uptake and internal transport dynamics of irrigation water in apple trees using deuterium-enriched water. Agric. Water Manag. 2023, 289, 108532. [Google Scholar]
- Jiang, C.; Lu, D.; Zu, C.; Shen, J.; Wang, S.; Guo, Z.; Zhou, J.; Wang, H. One-time root-zone N fertilization increases maize yield, NUE and reduces soil N losses in lime concretion black soil. Sci. Rep. 2018, 8, 10258. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.M.; Shao, H.X.; Chen, H.L.; Chang, T.T.; Wang, W.N.; Yang, Q.; Wang, Y.F. Study on Nitrogen Utilization Efficiency of Flue-Cured Tobacco with 15N Tracing Technique. Adv. Mater. Res. 2012, 1793, 520–524. [Google Scholar] [CrossRef]
- Haefele, S.M.; Jabbar, S.M.A.; Siopongco, J.; Tirol-Padre, A.; Amarante, S.T.; Sta Cruz, P.C.; Cosico, W.C. Nitrogen use efficiency in selected rice (Oryza sativa L.) genotypes under different water regimes and nitrogen levels. Field Crops Res. 2008, 107, 137–146. [Google Scholar] [CrossRef]
- Guo, J.; Fan, J.; Xiang, Y.; Zhang, F.; Yan, S.; Zhang, X.; Zheng, J.; Li, Y.; Tang, Z.; Li, Z. Coupling effects of irrigation amount and nitrogen fertilizer type on grain yield, water productivity and nitrogen use efficiency of drip-irrigated maize. Agric. Water Manag. 2022, 261, 107389. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; He, X.; Gong, P.; Lin, E. Water–nitrogen coupling and multi-objective optimization of cotton under mulched drip irrigation in arid northwest China. Agronomy 2019, 9, 894. [Google Scholar] [CrossRef]
- Badr, M.A.; El-Tohamy, W.A.; Zaghloul, A.M. Yield and water use efficiency of potato grown under different irrigation and nitrogen levels in an arid region. Agric. Water Manag. 2012, 110, 9–15. [Google Scholar] [CrossRef]
- Ma, Z.; Yin, J.; Yang, Y.; Sun, F.; Yang, Z. Effect of water and nitrogen coupling regulation on the growth, physiology, yield, and quality attributes and comprehensive evaluation of wolfberry (Lycium barbarum L.). Front. Plant Sci. 2023, 14, 1130109. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, H.M.A.A.; Wang, L.; Liu, H. Improved greenhouse cucumber production under deficit water and fertilization in Northern China. Int. J. Agric. Biol. Eng. 2018, 11, 42–46. [Google Scholar]
- Li, G.H.; Liu, P.P.; Zhao, B.; Dong, S.T.; Liu, P.; Zhang, J.W.; Tian, C.X.; He, Z.J. Effects of water conditions and controlled release urea on yield and leaf senescence physiological characteristics in summer maize. Ying Yong Sheng Tai Xue Bao 2017, 28, 571–580. [Google Scholar] [PubMed]
- Cui, Q.Q.; Dong, Y.H.; Li, M.; Zhang, W.D.; Liu, B.B.; Ai, X.Z.; Bi, H.G.; Li, Q.M. Effect of water-nitrogen coupling on photosynthesis and ultrastructure of cucumber leaves under CO2 enrichment. Ying Yong Sheng Tai Xue Bao 2017, 28, 1237–1245. [Google Scholar] [PubMed]
- Zhou, C.; Le, J.; Hua, D.; He, T.; Mao, J. Imaging Analysis of Chlorophyll Fluorescence Induction for Monitoring Plant Water and Nitrogen Treatments. Measurement 2018, 136, 478–486. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, M.; Li, Y.; Zhang, B.; Wu, X.; Shi, Y.; Liu, H. Evaluating the Combined Effects of Water and Fertilizer Coupling Schemes on Pear Vegetative Growth and Quality in North China. Agronomy 2023, 13, 867. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Tessier, J.T.; Raynal, D.J. Use of nitrogen to phosphorus ratios in plant tissue as an indicator of nutrient limitation and nitrogen saturation. J. Appl. Ecol. 2003, 40, 523–534. [Google Scholar] [CrossRef]
- Pan, N.; Shen, H.; Wu, D.M.; Deng, L.S.; Tu, P.F.; Gan, H.H.; Liang, Y.C. Mechanism of improved phosphate uptake efficiency in banana seedlings on acidic soils using fertigation. Agric. Water Manag. 2010, 98, 632–638. [Google Scholar] [CrossRef]
- Dai, Z.; Fei, L.; Huang, D.; Zeng, J.; Chen, L.; Cai, Y. Coupling effects of irrigation and nitrogen levels on yield, water and nitrogen use efficiency of surge-root irrigated jujube in a semiarid region. Agric. Water Manag. 2019, 213, 146–154. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Hu, T.; Geng, C.; Liu, J. Optimization of irrigation and nitrogen levels for a trade-off: Yield, quality, water use efficiency and environment effect in a drip-fertigated apple orchard based on TOPSIS method. Sci. Hortic. 2023, 309, 111700. [Google Scholar] [CrossRef]
- Cai, S.; Zheng, B.; Zhao, Z.; Zheng, Z.; Yang, N.; Zhai, B. Precision Nitrogen Fertilizer and Irrigation Management for Apple Cultivation Based on a Multilevel Comprehensive Evaluation Method of Yield, Quality, and Profit Indices. Water 2023, 15, 468. [Google Scholar] [CrossRef]
- He, Z.; Cao, H.; Hu, Q.; Zhang, Y.; Nan, X.; Li, Z. Optimization of apple irrigation and N fertilizer in Loess Plateau of China based on ANP-EWM-TOPSIS comprehensive evaluation. Sci. Hortic. 2023, 311, 111794. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.; Huang, D.; Dong, Q.; Li, P.; Ma, F. High-efficient utilization and uptake of N contribute to higher NUE of ‘Qinguan’ apple under drought and N-deficient conditions compared with ‘Honeycrisp’. Tree Physiol. 2019, 39, 1880–1895. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Zhong, F.; Jin, Q.; Liu, E.; Feng, J.; Wang, T.; Gao, Y. Fate of nitrogen-15 in the subsequent growing season of greenhouse tomato plants (Lycopersicon esculentum Mill) as influenced by alternate partial root-zone irrigation. RSC Adv. 2017, 7, 34392–34400. [Google Scholar] [CrossRef]
- Hartmut, K.; Alan, R.W. Determinations of total carotenoids and chlorophylls b of leaf extracts in different solvents. Analysis 1983, 4, 142–196. [Google Scholar]
- Kacar, B. Chemical analyses of plant and soil. II Bitki Analizleri. Agric. Fac. Publ. 1972, 453, 646. [Google Scholar]
- Kitson, R.E.; Mellon, M.G. Colorimetric determination of phosphorus as molybdivanadophosphoric acid. Ind. Eng. Chem. Anal. Ed. 1944, 16, 379–383. [Google Scholar] [CrossRef]
- Wang, C.; Isoda, A.; Li, M. Growth and eco-physiological performance of cotton under water stress conditions. Agric. Sci. China 2007, 6, 949–955. [Google Scholar] [CrossRef]
- Feng, X.; Li, Y.; Gu, J.; Zhuo, Y.; Yang, H. Error thresholds for quasispecies on single peak Gaussian-distributed fitness landscapes. J. Theor. Biol. 2007, 246, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Shi, Y.; Yin, B.; Zhou, S.; Li, Z.; Zhang, X.; Xu, J. Effect of different dwarfing interstocks on the vegetative growth and nitrogen utilization efficiency of apple trees under low-nitrate and drought stress. Sci. Hortic. 2022, 305, 111369. [Google Scholar] [CrossRef]
- Cabello, M.J.; Castellanos, M.T.; Romojaro, F.; Martínez-Madrid, C.; Ribas, F. Yield and quality of melon grown under different irrigation and nitrogen rates. Agric. Water Manag. 2008, 96, 866–874. [Google Scholar] [CrossRef]
- Khaembah, E.N.; Maley, S.; Gillespie, R.; George, M.; Michel, A.J.; Chakwizira, E.; de Ruiter, J.; Teixeira, E. Water and nitrogen stress effects on canopy development and biomass allocation in fodder beet (Beta vulgaris L.). N. Z. J. Agric. Res. 2022, 65, 63–81. [Google Scholar] [CrossRef]
- Yang, M.; Li, Z.; Liu, Y.; Huang, L.; Yang, Y.; Geng, F. Study on diurnal variation of photosynthesis in Magnolia grandiflora L. and Magnolia denudata Desr. Acta Hortic. 2008, 769, 433–440. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J. Effects of Water and Nitrogen Coupling on Photosynthesis and Chlorophyll Content of Hybrid Broussonetia papyrifera in Red Soil Area. J. Agric. Hortic. 2021, 3, 1–9. [Google Scholar]
- Zhao, D.; Glaz, B.; Comstock, C.J. Physiological and Growth Responses of Sugarcane Genotypes to Nitrogen Rate on a Sand Soil. J. Agron. Crop Sci. 2014, 200, 290–301. [Google Scholar] [CrossRef]
- Ahmed, U.S.; Senge, M.; Ito, K.; Adomako, T.J. Effects of Water Stress on Soil Plant Analytical Development (SPAD) Chlorophyll Meter Reading and its Relationship to Nitrogen Status and Grain Yield of Soybean under Different Soil Types. J. Rainwater Catchment Syst. 2010, 15, 33–38. [Google Scholar] [CrossRef]
- Han, X.; Sistla, S.A.; Zhang, Y.H.; Lü, X.T.; Han, X.G. Hierarchical responses of plant stoichiometry to nitrogen deposition and mowing in a temperate steppe. Plant Soil 2014, 382, 175–187. [Google Scholar] [CrossRef]
- Boardman, N.K. Comparative photosynthesis of sun and shade plants. Annu. Rev. Plant Physiol. 1977, 28, 355–377. [Google Scholar] [CrossRef]
- Takashima, T.; Hikosaka, K.; Hirose, T. Photosynthesis or persistence: Nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ. 2004, 27, 1047–1054. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K. Interspecific difference in the photosynthesis-nitrogen relationship: Patterns, physiological causes, and ecological importance. J. Plant Res. 2004, 117, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Mooney, H.A.; Field, C.; Gulmon, S.L.; Bazzaz, F.A. Photosynthetic capacity in relation to leaf position in desert versus old-field annuals. Oecologia 1981, 50, 109–112. [Google Scholar] [CrossRef]
- Gulias, J.; Flexas, J.; Mus, M.; Cifre, J.; Lefi, E.; Medrano, H. Relationship between maximum leaf photosynthesis, nitrogen content and specific leaf area in Balearic endemic and non-endemic Mediterranean species. Ann. Bot. 2003, 92, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Garnier, E.; Salager, J.L.; Laurent, G.; Sonié, L. Relationships between photosynthesis, nitrogen and leaf structure in 14 grass species and their dependence on the basis of expression. New Phytol. 1999, 143, 119–129. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S.; Uhl, C. Photosynthesis-nitrogen relations in Amazonian tree species: I. Patterns among species communities. Oecologia 1994, 97, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhao, X.; Li, Y. Relationship between the trait response of aboveground and belowground parts of dominant plant species to groundwater depth change in Horqin Sandy Land, eastern China. Ecol. Indic. 2023, 156, 111001. [Google Scholar] [CrossRef]
- Xiong, D.L.; Liu, X.; Liu, L.M.; Douthe, C.; Li, Y.; Peng, S.B.; Huang, J.L. Rapid responses of mesophyll conductance to changes of CO2 concentration, temperature and irradiance are affected by N supplements in rice. Plant Cell Environ. 2015, 38, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, Y.; Wu, G.; Wang, X.; Yuan, M.; Wang, J.; He, W.; Chen, F.; LeCocq, K.; Wang, L.; et al. Amendment with controlled release urea increases leaf morpho-physiological traits, grain yield and NUE in a double-cropping rice system in southern China. J. Sci. Food Agric. 2023, 103, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhang, Z.; Li, Y.; Zhang, Y.; Dong, H.; Fang, Y.; Han, L.; Xu, W.; Hu, L. Genetic improvement analysis of nitrogen uptake, utilization, translocation, and distribution in Chinese wheat in Henan Province. Field Crops Res. 2022, 277, 108406. [Google Scholar] [CrossRef]
- Ma, S.; Yue, J.; Wang, J.; Jia, Z.; Li, C.; Zeng, J.; Liu, X.; Zhang, J. Arbuscular Mycorrhizal Fungi Alleviate Salt Stress Damage by Coordinating Nitrogen Utilization in Leaves of Different Species. Forests 2022, 13, 1568. [Google Scholar] [CrossRef]
- Gu, M.R.; Shu, H.R.; Zhou, H.W. Apple N nutrition research IV. Storage 15N and distribution characteristics. Acta Hortic. Sin. 1986, 13, 25–30. [Google Scholar]
- Li, H.; Ge, S.; Jiang, Y.; Peng, F.; Wei, S.; Fang, X. Characteristics of absorption, utilization and distribution of 15N-urea application in different depths in Gala/Malus hupehensis. Agric. Sci. China 2011, 44, 1408–1414. [Google Scholar]
- Zhao, L.; Jiang, Y.; Peng, F.; Li, P.; Wang, L.; Li, H. Characteristics of absorption, utilization and distribution of spring soil 15N-urea application for Gala apple tree. Plant Nutr. Fertil. Sci. 2009, 15, 1439–1443. [Google Scholar]
- Zhang, L.; Han, M.; Zhao, C.; Liu, C.; Zhang, F.; Zhang, L.; Li, B. 15nitrogen study on absorption, distribution and utilization of nitrogen applied in early summer in red fuji apple. J. Plant Nutr. 2012, 35, 1557–1571. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Z.X.; Xing, Y.; Wu, X.X.; Ge, S.F.; Jiang, Y.M. Effects of split combined application of organic-inorganic fertilizers on plant growth, 15N absorption, utilization and loss of Gala apple tree. Ying Yong Sheng Tai Xue Bao 2019, 30, 1367–1372. (In Chinese) [Google Scholar] [PubMed]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Dong, S.; Fuchigami, L.H. Urea uptake and nitrogen mobilization by apple leaves in relation to tree nitrogen status in autumn. J. Hortic. Sci. Biotechnol. 2002, 77, 13–18. [Google Scholar] [CrossRef]
- Li, H.; Zhu, X.; Wang, J.; Wei, Y.; Nai, F.; Yu, H.; Wang, X. Unraveling differential characteristics and mechanisms of nitrogen uptake in wheat cultivars with varied nitrogen use efficiency. Plant Physiol. Biochem. 2023, 206, 108278. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Cao, H.; Liu, S.; Gu, X.; Cao, Y. Response of yield, quality, water and nitrogen use efficiency of tomato to different levels of water and nitrogen under drip irrigation in Northwestern China. J. Integr. Agric. 2016, 16, 1153–1161. [Google Scholar] [CrossRef]
- Scheer, C.; Rowlings, D.W.; Antille, D.L.; De Antoni Migliorati, M.; Fuchs, K.; Grace, P.R. Improving nitrogen use efficiency in irrigated cotton production. Nutr. Cycl. Agroecosyst. 2022, 125, 95–106. [Google Scholar] [CrossRef]
- Navnit, K.; Geeta, K.; Anil, K.; Sudhir, P. Influence of manuring and irrigation scheduling on system productivity, resource use efficiency, nutrient uptake and incidence of early shoot-borer (Chilo infuscatellus) in sugarcane (Saccharum spp. hybrid complex) and garlic (Allium sativum) intercropping system. Indian J. Agron. 2017, 61, 297–306. [Google Scholar]
- Liu, R.; Yang, Y.; Wang, Y.; Wang, S.Y.; Wang, X.C.; Rengel, Z.; Zhang, W.J.; Shu, L.Z. Alternate partial root-zone drip irrigation with nitrogen fertigation promoted tomato growth, water and fertilizer-nitrogen use efficiency. Agric. Water Manag. 2020, 233, 106049. [Google Scholar] [CrossRef]
- Wang, F.; Sha, J.; Chen, Q.; Xu, X.; Zhu, Z.; Ge, S.; Jiang, Y. Exogenous abscisic acid regulates distribution of 13C and 15N and anthocyanin synthesis in ‘Red Fuji’apple fruit under high nitrogen supply. Front. Plant Sci. 2020, 10, 504382. [Google Scholar] [CrossRef] [PubMed]
- San-Martino, L.; Sozzi, G.O.; San-Martino, S.; Lavado, R.S. Isotopically-labelled nitrogen uptake and partitioning in sweet cherry as influenced by timing of fertilizer application. Sci. Hortic. 2010, 126, 42–49. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Use of Isotope and Radiation Methods in Soil and Water Management and Crop Nutrition; Training Course Series 14; FAO/IAEA: Vienna, Austria, 2001. [Google Scholar]
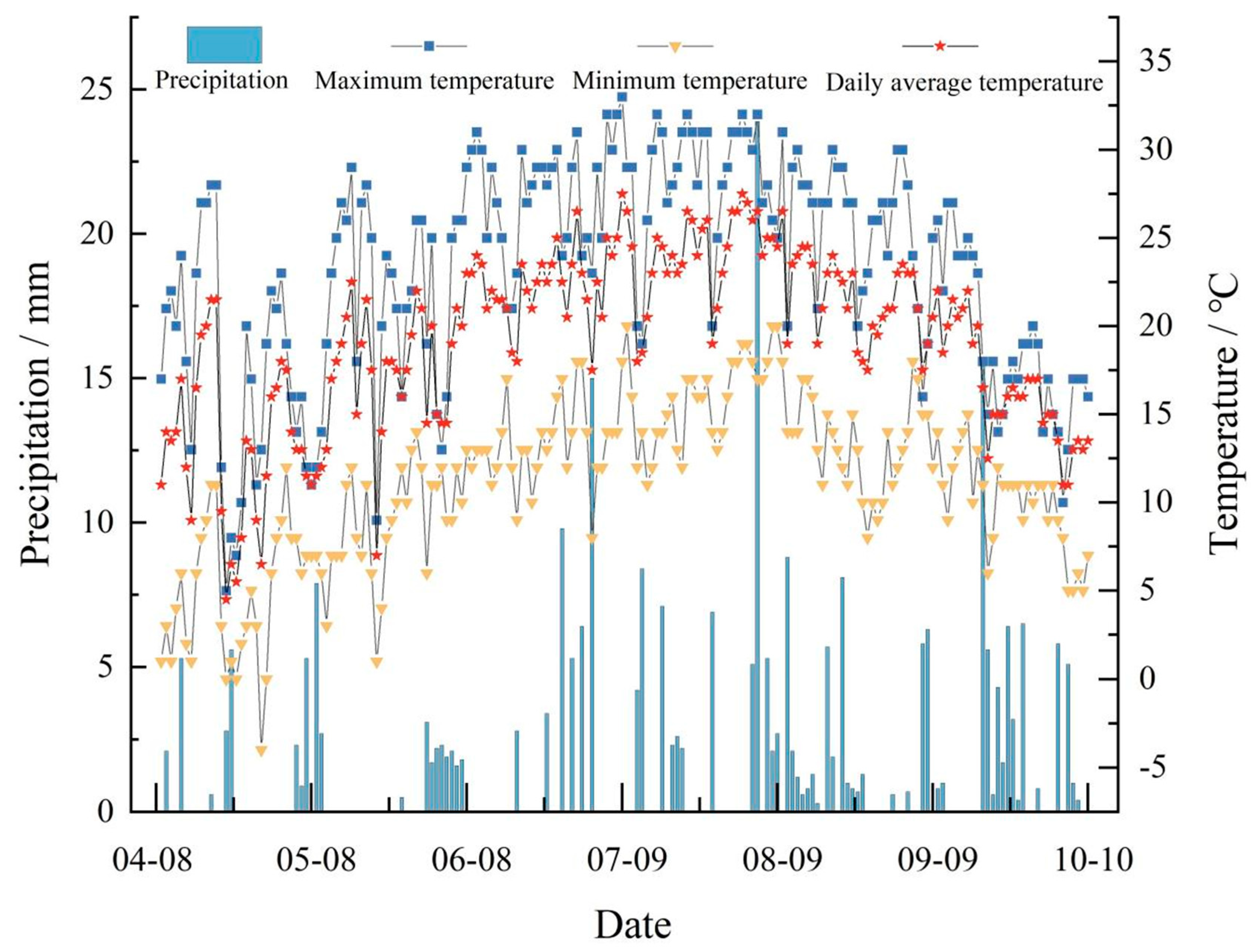
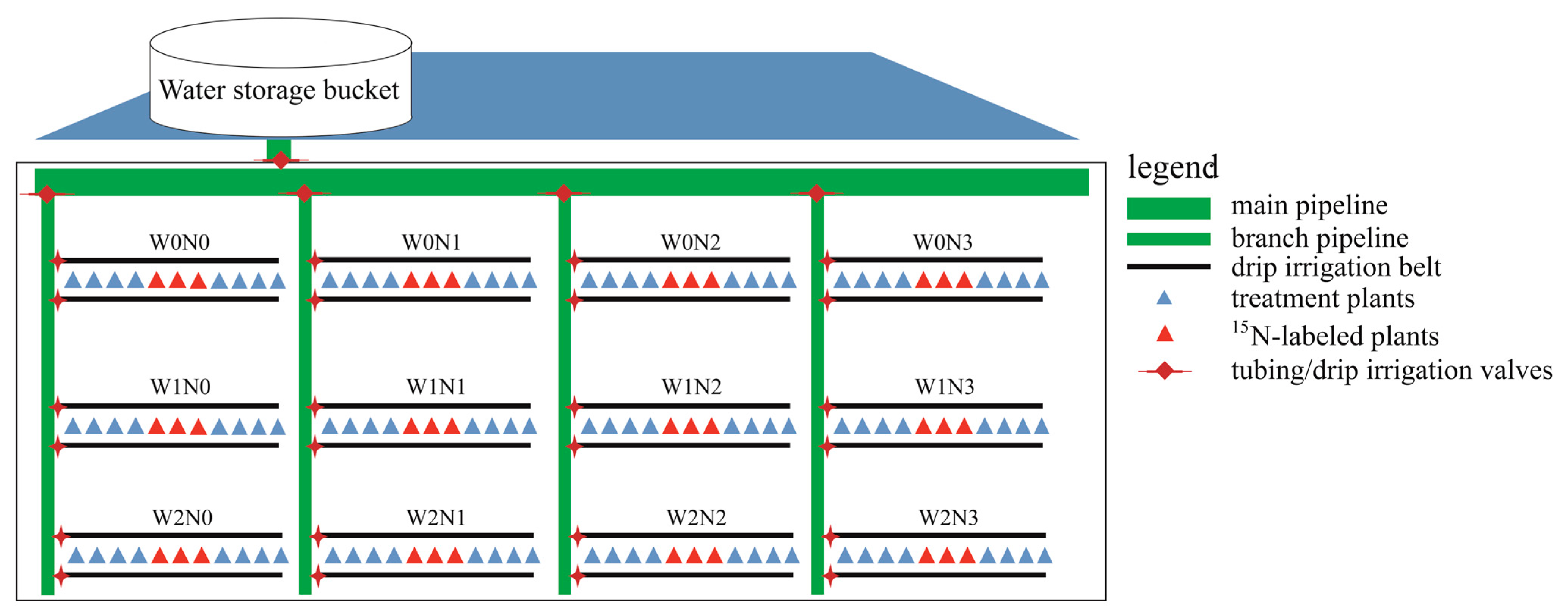
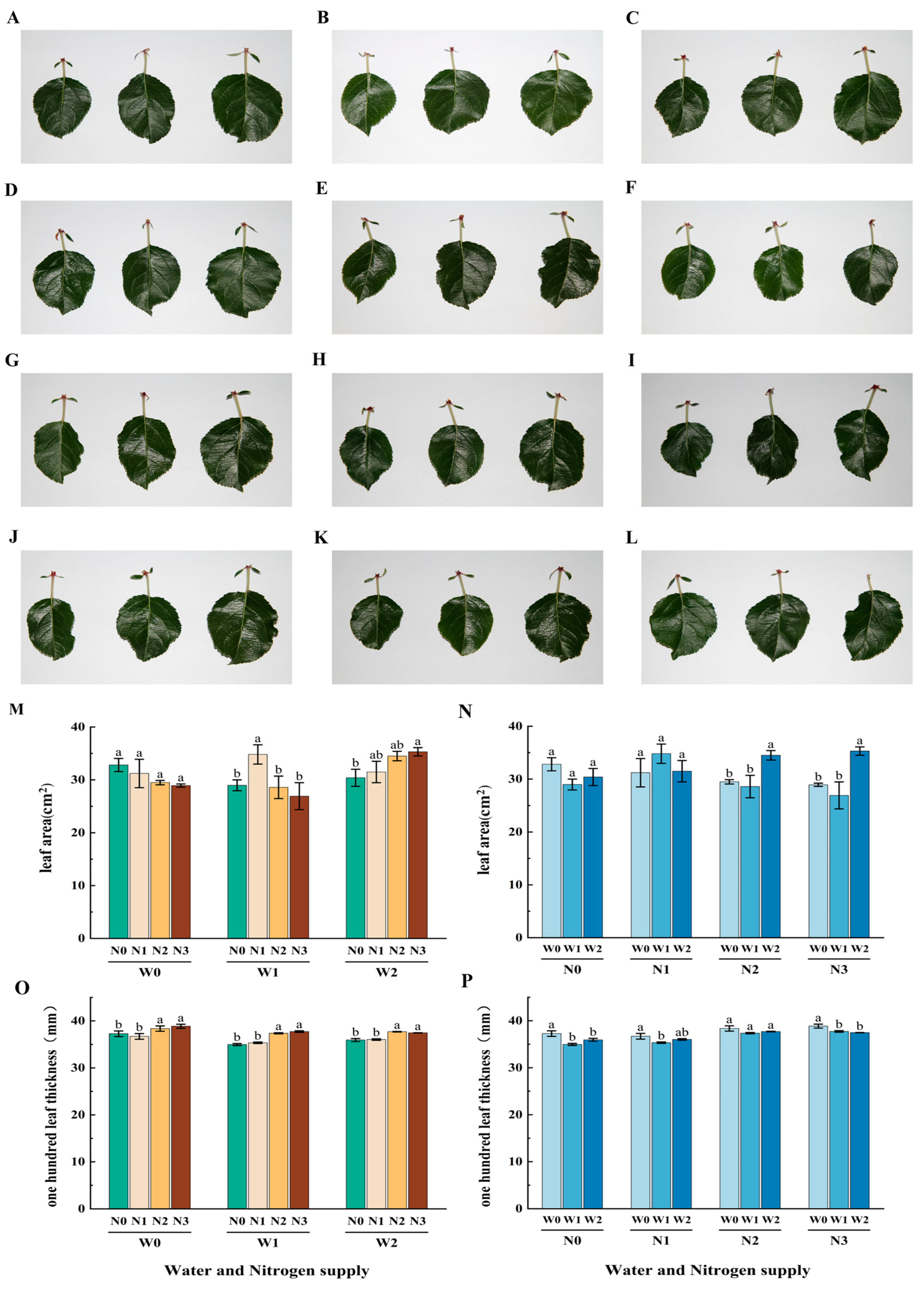
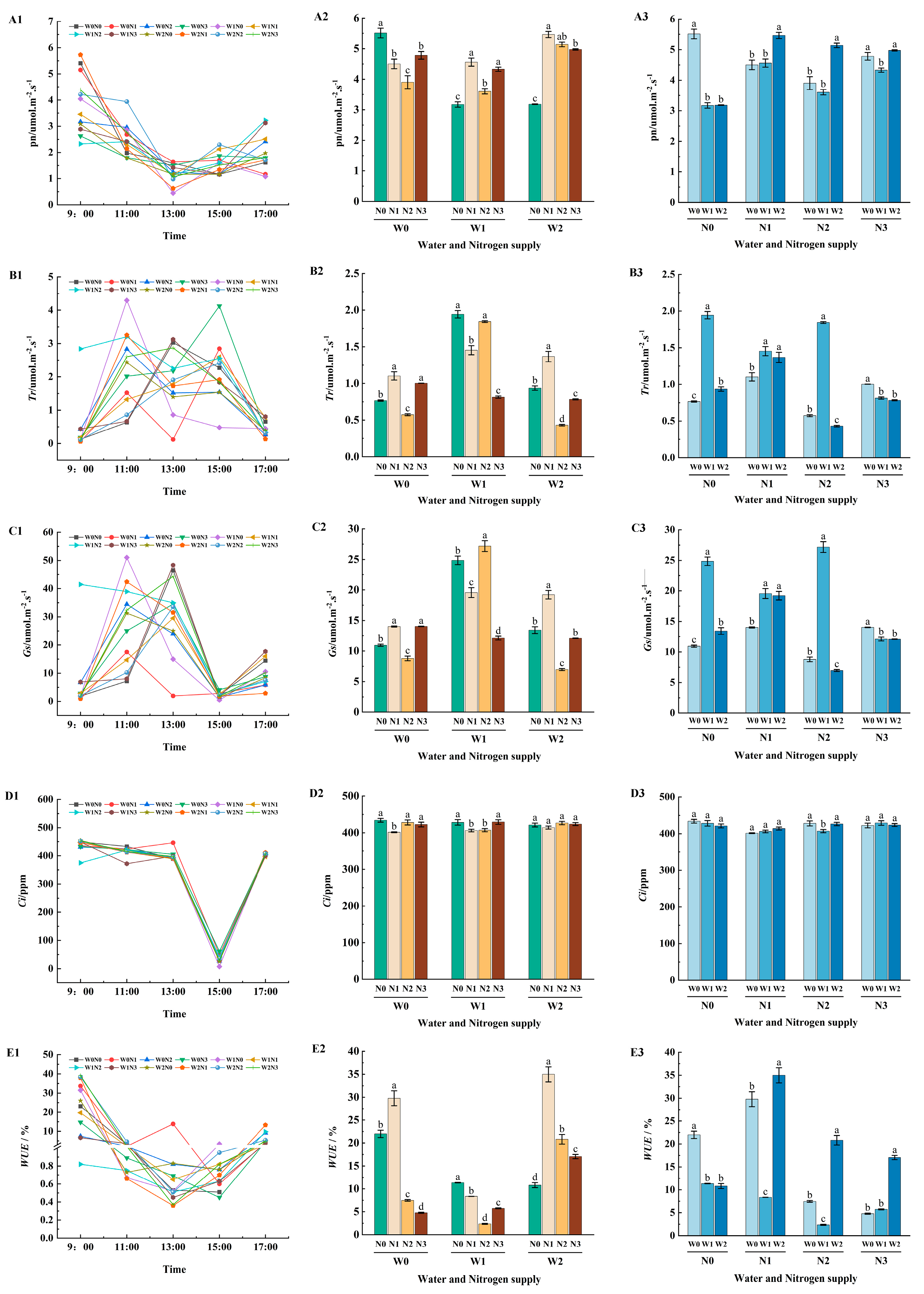

| Phenological Period | Name | Budding and Leafing Period | Flowering and Fruiting Period | Young Fruit Swelling Period | Fruit Rapid Expansion Period | Fruit Color Conversion Period | Whole Growth Period |
|---|---|---|---|---|---|---|---|
| Date | 4.8~5.2 | 5.3~5.22 | 5.23~6.30 | 7.1~8.24 | 8.25~10.10 | 4.8~10.10 | |
| Rainfall/mm | 16.4 | 19.1 | 38.5 | 126.8 | 74.7 | 275.5 | |
| Effective rainfall/mm | 10.9 | 13.2 | 25.6 | 100.7 | 57.2 | 207.6 | |
| Treatment | TN (g/kg) | TP (g/kg) | TK (g/kg) |
|---|---|---|---|
| W0N0 | 23.0 ± 0.01 d | 0.9 ± 0.4 abc | 14.6 ± 0.9 a |
| W0N1 | 26.0 ± 0.4 bc | 0.6 ± 0.1 c | 10.8 ± 1.6 bc |
| W0N2 | 25.8 ± 0.3 bc | 1.1 ± 0.1 a | 12.1 ± 0.4 b |
| W0N3 | 23.0 ± 0.6 d | 1.0 ± 0.3 abc | 7.9 ± 0.8 de |
| W1N0 | 25.0 ± 0.3 c | 0.6 ± 0.2 bc | 3.8 ± 0.8 g |
| W1N1 | 26.5 ± 0.1 b | 0.6 ± 0.1 bc | 8.0 ± 1.3 de |
| W1N2 | 28.9 ± 1.4 a | 1.0 ± 0.3 ab | 7.0 ± 0.8 ef |
| W1N3 | 26.7 ± 0.4 b | 0.7 ± 0.2 abc | 12.1 ± 0.8 b |
| W2N0 | 25.1 ± 0.5 c | 0.8 ± 0.3 abc | 7.3 ± 0.4 e |
| W2N1 | 27.9 ± 1.0 a | 0.7 ± 0.1 abc | 9.3 ± 0.9 cd |
| W2N2 | 28.5 ± 0.5 a | 0.7 ± 0.1 abc | 5.3 ± 0.5 fg |
| W2N3 | 23.3 ± 0.3 d | 0.6 ± 0.0 bc | 5.4 ± 1.7 fg |
| Treatment | Leaf | Annual Branches | Perennial Branches | Root | Fruit |
|---|---|---|---|---|---|
| W0N1 | 1.01 ± 0.12 a | 0.31 ± 0.02 b | 0.60 ± 0.04 b | 0.36 ± 0.07 ab | 0.06 ± 0.03 ab |
| W0N2 | 1.04 ± 0.15 a | 0.24 ± 0.04 b | 0.26 ± 0.04 d | 0.37 ± 0.08 ab | 0.06 ± 0.03 ab |
| W0N3 | 1.23 ± 0.11 a | 0.45 ± 0.06 a | 0.41 ± 0.03 c | 0.43 ± 0.06 a | 0.05 ± 0.03 ab |
| W1N1 | 1.12 ± 0.09 a | 0.45 ± 0.08 a | 0.36 ± 0.02 c | 0.41 ± 0.09 a | 0.03 ± 0.00 b |
| W1N2 | 1.22 ± 0.06 a | 0.28 ± 0.04 b | 1.16 ± 0.11 a | 0.42 ± 0.09 a | 0.08 ± 0.02 a |
| W1N3 | 1.09 ± 0.09 a | 0.31 ± 0.02 b | 0.16 ± 0.03 ef | 0.29 ± 0.01 b | 0.05 ± 0.01 ab |
| W2N1 | 1.13 ± 0.21 a | 0.29 ± 0.04 b | 0.12 ± 0.02 f | 0.37 ± 0.07 ab | 0.04 ± 0.02 ab |
| W2N2 | 1.04 ± 0.09 a | 0.30 ± 0.02 b | 0.24 ± 0.04 de | 0.35 ± 0.03 ab | 0.04 ± 0.02 ab |
| W2N3 | 1.20 ± 0.05 a | 0.06 ± 0.02 c | 0.18 ± 0.02 def | 0.36 ± 0.03 ab | 0.05 ± 0.01 ab |
| Treatment | TN Content of Plant/g | 15N Absorbed from 15N-Urea/mg | 15N-Urea Utilization Rate % |
|---|---|---|---|
| W0N1 | 68.98 ± 2.37 bc | 0.45 ± 0.06 c | 37.78 ± 5.29 a |
| W0N2 | 64.34 ± 3.86 cd | 0.44 ± 0.07 c | 18.27 ± 2.79 c |
| W0N3 | 75.24 ± 4.18 b | 0.6 ± 0.06 ab | 16.62 ± 1.62 c |
| W1N1 | 68.94 ± 1.23 bc | 0.52 ± 0.04 bc | 43.33 ± 3.6 a |
| W1N2 | 83.22 ± 3.13 a | 0.65 ± 0.02 a | 26.94 ± 0.71 b |
| W1N3 | 61.07 ± 2.93 d | 0.45 ± 0.05 c | 12.59 ± 1.48 c |
| W2N1 | 61.83 ± 4.84 d | 0.48 ± 0.1 c | 39.72 ± 8.31 a |
| W2N2 | 62.67 ± 2.77 cd | 0.43 ± 0.05 c | 17.88 ± 2.13 c |
| W2N3 | 59.96 ± 0.71 d | 0.5 ± 0.02 bc | 13.89 ± 0.6 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, G.; Feng, Y.; Ma, W.; Feng, T.; Lu, Y.; Mao, J.; Chen, B. 15N Isotope Labeled Tracking of the Nitrogen Utilization of Apple under Water and Nitrogen Coupling Application in Arid and Semiarid Areas. Agronomy 2024, 14, 1245. https://doi.org/10.3390/agronomy14061245
Lan G, Feng Y, Ma W, Feng T, Lu Y, Mao J, Chen B. 15N Isotope Labeled Tracking of the Nitrogen Utilization of Apple under Water and Nitrogen Coupling Application in Arid and Semiarid Areas. Agronomy. 2024; 14(6):1245. https://doi.org/10.3390/agronomy14061245
Chicago/Turabian StyleLan, Guanquecailang, Yongqing Feng, Weifeng Ma, Tong Feng, Yang Lu, Juan Mao, and Baihong Chen. 2024. "15N Isotope Labeled Tracking of the Nitrogen Utilization of Apple under Water and Nitrogen Coupling Application in Arid and Semiarid Areas" Agronomy 14, no. 6: 1245. https://doi.org/10.3390/agronomy14061245





