Screening of Miscanthus Genotypes for Sustainable Production of Microcrystalline Cellulose and Cellulose Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Extraction of MCC
2.3. Extraction of CNCs
2.4. Measurement of Chemical Composition and Physical Features
2.5. The Development of Extra Trees Models
2.6. Statistical Analysis
3. Results
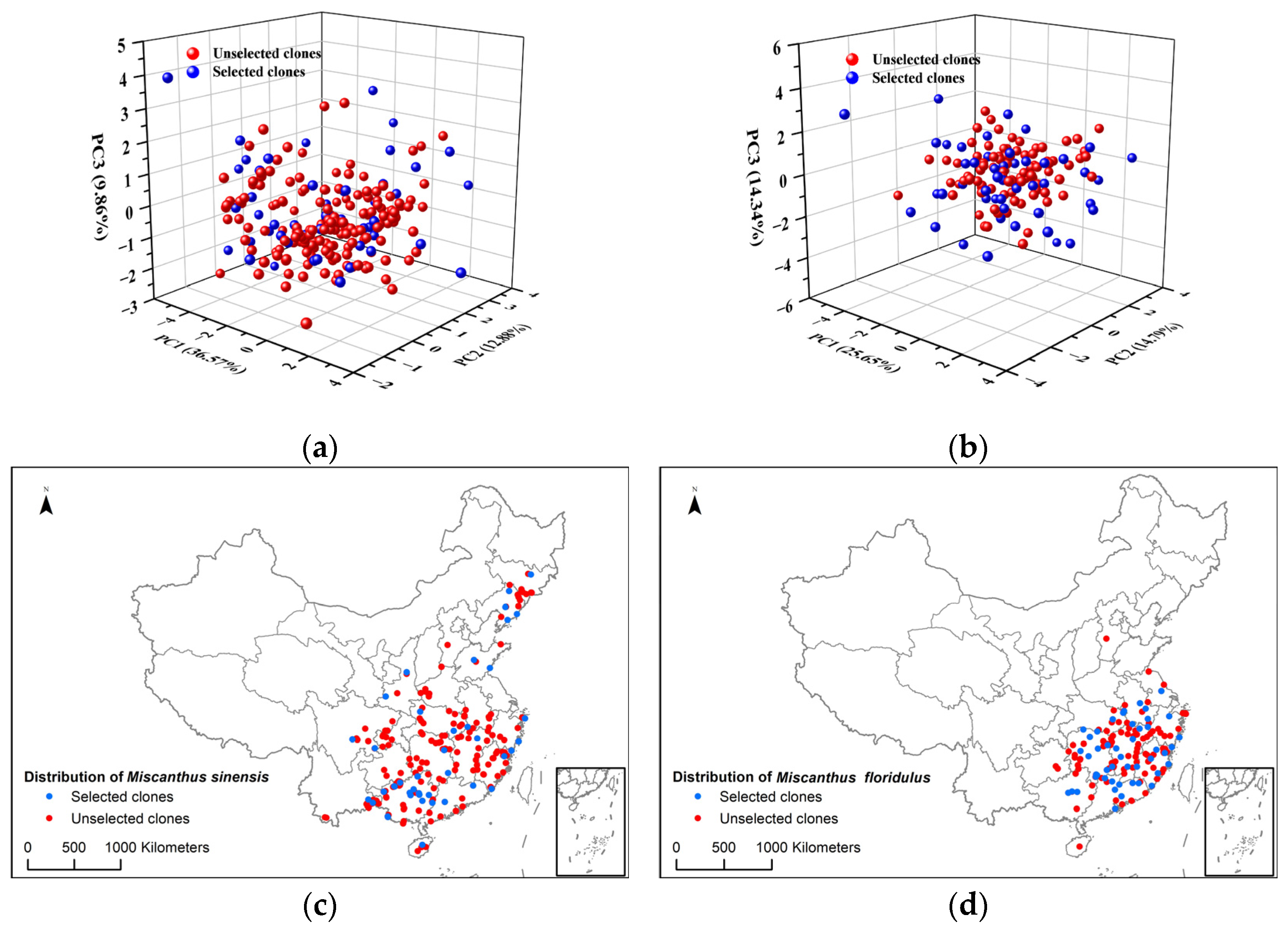
3.1. Selection of Representative Clones of M. sinensis and M. floridulus
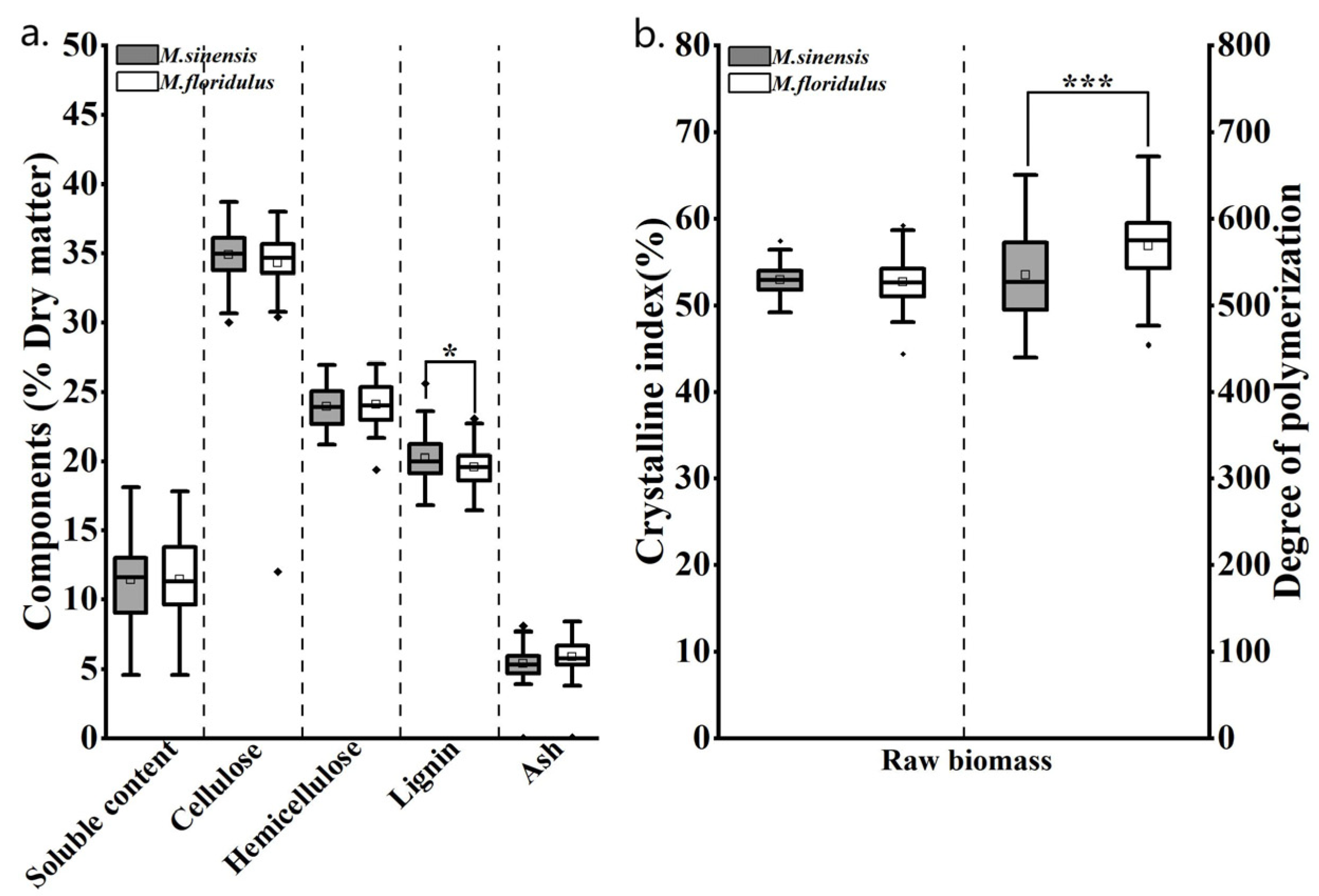
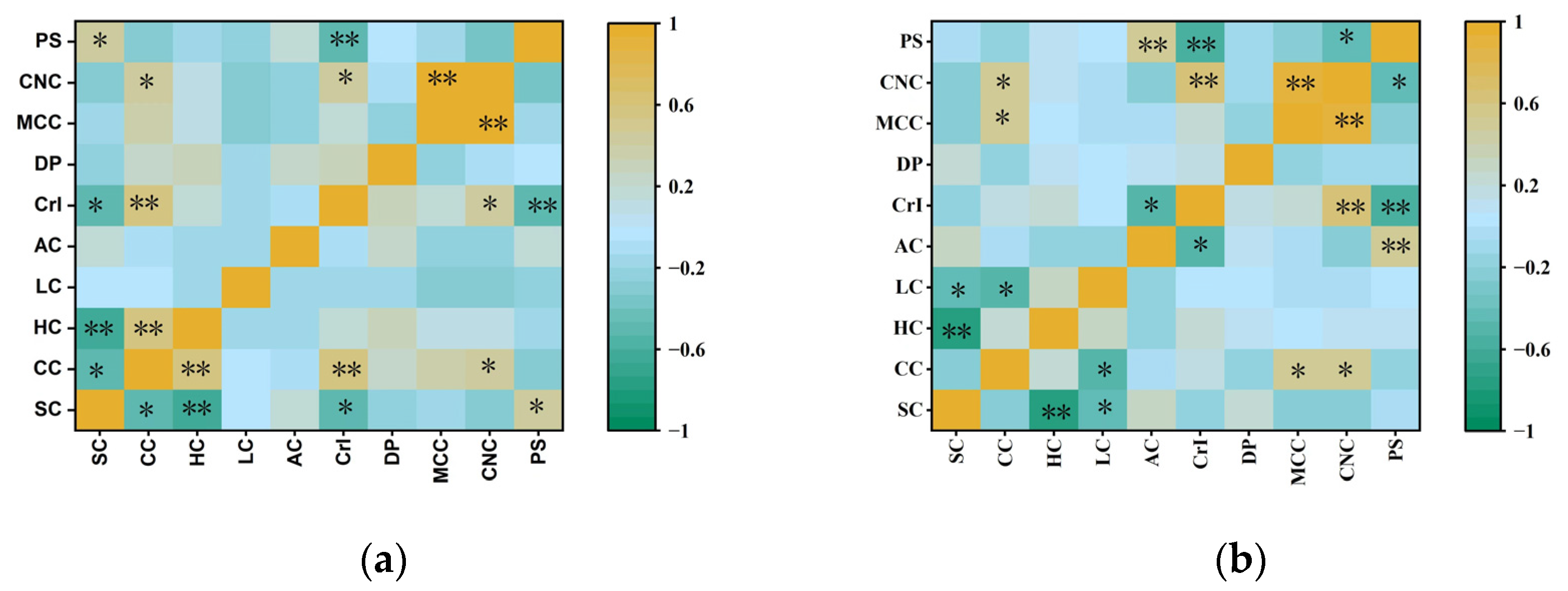
3.2. Diversity Analysis of Agronomic Traits and Biomass Quality Traits of Representative Clones of M. sinensis and M. floridulus
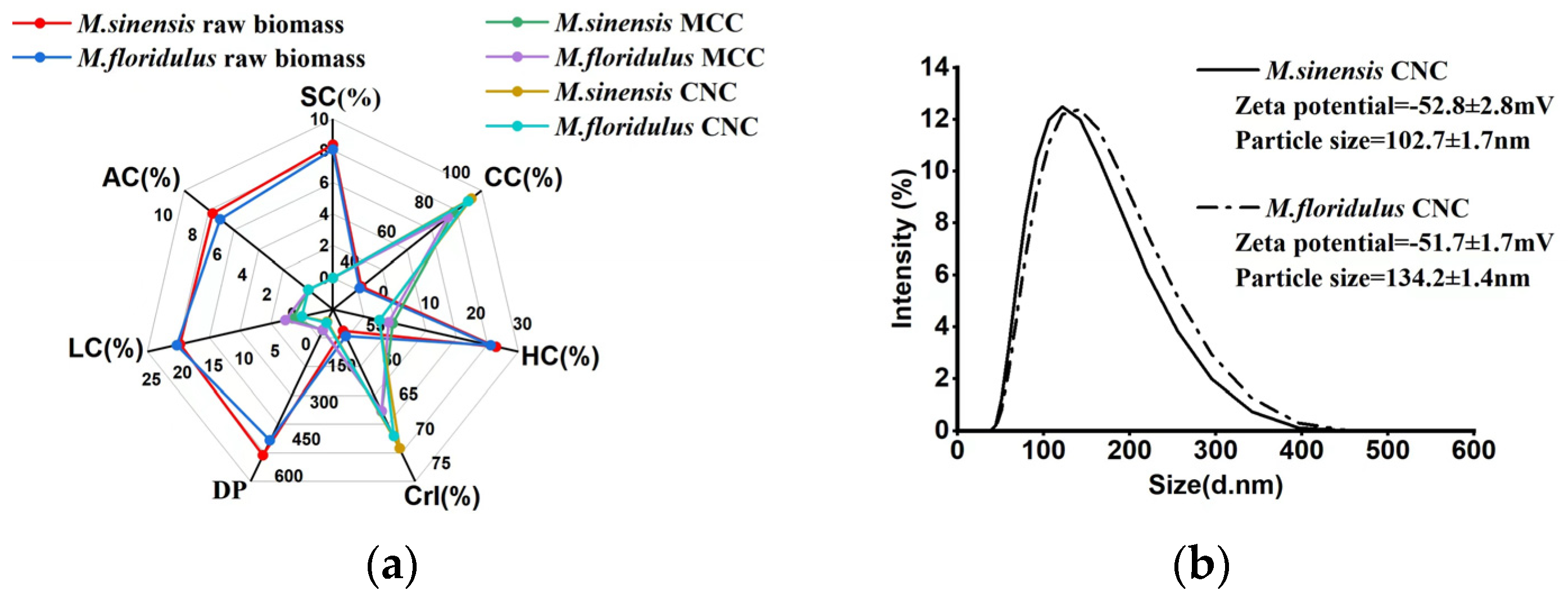
3.3. Analysis of the Yield Potentials of MCC and CNCs in M. sinensis and M. floridulus
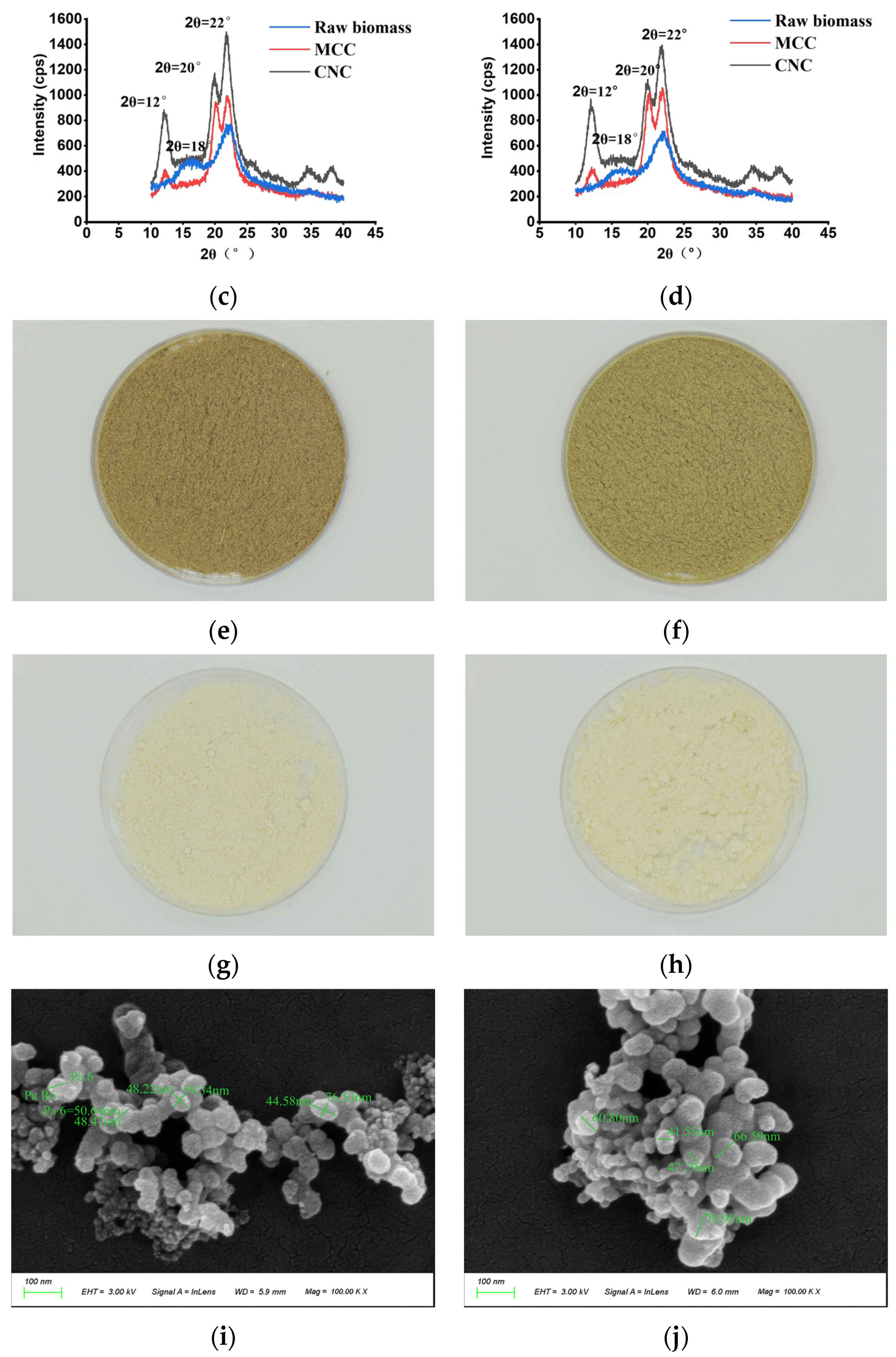
3.4. Comparison of Chemical Composition and Physical Features of MCC and CNCs in Representative Clones
3.5. Extra Trees Modeling for Yields of MCC and NCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karnaouri, A.; Chorozian, K.; Zouraris, D.; Karantonis, A.; Topakas, E.; Rova, U.; Christakopoulos, P. Lytic polysaccharide monooxygenases as powerful tools in enzymatically assisted preparation of nano-scaled cellulose from lignocellulose: A review. Bioresour. Technol. 2022, 345, 126491. [Google Scholar] [CrossRef] [PubMed]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose nanocrystals: Pretreatments, preparation strategies, and surface functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. [Google Scholar] [CrossRef] [PubMed]
- Kontturi, E.; Laaksonen, P.; Linder, M.B.; Nonappa; Gröschel, A.H.; Rojas, O.J.; Ikkala, O. Advanced Materials through Assembly of Nanocelluloses. Adv. Mater. 2018, 30, e1703779. [Google Scholar] [CrossRef] [PubMed]
- Randis, R.; Darmadi, D.B.; Gapsari, F.; Sonief, A.A. Isolation and characterization of microcrystalline cellulose from oil palm fronds biomass using consecutive chemical treatments. Case Stud. Chem. Environ. Eng. 2024, 9, 100616. [Google Scholar] [CrossRef]
- Xing, J.; Tao, P.; Wu, Z.; Xing, C.; Liao, X.; Nie, S. Nanocellulose-graphene composites: A promising nanomaterial for flexible supercapacitors. Carbohydr. Polym. 2019, 207, 447–459. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J. Chiral nematic cellulose nanocrystal composites: An organized review. Can. J. Chem. Eng. 2024, 25253. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind. Crops Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Babicka, M.; Woźniak, M.; Bartkowiak, M.; Peplińska, B.; Waliszewska, H.; Zborowska, M.; Borysiak, S.; Ratajczak, I. Miscanthus and Sorghum as sustainable biomass sourcesfor nanocellulose production. Ind. Crops Prod. 2022, 186, 115177. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, C.; Hu, H.; Li, Y.; Sun, D.; Wang, Y.; Peng, L. Genetic modification of plant cell walls to enhance biomass yield and biofuel production in bioenergy crops. Biotechnol. Adv. 2016, 34, 997–1017. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Sai Prasanna, N.; Mitra, J. Isolation and characterization of cellulose nanocrystals from Cucumis sativus peels. Carbohydr. Polym. 2020, 247, 116706. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, V.B.; Soetaredjo, F.E.; Putro, J.N.; Santoso, S.P.; Yuliana, M.; Sunarso, J.; Ju, Y.; Ismadji, S. Nanocelluloses: Sources, Pretreatment, Isolations, Modification, and Its Application as the Drug Carriers. Polymers 2021, 13, 2052. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, J.; Rouissi, T.; Brar, S.K. Miscanthus sp.—Perennial lignocellulosic biomass as feedstock for greener fumaric acid bioproduction. Ind. Crops Prod. 2022, 175, 114248. [Google Scholar] [CrossRef]
- El Achaby, M.; El Miri, N.; Hannache, H.; Gmouh, S.; Trabadelo, V.; Aboulkas, A.; Ben Youcef, H. Cellulose nanocrystals from Miscanthus fibers: Insights into rheological, physico-chemical properties and polymer reinforcing ability. Cellulose 2018, 25, 6603–6619. [Google Scholar] [CrossRef]
- Sakovich, G.V.; Skiba, E.A.; Gladysheva, E.K.; Golubev, D.S.; Budaeva, V.V. Miscanthus as a Feedstock for the Production of Bacterial Nanocellulose. Dokl. Chem. 2020, 495, 205–208. [Google Scholar] [CrossRef]
- Abreu, M.; Silva, L.; Ribeiro, B.; Ferreira, A.; Alves, L.; Paixao, S.M.; Gouveia, L.; Moura, P.; Carvalheiro, F.; Duarte, L.C.; et al. Low indirect land use change (ILUC) energy crops to bioenergy and biofuels—A Review. Energies 2022, 15, 4348. [Google Scholar] [CrossRef]
- Brosse, N.; Dufour, A.; Meng, X.; Sun, Q.; Ragauskas, A. Miscanthus: A fast-growing crop for biofuels and chemicals production. Biofuels Bioprod. Biorefining 2012, 6, 580–598. [Google Scholar] [CrossRef]
- Riseh, R.S.; Vazvani, M.G.; Hassanisaadi, M.; Thakur, V.K. Agricultural wastes: A practical and potential source for the isolation and preparation of cellulose and application in agriculture and different industries. Ind. Crops Prod. 2024, 208, 117904. [Google Scholar] [CrossRef]
- Xiang, W.; Xue, S.; Liu, F.; Qin, S.; Xiao, L.; Yi, Z. MGDB: A database for evaluating Miscanthus spp. to screen elite germplasm. Biomass Bioenergy 2020, 138, 105599. [Google Scholar] [CrossRef]
- Wang, S.; Yi, Z.; Iqbal, Y.; Chen, Z.; Xue, S.; Fu, T.; Li, M. Polyploid Miscanthus Lutarioriparius: A Sustainable and Scalable Biomass Feedstock for Cellulose Nanocrystal Preparation in Biorefinery. Agronomy 2022, 12, 1057. [Google Scholar] [CrossRef]
- Pires, J.R.A.; Souza, V.G.L.; Gomes, L.A.; Coelhoso, I.M.; Godinho, M.H.; Fernando, A.L. Micro and nanocellulose extracted from energy crops as reinforcement agents in chitosan films. Ind. Crops Prod. 2022, 186, 115247. [Google Scholar] [CrossRef]
- Cudjoe, E.; Hunsen, M.; Xue, Z.; Way, A.E.; Barrios, E.; Olson, R.A.; Hore, M.J.A.; Rowan, S.J. Miscanthus Giganteus: A commercially viable sustainable source of cellulose nanocrystals. Carbohyd. Polym. 2017, 155, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wei, H.; Himmel, M.E.; Jameel, H.; Kelley, S.S. NIR and Py-mbms coupled with multivariate data analysis as a high-throughput biomass characterization technique: A review. Front. Plant Sci. 2014, 5, 388. [Google Scholar] [CrossRef] [PubMed]
- Onsree, T.; Tippayawong, N.; Phithakkitnukoon, S.; Lauterbach, J. Interpretable machine-learning model with a collaborative game approach to predict yields and higher heating value of torrefied biomass. Energy 2022, 249, 123676. [Google Scholar] [CrossRef]
- Geurts, P.; Ernst, D.; Wehenkel, L. Extremely randomized trees. Mach. Learn. 2006, 63, 3–42. [Google Scholar] [CrossRef]
- Jose, D.M.; Vincent, A.M.; Dwarakish, G.S. Improving multiple model ensemble predictions of daily precipitation and temperature through machine learning techniques. Sci. Rep. 2022, 12, 4678. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Yang, Y.; Xie, G. Alkali-based pretreatments distinctively extract lignin and pectin for enhancing biomass saccharification by altering cellulose features in sugar-rich Jerusalem artichoke stem. Bioresour. Technol. 2016, 208, 31–41. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Ash in Biomass. Available online: https://www.nrel.gov/docs/gen/fy08/42622.pdf (accessed on 18 March 2022).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 18 March 2022).
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K.; Geng, A. Conversion of Lignocellulosic Biomass to Nanocellulose: Structure and Chemical Process. Sci. World 2014, 2014, 631013. [Google Scholar] [CrossRef]
- Dharmaraja, J.; Shobana, S.; Arvindnarayan, S.; Francis, R.R.; Jeyakumar, R.B.; Saratale, R.G.; Ashokkumar, V.; Bhatia, S.K.; Kumar, V.; Kumar, G. Lignocellulosic biomass conversion via greener pretreatment methods towards biorefinery applications. Bioresour. Technol. 2023, 369, 128328. [Google Scholar] [CrossRef]
- Smyth, M.; García, A.; Rader, C.; Foster, E.J.; Bras, J. Extraction and process analysis of high aspect ratio cellulose nanocrystals from corn (Zea mays) agricultural residue. Ind. Crops Prod. 2017, 108, 257–266. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Peng, W.; Karimi Sadaghiani, O. A review on the applications of machine learning and deep learning in agriculture section for the production of crop biomass raw materials. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 9178–9201. [Google Scholar] [CrossRef]
- Ge, H.; Zheng, J.; Xu, H. Advances in machine learning for high value-added applications of lignocellulosic biomass. Bioresour. Technol. 2023, 369, 128481. [Google Scholar] [CrossRef] [PubMed]


| CH (m) | MLL (cm) | MLW (cm) | SD (mm) | BD (cm) | TN (no.) | BC (cm) | MC (%) | DBW (kg) | L/S | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Max | 1.7 | 135.7 | 5.4 | 11.9 | 186.4 | 824.0 | 437.2 | 57.8 | 18.6 | 4.3 | |
| Min | 0.5 | 41.5 | 0.9 | 2.6 | 32.0 | 47.0 | 85.0 | 9.0 | 0.1 | 0.8 | |
| M. sinensis | AVG | 1.2 * | 86.5 * | 2.4 * | 6.4 * | 80.0 * | 230.7 * | 252.1 * | 41.2 | 4.4 * | 1.6 |
| (n = 50) | SD | 0.3 | 22.4 | 0.8 | 2.3 | 30.6 | 171.8 | 83.1 | 13.0 | 4.0 | 0.6 |
| CV | 27.1 | 25.9 | 34.9 | 35.5 | 38.3 | 74.5 | 33.0 | 31.5 | 91.3 | 39.3 | |
| Max | 2.5 | 140.3 | 5.7 | 13.5 | 292.3 | 783.0 | 849.7 | 62.7 | 29.7 | 8.3 | |
| Min | 0.9 | 85.5 | 0.9 | 5.9 | 42.3 | 23.0 | 100.0 | 10.7 | 0.9 | 0.9 | |
| M. floridulus | AVG | 1.7 * | 118.1 * | 3.3 * | 9.6 * | 164.1 * | 339.5 * | 439.2 * | 47.0 | 11.2 * | 1.9 |
| (n = 50) | SD | 0.4 | 12.3 | 0.8 | 1.6 | 60.0 | 166.7 | 168.3 | 8.9 | 7.1 | 1.11 |
| CV | 21.6 | 10.4 | 24.1 | 16.3 | 36.6 | 49.1 | 38.3 | 18.9 | 63.1 | 58.3 |
| Train MSE | Train RMSE | Train MAE | Train MAPE | Train R2 | Test MSE | Test RMSE | Test MAE | Test MAPE | Test R2 | Number of the Estimators | Max Depth of Tree | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CNC | 0.537 | 0.733 | 0.567 | 0.049 | 0.895 | 1.088 | 1.043 | 0.946 | 0.090 | 0.700 | 230 | 60 |
| MCC | 1.907 | 1.381 | 1.059 | 0.049 | 0.877 | 4.429 | 2.104 | 1.709 | 0.072 | 0.676 | 230 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; You, L.; Wang, S.; Li, J.; Chen, Z.; Si, B.; Iqbal, Y.; Xue, S.; Fu, T.; Yi, Z.; et al. Screening of Miscanthus Genotypes for Sustainable Production of Microcrystalline Cellulose and Cellulose Nanocrystals. Agronomy 2024, 14, 1255. https://doi.org/10.3390/agronomy14061255
Liu W, You L, Wang S, Li J, Chen Z, Si B, Iqbal Y, Xue S, Fu T, Yi Z, et al. Screening of Miscanthus Genotypes for Sustainable Production of Microcrystalline Cellulose and Cellulose Nanocrystals. Agronomy. 2024; 14(6):1255. https://doi.org/10.3390/agronomy14061255
Chicago/Turabian StyleLiu, Weiming, Lanqing You, Sheng Wang, Jie Li, Zhiyong Chen, Buchun Si, Yasir Iqbal, Shuai Xue, Tongcheng Fu, Zili Yi, and et al. 2024. "Screening of Miscanthus Genotypes for Sustainable Production of Microcrystalline Cellulose and Cellulose Nanocrystals" Agronomy 14, no. 6: 1255. https://doi.org/10.3390/agronomy14061255
APA StyleLiu, W., You, L., Wang, S., Li, J., Chen, Z., Si, B., Iqbal, Y., Xue, S., Fu, T., Yi, Z., & Li, M. (2024). Screening of Miscanthus Genotypes for Sustainable Production of Microcrystalline Cellulose and Cellulose Nanocrystals. Agronomy, 14(6), 1255. https://doi.org/10.3390/agronomy14061255






