Performance of ACCase-Resistant and ACCase-Susceptible Phenotypes of Sterile Oat Avena sterilis subsp. ludoviciana (Durieu) Nyman under Drought Conditions in the Greenhouse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Plant Growth Conditions
2.3. Experimental Design and Treatments
2.4. Statistical Analysis
3. Results and Discussion
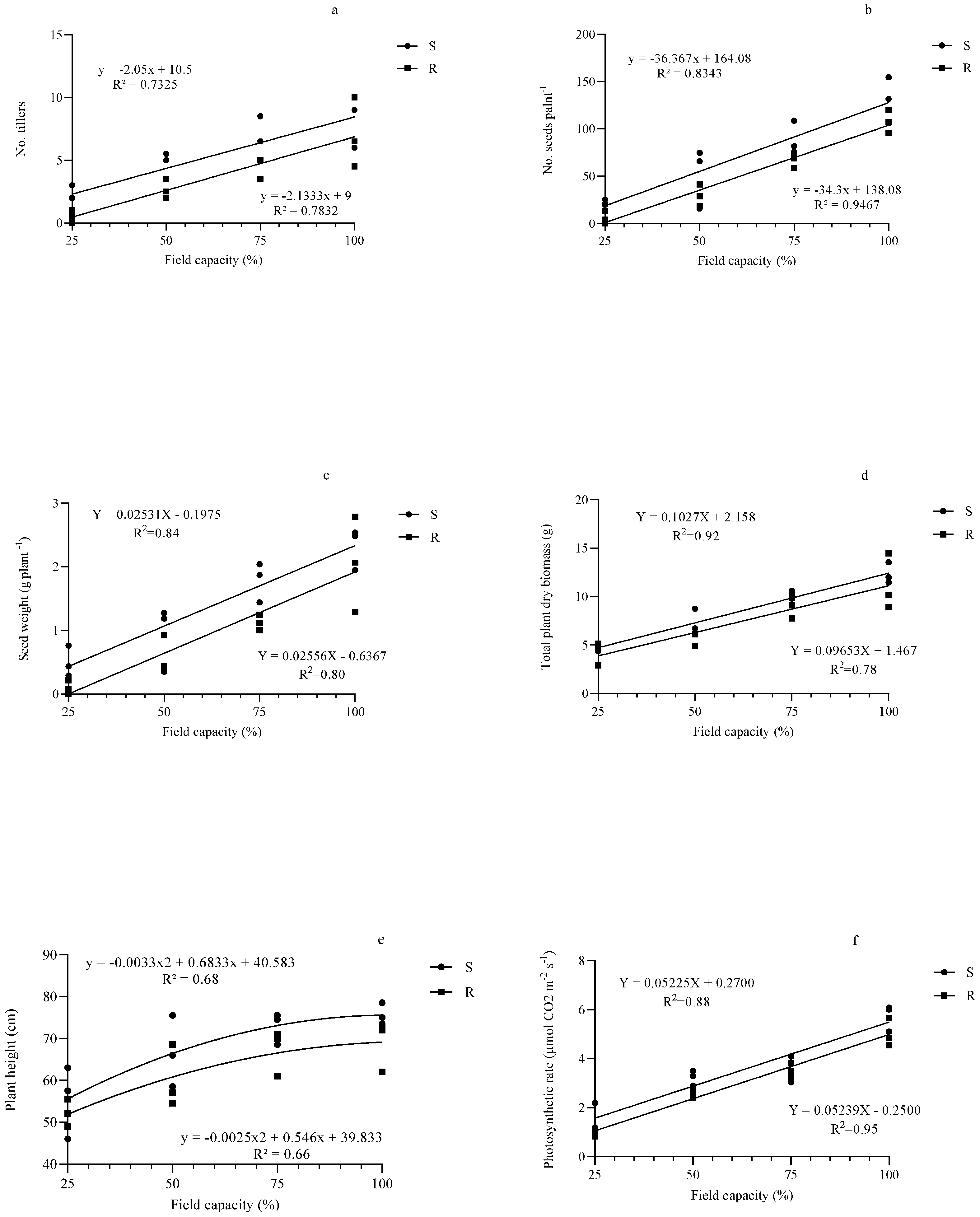
3.1. Number of Tillers and of Seeds per Plant
3.2. Seed Weight per Plant
3.3. Total Plant Dry Biomass and Height
3.4. Photosynthetic Rate
4. Conclusions and Recommendation
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Daugovish, O.; Thill, D.C.; Shafii, B. Competition between wild oat (Avena fatua) and yellow mustard (Sinapis alba) or canola (Brassica napus). Weed Sci. 2002, 50, 587–594. [Google Scholar] [CrossRef]
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds: Distribution and Biology; University Press of Hawaii: Honolulu, HI, USA, 1977. [Google Scholar]
- Sharma, M.; Born, W.V. Crop competition aids efficacy of wild oat herbicides. Can. J. Plant Sci. 1983, 63, 503–507. [Google Scholar] [CrossRef]
- CABI. Invasive Species Compedium; CAB International: Wallingford, UK, 2022. [Google Scholar]
- Mahajan, G.; Chauhan, B.S. Interference of wild oat (Avena fatua) and sterile oat (Avena sterilis ssp. ludoviciana) in wheat. Weed Sci. 2021, 69, 485–491. [Google Scholar] [CrossRef]
- Devine, M.; Shimabukuro, R. Resistance to acetyl coenzyme A carboxylase inhibiting herbicides. In Herbicide Resistance in Plants; Powles, S.B., Holtum, J.A.M., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 141–170. [Google Scholar]
- Benakashani, F.; Zand, E.; Naghavi, M.R.; Sasanfar, H.R. Mutations in Acetyl-CoA Carboxylase Enzyme, Mechanism of Cross Resistance in Wild Oat (Avena ludoviciana Deuri.) Biotypes to ACCase Inhibitor Herbicides. Iran. J. Weed Sci. 2014, 10, 179–190. [Google Scholar]
- Heap, I. The International Herbicide-Resistant Weed Database. 2023. Available online: https://www.weedscience.org (accessed on 19 October 2023).
- Jang, S.; Marjanovic, J.; Gornicki, P. Resistance to herbicides caused by single amino acid mutations in acetyl-C o A carboxylase in resistant populations of grassy weeds. New Phytol. 2013, 197, 1110–1116. [Google Scholar] [CrossRef]
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The challenge of herbicide resistance around the world: A current summary. Pest Manag. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef]
- Ofosu, R.; Agyemang, E.D.; Márton, A.; Pásztor, G.; Taller, J.; Kazinczi, G. Herbicide resistance: Managing weeds in a changing world. Agronomy 2023, 13, 1595. [Google Scholar] [CrossRef]
- Gherekhloo, J.; Oveisi, M.; Zand, E.; De Prado, R. A review of herbicide resistance in Iran. Weed Sci. 2016, 64, 551–561. [Google Scholar] [CrossRef]
- Zand, E.; Bana, K.F.; Soufizadeh, S.; Alizadeh, H.; Ramezane, K.; Makanali, S.; Fereydounpoor, M. Resistance to aryloxyphenoxypropionate herbicides in wild oat (Avena ludoviciana). Iran. J. Weed Sci. 2006, 2, 17–31. [Google Scholar]
- Kazemeini, S.A.; Naderi, R.; Aliabadi, H.K. Effects of different densities of wild oat (Avena fatua L.) and nitrogen rates on oilseed rape (Brassica napus L.) yield. J. Ecol. Environ. 2013, 36, 167–172. [Google Scholar] [CrossRef]
- Mobli, A.; Matloob, A.; Chauhan, B.S. The response of glyphosate-resistant and glyphosate-susceptible biotypes of annual sowthistle (Sonchus oleraceus) to mungbean density. Weed Sci. 2019, 67, 642–648. [Google Scholar] [CrossRef]
- Tang, W.; Xu, X.; Shen, G.; Chen, J. Effect of environmental factors on germination and emergence of aryloxyphenoxy propanoate herbicide-resistant and-susceptible Asia minor bluegrass (Polypogon fugax). Weed Sci. 2015, 63, 669–675. [Google Scholar] [CrossRef]
- Thompson, C.R.; Thill, D.C.; Shafii, B. Germination characteristics of sulfonylurea-resistant and-susceptible kochia (Kochia scoparia). Weed Sci. 1994, 42, 50–56. [Google Scholar] [CrossRef]
- Weller, S.; Florentine, S.; Mutti, N.; Jha, P.; Chauhan, B.S. Response of Chloris truncata to moisture stress, elevated carbon dioxide and herbicide application. Sci. Rep. 2019, 9, 10721. [Google Scholar] [CrossRef] [PubMed]
- Neve, P.; Diggle, A.; Smith, F.; Powles, S. Simulating evolution of glyphosate resistance in Lolium rigidum II: Past, present and future glyphosate use in Australian cropping. Weed Res. 2003, 43, 418–427. [Google Scholar] [CrossRef]
- Hassanpour-Bourkheili, S.; Gherekhloo, J.; Kamkar, B.; Ramezanpour, S.S. No fitness cost associated with Asn-2041-Ile mutation in winter wild oat (Avena ludoviciana) seed germination under various environmental conditions. Sci. Rep. 2021, 11, 1572. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Harrison, M.T.; Yan, H.; Liu, D.L.; Meinke, H.; Hoogenboom, G.; Wang, B.; Peng, B.; Guan, K.; Jaegermeyr, J. Silver lining to a climate crisis in multiple prospects for alleviating crop waterlogging under future climates. Nat. Commun. 2023, 14, 765. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Fonseca de Lima, C.F.; Vu, L.D.; De Smet, I. When drought meets heat–a plant omics perspective. Front. Plant Sci. 2023, 14, 1250878. [Google Scholar] [CrossRef] [PubMed]
- Antunović Dunić, J.; Mlinarić, S.; Pavlović, I.; Lepeduš, H.; Salopek-Sondi, B. Comparative analysis of primary photosynthetic reactions assessed by OJIP kinetics in three brassica crops after drought and recovery. Appl. Sci. 2023, 13, 3078. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882. [Google Scholar] [CrossRef]
- Wiese, A.F.; Vandiver, C.W. Soil moisture effects on competitive ability of weeds. Weed Sci. 1970, 18, 518–519. [Google Scholar] [CrossRef]
- Bhowmik, P.C. Weed biology: Importance to weed management. Weed Sci. 1997, 45, 349–356. [Google Scholar] [CrossRef]
- Beckie, H.J.; Harker, K.N. Our top 10 herbicide-resistant weed management practices. Pest Manag. Sci. 2017, 73, 1045–1052. [Google Scholar] [CrossRef]
- Sahil; Mahajan, G.; Loura, D.; Raymont, K.; Chauhan, B.S. Influence of soil moisture levels on the growth and reproductive behaviour of Avena fatua and Avena ludoviciana. PLoS ONE 2020, 15, e0234648. [Google Scholar] [CrossRef]
- Sasanfar, H.; Zand, E.; Baghestani, M.A.; Mirhadi, M.J.; Mesgaran, M.B. Cross-resistance patterns of winter wild oat (Avena ludoviciana) populations to ACCase inhibitor herbicides. Phytoparasitica 2017, 45, 419–428. [Google Scholar] [CrossRef]
- Samarah, N.H. Effects of drought stress on growth and yield of barley. Agron. Sustain. Dev. 2005, 25, 145–149. [Google Scholar] [CrossRef]
- Batool, A.; Cheng, Z.-G.; Akram, N.A.; Lv, G.-C.; Xiong, J.-L.; Zhu, Y.; Ashraf, M.; Xiong, Y.-C. Partial and full root-zone drought stresses account for differentiate root-sourced signal and yield formation in primitive wheat. Plant Methods 2019, 15, 75. [Google Scholar] [CrossRef]
- Gui, Y.-W.; Sheteiwy, M.S.; Zhu, S.-G.; Batool, A.; Xiong, Y.-C. Differentiate effects of non-hydraulic and hydraulic root signaling on yield and water use efficiency in diploid and tetraploid wheat under drought stress. Environ. Exp. Bot. 2021, 181, 104287. [Google Scholar] [CrossRef]
- Mahajan, G.; Mutti, N.K.; Walsh, M.; Chauhan, B.S. Effect of varied soil moisture regimes on the growth and reproduction of two Australian biotypes of junglerice (Echinochloa colona). Weed Sci. 2019, 67, 552–559. [Google Scholar] [CrossRef]
- Sarangi, D.; Irmak, S.; Lindquist, J.L.; Knezevic, S.Z.; Jhala, A.J. Effect of water stress on the growth and fecundity of common waterhemp (Amaranthus rudis). Weed Sci. 2016, 64, 42–52. [Google Scholar] [CrossRef]
- Yanniccari, M.; Vila-Aiub, M.; Istilart, C.; Acciaresi, H.; Castro, A.M. Glyphosate resistance in perennial ryegrass (Lolium perenne L.) is associated with a fitness penalty. Weed Sci. 2016, 64, 71–79. [Google Scholar] [CrossRef]
- Travlos, I.S. Competition between ACCase-inhibitor resistant and susceptible sterile wild oat (Avena sterilis) biotypes. Weed Sci. 2013, 61, 26–31. [Google Scholar] [CrossRef]
- Hassanpour-Bourkheili, S.; Heravi, M.; Gherekhloo, J.; Alcántara-de la Cruz, R.; De Prado, R. Fitness cost of imazamox resistance in wild poinsettia (Euphorbia heterophylla L.). Agronomy 2020, 10, 1859. [Google Scholar] [CrossRef]
- Du, L.; Qu, M.; Jiang, X.; Li, X.; Ju, Q.; Lu, X.; Wang, J. Fitness costs associated with acetyl-coenzyme A carboxylase mutations endowing herbicide resistance in American sloughgrass (Beckmannia syzigachne Steud.). Ecol. Evol. 2019, 9, 2220–2230. [Google Scholar] [CrossRef] [PubMed]
- Vila-Aiub, M.; Neve, P.; Steadman, K.; Powles, S. Ecological fitness of a multiple herbicide-resistant Lolium rigidum population: Dynamics of seed germination and seedling emergence of resistant and susceptible phenotypes. J. Appl. Ecol. 2005, 42, 288–298. [Google Scholar] [CrossRef]
- Wang, T.; Picard, J.; Tian, X.; Darmency, H. A herbicide-resistant ACCase 1781 Setaria mutant shows higher fitness than wild type. Heredity 2010, 105, 394–400. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Siddique, Z.; Jan, S.; Imadi, S.R.; Gul, A.; Ahmad, P. Drought stress and photosynthesis in plants. In Water Stress and Crop Plants: A Sustainable Approach; Wiley: Hoboken, NJ, USA, 2016; Volume 1, pp. 1–11. [Google Scholar]
- Li, L.; Mao, X.; Wang, J.; Chang, X.; Reynolds, M.; Jing, R. Genetic dissection of drought and heat-responsive agronomic traits in wheat. Plant Cell Environ. 2019, 42, 2540–2553. [Google Scholar] [CrossRef]
- Grieco, M.; Roustan, V.; Dermendjiev, G.; Rantala, S.; Jain, A.; Leonardelli, M.; Neumann, K.; Berger, V.; Engelmeier, D.; Bachmann, G. Adjustment of photosynthetic activity to drought and fluctuating light in wheat. Plant Cell Environ. 2020, 43, 1484–1500. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Díaz-Espejo, A.; Conesa, M.; Coopman, R.; Douthe, C.; Gago, J.; Gallé, A.; Galmés, J.; Medrano, H.; Ribas-Carbo, M. Mesophyll conductance to CO2 and Rubisco as targets for improving intrinsic water use efficiency in C3 plants. Plant Cell Environ. 2016, 39, 965–982. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.W.; Tezara, W. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Ann. Bot. 2009, 103, 561–579. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Neukermans, J.; Queval, G.; Noctor, G.; Harbinson, J. Photosynthetic control of electron transport and the regulation of gene expression. J. Exp. Bot. 2012, 63, 1637–1661. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Li, M.; Kim, C. Chloroplast ROS and stress signaling. Plant Commun. 2022, 3, 100264. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Fujita, K. Effects of drought stress on photosynthetic gas exchange, chlorophyll fluorescence and stem diameter of soybean plants. Biol. Plant. 2006, 50, 138–141. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-M.; Fang, Y.; Yang, H.-N.; Bai, L.-Y. Effects of drought-stress on seed germination and growth physiology of quinclorac-resistant Echinochloa crusgalli. PLoS ONE 2019, 14, e0214480. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Growth and reproduction of junglerice (Echinochloa colona) in response to water stress. Weed Sci. 2010, 58, 132–135. [Google Scholar] [CrossRef]
- Stout, D.G.; Simpson, G.M. Drought resistance of Sorghum bicolor. 1. Drought avoidance mechanisms related to leaf water status. Can. J. Plant Sci. 1978, 58, 213–224. [Google Scholar] [CrossRef]
- Vaghefi, S.A.; Keykhai, M.; Jahanbakhshi, F.; Sheikholeslami, J.; Ahmadi, A.; Yang, H.; Abbaspour, K.C. The future of extreme climate in Iran. Sci. Rep. 2019, 9, 1464. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, G.; Hickey, L.; Chauhan, B.S. Response of barley genotypes to weed interference in Australia. Agronomy 2020, 10, 99. [Google Scholar] [CrossRef]
- Aibar, J.; Ochoa, M.; Zaragoza, C. Field emergence of Avena fatua L. and A. sterilis ssp. ludoviciana (Dur.) Nym. in Aragon, Spain. Weed Res. 1991, 31, 29–32. [Google Scholar] [CrossRef]
- Ponce, R.; Santin, I. Competitive ability of wheat cultivars with wild oats depending on nitrogen fertilization. Agronomie 2001, 21, 119–125. [Google Scholar] [CrossRef]
- Moghaddam, H.; Oveisi, M.; Mehr, M.K.; Bazrafshan, J.; Naeimi, M.H.; Kaleibar, B.P.; Müller-Schärer, H. Earlier sowing combined with nitrogen fertilization to adapt to climate change effects on yield of winter wheat in arid environments: Results from a field and modeling study. Eur. J. Agron. 2023, 146, 126825. [Google Scholar] [CrossRef]
| Phenotype | No. Tillers Plant−1 | No. Seeds Plant−1 | Seed Weight Plant−1 | Photosynthesis Rate |
|---|---|---|---|---|
| Susceptible | 6.04 ± 0.70 a | 79.96 ± 10.2 a | 1.54 ± 0.24 a | 3.74 ± 0.27 a |
| Resistant | 3.54 ± 1.01 b | 51.54 ± 12.4 b | 0.96 ± 0.20 b | 3.01 ± 0.33 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naderi, R.; Bijani, F.; Chauhan, B.S.; Mueller-Schaerer, H. Performance of ACCase-Resistant and ACCase-Susceptible Phenotypes of Sterile Oat Avena sterilis subsp. ludoviciana (Durieu) Nyman under Drought Conditions in the Greenhouse. Agronomy 2024, 14, 1268. https://doi.org/10.3390/agronomy14061268
Naderi R, Bijani F, Chauhan BS, Mueller-Schaerer H. Performance of ACCase-Resistant and ACCase-Susceptible Phenotypes of Sterile Oat Avena sterilis subsp. ludoviciana (Durieu) Nyman under Drought Conditions in the Greenhouse. Agronomy. 2024; 14(6):1268. https://doi.org/10.3390/agronomy14061268
Chicago/Turabian StyleNaderi, Ruhollah, Farzad Bijani, Bhagirath S. Chauhan, and Heinz Mueller-Schaerer. 2024. "Performance of ACCase-Resistant and ACCase-Susceptible Phenotypes of Sterile Oat Avena sterilis subsp. ludoviciana (Durieu) Nyman under Drought Conditions in the Greenhouse" Agronomy 14, no. 6: 1268. https://doi.org/10.3390/agronomy14061268






