Elemental Analysis of Five Medicinal Plants Species Growing in North Ossetia Using Neutron Activation Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Neutron Activation Analysis
2.3. Data Evaluation
2.3.1. Contamination and Enrichment Factors
2.3.2. Transfer Factor
2.3.3. Daily Intake of Metal
2.3.4. Health Risk Index (HRI)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Elements Content in Medicinal Plants
3.2. Elemental Content of Soils
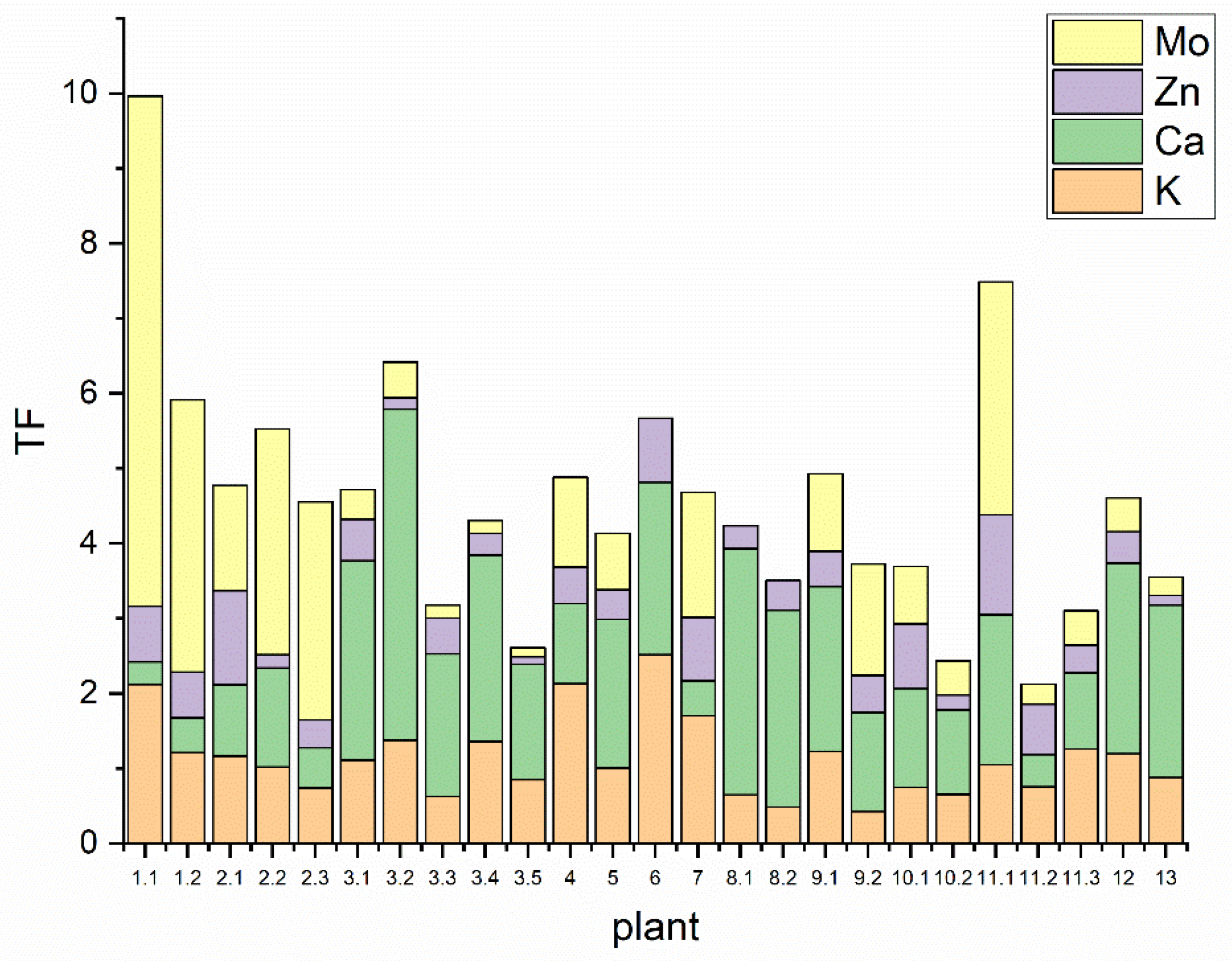
3.3. Metal Transfer from Soil in Plants
3.4. Principal Component and Discriminant Analyses
3.5. Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciocarlan, A.; Hristozova, G.; Aricu, A.; Dragalin, I.; Zinicovscaia, I.; Yushin, N.; Grozdov, D.; Popescu, V. Determination of the Elemental Composition of Aromatic Plants Cultivated Industrially in the Republic of Moldova Using Neutron Activation Analysis. Agronomy 2021, 11, 1011. [Google Scholar] [CrossRef]
- Tkachenko, K.G. Quality Control of Fruit and Seeds of Medicinal Plants. Maced. Pharm. Bull. 2022, 66, 39–40. [Google Scholar] [CrossRef]
- Hlihor, R.M.; Roșca, M.; Hagiu-Zaleschi, L.; Simion, I.M.; Daraban, G.M.; Stoleru, V. Medicinal Plant Growth in Heavy Metals Contaminated Soils: Responses to Metal Stress and Induced Risks to Human Health. Toxics 2022, 10, 499. [Google Scholar] [CrossRef]
- Luo, L.; Wang, B.; Jiang, J.; Fitzgerald, M.; Huang, Q.; Yu, Z.; Li, H.; Zhang, J.; Wei, J.; Yang, C.; et al. Heavy Metal Contaminations in Herbal Medicines: Determination, Comprehensive Risk Assessments, and Solutions. Front. Pharmacol. 2021, 11, 595335. [Google Scholar] [CrossRef] [PubMed]
- Kulhari, A.; Sheorayan, A.; Bajar, S.; Sarkar, S.; Chaudhury, A.; Kalia, R.K. Investigation of Heavy Metals in Frequently Utilized Medicinal Plants Collected from Environmentally Diverse Locations of North Western India. Springerplus 2013, 2, 676. [Google Scholar] [CrossRef] [PubMed]
- Oyebode, O.; Kandala, N.B.; Chilton, P.J.; Lilford, R.J. Use of Traditional Medicine in Middle-Income Countries: A WHO-SAGE Study. Health Policy Plan. 2016, 31, 984–991. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Lozano, R.; Gómez-Serranillos, M.P. Current Uses and Knowledge of Medicinal Plants in the Autonomous Community of Madrid (Spain): A Descriptive Cross-Sectional Study. BMC Complement. Med. Ther. 2020, 20, 306. [Google Scholar] [CrossRef]
- Brudzińska-Kosior, A.; Kosior, G.; Sporek, M.; Ziembik, Z.; Zinicovscaia, I.; Frontasyeva, M.; Dołhańczuk-Śródka, A. Nuclear Analytical Techniques Used to Study the Trace Element Content of Centaurium Erythraea Rafn, a Medicinal Plant Species from Sites with Different Pollution Loads in Lower Silesia (SW Poland). PLoS ONE 2023, 18, e0285306. [Google Scholar] [CrossRef]
- Abou-Arab, A.A.K.; Abou Donia, M.A. Heavy Metals in Egyptian Spices and Medicinal Plants and the Effect of Processing on Their Levels. J. Agric. Food Chem. 2000, 48, 2300–2304. [Google Scholar] [CrossRef]
- Dogheim, S.M.; Ashraf, E.M.M.; Alla, S.A.G.; Khorshid, M.A.; Fahmy, S.M. Pesticides and Heavy Metals Levels in Egyptian Leafy Vegetables and Some Aromatic Medicinal Plants. Food Addit. Contam. 2004, 21, 323–330. [Google Scholar] [CrossRef]
- Barthwal, J.; Nair, S.; Kakkar, P. Heavy Metal Accumulation in Medicinal Plants Collected from Environmentally Different Sites. Biomed. Environ. Sci. 2008, 21, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Melkaoui, C.; Cheriti, A.; Bouchekara, M. Assessment of Heavy Metals and Macromineral in Frequently Used Medicinal Plants from Algerian Sahara Traditional Ethnopharmacopeia. Ann. Pharm. Fr. 2023, 81, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Glavač, N.K.; Djogo, S.; Ražić, S.; Kreft, S.; Veber, M. Accumulation of Heavy Metals from Soil in Medicinal Plants. Arh. Hig. Rada Toksikol. 2017, 68, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, S.K.; Georgieva, A.; Peteva, Z.; Dimova, D. Trace Elements in Commonly Used Medicinal Plants from Varna Region, Bulgaria. Environ. Sci. Pollut. Res. 2021, 28, 59277–59283. [Google Scholar] [CrossRef] [PubMed]
- Lavrinenko, Y.; Plieva, A.; Zinicovscaia, I.; Hristozova, G.; Frontasyeva, M.; Tkachenko, K.; Dogadkin, D.; Gromyak, I.; Kolotov, V. Elemental Composition of Infusions of Herbs (Tisanes) of North Ossetia (the Caucasus). Agriculture 2021, 11, 841. [Google Scholar] [CrossRef]
- Tkachenko, K.; Frontasyeva, M.; Vasilev, A.; Avramov, L.; Shi, L. Major and Trace Element Content of Tribulus Terrestris l. Wildlife Plants. Plants 2020, 9, 764. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, K.; Kosareva, I.; Frontasyeva, M. The Influence of Manganese on Growth Processes of Hordeum l. (Poaceae) Seedlings. Plants 2021, 10, 1009. [Google Scholar] [CrossRef] [PubMed]
- Alla Kapelian, K.T.; Varfolomeeva, E. Search for Toxic Trace Elements in Rosa Rugosa Thunb. By Instrumental Neutron Activation Analysis: Accumulation and Responses to Exposure. J. Agric. Crop. 2023, 9, 503–513. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Gundorina, S.; Vergel, K.; Grozdov, D.; Ciocarlan, A.; Aricu, A.; Dragalin, I.; Ciocarlan, N. Elemental Analysis of Lamiaceae Medicinal and Aromatic Plants Growing in the Republic of Moldova Using Neutron Activation Analysis. Phytochem. Lett. 2020, 35, 119–127. [Google Scholar] [CrossRef]
- Gonçalves, E.P.R.; Soares, H.M.V.M.; Boaventura, R.A.R.; Machado, A.A.S.C.; Esteves da Silva, J.C.G. Seasonal Variations of Heavy Metals in Sediments and Aquatic Mosses from the Cávado River Basin (Portugal). Sci. Total Environ. 1994, 142, 143–156. [Google Scholar] [CrossRef]
- Fernández, J.A.; Carballeira, A. Evaluation of Contamination, by Different Elements, in Terrestrial Mosses. Arch. Environ. Contam. Toxicol. 2001, 40, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, R.L.; Gao, S. Composition of the Continental Crust; Elsevier Ltd.: Amsterdam, The Netherlands, 2003; Volume 3, ISBN 0-08-044338-9. [Google Scholar]
- Sałata, A.; Dąbek, L. Methods of Assessment of Stormwater Sediments Quality. In Proceedings of the E3S Web of Conferences, EDP Sciences, Boguszów-Gorce, Poland, 24 May 2017; Volume 17. [Google Scholar]
- Zinicovscaia, I.; Sturza, R.; Duliu, O.; Grozdov, D.; Gundorina, S.; Ghendov-Mosanu, A.; Duca, G. Major and Trace Elements in Moldavian Orchard Soil and Fruits: Assessment of Anthropogenic Contamination. Int. J. Environ. Res. Public Health 2020, 17, 7112. [Google Scholar] [CrossRef] [PubMed]
- Oladeji, O.M.; Kopaopa, B.G.; Mugivhisa, L.L.; Olowoyo, J.O. Investigation of Heavy Metal Analysis on Medicinal Plants Used for the Treatment of Skin Cancer by Traditional Practitioners in Pretoria. Biol. Trace Elem. Res. 2024, 202, 778–786. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency | US EPA. Available online: https://www.epa.gov/ (accessed on 17 April 2024).
- Markert, B.; Fränzle, S.; Wünschmann, S. Chemical Evolution: The Biological System of the Elements; Springer: Cham, Switzerland, 2015; pp. 1–282. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 517877. [Google Scholar] [CrossRef] [PubMed]
- Hamzah Saleem, M.; Usman, K.; Rizwan, M.; Al Jabri, H.; Alsafran, M. Functions and Strategies for Enhancing Zinc Availability in Plants for Sustainable Agriculture. Front. Plant Sci. 2022, 13, 1033092. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wei, X.; Ling, J.; Chen, J. Cobalt: An Essential Micronutrient for Plant Growth? Front. Plant Sci. 2021, 12, 768523. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, B.N.; Gridley, K.L.; Brady, J.N.; Phillips, T.; Tyerman, S.D. The Role of Molybdenum in Agricultural Plant Production. Ann. Bot. 2005, 96, 745. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Vinogradova, N.; Glukhov, A.; Chaplygin, V.; Kumar, P.; Mandzhieva, S.; Minkina, T.; Rajput, V.D. The Content of Heavy Metals in Medicinal Plants in Various Environmental Conditions: A Review. Horticulturae 2023, 9, 239. [Google Scholar] [CrossRef]
- Grosjean, N.; Purwadi, I.; Sirguey, C.; Chalot, M.; Le Jean, M.; van der Ent, A.; Blaudez, D. Rare Earth Elements in Plants: Transfer, Transport, Accumulation, Impacts and Perspectives. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2024; Volume 109, pp. 19–61. [Google Scholar]
- Ozturk, M.; Metin, M.; Altay, V.; Prasad, M.N.V.; Gul, A.; Bhat, R.A.; Darvash, M.A.; Hasanuzzaman, M.; Nahar, K.; Unal, D.; et al. Role of Rare Earth Elements in Plants. Plant Mol. Biol. Rep. 2023, 41, 345–368. [Google Scholar] [CrossRef]
- Sarma, H.; Deka, S.; Deka, H.; Saikia, R.R. Accumulation of Heavy Metals in Selected Medicinal Plants. Rev. Environ. Contam. Toxicol. 2011, 214, 63–86. [Google Scholar] [PubMed]
- Zhang, J.; Liu, C.L. Riverine Composition and Estuarine Geochemistry of Particulate Metals in China—Weathering Features, Anthropogenic Impact and Chemical Fluxes. Estuar. Coast. Shelf Sci. 2002, 54, 1051–1070. [Google Scholar] [CrossRef]
- Russia’s Federal Constituent Entities|Federation Council of the Federal Assembly of the Russian Federation. Available online: http://council.gov.ru/en/structure/regions/SE/ (accessed on 17 April 2024).
- Kabaloeva, D.V.; Tsirikhova, A.S.; Kisieva, M.T. Environmentally-related diseases and their prevention in the republic of north ossetia—Alania. Medical Pharm. J. Pulse 2020, 22, 60–64. [Google Scholar] [CrossRef]
- Arutyunyanz, A.A.; Salamova, N.A.; Simeonidi, D.D. Characterization of Condition of the Sewage Water and the Level of the Soil Pollution by Polygons of Waste Matter of Republic of North Ossetia—Alania. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 272, p. 032053. [Google Scholar]
- Zhong, G.; Wan, F.; Wu, S.; Jiang, X.; Tang, Z.; Zhang, X.; Huang, R.; Hu, L. Arsenic or/and Antimony Induced Mitophagy and Apoptosis Associated with Metabolic Abnormalities and Oxidative Stress in the Liver of Mice. Sci. Total Environ. 2021, 777, 146082. [Google Scholar] [CrossRef] [PubMed]
- Zaalishvili, V.; Burdzieva, O.; Kanukov, A.; Zaks, T. Eco-Geophysical and Geoecological Factors in Assessing the State of the Geological Environment Based on the Analysis of Spatial Databases of the Territory of the Republic of North Ossetia–Alania. Appl. Sci. 2022, 12, 2644. [Google Scholar] [CrossRef]
- Magometov, A.A.; Makoev, K.K.; Kebalova, L.A.; Toporkova, T.N. Problems of creation of a sanitary protection zone in the area of JSC "Electrozink"and OJSC “Pobedit”. Vladikavkaz 2020, 99. [Google Scholar]
- Mirecki, N.; Agič, R.; Šunić, L.; Milenković, L.; Ilić, Z.S. Transfer Factor as Indicator of Heavy Metals Content in Plants. Fresenius Environ. Bull. 2015, 24, 4212–4219. [Google Scholar]
- Sardans, J.; Peñuelas, J. Potassium Control of Plant Functions: Ecological and Agricultural Implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Barber, S.A. Mechanism of Potassium Absorption by Plants. Role Potassium Agric. 2015, 14, 293–310. [Google Scholar] [CrossRef]
- Pathak, R.K.; Singh, D.B.; Sharma, H.; Pandey, D.; Dwivedi, S. Calcium Uptake and Translocation in Plants. Calcium Transp. Elem. Plants 2021, 21, 373–386. [Google Scholar] [CrossRef]
- Zárate-Quiñones, R.H.; Custodio, M.; Orellana-Mendoza, E.; Cuadrado-Campó, W.J.; Grijalva-Aroni, P.L.; Peñaloza, R. Determination of Toxic Metals in Commonly Consumed Medicinal Plants Largely Used in Peru by ICP-MS and Their Impact on Human Health. Chem. Data Collect. 2021, 33, 100711. [Google Scholar] [CrossRef]
- Izah, S.C.; Aigberua, A.O.; Richard, G. Concentration, Source, and Health Risk of Trace Metals in Some Liquid Herbal Medicine Sold in Nigeria. Biol. Trace Elem. Res. 2022, 200, 3009–3022. [Google Scholar] [CrossRef] [PubMed]
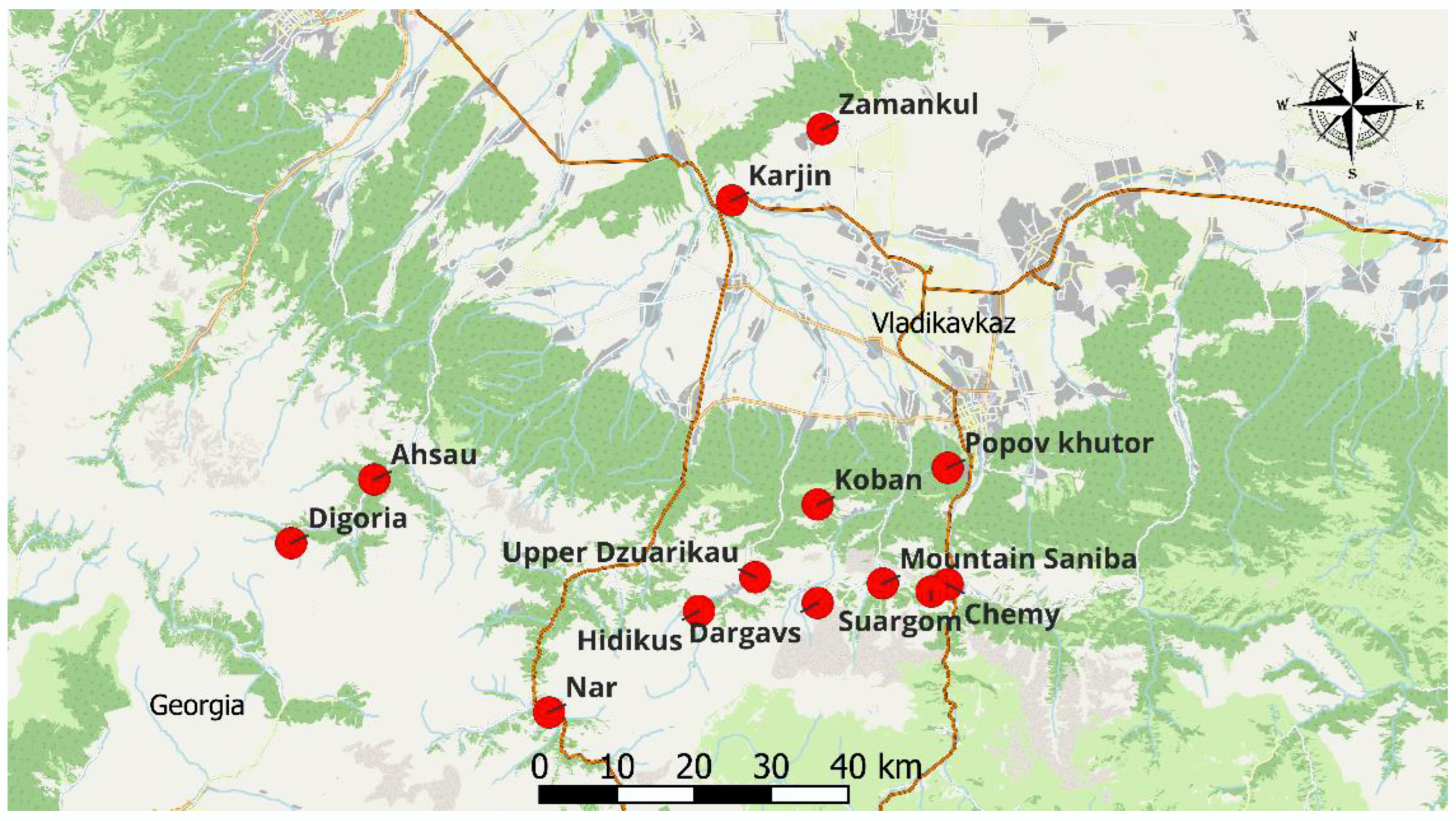



| Site | Site Name | Latitude | Longitude | Species | Shorthand | Short Name | Soil Type |
|---|---|---|---|---|---|---|---|
| 1 | Zamankul | 43.359769 | 44.432536 | Mentha longifolia | 1.1 | Brook-mint | ordinary chernozems |
| Origanum vulgare | 1.2 | oregano | ordinary chernozems | ||||
| 2 | Koban | 42.9278 | 44.42503 | Mentha longifolia | 2.1 | Brook-mint | brown mountain-wood |
| Hypеricum perforаtum | 2.2 | St. John’s wort | alpine-meadow | ||||
| Oríganum vulgare | 2.3 | oregano | brown mountain-wood | ||||
| 3 | Dargavs | 42.81393 | 44.42507 | Mentha longifolia | 3.1 | Brook-mint | alpine-meadow |
| Thymus daghestanicus | 3.2 | thyme | alpine-meadow | ||||
| Hypеricum perforаtum | 3.3 | St. John’s wort | brown mountain-wood | ||||
| Oríganum vulgare | 3.4 | oregano | alpine-meadow | ||||
| Chamaenerion angustifolium | 3.5 | fireweed | alpine-meadow | ||||
| 4 | Mountain Saniba | 42.836477 | 44.527862 | Mentha longifolia | 4 | Brook-mint | alluvial |
| 5 | Ahsau | 42.95672 | 43.72495 | Mentha longifolia | 5 | Brook-mint | mountain-wood |
| 6 | Popov khutor | 42.970175 | 44.630214 | Mentha longifolia | 6 | Brook-mint | brown mountain-wood |
| 7 | Karjin | 43.277963 | 44.289759 | Thymus daghestanicus | 7 | thyme | ordinary chernozems |
| 8 | Nar | 42.687258 | 44.001003 | Thymus daghestanicus | 8.1 | thyme | alpine-meadow |
| Hypеricum perforаtum | 8.2 | St. John’s wort | alpine-meadow | ||||
| 9 | Chemy | 42.835549 | 44.629982 | Thymus daghestanicus | 9.1 | thyme | alpine-meadow |
| Hypеricum perforаtum | 9.2 | St. John’s wort | alpine-meadow | ||||
| 10 | Hidikus | 42.80478 | 44.2377 | Thymus daghestanicus | 10.1 | thyme | alpine-meadow |
| Chamaenerion angustifolium | 10.2 | fireweed | alpine-meadow | ||||
| 11 | Digoria | 42.88327 | 43.59385 | Thymus daghestanicus | 11 | thyme | brown forest |
| 12 | Upper Dzuarikau | 42.843854 | 44.326609 | Hypеricum perforаtum | 12.1 | St. John’s wort | alpine-meadow |
| Oríganum vulgare | 12.2 | oregano | alpine-meadow | ||||
| Thymus daghestanicus | 12.3 | thyme | alpine-meadow | ||||
| 13 | Suargom | 42.82717 | 44.60466 | Chamaenerion angustifolium | 13 | fireweed | humus-carbonate |
| Brook-Mint | Oregano | St. John’s Wort | ||||
|---|---|---|---|---|---|---|
| Element | Range | Mean ± SD | Range | Mean ± SD | Range | Mean ± SD |
| Na | 134.0–545.0 | 285 ± 198 | 82–179 | 134.8 ± 34.5 | 41–229 | 89.7 ± 78.5 |
| Mg | 2967–4529 | 3520 ± 543 | 1990–3277 | 2734 ± 500 | 1229–2966 | 2196 ± 680 |
| Al | 470–3040 | 1185 ± 989 | 276–939 | 614 ± 235 | 78–1070 | 375 ± 395 |
| K | 19,400–49,500 | 33,750 ± 10,487 | 21,700–30,900 | 26,580 ± 3887 | 12,800–27,300 | 16,220 ± 6210 |
| Ca | 13,700–20,000 | 16,550 ± 2550 | 10,100–17,000 | 1352 ± 28,230 | 5400–13,600 | 8076 ± 3347 |
| Sc | 0.11–0.77 | 0.29 ± 0.25 | 0.08–0.21 | 0.15 ± 0.05 | 0.02–0.30 | 0.10 ± 0.12 |
| V | 0.71–3.80 | 1.63 ± 1.21 | 0.45–1.26 | 0.91 ± 0.32 | 0.34–1.70 | 0.74 ± 0.65 |
| Mn | 36.6–117.0 | 60.8 ± 28 | 33.4–49.0 | 42.6 ± 6.2 | 27.8–190 | 100.4 ± 65 |
| Fe | 339–2080 | 829 ± 680 | 269–591. | 444.6 ± 121 | 54.0–798 | 269.8 ± 300 |
| Co | 0.18–0.78 | 0.39 ± 0.3 | 0.19–0.28 | 0.25 ± 0.03 | 0.17–1.73 | 0.59 ± 0.64 |
| Ni | 2.3–4.1 | 3.17 ± 0.9 | 1.44–4.7 | 2.56 ± 1.88 | 0.82–4.7 | 2.98 ± 1.66 |
| Zn | 26.1–142 | 55.3 ± 44 | 46–101 | 78.9 ± 22 | 48.0–87 | 64.8 ± 17 |
| As | 0.1–0.69 | 0.34 ± 0.21 | 0.08–0.92 | 0.30 ± 0.35 | 0.05–0.36 | 0.13 ± 0.13 |
| Rb | 2.3–37 | 12.9 ± 12.5 | 5.0–19.0 | 10.1 ± 5.9 | 2.5–87.0 | 22.2 ± 36 |
| Sr | 26.3–116 | 65.7 ± 31 | 19.2–80 | 53.9 ± 26 | 8.4–79.0 | 39.2 ± 26 |
| Mo | 0.45–4.9 | 2.49 ± 1.78 | 0.29–2.9 | 1.58 ± 1.1 | 0.16–3.40 | 1.30 ± 1.5 |
| Sb | 0.02–0.26 | 0.08 ± 0.09 | 0.02–0.06 | 0.04 ± 0.02 | 0.02–0.08 | 0.03 ± 0.03 |
| Cs | 0.05–0.32 | 0.14 ± 0.11 | 0.04–0.12 | 0.08 ± 0.03 | 0.03–0.38 | 0.13 ± 0.15 |
| Ba | 23.2–78 | 39.5 ± 19.7 | 19.2–59 | 30.9 ± 16 | 5.4–79.0 | 29.9 ± 22 |
| La | 0.40–1.46 | 0.87 ± 0.54 | 0.19–0.45 | 0.33 ± 0.1 | 0.08–0.63 | 0.24 ± 0.26 |
| Sm | 0.05–0.28 | 0.11 ± 0.09 | 0.03–0.09 | 0.06 ± 0.02 | 0.01–0.13 | 0.04 ± 0.05 |
| Th | 0.08–0.61 | 0.22 ± 0.2 | 0.05–0.14 | 0.11 ± 0.04 | 0.03–0.21 | 0.08 ± 0.09 |
| U | 0.02–0.15 | 0.07 ± 0.05 | 0.01–0.03 | 0.02 ± 0.01 | 0.01–0.04 | 0.02 ± 0.02 |
| Thyme | Fireweed | ||||
|---|---|---|---|---|---|
| Range | Mean ± SD | Range | Mean ± SD | RP | |
| Na | 73–219 | 139.5 ± 56 | 42.9–102 | 64 ± 25 | 150 |
| Mg | 2422–3986 | 3067 ± 700 | 702–3616 | 2221 ± 1192 | 2000 |
| Al | 192–1270 | 612 ± 400 | 150–396 | 260 ± 126 | 80 |
| K | 9870–34,500 | 2187 ± 9340 | 9438–19,400 | 15,084 ± 5050 | 19,000 |
| Ca | 7300–14,900 | 11,566 ± 2671 | 2671–12,200 | 8167 ± 4050 | 10,000 |
| Sc | 0.06–0.3 | 0.15 ± 0.09 | 0.03–0.08 | 0.06 ± 0.03 | 0.02 |
| V | 0.39–1.85 | 0.97 ± 0.52 | 0.18–0.55 | 0.35 ± 0.2 | 0.5 |
| Mn | 42–148 | 83 ± 39 | 39.2–96 | 64.2 ± 24 | 200 |
| Fe | 233–890 | 449 ± 260 | 153–278 | 215 ± 63 | 150 |
| Co | 0.16–0.42 | 0.27 ± 0.1 | 0.10–0.14 | 0.11 ± 0.01 | 0.2 |
| Ni | 1.76–3.8 | 2.83 ± 0.89 | 0.89–2.05 | 1.6 ± 0.5 | 1.5 |
| Zn | 40–74 | 56 ± 13 | 12.8–31.4 | 21.3 ± 7.8 | 50 |
| As | 0.07–0.59 | 0.23 ± 0.2 | 0.05–0.2 | 0.13 ± 0.08 | 0.1 |
| Rb | 4.1–32 | 11.8 ± 10.3 | 4.6–10.3 | 7.8 ± 2.4 | 50 |
| Sr | 47–89 | 67.3 ± 14 | 14.0–59 | 40 ± 18 | 50 |
| Mo | 0.15–2.5 | 1.1 ± 0.9 | 0.1–0.8 | 0.413735 | 0.5 |
| Sb | 0.03–0.06 | 0.04 ± 0.01 | 0.01–0.02 | 0.01 ± 0.003 | 0.1 |
| Cs | 0.05–0.13 | 0.08 ± 0.03 | 0.03–0.09 | 0.05 ± 0.03 | 0.2 |
| Ba | 8.5–104 | 53.8 ± 35 | 6.7–35 | 15.6 ± 13 | 40 |
| La | 0.15–0.74 | 0.38 ± 0.24 | 0.09–0.24 | 0.17 ± 0.07 | 0.2 |
| Sm | 0.03–0.13 | 0.070 ± 0.03 | 0.02–0.04 | 0.03 ± 0.01 | 0.04 |
| Th | 0.05–0.27 | 0.12 ± 0.08 | 0.02–0.8 | 0.04 ± 0.03 | 0.005 |
| U | 0.01–0.07 | 0.05 ± 0.03 | 0.01 | ||
| Element | Min | Max | Mean | Med | SD | MAC | UCC |
|---|---|---|---|---|---|---|---|
| Na | 7200 | 28,000 | 11,186 | 9190 | 5005.7 | 24,259 | |
| Mg | 10,685.32 | 30,400 | 20,427 | 21,400 | 6044.9 | 14,957 | |
| Al | 62,000 | 95,000 | 79,176 | 82,000 | 11,424.2 | 81,505 | |
| Si | 156,000 | 350,000 | 242,000 | 231,000 | 51,085.7 | 313,315 | |
| K | 13,300 | 28,700 | 21,904 | 22,100 | 4447.0 | 23,244 | |
| Ca | 3100 | 66,100 | 11,657 | 7600 | 13,108.9 | 25,568 | |
| Sc | 6.72 | 20.2 | 14 | 15.3 | 3.5 | 14 | |
| Ti | 2070 | 5900 | 4532 | 4600 | 940.9 | 383 | |
| V | 28 | 166 | 113 | 120 | 30.7 | 150 | 97 |
| Cr | 28.3 | 107 | 81 | 89 | 22.6 | 100 | 92 |
| Mn | 168 | 3030 | 972 | 831 | 594.8 | 1500 | 774 |
| Fe | 16,300 | 53,000 | 40,892 | 41,000 | 9286.0 | 39,176 | |
| Co | 4.7 | 28 | 15 | 15 | 5.3 | 50 | 17 |
| Ni | 8.4 | 80 | 43 | 43.9 | 16.4 | 80 | 47 |
| Zn | 64 | 224 | 131 | 125 | 45.5 | 23 | 67 |
| As | 6.3 | 88 | 30 | 23.9 | 18.8 | 2 | 4.8 |
| Rb | 65 | 162 | 118 | 120 | 23.2 | 84 | |
| Sr | 72 | 243 | 122 | 98 | 45.1 | 320 | |
| Zr | 149 | 273 | 204 | 204 | 25.5 | 193 | |
| Mo | 0.72 | 3.44 | 1 | 0.97 | 0.5 | 0.07 | 1.1 |
| Sb | 0.51 | 6 | 2 | 1.51 | 1.1 | 4.5 | 0.4 |
| Cs | 3.54 | 11.7 | 8 | 7.9 | 2.5 | 4.9 | |
| Ba | 304 | 680 | 446 | 427 | 87.1 | 628 | |
| La | 20.1 | 43.7 | 34 | 35.6 | 6.6 | 31 | |
| Ce | 40 | 88 | 69 | 72 | 13.5 | 63 | |
| Nd | 16.6 | 39 | 31 | 32 | 6.8 | 27 | |
| Sm | 3.2 | 7.8 | 6 | 6.55 | 1.2 | 4.7 | |
| Eu | 0.71 | 1.77 | 1 | 1.34 | 0.3 | 1 | |
| Tb | 0.427 | 1.1 | 1 | 0.85 | 0.2 | 0.7 | |
| Yb | 1.24 | 4.1 | 3 | 2.92 | 0.7 | 2 | |
| Lu | 0.18 | 0.7 | 0 | 0.44 | 0.1 | 0.31 | |
| Hf | 3.22 | 7.1 | 5 | 5.4 | 0.8 | 5.3 | |
| Ta | 0.55 | 1.33 | 1 | 0.96 | 0.2 | 0.9 | |
| W | 0.88 | 5.7 | 2 | 2.2 | 0.9 | 1.9 | |
| Th | 6.4 | 16.6 | 12 | 12.7 | 2.7 | 10.5 | |
| U | 1.8 | 63 | 5 | 2.9 | 12.0 | 2.7 |
| DIM | HRI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| № | Location | Ni | Zn | As | Sb | Ni | Zn | As | Sb | |
| 1 | Zamankul | 1.1 | 2.82 × 10−2 | 5.81 × 10−5 | 1.25 × 10−5 | 9.4 × 10−2 | 1.9 × 10−1 | 4.2 × 10−3 | ||
| 1.2 | 2.70 × 10−2 | 8.51 × 10−5 | 2.41 × 10−5 | 9.0 × 10−2 | 2.8 × 10−1 | 8.0 × 10−3 | ||||
| 2 | Koban | 2.1 | 8.45 × 10−4 | 5.93 × 10−2 | 1.34 × 10−4 | 3.29 × 10−5 | 4.2 × 10−2 | 2.0 × 10−1 | 4.5 × 10−1 | 1.1 × 10−3 |
| 2.2 | 1.35 × 10−3 | 1.76 × 10−2 | 1.24 × 10−4 | 2.11 × 10−5 | 6.8 × 10−2 | 5.9 × 10−2 | 4.1 × 10−1 | 7.0 × 10−3 | ||
| 2.3 | 4.81 × 10−3 | 3.16 × 10−2 | 4.76 × 10−5 | 1.16 × 10−5 | 2.4 × 10−2 | 1.1 × 10−1 | 1.6 × 10−1 | 3.9 × 10−3 | ||
| 3 | Dargavs | 3.1 | 2.76 × 10−3 | 4.08 × 10−2 | 4.93 × 10−5 | 1.46 × 10−5 | 1.4 × 10−1 | 1.4 × 10−1 | 1.6 × 10−1 | 4.9 × 10−3 |
| 3.2 | 1.82 × 10−3 | 1.53 × 10−2 | 1.29 × 10−4 | 2.35 × 10−5 | 9.1 × 10−2 | 5.1 × 10−2 | 4.3 × 10−1 | 7.8 × 10−3 | ||
| 3.3 | 2.76 × 10−3 | 5.11 × 10−2 | 4.93 × 10−5 | 1.06 × 10−5 | 1.4 × 10−1 | 1.7 × 10−1 | 1.6 × 10−1 | 3.5 × 10−3 | ||
| 3.4 | 2.23 × 10−3 | 2.74 × 10−2 | 6.16 × 10−5 | 1.76 × 10−5 | 1.1 × 10−1 | 9.1 × 10−2 | 2.1 × 10−1 | 5.9 × 10−3 | ||
| 3.5 | 1.20 × 10−3 | 1.10 × 10−2 | 2.82 × 10−5 | 8.22 × 10−6 | 6.0 × 10−2 | 3.7 × 10−2 | 9.4 × 10−2 | 2.7 × 10−3 | ||
| 4 | Mountain Saniba | 4 | 2.99 × 10−2 | 2.76 × 10−4 | 4.23 × 10−5 | 1.0 × 10−1 | 9.2 × 10−1 | 1.4 × 10−3 | ||
| 5 | Ahsau | 5 | 2.41 × 10−3 | 2.05 × 10−2 | 4.05 × 10−4 | 4.17 × 10−5 | 1.2 × 10−1 | 6.8 × 10−2 | 1.4 | 1.4 × 10−2 |
| 6 | Popov khutor | 6 | 8.34 × 10−2 | 2.00 × 10−4 | 1.53 × 10−4 | 2.8 × 10−1 | 6.7 × 10−1 | 5.1 × 10−2 | ||
| 7 | Karjin | 7 | 4.34 × 10−2 | 4.34 × 10−5 | 1.91 × 10−5 | 1.4 × 10−1 | 1.4 × 10−1 | 6.4 × 10−3 | ||
| 8 | Nar | 8.1 | 1.91 × 10−3 | 2.35 × 10−2 | 9.28 × 10−5 | 3.35 × 10−5 | 9.6 × 10−2 | 7.8 × 10−2 | 3.1 × 10−1 | 1.1 × 10−2 |
| 8.2 | 1.57 × 10−3 | 2.82 × 10−2 | 5.17 × 10−5 | 2.15 × 10−5 | 7.9 × 10−2 | 9.4 × 10−2 | 1.7 × 10−1 | 7.2 × 10−3 | ||
| 9 | Chemy | 9.1 | 3.29 × 10−2 | 2.11 × 10−4 | 4.70 × 10−5 | 1.1 × 10−1 | 7.0 × 10−1 | 1.6 × 10−2 | ||
| 9.2 | 3.17 × 10−2 | 6.16 × 10−5 | 2.05 × 10−5 | 1.1 × 10−1 | 2.1 × 10−1 | 6.8 × 10−3 | ||||
| 10 | Hidikus | 10.1 | 1.03 × 10−3 | 3.42 × 10−2 | 3.46 × 10−5 | 3.58 × 10−5 | 5.2 × 10−2 | 1.1 × 10−1 | 1.2 | 1.2 × 10−2 |
| 10.2 | 1.16 × 10−3 | 1.84 × 10−2 | 8.57 × 10−5 | 5.8 × 10−2 | 6.1 × 10−2 | 2.9 × 10−3 | ||||
| 11 | Digoria | 11 | 8.98 × 10−4 | 4.99 × 10−2 | 6.40 × 10−5 | 1.24 × 10−5 | 4.5 × 10−2 | 1.7 × 10−1 | 2.1 × 10−1 | 4.1 × 10−3 |
| 12.1 | 2.17 × 10−3 | 4.64 × 10−2 | 2.76 × 10−5 | 8.98 × 10−5 | 1.1 × 10−1 | 1.5 × 10−1 | 9.2 × 10−2 | 3.0 × 10−3 | ||
| 12.2 | 1.47 × 10−3 | 4.02 × 10−2 | 2.26 × 10−4 | 3.17 × 10−5 | 7.3 × 10−2 | 1.3 × 10−1 | 7.5 × 10−1 | 1.1 × 10−2 | ||
| 12 | Upper Dzuarikau | 12.3 | 5.46 × 10−2 | 5.40 × 10−4 | 3.52 × 10−5 | 1.8 × 10−1 | 1.8 | 1.2 × 10−2 | ||
| 13 | Suargom | 13 | 8.63 × 10−4 | 1.31 × 10−2 | 8.10 × 10−5 | 1.26 × 10−5 | 4.3 × 10−2 | 4.4 × 10−2 | 2.7 × 10−1 | 4.2 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavrinenko, Y.; Plieva, A.; Chaligava, O.; Grozdov, D.; Frontasyeva, M.; Tkachenko, K.; Zinicovscaia, I. Elemental Analysis of Five Medicinal Plants Species Growing in North Ossetia Using Neutron Activation Analysis. Agronomy 2024, 14, 1269. https://doi.org/10.3390/agronomy14061269
Lavrinenko Y, Plieva A, Chaligava O, Grozdov D, Frontasyeva M, Tkachenko K, Zinicovscaia I. Elemental Analysis of Five Medicinal Plants Species Growing in North Ossetia Using Neutron Activation Analysis. Agronomy. 2024; 14(6):1269. https://doi.org/10.3390/agronomy14061269
Chicago/Turabian StyleLavrinenko, Yulia, Anna Plieva, Omari Chaligava, Dmitrii Grozdov, Marina Frontasyeva, Kirill Tkachenko, and Inga Zinicovscaia. 2024. "Elemental Analysis of Five Medicinal Plants Species Growing in North Ossetia Using Neutron Activation Analysis" Agronomy 14, no. 6: 1269. https://doi.org/10.3390/agronomy14061269






