Abstract
The low solubility and enhanced fixation of zinc (Zn) in semi-arid and dry climates limits Zn uptake in plants. Zn deficiency in soil impairs crop production and human health, necessitating agricultural biofortification. A pot experiment was conducted to evaluate the effect of Zn and various types of manure on the Zn biofortification of lentils. The treatments, consisting of a control (Con), normal manure (NM), composted manure (CM), and acidified manure (AM), were applied under saline soil (EC 8.00 dS m−1) and non-saline soil (EC 1.48 dS m−1) conditions along with two levels of Zn, including Zn at 0 kg ha−1 (native soil Zn = 2.2 mg kg−1) and Zn at 25 kg ha−1 (62.2 mg Zn kg−1 soil was achieved). The AM was prepared by adding sulfur and sulfur-oxidizing bacteria to the composted manure. All the manures were applied at 1% (w/w), and ZnSO4 (33% Zn) was used as a Zn source. Lentil variety Masoor 2021 was cultivated as a test crop in five replications of each treatment arranged in a completely randomized design. Applying AM with Zn considerably increased the lentils’ growth, yield, and Zn content under saline and non-saline conditions. Under non-saline soils, the treatment of AM + Zn significantly promoted the Zn content in the root (132.5%), shoot (91.7%), grain (49.1%), root length (79.7%), plant height (33.7%), and SPAD value (29.9%). Under saline conditions, application of AM + Zn promoted uptake of Zn in the root (218.5%), Zn content in the shoot (175.7%), Zn accumulation in the grain (107.7%), root length (109.7%), plant height (37.8%), and SPAD value (52.8%) compared to the control. According to the results, lentils should be grown with AM and Zn to increase their growth, yield, and Zn content significantly. This is a cost-effective and sustainable way to combat Zn deficiency in lentils.
1. Introduction
The ever-increasing global population has resulted in a massive increase in land-based resource use, converting agricultural land into agricultural determinations that farmers cannot access [1]. As a result, producers are under pressure to produce more food to feed the world’s growing population, and the goal of agriculture has shifted from human health and nutrition to producer profitability [2]. Because of this, more than half of the world’s population is deficient in zinc (Zn), iron (Fe), iodine (I), and selenium (Se) [3]. Zinc malnutrition affects 50% of the world’s population [2,4,5], including nearly a third of Pakistan’s population [6,7].
Zinc is a micronutrient vital for human, animal, and plant life cycles [8]. Its deficiency has detrimental effects on cell growth, the reproductive system, and the immune system, and causes around 800,000 deaths every year in the world’s poor population [3]. Zinc shortages in plants result in tiny chlorotic leaves and reduced photosynthetic activity. Pakistan’s soils are inherently alkaline and calcareous, with low organic matter and a high pH, resulting in lower plant Zn accessibility. Zinc precipitation occurs in calcareous soils, rendering it inaccessible to plants [9]. Its deficiency is the most limiting factor after nitrogen (N) and phosphorus (P) in soils of high pH, like alkaline calcareous soils [10,11]. High calcium carbonate (CaCO3) and low organic matter are the main reasons for Zn deficiency in arid and semi-arid regions [12]. Other reasons for low Zn in soil are parent material with less Zn concentration [13,14], high pH of the soil [15], more P in soil [11], salt content [16], low manure application [17], and the formation of complexes with the soil [18].
Food legumes are vital and diverse in farming systems and poor people’s diets worldwide. They are perfect crops for attaining three developmental goals in a specific community simultaneously: poverty reduction, improved human health and nutrition, and increased environmental resilience [19]. Compared to other staple foods, lentils are considered a relatively inexpensive source of whole foods, with a high protein and mineral content in food and feed [20]. Despite varying climatic conditions, lentils are the most widely cultivated legumes in many developing countries [21]. This crop is primarily used as a low-cost, high-quality protein component in the anthropological diet [22]. Thus, bio-fortifying lentils with Zn can be a valuable tactic to counteract human Zn insufficiency while increasing profits. This crop can be a Zn biofortification competitor, helping alleviate Zn deficiency in developed and developing countries. Thus, it is crucial to solve this issue, which influences every third offspring in Pakistan. Various procedures such as breeding and modern biotechnology (to provide micronutrients in crops) [23], the addition of micronutrients to food sources, toppings, or drinks [24], administering micronutrients through tablets and syrups [25], and the utilization of micronutrient-containing fertilizers [26] are being investigated. Still, practical solutions are limited due to financial constraints and the nature of Pakistan soil (which has more CaCO3, i.e., >3%, less organic matter, and a high pH).
Biofortification or increasing inanimate Zn in pulse grains through organic amendments is a new, effective, and cost-efficient technique for treating Zn insufficiency [17]. Combining organic and inorganic amendments or nutrient sources to increase mineral nutrient concentration has recently gained prominence in agriculture [27]. It is also helpful in overcoming the problem of Zn insufficiency in developing countries. It acts as a substitute for synthetic chelates, and using natural chelates rather than artificial chelates is a long-term approach. The application of Zn ameliorated salinity by promoting plant photosynthetic capacity and antioxidant enzymes, as well as promoting the phytoavailability of Zn for plant uptake. Many researchers have studied the effect of organic amendments on plant growth, development, yield, and the biofortification of zinc [28,29]. However, little has been observed on the impact of different types of processed animal manure on Zn biofortification. We hypothesized that processed manure and Zn application could promote plant growth, yield, and Zn biofortification in lentils under saline and non-saline conditions. The current study aimed to investigate the effect of processed manures and Zn on lentil growth, physiology, and yield, and to biofortify Zn in lentil grains under saline and non-saline conditions.
2. Materials and Methods
2.1. Experimental Site and Soil Analysis
A pot study was conducted in October 2020 in the net house of the Institute of Soil and Environmental Sciences (ISES), University of Agriculture, Faisalabad (UAF), Pakistan. A composite sample of the prepared soil was subjected to several physiochemical analyses before the experiment. Almost 250 g of prepared soil sample was used to make a saturated soil paste to measure soil pH, electrical conductivity (EC), and saturation percentage. After preparing the saturated paste, the pH was determined using a digital pH meter. After calibrating the EC meter with a 0.01 N solution of potassium chloride (KCl), the EC of the extract was determined with an EC meter (Jenway, London, UK) in dSm−1. The soil saturation percentage was measured after measuring the oven-dried weight of the paste and the china dish. For the soil texture analysis, the Bouyoucos hydrometer technique was used. The Walkley and Black technique was used to measure the organic carbon content in the soil [30]. To extract the zinc (Zn), a solution of AB-DTPA (20 mL) was poured into 10 g of soil and shaken for fifteen minutes. The filtrate was collected using Whatman No. 42 filter paper. Following the method of [31], the extract was analyzed using an atomic absorption spectrophotometer. Total nitrogen (N) from the experimental soil sample was determined following the standard Kjeldahl process [32]. Extractable potassium (K) determination was carried out through a flame photometer, and phosphorus (P) was measured by a sodium bicarbonate solution, which is called Olsen P [33] (see Table 1).

Table 1.
Characteristics of the experimental soil and normal, composted, and acidified manures.
2.2. Preparation of Processed/Acidified Animal Manure and Composts
Animal manure was taken from the Directorate of Farms of the University of Agriculture, Faisalabad. It was divided into three parts, i.e., one part was processed to prepare acidified manure (AM) by treating the collected animal manure with elemental sulfur and microbes (bioaugmentation) to lower the pH to 2.0–2.5; the second part of the animal manure was processed for composting (composted manure; CM); the remaining third part of the animal manure was used without processing (fresh manure: FM). Sulfur-oxidizing bacterial (SOB) Acidithiobacillus thiooxidan, was previously isolated and evaluated for lowering the pH of composted manure. For the SOB A. thiooxidan purification, the isolate was inoculated into a thiosulphate agar medium. The SOB A. thiooxidan was cultivated, and the results of the sulfate concentration tests were compared, revealing the presence of sulfur-oxidizing bacteria. Sulfate concentrations of 12–80 mmol/L were detected in the A. thiooxidan cultures after 7–23 days of incubation (final pH 1.5–2.5) using the barium sulfate precipitation method, with up to 424 mmol/L after 45 days (final pH 1.5). Then, acidified manure was prepared using bioaugmentation of elemental sulpher (So) and adding cow dung with sulfur-oxidizing bacteria. Different amounts of So were blended with the cow manure, and after an incubation period, the formulation with the lowest pH result was chosen for further research. On the 15th day of incubation, an amendment of 2–2.5 pH was achieved at 25 °C temperature, 60–65 percent moisture, and 0.40 percent molasses in the presence of effective SOB A. thiooxidan. Then, under pot conditions, the acidified manure (AM) was chosen for further investigation to improve lentil growth, yield, and biofortification with zinc.
For the compost preparation, this study used a self-heating composter, due to the breakdown of the cow manure, in which the temperature was regulated. The composter was a rectangular container 15 cm long, 15 cm wide, and 30 cm high. Natural ventilation was achieved by perforating the container with two holes (0.5 cm) on the side of the insulating wall. Urea was employed as the N source to modify the C/N ratio. To adapt the C/N ratio to 20:1–30:1, fresh manure and urea were mixed at a 300:1 (w/w) ratio. In the composter, fifteen kilograms (15 kg) of mixed material was placed. Adding tap water adjusted the initial moisture level to roughly 60%. The moisture content of the compost was uncontrolled during the composting process. In the first 18 days, the mixture was physically turned twice a week, then once a week until the end. Composting was judged complete when the temperature of the composters reached room temperature for more than 15 days. Samples were taken once after the composting process was completed to evaluate the pH. After blending, 20 g of moist sample was collected from the container at three points. The sample was tested for pH (1:2.5) on a dilution base.
2.3. Experimental Design and Treatments
The pot experiment was conducted during the 2020–2021 lentil cropping season in the ISES, UAF warehouse. The soil was collected in a bulk quantity, then dried and ground. After grinding the soil, it was passed through a 2 mm sieve. The experimental treatment comprised the control, fresh manure (FM), composted manure (CM), and acidified manure (AM) and was applied along with ZnSO4 25 kg ha−1 and without Zn application (native soil Zn). The application of 25 kg Zn ha−1 was selected using recommendations for Pakistan soil [34]. In total, 96 pots were used, including 48 each for the non-saline and saline soils. One group of 48 pots was filled with eight kilograms of soil from a field with saline soil, and eight kilograms of regular soil was used in the other 48 pots for the experiment. Before sowing, urea for nitrogen (N), di-ammonium phosphate (DAP) for phosphorous (P), and sulfate of potash (SOP) for potassium (K) were mixed in the soil as per recommended dose (32:57:59 kg ha−1). Lentil variety Masoor 2021 seeds were taken from the Pulses Section of the Ayub Agriculture Research Institute in Faisalabad. Six seeds were planted in each pot. Tap water was applied for irrigation. The pots were arranged in a completely randomized design (CRD) with six replicates of each treatment. When planting, water was filled in the pots up to each pot’s maximum capacity. After this, irrigation was applied at appropriate intervals until the crop’s maturity, depending upon the plant’s requirement. For plant protection, all possible measures were considered when needed. No weedicide was applied; the weeds were removed manually instead of spraying the weeds.
2.4. Agronomic and Physiological Attributes
The data linked to the yield and growth of the lentil crop was observed at maturity and after harvesting the crop. Root length, shoot length, and pod length were measured after being separated from the lentil plant using a meter rod. The fresh weight of the lentil shoot, root, and pod was measured using a digital weighing balance after harvesting. For the shoot and root dry weight, shoots and roots were cut into small pieces using scissors and put into paper bags with labels. Afterward, they were placed under the sun to dry for 3–4 days. Then, these bags were put in the oven at 65 °C until achieving their constant weight. The pods were shaded and sun-dried for three days before being dried in an oven for 24 h at 65 °C to determine their dry weight. After oven-drying, the samples were weighed using a weighing balance. Grains were separated from the pods by manually threshing each replication separately after drying. The number of grains per pod from each treatment was counted. After removing the grains, the husk weight was calculated. One hundred grains of each replication were calculated, and their weight was measured using a weighing balance. After harvesting the plants, the shoots, leaves, and pods were weighed together to determine bio-yield. At the vegetative stage, three of the six replications were harvested to count the number of nodules in the lentil crop.
An infrared gas analyzer (IRGA) was used to note and determine the physiological parameters of the lentils at the flowering stage of the crop. The conductance of stomata, internal CO2 of sub-stomata, and rate of transpiration, evaporation, photosynthesis, and respiration were measured using the IRGA in the presence of sunlight. The chlorophyll content was measured by taking the SPAD value using a chlorophyll meter. Samples of 0.5 g of leaves from each treatment were homogenized with 80 percent acetone (v/v) and filtered through a filter paper for chlorophyll pigments. A spectrophotometer was used to measure the absorbance of the resultant solution at 663, 645, and 480 nm for chlorophyll a, b, and carotenoids, respectively [35]. The relative water content was determined by adopting the method reported by Teulat et al. [36].
Electrolyte leakage was determined using the method of Lutts et al. [37], which involves cutting uniform leaf discs from each treatment plant using a sharp cork borer. The leaf discs were inserted into a test tube with 5 mL of distilled water separately for each treatment replication. By inserting the conductivity meter’s probe into the solution, the conductivity of the solution was determined. The ion leakage from the leaf discs was shown in this way (Reading1). The leaf disc-containing solution was autoclaved. The conductivity of the solution was measured after the liquid had cooled down, and the total ion concentration in the leaf discs was determined (Reading 2). Ion leakage was calculated as a proportion of total ions leaked (Reading1/Reading2 × 100).
2.5. Grain Quality Assessment
The protein content of the grains was measured from the nitrogen content of the grains [38]. Ábrahám et al.’s [39] method was adopted to determine the free proline content. One gram of leaves was homogenized in 3% sulfosalicylic acid and filtered through Whatman filter paper No. 2. The mixture was heated in a water bath at 100 °C for one hour after adding acid ninhydrin and glacial acetic acid, and the process was stopped with an ice bath. The absorbance of the filtrate was measured at 520 nm after extraction with toluene. The concentration of proline was calculated using a standard curve and represented as a percentage (%). Standard procedures were followed to determine NPK and Zn in the grains, husks, shoots, and roots. Samples of plant shoots, roots, husks, and grains were prepared for digestion after sun drying, followed by oven drying at 65 °C, and grinding each treatment replication. The lentil husks from the grains were separated manually and also subjected to chemical analysis. For the determination of Zn, P, and K in the different plant parts, the Wolf [40] digestion procedure was followed. These three nutrients can be determined from the same digest. To determine nitrogen (N) in different plant parts, the samples were digested using concentrated sulfuric acid and hydrogen peroxide (2:1).
Zinc (Zn) analysis from the grains, husks, shoots, and roots was carried out using an atomic absorption spectrophotometer (AAS). The Olsen and Sommers [33] method was used to determine the amount of phosphorus (P) in the grains, husks, shoots, and roots. Potassium analysis from grains, husks, shoots, and roots was performed through a flame photometer. The nitrogen content of the grains, husks, shoots, and roots was measured using the digested samples in the Kjeldahl apparatus [32].
2.6. Statistical Analysis
Statistix 8.1 software was used for statistical analysis. The principal component analysis (PCA) was conducted using XLSTAT software version 2018, and the correlation graph was created using R-studio-2023. For a complete randomized design, the data in the experiment were analyzed using a two-factor ANOVA approach. At p < 0.05, Fisher’s protected least significant difference (LSD) was used to separate the means [41]. Microsoft Excel 2013 was used to create the graphs and determine standard errors (Microsoft, Redmond, WA, USA). The response of Zn concentration in the root, shoot, husk, and grain to soil Zn treatment and organic amendments was evaluated using general linear models.
3. Results
The characteristics of the three types of tested manures, including FM, CM, and AM, are given in Table 1. Among the tested manures, the lowest pH of 2.2 was shown by the AM, and the FM showed a pH of 7.5. The AM manure had the highest carbon of 56.30%, phosphorus of 0.69%, and zinc of 47 mg kg−1. The CM manure reported higher nitrogen contents of 0.52% and potassium contents of 0.27%.
3.1. Agronomic Attributes of Crop
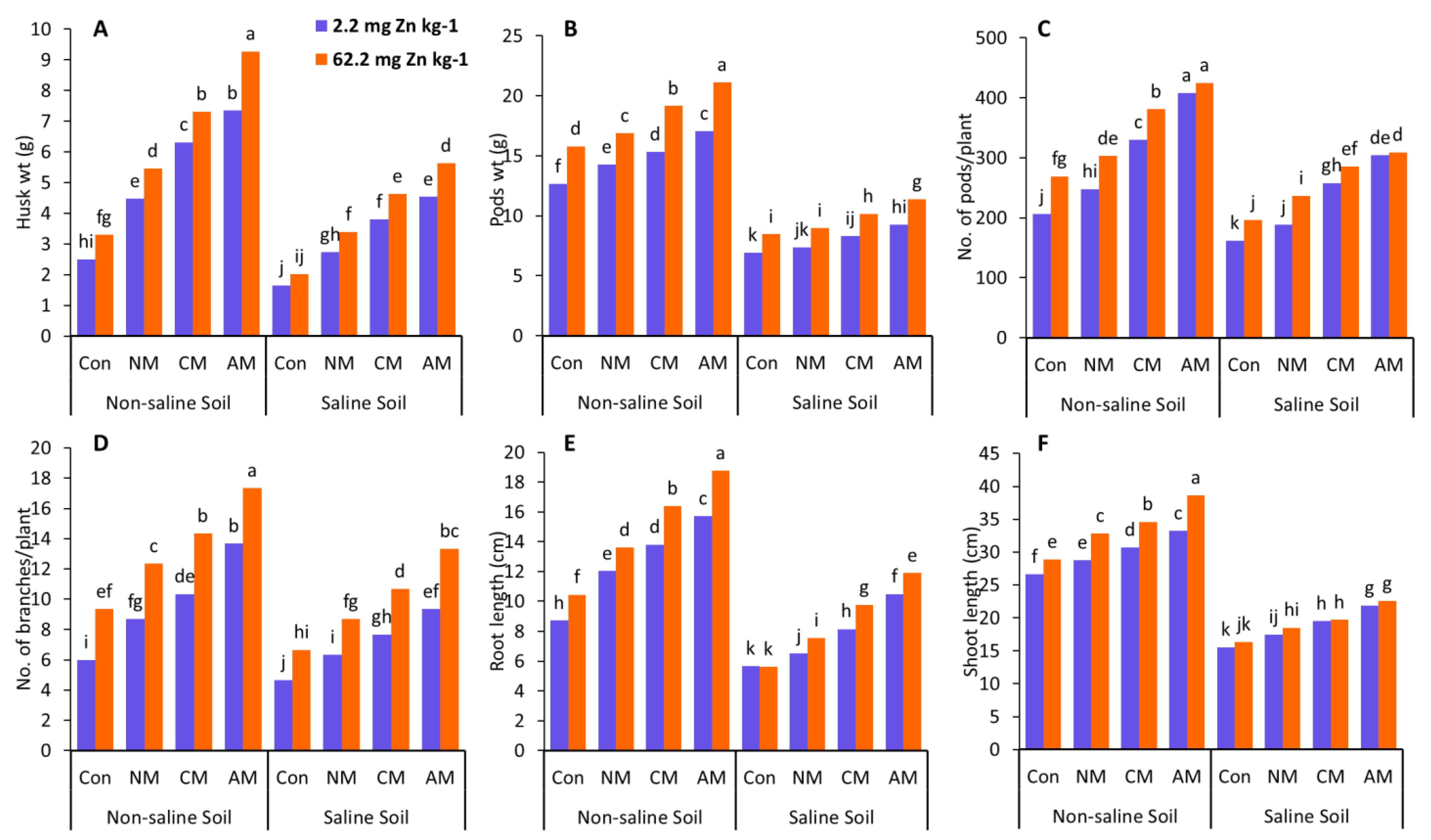
Zn significantly influenced the shoot and root length in non-saline and saline soils compared to the control (Figure 1). In non-saline soil, all the Zn treatments considerably increased shoot and root lengths compared to the control, with a 45% increase in shoot length and a 115.5% increase in root length found in the AM with Zn treatment compared to the control (without Zn). In saline soil, a similar trend was observed, with a 45% increase in shoot length and a 110% increase in root length, respectively (Figure 1). In the same way, the highest increases in shoot length (25%, 40.5%) and root length (81%, 85%) were reported without Zn treatment in both non-saline and saline soils compared to their control. The CM with Zn also substantially influenced the pod number and the number of branches compared to the control and the NM in both non-saline and saline soils (Figure 1). When compared to the control (without Zn), adding CM with Zn resulted in a considerable increase in the number of pods and branches in both non-saline and saline soils, with the most significant increase in the number of pods (84.9%, 76.1%) and branches (138.9%, 128.6%). In the treatment where the CM without Zn was employed, the most considerable values of the number of pods (60.19%, 59.26%) and the number of branches (72.22%, 64.27%) were seen in both non-saline and saline soils, when compared to the control.
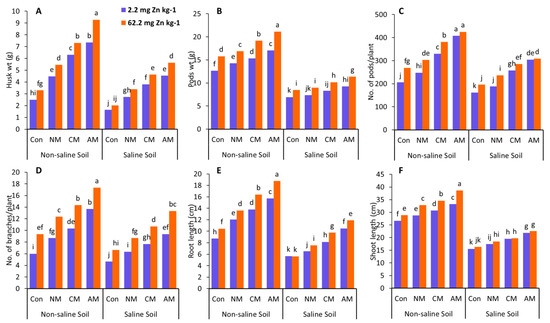
Figure 1.
Effects of using different organic amendments with zinc (Zn) application on husks weight (A), pods weight (B), No. of pods/plant (C), No. of branches/plant (D), root length (E), and shoot length (F) under saline and non-saline soil conditions. At the 5% probability level, means with different letters are significantly different according to LSD. (Con: control; NM: normal manure; CM: compost manure; AM: acidified manure).
3.2. Physiological Attributes of Crop
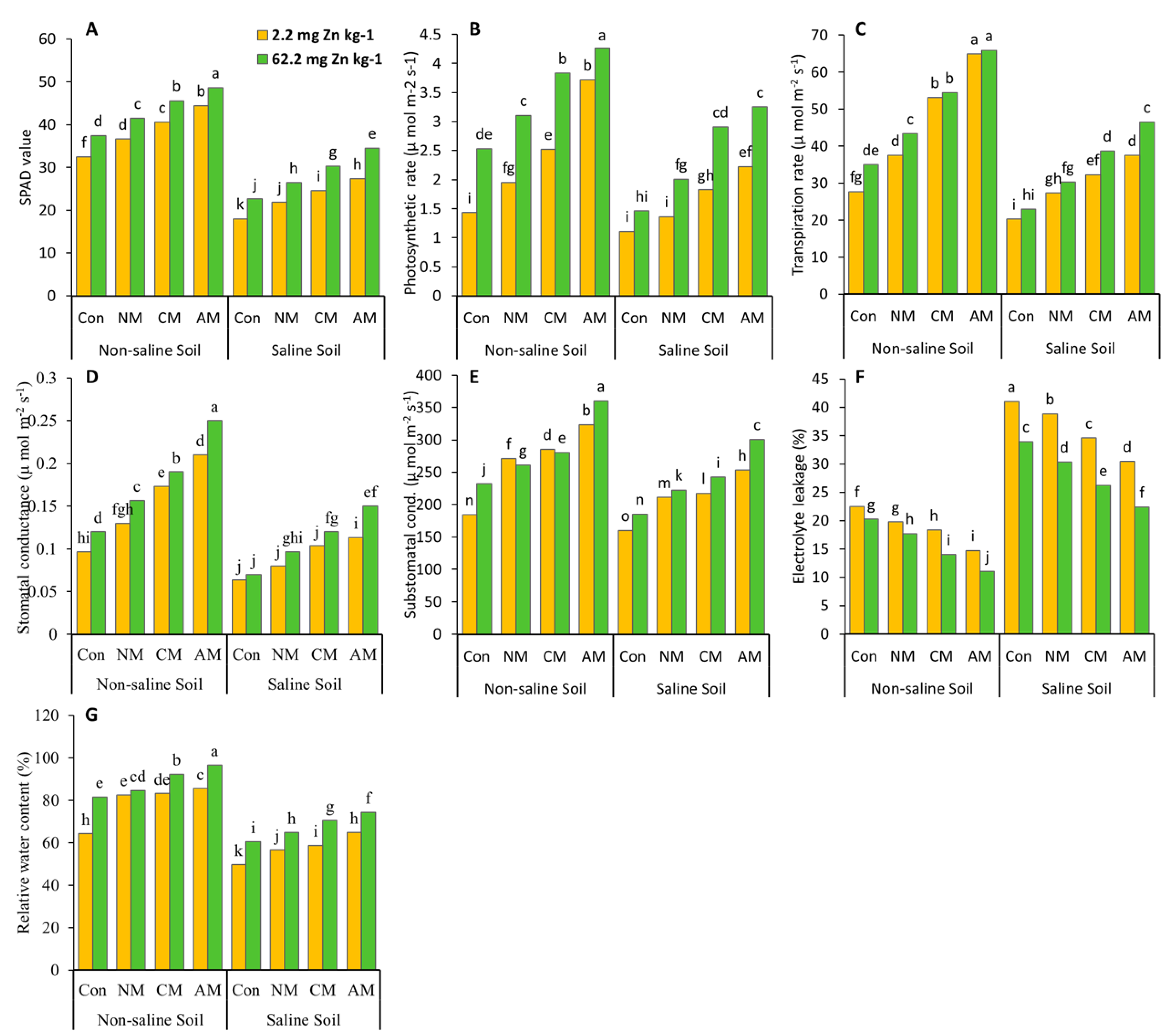
The relative water contents, stomatal conductance, sub-stomatal conductance, photosynthetic rate, and transpiration rate were much higher in the condition in which AM with Zn was applied than in the control and all the other treatments in both non-saline and saline soils (Figure 2). The relative water content (50%, 49.5%), stomatal conductance (160%, 138%), sub-stomatal conductance (95.5%, 88%), photosynthesis rate (199%, 193%), and transpiration rate (138%, 129.5%) were all significantly increased in both the non-saline and saline soils, compared to the control (without Zn), when AM with Zn was applied. In the treatment when AM without Zn was used, the maximum values of relative water content (33%, 31%), stomatal conductance (119%, 83%), sub-stomatal conductance (75.5%, 58%), photosynthetic rate (160%, 98%), and transpiration rate (134%, 85%) were found in non-saline and saline soils, when compared to the control.
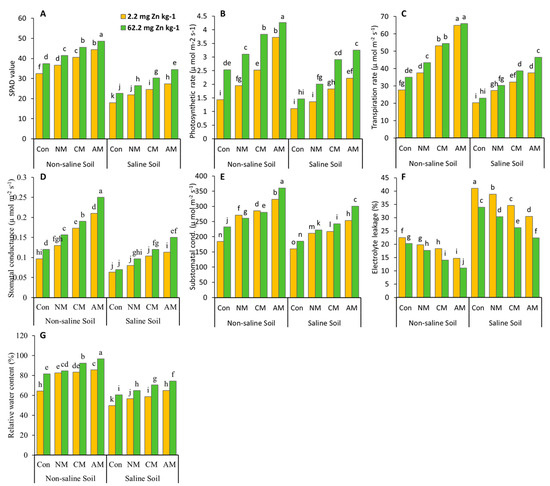
Figure 2.
Effects of using different organic amendments and zinc (Zn) application on SPAD value (A), photosynthesis rate (B), transpiration rate (C), stomatal conductance (D), sub-stomatal conductance (E), chlorophyll value, electrolyte leakage (F), and relative water content (G) under saline and non-saline soil conditions. At the 5% probability level, the means with different letters are significantly different according to LSD. (Con: control; NM: normal manure; CM: compost manure; AM: acidified manure).
There was a significant increase in the SPAD value in both non-saline and saline soils in the AM with Zn treatment compared to the control and all the other treatments with and without Zn, and a maximum increase in the SPAD value (50%) was found in the non-saline soil (Figure 2) when compared to the control. Similarly, in the saline soil, the maximum SPAD value (49.31%) was seen in the treatment where AM without Zn was applied compared to the control. Introducing AM with Zn in non-saline soil decreased the electrolyte leakage content (45.46%). In contrast, saline soil reduced electrolyte leakage content (34.18%) compared to the control. When AM without Zn was applied to non-saline and saline soils, the lowest value of electrolyte leakage content (34.46%, 25.77%) was observed when compared to the control.
3.3. Grain Quality and Nutrient Analyses
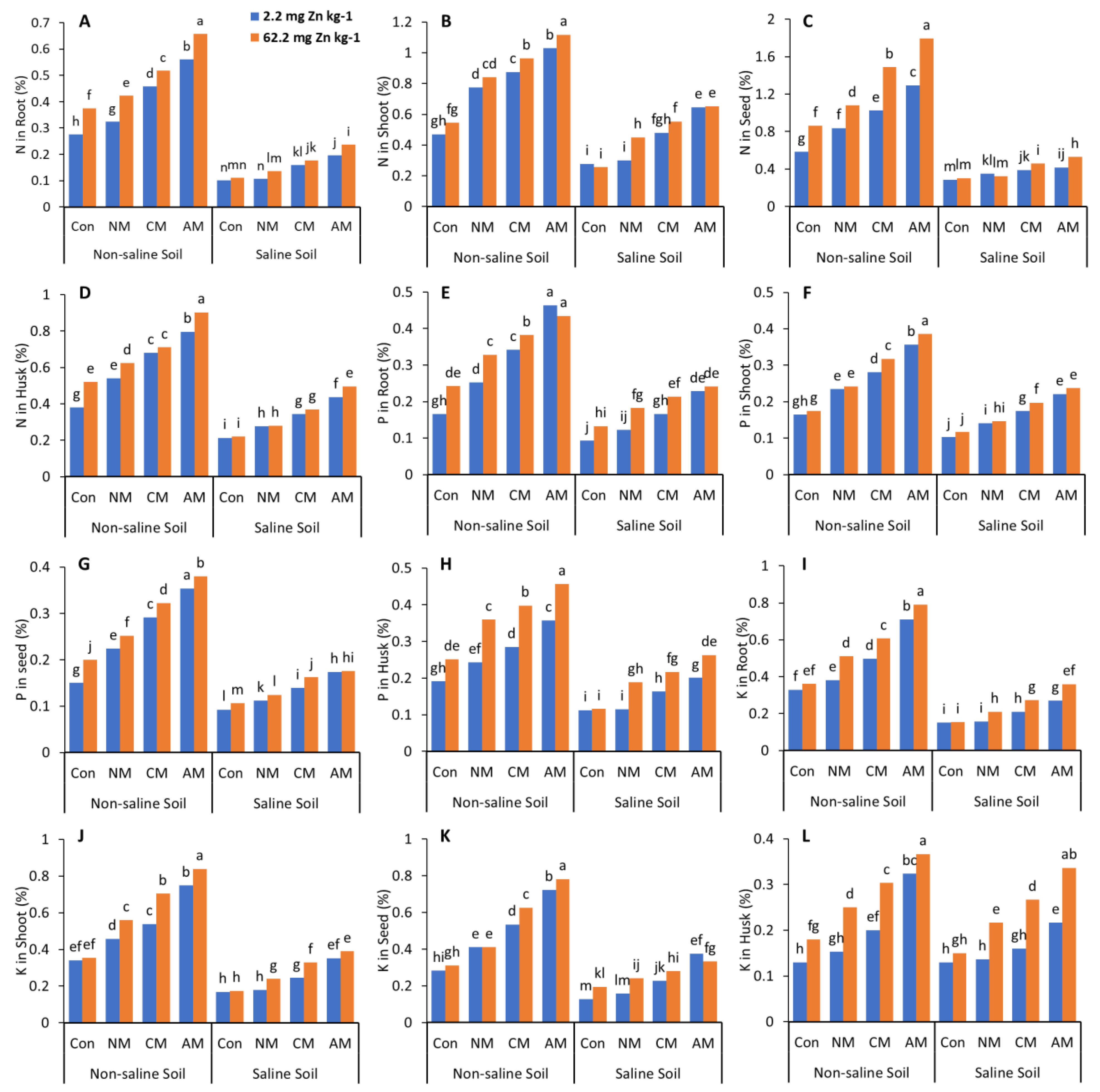
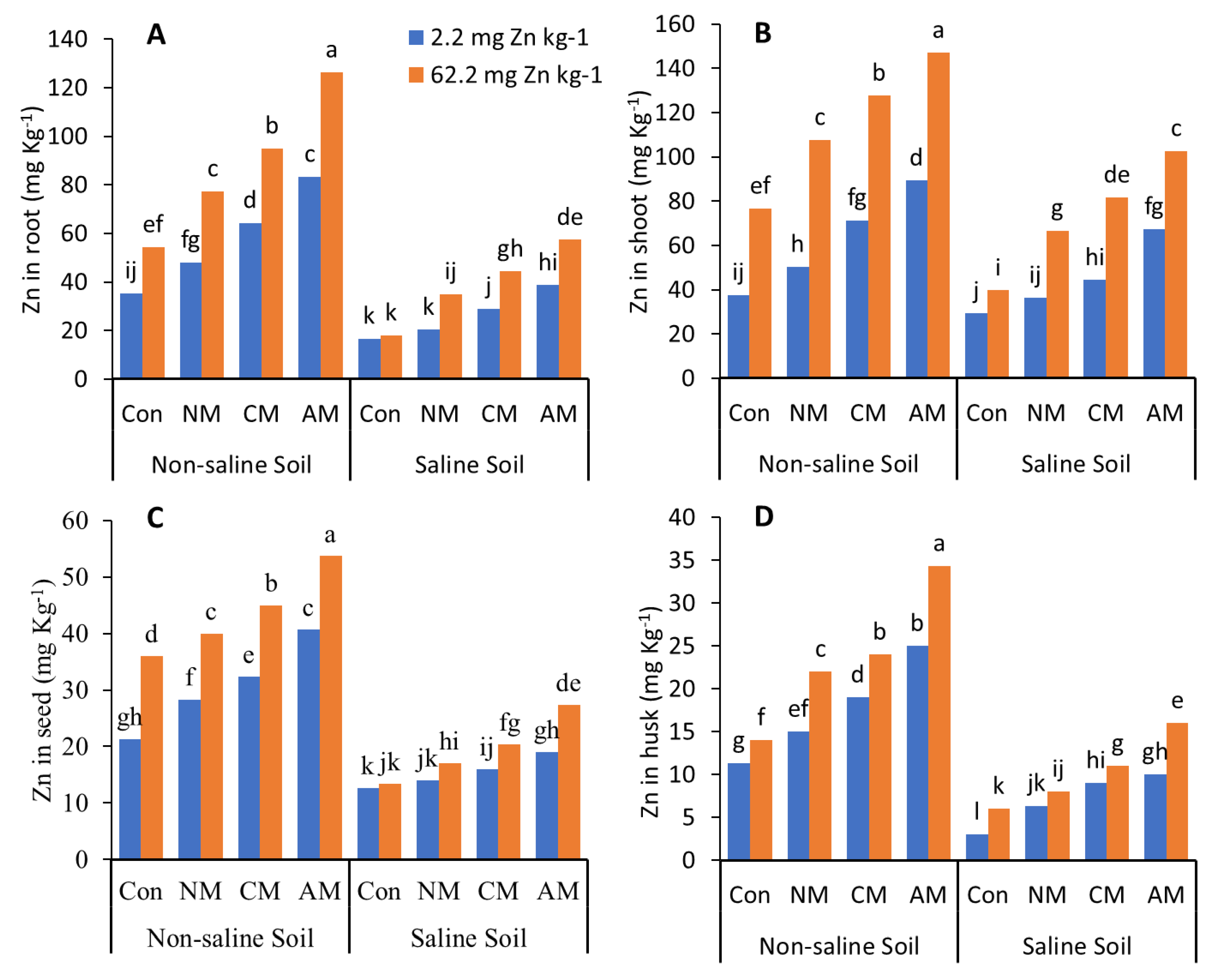
In both non-saline and saline soils, applying NM with and without Zn to the soil significantly decreased the N, P, K, and Zn content in the roots, shoots, husks, and pods compared to all the other treatments except the control (Figure 3 and Figure 4). In non-saline soil, adding AM with Zn increased the N, P, K, and Zn content compared to the control and all the other treatments with and without Zn, with maximum values of 162.4%, 127.8%, 177.1%, and 151.6%, respectively, in the grain when compared to the control, whereas in saline soil, the AM with Zn increased the N, P, K and Zn content compared to the control, with maximum values of 86.4%, 81.5%, 161.5%, and 115.8%, respectively, in the grain. Adding Zn to the treatments affects the N, P, K, and Zn content in the roots, shoots, husks, and pods. While in both non-saline and saline soils, the CM with Zn increased the N, P, K, and Zn content by 88.1% and 74.08%, 93.4% and 45.3%, 121.9% and 118.9%, 110.9% and 60.52% in the grain, respectively.
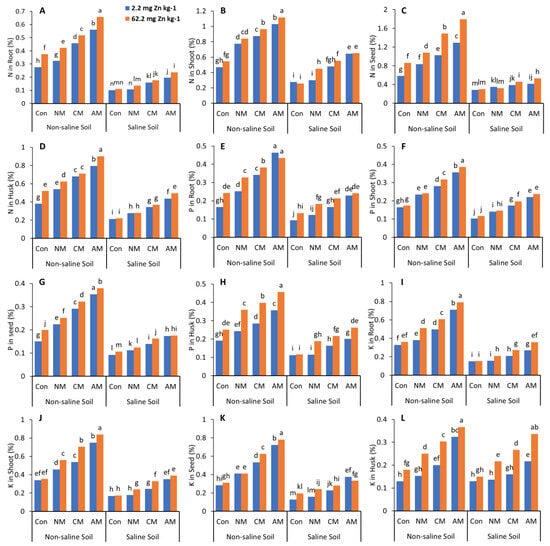
Figure 3.
Effects of different organic amendments and zinc (Zn) on N content in roots (A), shoots (B), seeds (C), and husks (D); P content in roots (E), shoots (F), seeds (G), and husks (H); and K content in roots (I), shoots (J), seeds (K), and husks (L) under saline and non-saline soil conditions. At the 5% probability level, means with different letters are significantly different according to LSD; (Con: control; NM: normal manure; CM: compost manure; AM: acidified manure).
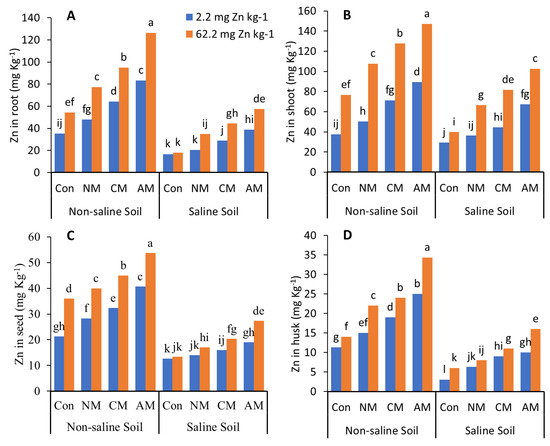
Figure 4.
Effects of different organic amendments with and without zinc (Zn) as well as their control on the Zn in roots (A), shoots (B), seeds (C), and husks (D) under saline and non-saline soil conditions. At the 5% probability level, means with different letters are significantly different according to LSD; (Con: control; NM: normal manure; CM: compost manure; AM: acidified manure).
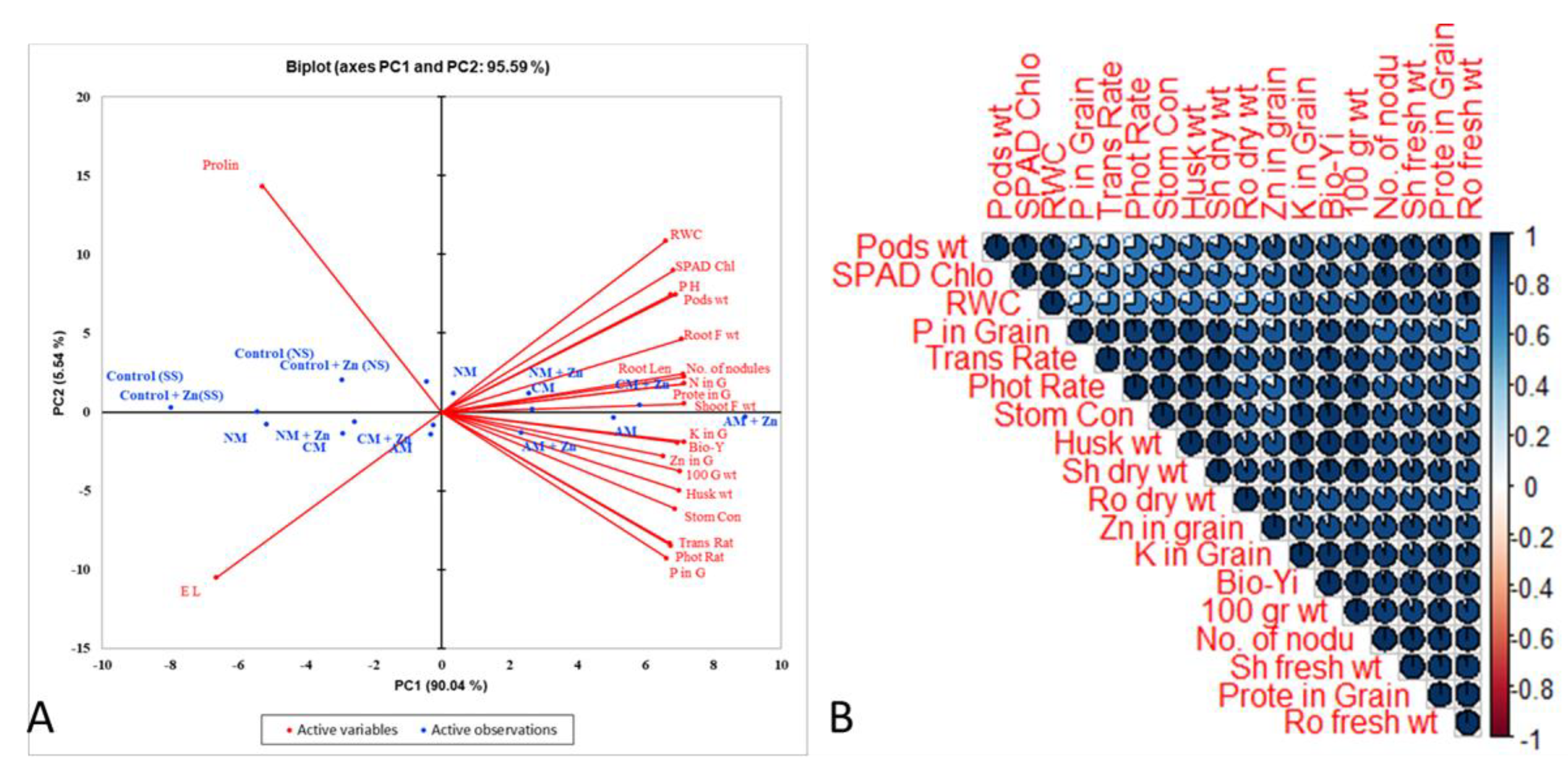
3.4. Results from PCA and Correlation
Agronomic (biological yield, plant height, root length, number of nodules, husk weight, pod weight, shoot fresh weight, root fresh weight, and 100-grain weight), physiological (photosynthesis rate, transpiration rate, stomatal conductance, relative water content, electrolyte leakage, and SPAD index), biochemical (protein and proline), and chemical parameters (nitrogen, phosphorus, potash, and zinc in the grain) were found to have substantial positive and negative correlations (Figure 5). The score and loading plots of the principal component analysis are shown in the Figure (PCA). Within the dataset, the first two PCA components revealed the highest variance (95.5%) of all the parameters studied, with PC1 accounting for 90.04 percent of the variation and PC2 for 5.54 percent. Furthermore, the first two components successfully displaced all of the applied treatments. This treatment experiment demonstrated that the application of acidified manure, alone or in combination with zinc, had a substantial ameliorative effect on all of the examined lentil plant characteristics compared to the control (Figure 5). The PCA variables with parameters had a beneficial impact on PC1 (relative water contents, SPAD value, pH, pods weight, root fresh weight, root length, No. of nodules, nitrogen in grain, protein grain, shoot fresh weight, potassium in grain, Biological yield, Zn in grain, 100 grains weight, husk weight, stomatal conductance, transpiration rate, photosynthetic rate, and phosphorus in grain), whereas PC2 was positively influenced in the PCA observations of Con SS, Con NS, Con + Zn (NS), Con + Zn (SS), NM, NM + Zn, CM, CM + Zn, and AM. Furthermore, the parameters of PC1 and PC2 were found to have a solid negative connection.
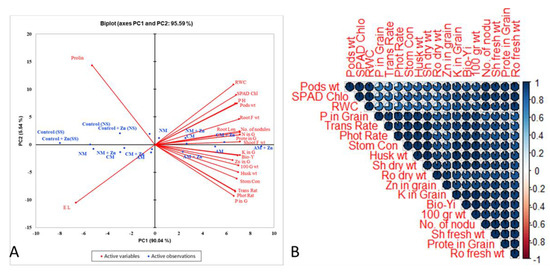
Figure 5.
(A) In the principal component analysis of observations and variables, the first two components revealed 95.59% of the variability between the used treatments. They investigated the parameters of lentil plants under different organic amendments with and without Zn in normal and saline soil. Observations are as follows: NM = normal manure; CM = composted manure; AM = acidified manure; NM + Zn = normal manure + zinc; CM + Zn = composted manure + zinc; AM + Zn = acidified manure + zinc. (B) A statistically significant relationship exists between lentil agronomic, physiological, chemical, and biochemical parameters and their grain Zn concentrations (p = 0.05) in non-saline and saline soil under different organic amendments with or without Zn. Trans Rate: transpiration rate; Stom Con: stomatal conductance; Sh fresh wt: shoot fresh weight; Sh dry wt: shoot dry weight; Ro fresh wt: Root fresh weight; Ro dry wt: root dry weight; RWC: relative water contents; Prote in grain: protein in grain; Pods wt: pod weight; Phot Rate: photosynthesis rate; P in grain: phosphorus in grain; K in grain: potassium in grain; Husk wt: husk weight; SPAD Chlo: SPAD chlorophyll; No. of nodu: number of nodules; 1000 G.W: 1000 grain weight; Bio Yi: biological yield.
4. Discussion
Increased plant growth indicates the best effects from the acidified manure, which, by improving the pH, activates the soil’s micronutrients (i.e., Zn) and makes them available for the plant. This may be due to various mechanisms, including the pH of the soil, to make the nutrients mobilize in the soil and more available to the plant [42]; manure contains substances that increase the number of nutrients in the soil that regulate plant growth by reducing the ammonia volatilization [43,44]. In both non-saline and saline soils, the physiological parameters like photosynthetic rate, transpiration rate, and chlorophyll were much higher when AM with Zn was applied than in the control and all the other treatments in both non-saline and saline soils. This is because micronutrients play a crucial role in many of the plant’s physiological processes, like enzyme activity [45], photosynthesis [46], and physiology, which are essential for the growth and development of plants [47].
Adding acidified manure to the soil improved the physiological properties of maize grown in tannery-polluted soil [48]. By disorganizing chloroplasts and halting the electron transport chain reaction, chromium toxicity in soil has a detrimental impact on chlorophyll concentration, electrolyte leakage, and relative water content in leaves [49]. Protoporphyrin binding is disrupted due to decreased iron availability caused by chromium poisoning, and ROS generation destroys protein complexes and hinders chlorophyll synthesis [50]. Biochar significantly improved chlorophyll content, photosynthetic rate, transpiration rate, and stomatal conductance when coupled with solid acidified manure and lowered electrolyte leakage [48]. These findings can be explained by the fact that after adding acidified manure to polluted soil, the concentration of AB-DTPA Cr in the soil was significantly reduced.
Zinc spraying boosted chlorophyll production in safflower, according to research, demonstrating the importance of zinc in nitrogen metabolism and chlorophyll formation [51]. Zinc caused chlorophyll to be produced due to sulfhydryl group protection [52]. In reality, zinc is essential for the functioning of enzymes involved in the manufacture of chlorophyll [53]. The fall in chlorophyll content was due to a lack of water. The highest chlorophyll concentration was found in zinc oxide during blooming, heading, and grain-filling stages, whereas the lowest was found in the control. Behtash et al.’s [54] findings on red beet embryos reveal that zinc prevented cadmium from destroying chlorophyll and substantially influenced chlorophyll index and chlorophyll content compared to the control treatment.
When stressed, plants close their stomata to limit their transpiration rate, reducing water loss to the environment. The signaling molecule, abscisic acid, produced in the roots, regulates this process. The generation of reactive oxygen species (ROS) is slowed by limiting transpiration [55]. In reaction to drought and salinity stress, stomata close due to reduced leaf turgor, air vapor pressure, and root-generated chemical signals [56]. Thus, mesophyll conductance and stomatal closure reduction in tense situations are typically linked to a decreased photosynthetic rate [56]. To preserve water, plants grown in drought have decreased stomatal conductance. According to several studies, stomata shut during the early phases of drought stress, resulting in higher water use efficiency. Zn shortage reduces photosynthetic capacity due to a decrease in stomatal conductance, according to Wang and Jin [57]. Stomata closure has been shown to restrict water transpiration more than CO2 diffusion into leaf tissues [58]. According to Saboor et al. [59], biofertilizers and zinc application increase stomatal conductance in the flowering, heading, and grain-filling stages compared to the control under the same water limitation level. This could be due to acidified manure, which lowered soil pH and increased plant zinc availability.
When zinc was added, the electrical conductivity content dropped considerably [60]. Plant membranes undergo alterations in response to environmental stresses, typically linked to increased permeability and integrity loss [60]. Plants’ ability to preserve membrane integrity under drought impacts their drought tolerance. Drought-stressed maize plants have more electrolyte leakage than plants grown under control conditions, according to Quan et al. [61]. Zn also protects the bio-membrane from oxidative and peroxidative damage, loss of plasma membrane integrity, and changes in membrane permeability by stabilizing and protecting it [52]. These findings are comparable to those of navy beans and spinach [62]. Enzyme activity rose linearly as Zn concentration in leaf blades increased, indicating that enzyme activity and Zn concentration were closely linked. According to Huang [63], Zn closely interacts with enzymes. The extent of net photosynthesis and chlorophyll synthesis suppression was determined by the blade’s Zn status [64]. Fu et al. [65] found that a Zn shortage in apple leaves reduced net photosynthesis. Photosynthesis has been connected to Zn content in the leaves of plants that grow in higher Zn-supplied soils, with a critical Zn content of 14 g/g [64]. According to our findings, as zinc concentration rises, enzyme activity increases, resulting in a higher rate of photosynthesis.
Agronomic parameters, like the number of nodules, biological yield, root–shoot fresh weight, and root–shoot length, were greatly enhanced when zinc was used with the AM. Pedersen et al. [66] recently found that applying sulfuric acid-acidified manure slurry to sandy soil increased nutrient bioavailability and plant vegetative development. Other researchers agreed that micronutrients improved plant growth characteristics such as leaf numbers per plant [67], plant biological yield [68], the diameter of the stem, plant length [69], and other production-related parameters [45]. Substantial increase in the amount of Zn and growth stage improvement increases the number of nodules [70] per plant, productive number of branches [71], and dry weight of the shoot [72]. In the case of 100-grain weight, a combined application of zinc + AM exhibited the most significant increase, which was related to improved nutrient uptake because of a decrease in pH, faster growth of roots, and thus higher 100-grain weight and plant biomass. Zhao et al. [18] discovered that soil pH was the most critical factor in controlling the solubility of nutrients from soil surfaces, mineral diversification, and the ultimate accessibility and transport of essential micronutrients in the soil. According to Uprety et al. [73], applying acidic compost to the soil can significantly boost the concentration of micronutrients in soil; manure was used as a basic material in this case, which increased micronutrient availability for a maize plant and its development and production [74]. Our findings agreed with those of Tariq et al. [67] and Lana et al. [75], who found that micronutrients were important in achieving greater yields and growth of plants. Other crops have seen significant increases in grain production after the Zn application [52].
The uptake of nitrogen, phosphorus, potassium, and zinc was measured in the roots, shoots, husks, and grains of lentils, with the best results coming from treatments that included both acidified manure and zinc. As a result of So oxidation, acid was produced, lowering the pH of the soil and permitting the dissolution of adsorbed and precipitated nutrients [76,77]. It was also hypothesized that So oxidation would desorb the valuable nutrients from the surfaces of the minerals and substitute micronutrients such as Zn, Fe, Mn, etc., from minerals surfaces like aluminum hydroxide and organic compounds [78,79]. Moreover, the sulfate formed by So oxidation has sites with a negative charge where metal complexation occurs, which seems easy for plant roots to access. Correcting pH with acids like organic acids (gallic, oxalic, and citric acids), HNO3, and acetic acid improved nutrient availability and explored the effect that the addition of acids to soil mobilizes nutrients from minerals through carbonate dissolution, which aids in the soils’ buffering [80,81,82]. The acids aid in dissolving calcium minerals, and the amount and kind of carbonate present; these, in addition to particle size and sample size, all influence solubility. Consequently, these improve micronutrient uptake by plants and the properties of salt-affected soil [83]. Finally, in both non-saline and saline soils, the application of acidified manure with zinc improved all lentil growth, yield, and nutrient uptake metrics compared to the control and other treatments. Further, acidified manure (1% w/w of soil) with the recommended dose of zinc ensures there is enough for plants without additional input, as plants did not respond well to NM and composted manure with the recommended dose of zinc in non-saline and saline soils compared to the acidified manure. In the case of the acidified manure, the greatest response was attributed to factors such as the solubilization of minerals by H2SO4 and bacterial production of organic acids, both of which aid in the dissolution and absorption of micronutrients, as well as micronutrient buildup in plants and their grains.
5. Conclusions
- Saline conditions most significantly affected plant growth, yield, and nutrient uptake in lentils. Applying zinc with various types of manure alleviated saline toxicity in lentils.
- Acidified manure with zinc (62.2 mg kg−1) showed the most significant increase in plant growth, physiology, yield, and nutrient uptake in lentils under saline and non-saline conditions.
- The treated zinc and acidified manure yielded higher N, P, K, and Zn contents, but the difference between sole and combined applications of these treatments was negligible. The Zn and acidified manure allowed plants to absorb the nutrients necessary to attain their sufficiency level.
- The pH of the soil dropped for a shorter length of time due to acidification by S oxidation, and this pH shock mobilized soil nutrients and made them highly accessible to the plants.
- On the other hand, the tested soil was equipped with a capacity for buffering pH changes; therefore, this product would be better used when the crop has its highest nutritional requirement.
Author Contributions
Conceptualization, N.Y. and M.N. (Muhammad Naveed); methodology, N.Y. and M.Y. (Madeeha Younas); software, M.Y. (Madeeha Younas) and M.Z.M.; validation and formal analysis, M.H.B., M.N. (Muhammad Naveed), S.S. and A.M.; resources, M.N. (Muhammad Nadeem) and M.Y. (Muhammad Yaseen); data curation, N.Y.; writing—original draft preparation, N.Y., A.M. and M.Z.M.; writing—review and editing, M.Z.M., A.M., S.S. and I.A.-A.; funding acquisition, S.S. and I.A.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R298), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nawaz, A.; Farooq, M.; Lal, R.; Rehman, A.; Rehman, H. Comparison of conventional and conservation rice-wheat systems in Punjab, Pakistan. Soil Tillage Res. 2017, 169, 35–43. [Google Scholar] [CrossRef]
- Mayer, J.E.; Pfeiffer, W.H.; Beyer, P. Biofortified crops to alleviate micronutrient malnutrition. Curr. Opin. Plant Biol. 2008, 11, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Khush, G.S.; Lee, S.; Cho, J.I.; Jeon, J.S. Bio-fortification of crops for reducing malnutrition. Plant Biotech. Rep. 2012, 6, 195–202. [Google Scholar] [CrossRef]
- Kenzhebayeva, S.; Abekova, A.; Atabayeva, S.; Yernazarova, G.; Omirbekova, N.; Zhangand, G.; Wang, Y. Mutant lines of spring wheat with increased iron, zinc, and micronutrients in grains and enhanced bioavailability for human health. BioMed Res. Int. 2019, 2019, 9692053. [Google Scholar] [CrossRef] [PubMed]
- Veena, M.; Puthur, J.T. Seed nutripriming with zinc is an apt tool to alleviate malnutrition. Environ. Geochem. Health 2022, 44, 2355–2373. [Google Scholar] [CrossRef] [PubMed]
- World Health Report. Reducing Risks, Promoting Healthy Life; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Bhutta, Z.A.; Jiwani, A.; Feroze, A.; Kissana, N.; Monasterio, I.O. Assessment of human zinc deficiency and determinants in Pakistan: Implications for interventions. Proceeding of the International Zinc Association Conference Zinc Crops 2007—Improving Crop Production and Human Health, Istanbul, Turkey, 24–26 May 2007. [Google Scholar]
- Hafeez, B.; Kanif, Y.M.; Saleem, M. Role of Zinc in plant nutrition—A review. Am. J. Exp. Agric. 2013, 3, 374–391. [Google Scholar] [CrossRef]
- Morshedi, A.; Farahbakhsh, H. Effects of potassium and zinc on grain protein contents and yield of two wheat genotypes under soil and water salinity and alkalinity stresses. Plant Ecophys. 2011, 2, 67–72. [Google Scholar]
- Rashid, A.; Ryan, J. Micronutrient constraints to crop production in soils with Mediterranean-type characteristics: A review. J. Plant Nutr. 2008, 27, 959–975. [Google Scholar] [CrossRef]
- Alloway, B.J. Zinc in Soils and Plant Nutrition, 2nd ed.; International Fertilizer Industry Association: Paris, France, 2008. [Google Scholar]
- Imran, M.; Arshad, M.; Khalid, A.; Kanwal, S.; Crowley, D.E. Perspectives of rhizosphere microflora for improving Zn bioavailability and acquisition by higher plants. Int. J. Agric. Biol. 2014, 16, 653–662. [Google Scholar]
- Kochian, L.V. Molecular physiology of mineral nutrient acquisition, transport, and utilization. Biochem. Mol. Biol. Plant 2000, 1204–1249. [Google Scholar]
- Hussain, S.; Maqsood, M.A.; Rahmatullah, M. Increasing grain zinc and yield of wheat for the developing world: A review. Emir. J. Food Agric. 2010, 22, 326–339. [Google Scholar] [CrossRef]
- Alloway, B.J. Zinc in Soils and Crop Nutrition; International Zinc Association: Brussels, Belgium, 2004. [Google Scholar]
- Kausar, M.; Chaudhry, F.; Rashid, A.; Latif, A.; Alam, S. Micronutrient availability to cereals from calcareous soils. Plant Soil 1976, 45, 397–410. [Google Scholar] [CrossRef]
- Cakmak, I. Bio-fortification of cereals with zinc and iron through fertilization strategy. In Proceedings of the 19th World Congress of Soil Science, Brisbane, Australia, 1–6 August 2010; pp. 1–6. [Google Scholar]
- Zhao, K.; Selim, H.M. Adsorption-desorption kinetics of Zn in soils: Influence of phosphate. Soil Sci. 2010, 175, 145–153. [Google Scholar] [CrossRef]
- Siddiq, M.; Uebersax, M.A.; Siddiq, F. Global production, trade, processing and nutritional profile of dry beans and other pulses. In Dry Beans and Pulses: Production, Processing, and Nutrition; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2022; pp. 1–28. [Google Scholar]
- Migliozzi, M.; Thavarajah, D.; Thavarajah, P.; Smith, P. Lentil and kale: Complementary nutrient-rich whole food sources to combat micronutrient and calorie malnutrition. Nutrients 2015, 7, 9285–9298. [Google Scholar] [CrossRef] [PubMed]
- Pirhayati, M.; Soltanizadeh, N.; Kadivar, M. Chemical and microstructural evaluation of ‘hard-to-cook’phenomenon in legumes (pinto bean and small-type lentil). Int. J. Food Sci. Techn. 2011, 46, 1884–1890. [Google Scholar] [CrossRef]
- Roy, F.; Boye, J.I.; Simpson, B.K. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res. Int. 2010, 43, 432–442. [Google Scholar] [CrossRef]
- Bouis, H.E.; Welch, R.M. Bio-fortification a sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crops Sci. 2010, 50, 20–32. [Google Scholar]
- Anonymous. Zinc in Fertilizers, Essential for Crops; International Zinc Association: Brussels, Belgium, 2007. [Google Scholar]
- Strand, T.A. Zinc and Infectious Disease—Studies of Mice and Men. Doctoral Dissertation, Center for International Health, University of Bergen, Bergen, Norway, 2003. [Google Scholar]
- Imtiaz, M.; Alloway, B.J.; Khan, P.; Memon, M.Y.; Siddiqui, S.H.; Aslam, M.; Shah, K.H. Zinc deficiency in selected cultivars of wheat and barley as tested in solution culture. Commun. Soil Sci. Plant Anal. 2006, 23, 1703–1721. [Google Scholar] [CrossRef]
- Atilgan, A.; Coskan, A.; Alagoz, T.; Oz, H. Application level of chemical and organic fertilizers in the greenhouses of Mediterranean Region and its possible effects. Asian J. Chem. 2008, 20, 3702–3714. [Google Scholar]
- Naveed, S.; Rehim, A.; Imran, M.; Bashir, M.A.; Anwar, M.F.; Ahmad, F. Organic manures: An efficient move towards maize grain bio-fortification. Int. J. Recycl. Org. Waste Agric. 2018, 7, 189–197. [Google Scholar] [CrossRef]
- Hussain, A.; Zahir, Z.A.; Ditta, A.; Tahir, M.U.; Ahmad, M.; Mumtaz, M.Z.; Hayat, K.; Hussain, S. Production and implication of bio-activated organic fertilizer enriched with zinc-solubilizing bacteria to boost up maize (Zea mays L.) production and bio-fortification under two cropping seasons. Agronomy 2019, 10, 39. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996; Volume 5, pp. 961–1010. [Google Scholar]
- Soltanpour, P.N.; Workman, S.M.; Schwab, A.P. Use of inductively-coupled plasma spectrometry for the simultaneous determination of macro-and micronutrients in NH4HCO3-DTPA extracts of soils. Soil Sci. Soc. Am. J. 1979, 43, 75–78. [Google Scholar] [CrossRef]
- Jackson, P.E.; Krol, J.; Heckenberg, A.L.; Mientijes, M.; Staal, W. Determination of total nitrogen in food, environmental and other samples by ion chromatography after Kjeldahl digestion. J. Chromatogr. A 1991, 546, 405–410. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis: Part 2, 2nd ed.; Page, A.L., Ed.; Agronomy Monograph 9; American Society of Agronomy; Soil Science Society of America: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Rashid, A.; Zia, M.; Ahmad, W. Micronutrient Fertilizer Use in Pakistan: Historical Perspective and 4r Nutrient Stewardship; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Teulat, B.; Zoumarou-Wallis, N.; Rotter, B.; Ben Salem, M.; Bahri, H.; This, D. QTL for relative water content in field-grown barley and their stability across Mediterranean environments. Theor. Appl. Genet. 2003, 108, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 2003, 78, 389–398. [Google Scholar] [CrossRef]
- Jones, D.B. Factors for Converting Percentages of Nitrogen in Foods and Feeds into Percentages of Proteins (No. 183); US Department of Agriculture: Washington, DC, USA, 1931. [Google Scholar]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. In Plant Stress Tolerance: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2010; pp. 317–331. [Google Scholar]
- Wolf, B. The determination of boron in soil extracts, plant materials, composts, manures, water and nutrient solutions. Commun. Soil Sci. Plant Anal. 1971, 2, 363–374. [Google Scholar] [CrossRef]
- Steel, E.A.; Kennedy, M.C.; Cunningham, P.G.; Stanovick, J.S. Applied statistics in ecology: Common pitfalls and simple solutions. Ecosphere 2013, 4, 1–13. [Google Scholar] [CrossRef]
- Kimetu, J.M.; Lehmann, J.; Ngoze, S.O.; Mugendi, D.N.; Kinyangi, J.M.; Riha, S.; Verchot, L.; Recha, J.W.; Pell, A.N. Reversibility of soil productivity decline with organic matter of differing quality along a degradation gradient. Ecosystems 2008, 11, 726–739. [Google Scholar] [CrossRef]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of animal slurry—A review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef]
- Christensen, M.L.; Hjorth, M.; Keiding, K. Characterization of pig slurry with reference to flocculation and separation. Water Res. 2009, 43, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, D.D.; Barros, P.M.; Cordeiro, A.M.; Serra, T.S.; Lourenço, T.; Chander, S.; Oliveira, M.M.; Saibo, N.J. Seven zinc-finger transcription factors are novel regulators of the stress responsive gene OsDREB1B. J. Exp. Bot. 2012, 63, 3643–3656. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plant, 2nd ed.; Academic Press: New York, NY, USA, 1995; p. 890. [Google Scholar]
- George, T.S.; French, A.S.; Brown, L.K.; Karley, A.J.; White, P.J.; Ramsay, L.; Daniell, T.J. Genotypic variation in the ability of landraces and commercial cereal varieties to avoid manganese deficiency in soils with limited manganese availability: Is there a role for root-exuded phytases? Physiol. Plant 2014, 151, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Azeem, M.; Naveed, M.; Latif, A.; Bashir, S.; Ali, A.; Ali, L. Synergistic use of biochar and acidified manure for improving growth of maize in chromium contaminated soil. Int. J. Phytorem. 2020, 22, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Sun, X.; Zhang, L.; Sakamoto, W. Cooperative D1 degradation in the photosystem II repair mediated by chloroplastic proteases in Arabidopsis. Plant Physiol. 2012, 159, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H. Chromium as an environmental pollutant: Insights on induced plant toxicity. J. Botany 2012, 2012, 375843. [Google Scholar] [CrossRef]
- Movahhedi-Dehnavi, M. Effect of Foliar Application of Micronutrients (Zinc and Manganese) on the Quantitative and Qualitative Yield of Different Autumn Safflower Cultivars under Drought Stress in Isfahan. Ph. D. Thesis, Tarbiat Modarres University, Tehran, Iran, 2004. [Google Scholar]
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, N.; Timco, P.M. Protochlorophyllide photoreduction. Photosynth. Res. 1998, 58, 5–23. [Google Scholar] [CrossRef]
- Behtash, F.; Tabatabai, J.; Malakoti, M.; Sarvaradin, M.; Oostan, S.H. The effect of zinc and cadmium on growth, chlorophyll, photosynthesis, cadmium concentration in Red Beet. J. Soil Res. 2010, 24, 31–41. [Google Scholar]
- Monneveux, P.; Rekika, D.; Acevedo, E.; Merah, O. Effect of drought on leaf gas exchange, carbon isotope discrimination, transpiration efficiency and productivity in field grown durum wheat genotypes. Plant Sci. 2006, 170, 867–872. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jin, J.Y. Photosynthetic rate, chlorophyll fluorescence parameters, and lipid peroxidation of maize leaves as affected by zinc deficiency. Photosynthetica 2005, 43, 591–596. [Google Scholar] [CrossRef]
- Sikuku, P.A.; Netondo, G.W.; Onyango, J.C.; Musyimi, D.M. Chlorophyll fluorescence, protein and chlorophyll content of three nerica rainfed rice varieties under varying irrigation regimes. Sci. Agric. 2010, 10, 84–94. [Google Scholar]
- Saboor, A.; Ali, M.A.; Ahmed, N.; Skalicky, M.; Danish, S.; Fahad, S.; Datta, R. Biofertilizer-based zinc application enhances maize growth, gas exchange attributes, and yield in zinc-deficient soil. Agriculture 2021, 11, 310. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Quan, R.; Shang, M.; Zhang, H.; Zhao, Y.; Zhang, J. Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechn. J. 2004, 2, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.E.; Mohamed, A.K. Reduction in carbonic anhydrase activity in zinc deficient leaves of Phaseolus vulgaris L. Crop Sci. 1973, 13, 351–354. [Google Scholar] [CrossRef]
- Huang, Y.H.; Shih, C.M.; Huang, C.J.; Lin, C.M.; Chou, C.M.; Tsai, M.L.; Liu, T.P.; Chiu, J.F.; Chen, C.T. Effects of cadmium on structure and enzymatic activity of Cu, Zn-SOD and oxidative status in neural cells. J. Cell. Biochem. 2006, 98, 577–589. [Google Scholar] [CrossRef]
- Ohki, K. Effect of zinc nutrition on photosynthesis and carbonic anhydrase activity in cotton. Physiol. Plant. 1976, 38, 300–304. [Google Scholar] [CrossRef]
- Fu, C.; Li, M.; Zhang, Y.; Zhang, Y.; Yan, Y.; Wang, Y.A. Morphology, photosynthesis, and internal structure alterations in field apple leaves under hidden and acute zinc deficiency. Sci. Hortic. 2015, 193, 47–54. [Google Scholar] [CrossRef]
- Pedersen, I.F.; Rubæk, G.H.; Sørensen, P. Cattle slurry acidification and application method can improve initial phosphorus availability for maize. Plant Soil 2017, 414, 143–158. [Google Scholar] [CrossRef]
- Tariq, A.; Anjum, S.A.; Randhawa, M.A.; Ullah, E.; Naeem, M.; Qamar, R.; Ashraf, U.; Nadeem, M. Influence of zinc nutrition on growth and yield behaviour of maize (Zea mays L.) Hybrids. Am. J. Plant Sci. 2014, 5, 2646–2654. [Google Scholar] [CrossRef]
- Verma, D.; Meena, R.H.; Sukhwal, A.; Jat, G.; Meena, S.C.; Upadhyay, S.K.; Jain, D. Effect of ZSB with graded levels of zinc fertilizer on yield and zinc uptake under maize cultivation. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023, 93, 379–385. [Google Scholar] [CrossRef]
- Ziaeyan, A.H.; Rajaie, M. Combined effect of zinc and boron on yield and nutrients accumulation in corn. Int. J. Plant Prod. 2009, 3, 35–44. [Google Scholar]
- Reid, D.E.; Ferguson, B.J.; Hayashi, S.; Lin, Y.H.; Gresshoff, P.M. Molecular mechanisms controlling legume autoregulation of nodulation. Ann. Bot. 2011, 108, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.J.; Athokpam, H.S.; Patel, K.P.; Meena, M.C. Effect of nitrogen and phosphorus in conjunction with organic and micronutrients on yield and nutrient uptake by maize-wheat cropping sequences and soil fertility. Environ. Ecol. 2009, 27, 25–31. [Google Scholar]
- Somani, L.L. Micronutrients for Soil and Plant Health; Agrotech Publishing Academy: Udaipur, India, 2008; pp. 14–74. [Google Scholar]
- Uprety, D.; Hejcman, M.; Szakova, J.; Kunzova, E.; Tlustos, P. Concentration of trace elements in arable soil after long-term application of organic fertilizers. Nutr. Cycl. Agroecosyst. 2009, 85, 241–252. [Google Scholar] [CrossRef]
- Lisuma, J.B.; Semoka, J.M.R.; Semu, E. Maize yield response and nutrient uptake after micronutrient application on a volcanic soil. J. Agron. 2006, 98, 402–406. [Google Scholar] [CrossRef]
- Lana, A.M.Q.; Lana, R.M.Q.; Frigoni, A.S.; Trevisan, L.R. Dosages, sources and application period of micronutrients in corn crop. Magistra 2007, 19, 76–81. [Google Scholar]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers, 8th ed.; Mc Millon Publishing Co.: New York, NY, USA, 2016. [Google Scholar]
- Ullah, I.; Jilani, G.; Khan, K.S.; Akhtar, M.S.; Rasheed, M. Sulfur oxidizing bacteria from sulfur rich ecologies exhibit high capability of phosphorous solubilization. Int. J. Agric. Biol. 2014, 16, 550. [Google Scholar]
- Iqbal, M.; Puschenreiter, M.; Oburger, E.; Santner, J.; Wenzel, W.W. Sulfur aided phytoextraction of Cd and Zn by Salix smithiana combined with in situ metal immobilization by gravel sludge and red mud. Environ. Pollut. 2012, 170, 222–231. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.J.; Andrew, S.J.; Smart, M.K.; Smolders, E. Effects of sulfate on cadmium uptake by Swiss chard: I. Effects of complexation and calcium competition in nutrient solutions. Plant Soil. 1998, 202, 211–216. [Google Scholar] [CrossRef]
- Renella, G.; Landi, I.I.; Nannipieri, P. Degradation of low molecular weight organic acids complexed with heavy metals in soil. Geoderma 2004, 122, 311–315. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Schwertmann, U.; Susser, P.; Natsher, L. Proton buffer compounds in soil. J. Plant Nutr. Soil Sci. 1987, 150, 174–178. [Google Scholar] [CrossRef]
- Kayser, A.; Wenger, K.; Keller, A.; Attinger, W.; Felix, H.R.; Gupta, S.K.; Schulin, R. Enhancement of phytoextraction of Zn, Cd, and Cu from calcareous soil: The use of nta and sulfur amendments. Environ. Sci. Technol. 2000, 34, 1778–1783. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).