Abstract
The Northeast China Plain (NCP) is the country’s most important grain-producing area. Unraveling how bacterial communities in this region assemble and distribute according to soil type is essential for sustainable agricultural development and optimizing the precise management of soil resources. In this study, 106 soil samples were collected from three typical zonal soil types (black calcium soil (BCS), black soil (BS), and dark brown soil (DBS)) spanning from west to east in the NCP. By combining soil field surveys and high-throughput microbial sequencing analysis, we found that bacterial diversity and community structure differed significantly by soil type. Proteobacteria, Gemmatimonadetes, and Acidobacteria were enriched in BCS, BS, and DBS, respectively. Compared to BSC and DBS, BS had the highest nutrient concentration and most neutral pH values, which may recruit more diverse bacterial communities and construct a more connected ecological network. Network analysis further identified Burkholderiales, Sphingomonadales, and SC_I_84 as key hubs in BS, BCS, and BCS, respectively. The majority of classified hubs consistent with the results of the linear discriminant analysis effect size belonged to the predominant biomarkers. Redundancy and Mantel test analyses revealed that the bacterial composition in various soil types showed distinctive responses to heterogeneity in soil physicochemical properties. Soil pH and TP were the primary factors shaping the soil bacterial community structure in these three soil types on the NCP. Moreover, bacterial composition and diversity were strongly related to changes in soil multifunctionality in BCS, and the relative abundances of three classes (TM1, Opitutae, and Deinococci) were the most important biotic variables for predicting BCS ecosystem multifunctionality. In summary, our results suggest that soil type variation has a strong influence in terms of shaping bacterial community structure and affecting soil multifunctionality. Correspondingly, diverse co-occurrence patterns were observed in different soil types.
1. Introduction
Soil is a heterogeneous and dynamic environment containing diverse microbes that aggregate into intricate community structures and play important roles in maintaining soil ecosystem function [1]. Due to the highly heterogeneous nature of soil, multiple soil characteristics, including organic matter content, pH, essential nutrient content, soil texture, and soil aggregates, are generally distinct among different soil types [2]. The formation of zonal soils is primarily classified by climate, while intrazonal and azonal soils are classified based on local factors, such as the characteristics of the parent material [3]. Black calcium soils (calcium-ustic isohumisols, BCSs), black soils (Hapli-Udic isohumosols, BSs), and dark brown soils (mol-boric argosols, DBSs) are the three main zonal soil types in the Northeast China Plain (NCP) [4,5,6]. Among these three types, BCSs formed during the transition from temperate subhumid to semiarid and meadow steppe plants, with obvious humification and calcification. They are mainly distributed in the western part of Heilongjiang Province, with lower precipitation and higher temperatures [5]. BSs are mainly distributed in the temperate and semihumid climatic zones. Coupled with long, cold winters, the decomposition of organic matter by microorganisms in these areas is limited [7]. DBSs are mainly characterized by the accumulation of weakly acidic humus matter, mild leaching, and clayization [8].
Soil microorganisms play vital roles in terrestrial ecosystems and regulate critical soil processes, such as aggregation, nutrient cycling, and pollutant degradation [9]. Several studies have demonstrated that soil type is a principal driver in shaping microbial communities, as well as the main factor influencing soil microbial community structure and population density, and that various types of soils recruit specific microbial taxa [10,11]. A comparison of natural forests and cropped vineyards under four soil types with varying depths in Australia revealed that soil microbial communities are governed by soil type and altered by land use practices. Similar findings were reported by Zhao et al. [3], who examined bacterial and fungal community structures, as well as microbial functional genes, in three major zonal soil types ranging from the cold temperate to subtropical climate zones. Some studies have suggested that it is difficult to attribute the influence of soil type on the microbial community to a single factor because soil type results from complex interactions between soil parent materials and historical and present climatic conditions [3,12]. However, compared to the large body of pedodiversity and microbial diversity, the identification of subsoil microbial communities based on soil type on a regional scale remains incomplete. Therefore, it is crucial to identify the differences in soil microorganisms among various soil types and their potential significance.
In recent years, it was suggested that the analysis of the associations among microorganisms within a community may be more important for ecological functioning than microbial diversity [13,14]. Moreover, most studies have shown that both the diversity and associations of soil microbiota are sensitive to environmental changes and are closely related to the essential ecosystem services provided by microorganisms [15,16,17,18]. Network analysis [13] and random matrix theory [19] can predict potential microbial associations from vast amounts of sequencing data [20,21] and provide more information on the complex interactions between taxa, such as mutualism, commensalism, parasitism, predation, and competition. Topological network parameters, including node degrees, clustering coefficients, and modularity, reflect environmental fluctuations, community stability, and network complexity in multiple ecosystems [22,23,24]. Species in the network with a higher node degree value or centrality can be statistically identified as keystone species [25], which may cause dramatic shifts in microbial community composition and function when removed [26]. Recent studies have shown that network structure and topological features are potential indicators of environmental sensitivity because they are significantly influenced by cropping practices, soil types, and climate change at local or global scales [27,28]. Therefore, exploring the geographical patterns of the co-occurrence network topological features for different soil microbiota in typical agroecosystems can provide insights into the response of the soil microbial community assembly to various soil types at regional spatial scales. Additionally, it can provide valuable information on soil resource management and agroecosystem multifunctioning at a regional scale.
To enable firm conclusions to be made regarding the effect of soil type on microbial communities and functions, it is important to assess the combined effects of multiple factors on various processes, particularly as soil ecosystem multifunctionality has been used as a powerful indicator in many studies. It is widely recognized that soil microbes play critical roles in maintaining multiple ecosystem functions and services. However, further empirical evidence is required to investigate the influence of soil type on the relationship between microbial traits and multifunctionality. In this study, we collected 106 samples representing three different soil types from northeastern China. We primarily focused on the following research questions: (1) What are the differences in soil properties, soil bacterial biodiversity, and community composition among these three typical soil types? (2) How do microbial co-occurrence patterns and key hubs vary among different soil types? and (3) What environmental features influence bacterial community structure, and which bacterial taxa may affect potential soil multifunctionality across different soil types?
2. Materials and Methods
2.1. Sample Collection
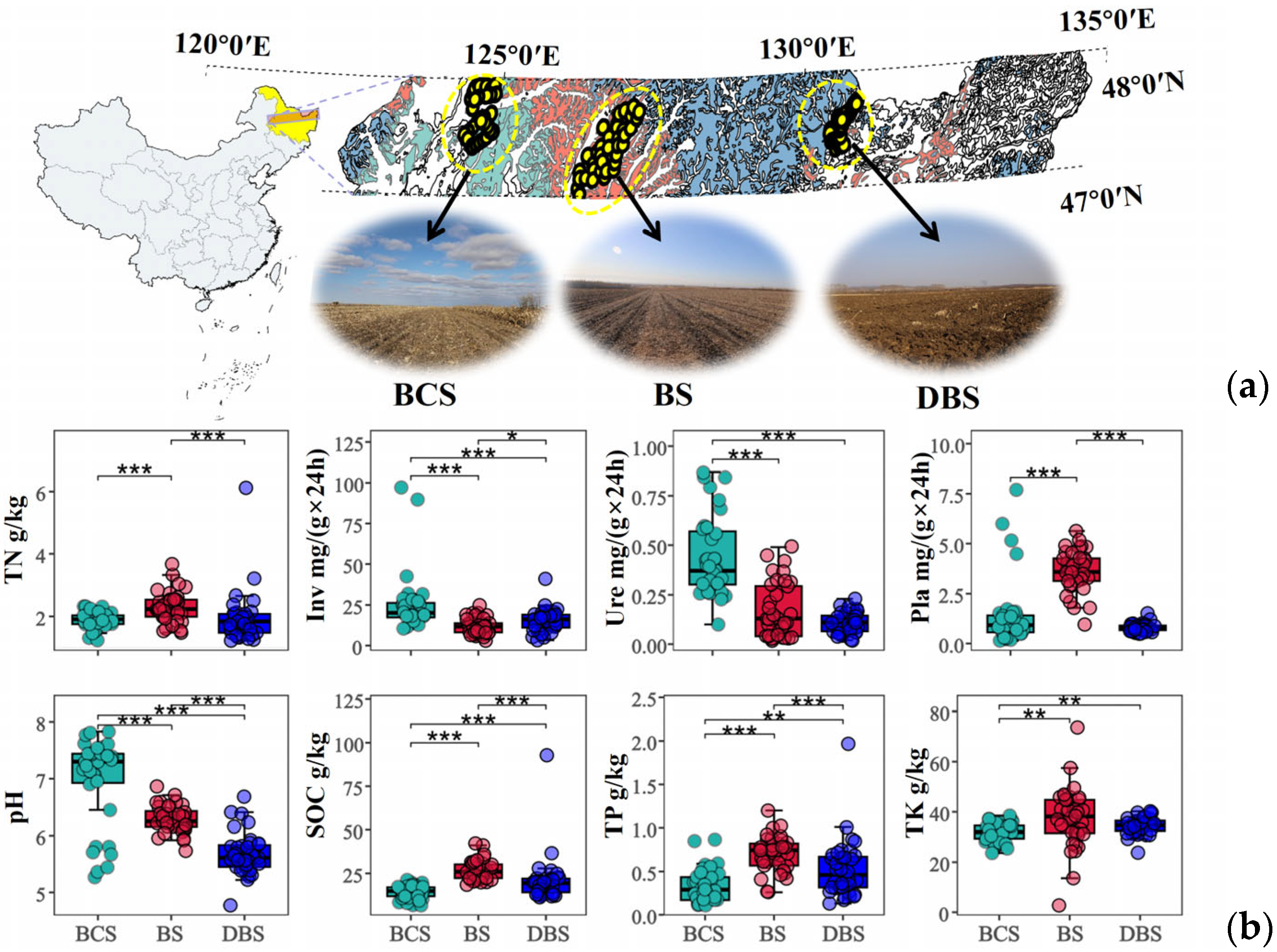
The three types of soil utilized in this study were black calcium soil (BCS), typical black soil (BS), and dark brown soil (DBS), according to the Genetic Soil Classification of China (GSCC), and they exhibited a zonal distribution from east to west in Heilongjiang Province. A total of 106 soil sites were selected and sampled from arable land containing these three typical soil types in the longitude zone (120°0′~135°0′ E; 47°0′~48°0′ N) in Northeast China (Figure 1a). The mean annual temperatures of BCS, BS, and DBS are 4–5 °C, 0.5–4 °C, and −2–5 °C, respectively, with corresponding rainfall between approximately 350 and 500 mm, 500 and 600 mm, and 500 and 700 mm. The basic information of soil sample sites is shown in Supplementary Table S1. Soil samples from the different soil types were collected close to the maturity growth stage of soybean or maize in October 2022. At each site, eight soil cores were collected away from the plant root zone to minimize the influence of the rhizosphere on the microbial community composition. The samples were obtained from a depth of 0~20 cm and combined within an area of approximately 100 m2. Subsequently, the soil samples were transported to the laboratory in containers with ice within 12 h and then passed through a 2 mm sieve. All samples were divided into two sections, with one section stored at −80 °C for biological analysis and the other at 4 °C for physicochemical property analysis.
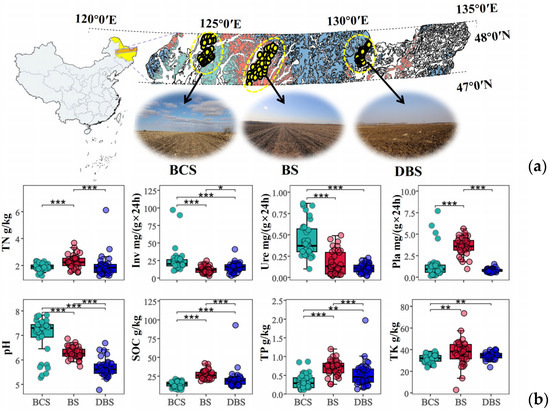
Figure 1.
Regional soil map of the study area and basic properties of soils. (a) Distribution of sampling sites in the latitude zone of Northeast China with typical agricultural landscape photographs. (b) Comparisons of soil basic properties among three types of soil. *, **, and *** indicate significant differences among the three soil types based on the t-test results at confidence levels of p < 0.05, p < 0.01, and p < 0.001, respectively. The different colors represent the different soil types. Inv: soil invertase activity; Pla: soil phosphatase activity; SOC: soil organic carbon; TK: soil total potassium content; TN: soil total nitrogen content; TP: soil total phosphorus content; Ure: soil urease activity. BCS: black calcium soil; BS: black soil; DBS: dark brown soil.
2.2. Soil Property and Enzyme Activity Analyses
Soil pH was measured by a pH meter (Mettler Toledo FE20, Shanghai, China) after stirring a soil–water suspension (1:2.5; w/v) for 30 min. Soil organic carbon (SOC) was determined by the oxidation of potassium dichromate method (HJ615–2011, Chinese Standards for Determination of Soil Organic Carbon) [29]. Total nitrogen content (TN) in soil was analyzed using an elemental analyzer (VarioEL III, Elementar Analysensysteme GmbH, Langenselbold, Germany). Total P (TP) content in the soil was measured by the molybdenum blue method after digestion with hydrofluoric acid–perchloric acid. Soil potassium content (TK) was determined using a flame photometry instrument (FP640, Shanghai Precision Instrument Co., Shanghai, China) after digestion with hydrofluoric perchloric acid.
Soil urease activity (Ure) was assessed using the colorimetric analysis of the sodium phenate–sodium hypochlorite method [30]. Soil invertase activity (Inv) was determined by the 3,5-dinitrosalicylic acid method [31]. Soil phosphatase activity (Pho) was estimated by disodium phenyl phosphate colorimetry, with the phosphatase activity of DBS, BS, and BCS determined using acetate buffer (pH = 5.0~5.4), citrate buffer (pH = 7.0), and borate buffer (pH = 9~10) based on soil pH [32].
2.3. High-Throughput Sequencing
Total DNA was extracted from 0.5 g of soil using the FastDNA SPIN Kit for soil (MP Biomedicals, Solon, OH, USA), following the manufacturer’s protocol. DNA concentration and purity were measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). PCR amplification of the bacterial 16S rRNA genes targeting the V3-V4 region was carried out using the primer 341F (5′-CCTAYGGGRBGCASCAG-3′) and the reverse primer 806R (5′-GGACTACNNGGGTATCTAAT-3′). In all PCR reactions, 15 µL of Phusion® High-Fidelity PCR Master Mix (New England Biolabs, Ipswich, MA, USA), 2 µM of forward and reverse primers, and approximately 10 ng of template DNA (Wekemo Tech Group Co., Ltd. Shenzhen, China) were used. The PCR thermal cycling procedure involved an initial denaturation step at 98 °C for 1 min, followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, and elongation at 72 °C for 30 s and finally at 72 °C for 5 min. Afterward, we mixed 1XTAE buffer with the PCR products in equal volumes and then performed electrophoresis on 2% agarose gel. A mixture of PCR products at equal densities was prepared. Subsequently, the PCR products were purified using the Qiagen Gel Extraction Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. Sequencing libraries were generated using the TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, San Diego, CA, USA), following the manufacturer’s recommendations, with index codes added post-library generation. The quality of the library was assessed using a Qubit@2.0 Fluorometer (Thermo Scientific, Waltham, MA, USA). Lastly, Illumina NovaSeq was used to generate 250 bp paired-end reads from the library. The sequence data were deposited in the Sequence Read Archive (SRA) of the National Center for Biotechnology Information (NCBI) under the accession number PRJNA1099395.
2.4. Bioinformatics and Statistical Analyses
The raw data of FASTQ files were converted into a format that can be operated by the QIIME2 pipeline (https://docs.qiime2.org/2019.1/, accessed on 4 May 2023). A feature table of operational taxonomic units (OTUs) was generated based on the demultiplexed sequences from each sample by quality filtering, trimming, denoising, merging, and identifying chimeric sequences [33]. The taxonomic classification was performed using the GREENGENES (13_8) database [34].
2.5. Network Analyses
We constructed 3 co-occurrence networks (DBS\BCS) among bacterial taxa across all soil samples of the three soil types to better understand bacterial connectivity. Each network was constructed by calculating the co-occurrence among 35 replicates, except for one network (BS) with 36 replicates. The networks were constructed using the Molecular Ecological Network Analyses Pipeline (MENAP, http://ieg4.rccc.ou.edu/MENA/, accessed on 12 May 2023), based on random matrix theory (RMT), as described by Zhou et al. [13,35] and Deng et al. [20].
In brief, the OUT data were uploaded according to the pipeline’s requirements and submitted to construct the network with default settings. Subsequently, analyses of “global network properties”, “individual node centrality”, and “module separation and modularity” were performed. The relevant network files for network analysis were explored, and some topological parameters of each constructed co-occurrence network were analyzed using the Network Analyzer plugin within Cytoscape. The network graph was visualized using the Gephi (0.9.2) platform for network visualization [36].
Network topological properties were also calculated using MENAP, including similarity thresholds, total nodes, total links, average degrees, average path distances, average clustering coefficients, r square of power-law, and modularity. Additionally, we identified the keystone taxa using within-module connectivity (Zi) and among-module connectivity (Pi) at a threshold of 2.5 and 0.62, respectively [20,35,37]. Based on the value of Zi-Pi, all the nodes were classified into four different categories, including peripheral nodes (Zi ≤ 2.5, Pi ≤ 0.62), connectors (Zi ≤ 2.5, Pi > 0.62), module hubs (Zi > 2.5, Pi ≤ 0.62), and network hubs (Zi > 2.5, Pi > 0.62). Network hubs, module hubs, and connectors were considered to play important roles in the stability and resistance of microbial communities. In this study, the nodes belonging to network hubs and module hubs were defined as representative taxa of these networks in the respective ecological groups of the three soil types.
2.6. Soil Ecosystem Multifunctionality
Soil ecosystem multifunctionality (SEMF) refers to the ability of soil ecosystems to simultaneously provide and maintain multiple ecological functions and services, including the improvement of soil physical and chemical properties, soil nutrient storage, nutrient cycling, soil nitrogen conversion, and litter decomposition. In this study, nine soil ecosystem functions related to C, N, and P were selected for assessment, including soil pH, nutrient cycling index (soil SOM, TN, TP, and TK), and soil enzyme activity (soil sucrase activity, urease activity, and phosphatase activity). Z-scores were computed for all variables evaluated using the averaging method, with SEMF quantified as the average Z-score for all functions measured for each sample [38,39]. The calculation formulae were as follows:
where Zij represents the Z-score of the j-th function index in the i-th soil sample, xij is the value of the j-th function index in the i-th soil sample, μj represents the average value of the j-th function index in all samples, and σj represents the standard deviation of the j-th soil function index in all samples. Additionally, SEMF represents the soil ecosystem multifunctional index of sample i.
Zij = (xij − μj)/σj,
2.7. Statistical Analyses
All statistical analyses for soil characteristics and bacterial diversity were performed using the R environment (version 3.6.2; https://www.r-project.org/, accessed on 10 May 2023) [40]. For the multiple comparisons of soil properties among the different soil types and treatments, one-way ANOVA was used along with the post hoc Tukey test. Shannon and richness indices were used to characterize alpha diversity in each sample. The distribution ratio of dominant bacterial phyla in the different soil types was visualized using Circos with the R package ‘circlize’ (v. 0.4.16), and the differences among the relative abundances of major taxa (top 12) in various types of soil bacterial communities at the genus level were assessed using the Wilcoxon tests. Biomarkers with varying abundances were identified using the linear discriminant analysis effect size (LEfSe) method on the Galaxy platform (http://galaxy.biobakery.org/). Principal coordinate analysis (PCoA), Adonis tests, random forest analysis, and Sepearman correlation analysis were conducted using R3.5.2 with the ‘vegan’ (v. 2.6-4) [41], ‘randomForest’ (v. 4.7-1.1) [42], and ‘ggpmisc’ packages (v. 0.5.6) [43], and the results were visualized using the ‘ggplot2’ package (v. 3.5.0). Microbial community relationships with environmental factors were analyzed using redundancy analysis (RDA) with the ‘vegan’ package (v. 2.6-4). Practical Mantel tests and Spearman correlations were used to evaluate the linkages between the relative abundances of major taxa (top 12) at the genus level and environmental factors.
3. Results
3.1. Variation in Soil Properties and Extracellular Enzyme Activities by Soil Type
Distinct soil properties and enzyme activities were observed among the three soil types investigated across the NCP (Figure 1b). The ANOVA results showed that soil nutrients, including soil organic carbon (SOC), total phosphorus (TP), and total potassium (TK), were significantly higher in BS and generally showed the following decreasing trend: BS > DBS > BCS (p < 0.01). Soil pH, which is key to determining nutrient availability in soils, differed significantly among the three soil types. BCS had the highest pH, ranging from 5.27 to 7.83, which was on average 0.11 and 0.23 times higher than that in BS and DBS (p < 0.001). Notably, BCS had the lowest soil nutrient levels, while its extracellular enzyme activities, including soil urease and invertase activities, were the highest among the three soils, except for phosphatase activity. Soil phosphatase activity was highest in BS (p < 0.001), which was 1.51 and 3.54 times that in DBS and BCS, respectively.
3.2. Variation in Bacterial Diversity and Community Composition by Soil Type
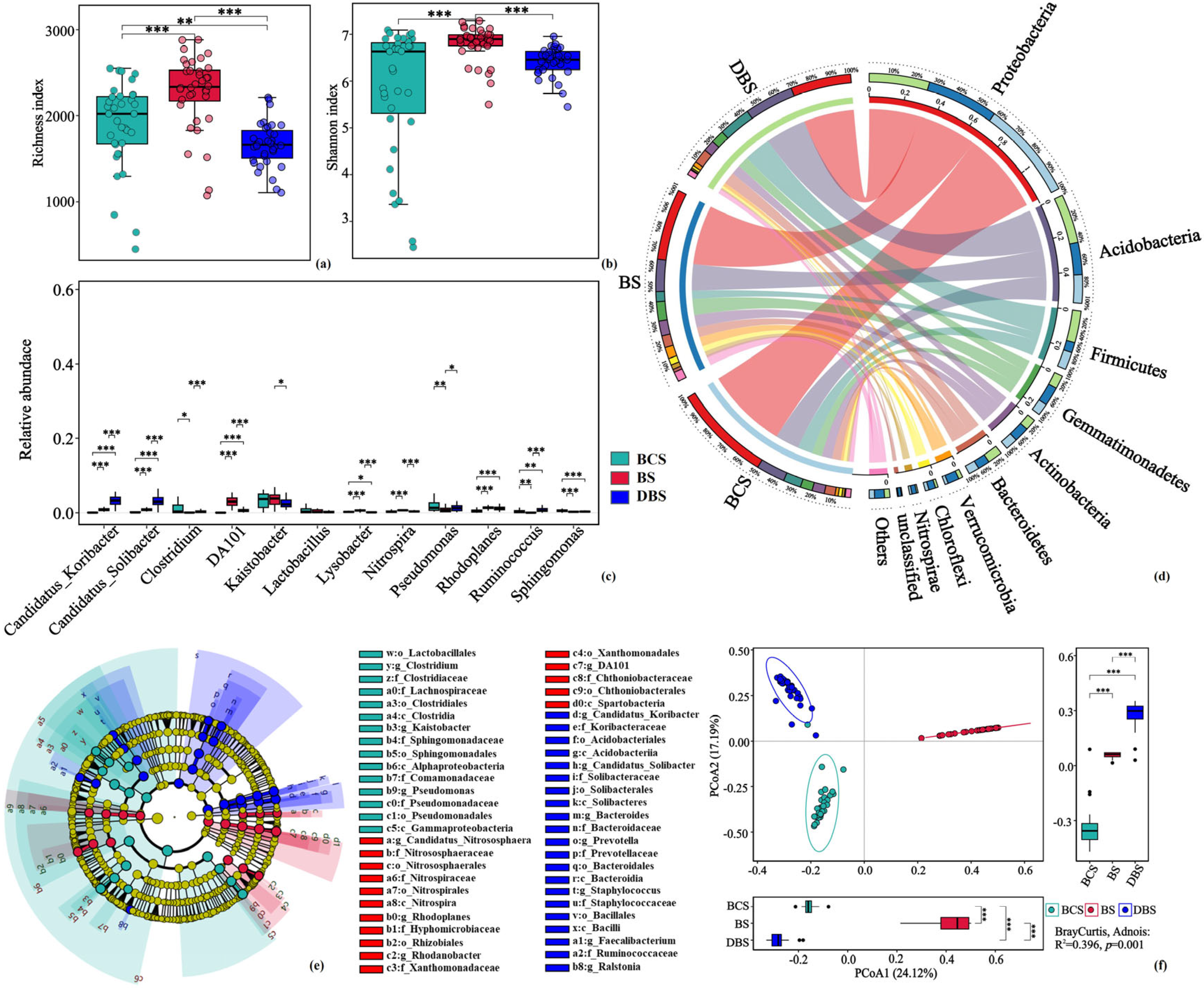
The sequencing data of the 106 soil samples were processed following the procedure detailed in the Materials and Methods, recognizing 103,867 unique bacterial taxa. Sequences were assigned to 72 bacterial phyla, 194 classes, 299 orders, 365 families, and 805 genera. Among the three soil types, the most abundant phyla in all soil samples were Proteobacteria, Acidobacteria, Firmicutes, Gemmatimonadetes, and Actinobacteria, accounting for an average of 82.33% of the bacterial sequences from each soil type (Figure 2d, Table S2). However, the relative abundance of the dominant phyla varied significantly in the different soil types. The relative abundance of Proteobacteria was highest in the BCS samples and significantly lower in the DBS samples (p < 0.05). The relative abundances of Acidobacteria and Firmicutes were significantly higher in DBS, and other phyla, including Gemmationadates, Actinobacteria, and Verrucomicrobia, had the highest relative abundances in BS (p < 0.05). At the genus level, the dominant genera that showed significant differences across the three soil types were Candidatus Koribacter, Candidatus Solibacter, DA101, Lysobacter, and Ruminococcus (p < 0.05; Figure 2c). The relative abundances of Candidatus Koribacter, Candidatus Solibacter, and Ruminococcus were significantly higher at the DBS sites than at the BS and BCS sites (p < 0.01). However, DA101 and Lysobacter were most abundant at the BS sites (p < 0.05).
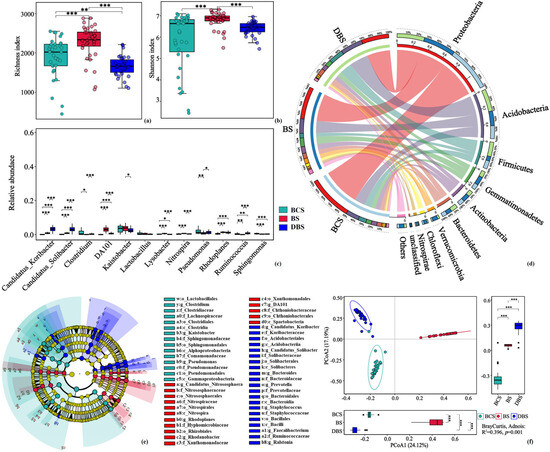
Figure 2.
Diversity, relative abundance and structure of soil bacterial communities in BCS, BS, and DBS. (a,b) Average microbial α-diversity (richness indices (a) and Shannon indices (b)) of samples from the three soil types. (c) Differences in the relative abundance of the top 10 bacterial taxa at the genus level among the soil types. (d) Circos plot showing the relative abundance of major taxa (top 10) in the bacterial communities at the phylum level. (e) Linear discriminant analysis (LDA) effect size analysis cladograms of the most abundant biomarkers in the three soil types. Taxa with LDA scores >4.0 and p-values < 0.05 were identified as biomarkers, and all the detected taxa were assigned to phyla (the outermost), classes, orders, families, genera, and species. (f) Results of the principal coordinate analysis (PCoA) based on weighted UniFrac distances among the three soil types. The Adonis analysis result is at the bottom right of the figure. Significance test: *, p < 0.05; **, p < 0.01; ***, p < 0.001. Different colors represent samples from the different soil types.
To explore the changes in bacterial community structure in the different soil types, we examined microbial taxonomic diversity and found that there were significant structural differences in microbial α- and β-diversity in the three soil types. Soil bacterial α-diversity estimated by the richness and Shannon indices was significantly higher in the BS sites than in the other soil types (ANOVA, p < 0.001; Figure 2a,b). The principal coordinate analysis (PCoA) results revealed that the microbes of the three soil types could be clearly separated; PC1 and PC2 explained 35.0% and 18.3% of the variation, respectively, suggesting that soil type reshaped the soil microbiomes (Adonis: R2 = 0.396, p = 0.001; Figure 2f).
Linear discriminant analysis effect size (LEfSe) analysis identified 52 discriminant taxa in the bacterial community among the three soil types (LDA score > 4.0, p < 0.05). There were 15, 16, and 21 biomarkers distinguished in BCS, BS, and DBS, respectively (Figure 2e). In general, the phyla Proteobacteria, especially the classes Alphaproteobacteria, Gemmaproteobacteria, and Firmicutes, were significantly enriched in BCS, whereas Nitrospira and Spartobacteria were significantly enriched in BS. In DBS, Acidobacteria (classes Acidobacteria and Solibacteres) and Bacteroidia (class Bacteroidia) were dominant compared to the other soil types.
3.3. Variation in Co-Occurrence Networks with Bacterial Communities by Soil Type
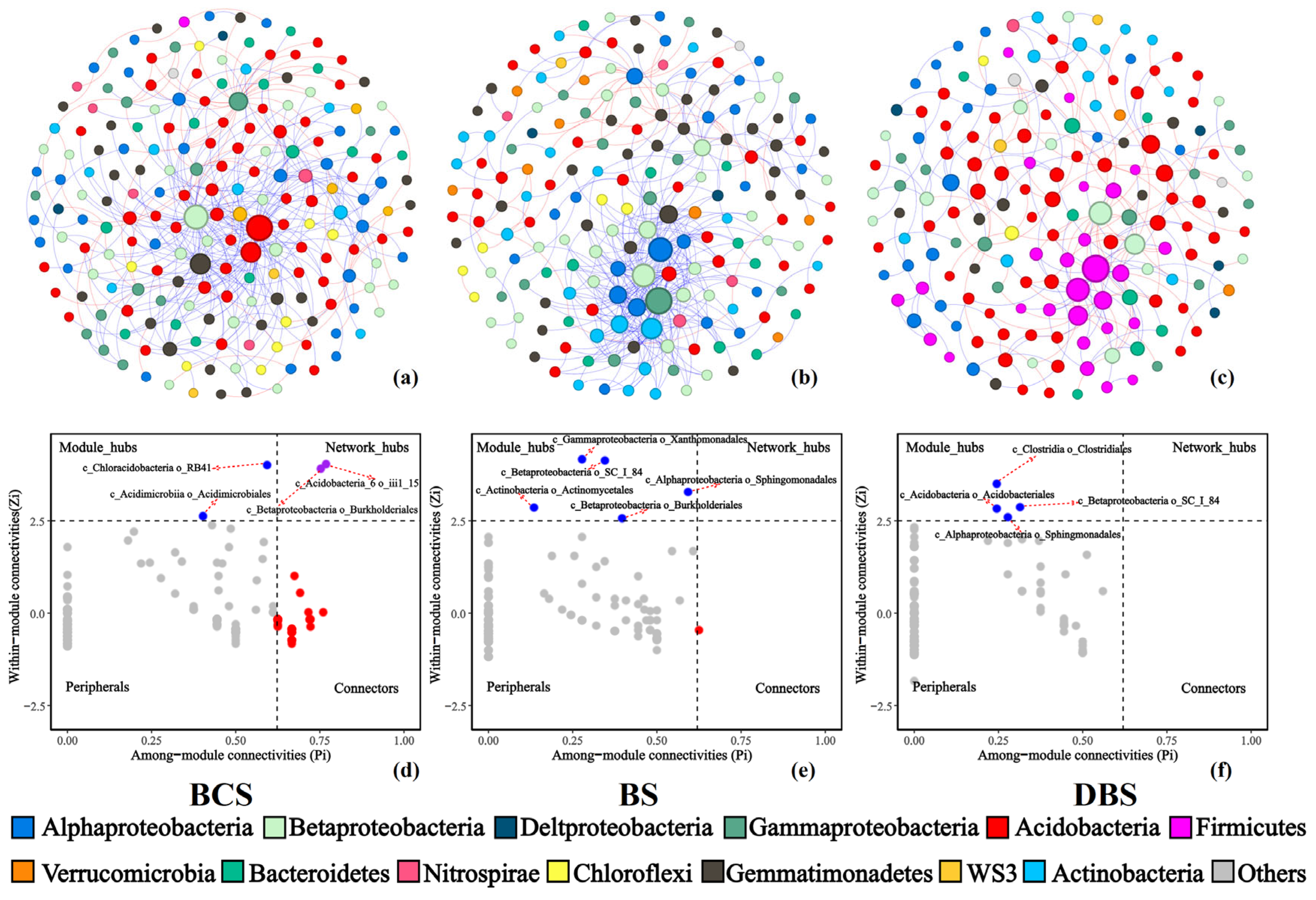
Co-occurrence network analyses were employed to explore the role of biotic interactions in community assembly and to identify potential keystone species that showed distinct patterns among the three soil types (Figure 3, Table 1). In the bacterial network, >90% of the nodes in the three soil types belonged to the phyla Proteobacteria, Acidobacteria, Gemmatimondetes, Bacteroidetes, Firmicutes, and Actinobacteria. Interestingly, Acidobacteria dominated the DBS co-occurrence network, whereas Proteobacteria dominated those of BS and BCS. The bacterial network of BS was significantly larger (e.g., more nodes and edges) and more complex (e.g., higher average degree, average clustering coefficient, and density) than those of BCS and DBS (Table 1). The highest ratio of positive to negative links in BS was >1.6 times higher than in the other soil types (BCS = 3.98, BS = 6.50, and DBS = 1.51). For these three networks, the clustering coefficients increased in the following order: DBS < BCS < BS (p < 0.05).
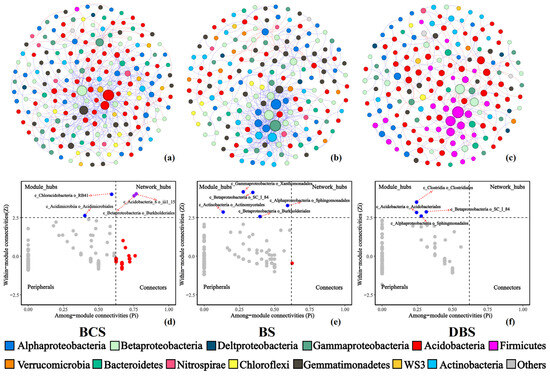
Figure 3.
Network co-occurrences of the BCS (a), BS (b), and DBS (c) communities and topological roles of nodes in the BCS (d), BS (e), and DBS (f) networks. Node size is proportional to the relative abundance. Edges between nodes represent the relationships among OTUs. Node colors represent the different major genera. Blue links indicate positive relationships, and red links indicate negative relationships.

Table 1.
Parameters of molecular ecological networks of bacterial communities within the three soil types and comparison of the topological properties of their subnetworks.
Furthermore, we determined the topological role of each OTU in the microbial networks of the three soil types using a Zi-Pi plot to assess whether taxa occupied key positions in each network, which showed that the majority of genera were peripherals (Figure 3d–f). In this study, 13 bacterial taxa were classified as module hubs, and the network hubs for the three networks were affiliated with Proteobacteria (Alpha, Beta, and Gamma), Acidobacteria, Actinobacteria, and Firmicutes. In the BS and DBS networks, no network hub was identified, whereas in the BCS network, two network hubs belonged to Acidobacteria (Acidobacteria_6) and Proteobacteria (Burkholderiales), and two module hubs belonged to Actinobacteria (Acidimicrobiales) and Acidobacteria (Chloracidobacteria). For BS, five OTUs from the genera Kaistobacter, Lysobacter, Methylibium, and Arthrobacter and the class Betaproteobacteria were identified as module hubs. For DBS, there were generally four OTUs from the genera Kaistobacter and Finegoldia, the family Koribacteraceae, and the class Betaproteobacteria. Table S3 displays the taxonomy of connectors, module hubs, and network hubs.
3.4. Environmental Variables Shaping Soil Bacterial Community Structure
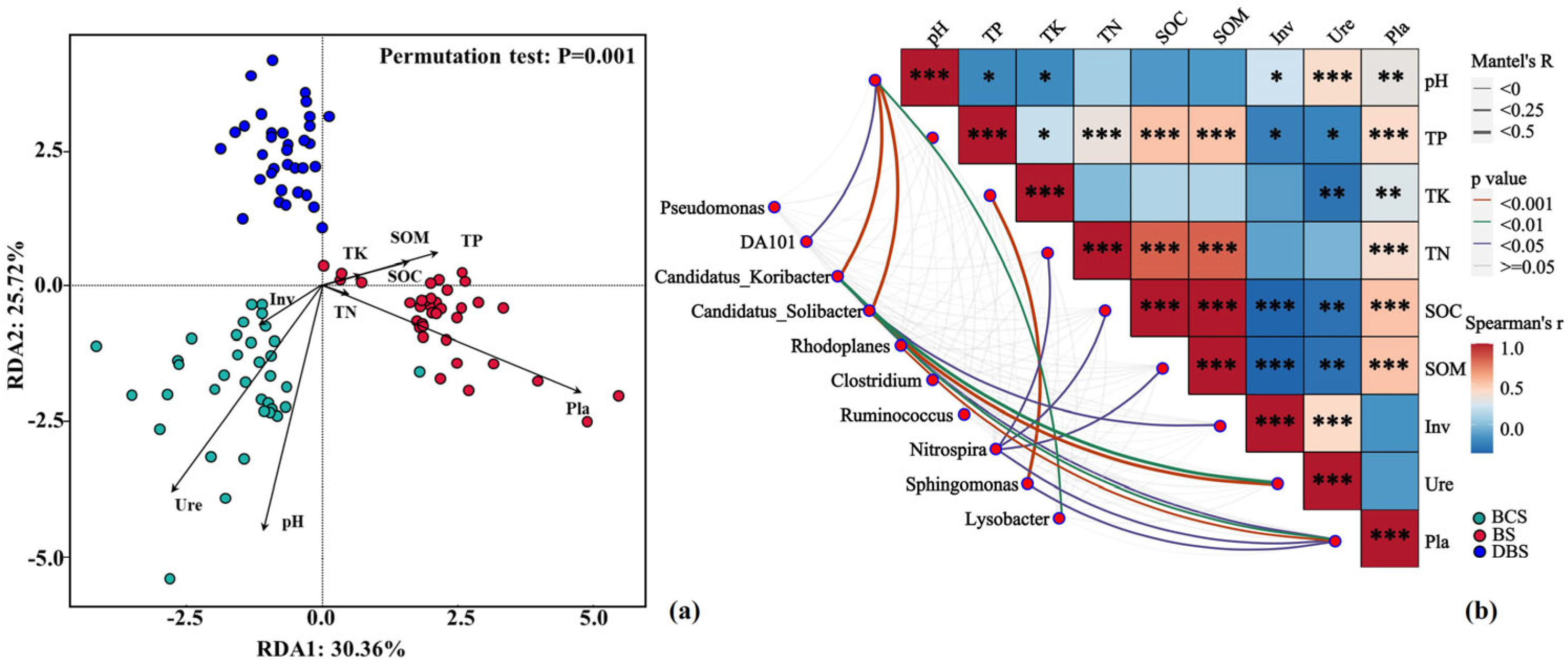
The redundancy analysis (RDA) ordination plot showed an overall pattern of soil properties and extracellular enzyme activities being associated with shifts in bacterial community composition among the three soil types (explained by 56.08% of the variance; Figure 4a). Both pH and TP were the main drivers of variation in bacterial community composition, followed by SOC, TK, and TN. Notably, the BS samples demonstrated a strong positive correlation with soil Pla, whereas the DBS samples showed the opposite. BCS was positively associated with pH and Ure and negatively associated with TP and SOM. The Mantel test analysis demonstrated that most of the relative abundances of the top 10 bacterial genera were significantly correlated with soil pH and Pla, and Nitrospira was significantly correlated with TK, TN, and SOC, which also confirmed the RDA results (Figure 4b). Inv and Ure showed strong negative associations with TP and SOC, whereas soil Pla showed strong positive correlations with TP, TN, and SOC. Soil pH showed strong negative associations with TP and TK.
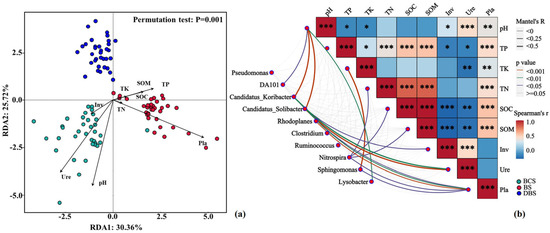
Figure 4.
Soil chemical properties associated with the three soil types. (a) Redundancy analysis (RDA) demonstrating soil bacterial community structures of the three soil types constrained by various soil properties. (b) Mantel test relationships between the relative abundance of the top 10 bacterial genera and soil properties, and the Spearman correlations among soil properties. The width of each edge matches with Mantel’s r statistic, and the line color denotes the statistical significance based on 999 permutations. Pairwise comparisons of soil properties are also shown, with a color gradient denoting Spearman’s correlation coefficient. Significance levels: *** p < 0.001, ** p < 0.01, * p < 0.05. Inv: soil invertase activity; Pla: soil phosphatase activity; SOC: soil organic carbon; TK: soil total potassium content; TN: soil total nitrogen content; TP: soil total phosphorus content; Ure: soil urease activity.
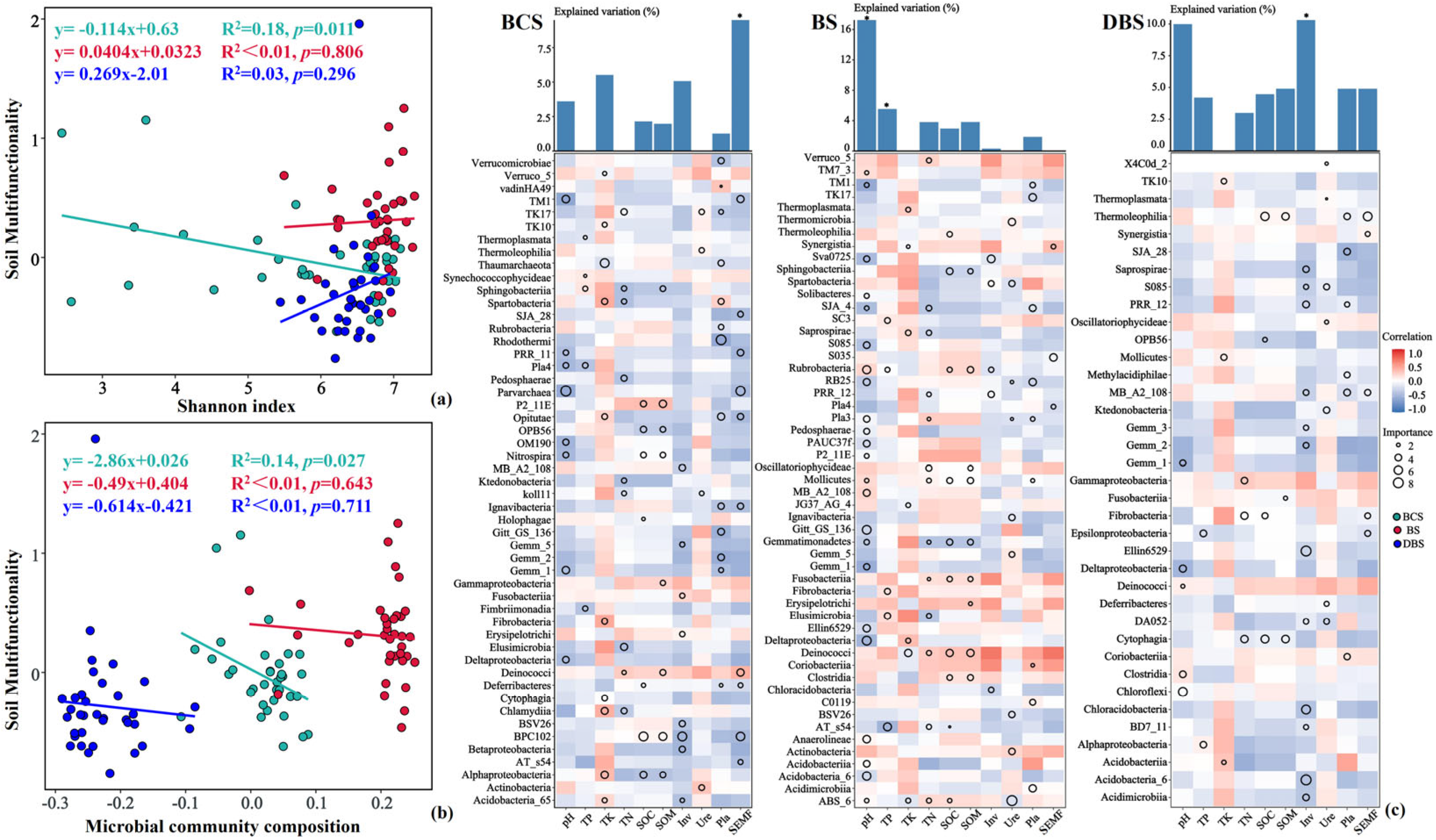
For the averaging approach, ordinary least-squares (OLS) regression models revealed that the microbial community composition and Shannon diversity had a significant and negative linear relationship with soil ecosystem multifunctionality at the BCS sites only. However, the microbial diversity and composition in the BS and DBS did not show a significant relationship with SEMF (Figure 5a,b). To identify the correlations between the environmental variables and belowground bacterial community assembly in the different soil types, we further examined the relative contribution of the class-level core microbiota to soil properties using correlation and random forest analysis. Overall, there were significant correlations between bacterial α-diversity and environmental parameters, and we found that different bacterial classes contributed to the variations in the properties of the different soil types (Figure 5c).
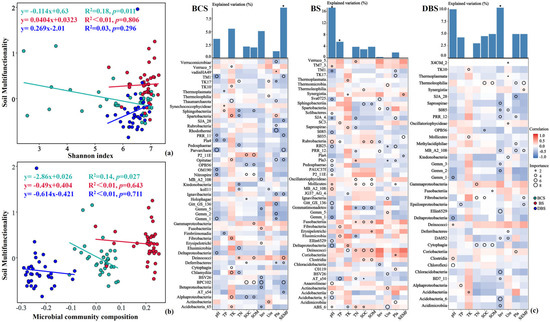
Figure 5.
Linear relationships between soil multifunctionality and soil diversity ((a) Shannon indices) or microbial community composition ((b) network clustering coefficients) of the three soil types. Statistical analysis was performed using ordinary least-squares linear regressions. (c) Potential biological contributions of the class-level core microbiota to soil properties in the three soil types based on correlations and random forest models. The bar plots show the mean explained variation in soil properties and SEMF with soil bacterial α-diversity using random forest modeling. The circle size represents the variable’s importance. Colors represent Spearman correlations. *, p < 0.05. SEMF: soil ecosystem multifunctionality; Inv: soil invertase activity; Pla: soil phosphatase activity; SOC: soil organic carbon; TK: soil total potassium content; TN: soil total nitrogen content; TP: soil total phosphorus content; Ure: soil urease activity.
In this study, soil ecosystem multifunctionality was the most important predictor of BSC soil bacterial diversity. Consistent with the linear regression results, TM1 (p_Acidobacteria), Opitutae (p_Verrucomicrobia), and Deinococci (p_Thermi) abundances were the most important variables for predicting soil ecosystem multifunctionality in the BCS. For the BS, soil pH and TP played the most important roles in variations in soil bacterial community diversity. The important variables for predicting soil pH and TP in the BS were Rubrobacteria, Pedosphaerae, MB_A2_108, Deltaproteobacteria, Anaerolineae, Acidobacteria, and Acidobacteria_6 for pH and Fibrobacteria and Elusimicrobia abundances for TP. In DBS, soil physicochemical properties explained 46.67% of the variation in bacterial diversity, and soil invertase activity was the most vital predictor of the DBS soil bacterial community. The relative abundances of Chloracidobacteria, Acidobacteria_6 from the Acidobacteria phylum, and Acidimicrobiia and MB_A2_108 from the Actinobacteria phylum were the most important predictors of soil invertase activity.
4. Discussion
Northeastern China is the primary grain production base for the country, accounting for approximately 17.63% of the total cultivated area. Heilongjiang Province is a typical agricultural area in northeastern China and has the largest cropland area in the country. Considering the vital role of cropland soils in global food production, it is important to understand the microbial distribution patterns in agricultural soils at a regional scale [44,45]. In the present study, we investigated soil bacterial distributions across three distinct soil types in northeastern China, BCS, BC, and DBS. Our findings revealed clear differences in the soil properties, bacterial diversity, and community structure among the three soil types, with specific bacterial subset taxa for each soil type (Figure 1 and Figure 2). The bacterial communities in the different soil types exhibited distinct co-occurrence patterns (Figure 3, Table 1). The driving factors of the soil bacterial communities varied among the soil types, with different bacterial classes contributing to the variation in properties across the soil types (Figure 4 and Figure 5).
4.1. Distinct Soil Types Determine the Physiochemical Properties of Soil
Soil type is considered the primary driver influencing ecological dynamics and plays a key role in shaping soil microbial communities. Many studies have demonstrated that the physicochemical properties of different soil types can significantly influence the diversity and structure of bacterial and fungal communities, with specific microbial taxa being recruited by different soil types [46,47,48]. The BCS area was situated in the western part of the study area (Figure 1), with obvious humification and calcification. This soil type displays lime reactions and is characterized by a higher soil pH, which also reflects calcium accumulation in the soil formation process. Therefore, it is unsurprising that the soil pH of BCS (5.27–7.83) was higher than those of BS (5.73–6.86) and DBS (4.77–6.68). Humification is the most important pedogenetic process in black soil formation. The decomposition of organic matter by microorganisms was limited in the BS, resulting in higher organic carbon, total nitrogen, total phosphorus, and total potassium contents than in the other two soil types (p < 0.05) [49]. In dark-brown soils, weakly acidic humus matter, mild leaching, and clayization contributed to the lowest soil pH among the three soil types [50,51].
Soil enzyme activity plays an important role in soil material cycling and energy conversion and is a vital aspect of studying microbial communities in terrestrial ecosystems [52]. It is closely linked to the physical and chemical properties of the soil, soil type, fertilization, tillage, and other agricultural management practices [53]. Invertase, urease, and phosphatase activities were closely related to C, N, and P cycling in the soil, and their activities varied significantly across the three soil types [54]. Phosphatase activity was the highest in BS, whereas the highest values for the other two soil enzymes were observed for DBS.
4.2. Effects of Soil Type on the Variation in Soil Bacterial Diversity, Composition, and Community Structure
The importance of soil type as the primary factor shaping microbial community composition is well documented [6,55]. Many studies have indicated that dominant bacterial taxa vary among different soil environments [11,56,57,58]. Along with significant indigenous differences in soil physiochemical properties among the three soil types (Figure 1; p < 0.05), soil bacterial communities exhibited distinct responses to soil type, with variations observed among different phyla or genera (Figure 2). Proteobacteria, Acidobacteria, Firmicutes, Gemmationadates, Actinobacteria, and Verrucomicrobia were the dominant phyla in this study, which is consistent with previous findings demonstrating their dominance as major soil bacterial groups in diverse environments and exhibiting significant differences in abundance among different soil types [59,60]. The phylum Proteobacteria is known for its ability to utilize a wide range of carbon substrates for rapid growth [61] and prefers to multiply in medium to alkaline soils [62]. These characteristics of Proteobacteria may account for their highest relative abundance being observed in the BCS with the highest soil pH.
In contrast, soil Acidobacteria and Firmicutes were relatively abundant in DBS, which had the lowest soil pH. This finding is consistent with that of our previous study, indicating significant enrichment of these phyla in the DBS profiles [63]. Previous studies have noted the importance of the phylum Acidobacteria in maintaining ecological balance and driving soil ecosystem functions [64,65]. This phylum is one of the most widely distributed and diverse bacterial phyla in the soils of northeastern China, accounting for approximately 20–50% of total soil bacterial communities [66,67]. Some studies have also shown a significant negative relationship between the relative abundance of Acidobacteria and soil pH [68,69,70]. The highest abundance was observed in soils with lower pH values, which explains their enrichment in DBS at the lowest pH in this study. Conversely, Gemmatimonadetes, Actinobacteria, and Verrucomicrobia were more likely to be abundant in the BS, as their relative abundances were reported to be positively correlated with soil nutrients.
At the same time, we assessed the α-diversity of the soil bacterial community by quantifying the richness and Shannon indices across the three soil types. These two indices are commonly used to measure the species richness and diversity in communities. A higher richness index indicates a greater abundance of species within the community [71,72], whereas the Shannon index is more sensitive to variations in rare species, reflecting both richness and evenness. A higher Shannon diversity index indicates a higher level of community diversity [73,74]. In our study, the α-diversity of soil bacterial communities exhibited significant variation across the three soil types. For BS, both the richness and Shannon indices were higher than those of the other two soil types. Additionally, soil bacterial richness was significantly higher in BCS compared to DBS, whereas it had no significant effect on soil bacterial diversity. This result suggests that the variation in soil bacterial α-diversity between BCS and DBS was mainly caused by increases in species numbers rather than by changes in the relevant abundance of specific bacteria, promoting the Shannon diversity index. Furthermore, PCoA and LEfSe indicated significant differences in soil microbial communities among the three soil types, with distinct enriched groups for each type. These findings corroborate the observations of Xue et al. [75], who suggested that soil microbial communities are governed by soil type and are altered by land use.
4.3. Stability of Soil Bacterial Networks and Specific Hubs in Different Soil Types
To better understand the interactions among soil microorganisms, identify key species that influence communities, and determine the aggregation status of microorganisms in ecosystems, we constructed three co-occurrence networks of microorganisms (OTU levels) in the NCP. These networks were based on significant correlations and highlighted the effects of soil type on the microbial community. In this study, we found that the bacterial network in BS exhibited greater complexity and stability than those of BCS and DBS in terms of its topological properties, including higher numbers of nodes, edges, and clustering coefficients (Table 1). This may indicate that the bacterial community in BS is more adaptable to environmental conditions and has greater ecological stability. Increased interactions among bacterial species in BS can facilitate various functions, including nutrient cycling and promoting plant growth [76].
Furthermore, the modularity value was higher in the BS network than in the other two networks. Previous studies have interpreted modules as niches [77,78]. The modularity of this microbial co-occurrence network varied with the environment, with higher values linked to stronger niche variations. Therefore, compared with the bacterial communities in BCS and DBS, the presence of more niches in BS provided greater opportunities for different species to interact with each other.
Unlike BS, BCS and DBS exhibited relatively low network complexity, which may reflect the unstable network characteristics of their bacterial communities. In the present study, the BCS network had a shorter average path length (Table 1), suggesting that it is a small-world network [79]. Previous studies interpreted networks with small path lengths as small-world networks, which have been linked to the quick responses of ecosystems to disturbances [13]. Surprisingly, the BCS network had a lower average path length but a higher number of positive links than DBS. A possible explanation for this result is the positive correlation between the microorganisms in these two kingdoms, which may be due to their ecological interactions (i.e., commensalism and mutualism) [26]. More positive interactions suggested greater cooperation and stronger syntrophic relationships in complex microbial communities [80]. Therefore, in DBS, which has low nutrient and pH values, stronger antagonistic associations exist among species, such as predation and/or competition for limited resources.
Microbial co-occurrence network analysis was used to identify the species that play important roles in shaping network structures. These keystone species can be detected as network hubs, module hubs, and connectors based on network topology [81,82,83]. They are the most influential and essential members of the soil microbial community and play vital roles in maintaining network stability [84]. Topologically, the module hubs had a high Z-score but a low p-value, indicating a strong connection with many species within their modules. They may mediate important functions but tend to operate at a lower level within the overall community. Network hubs and connectors are the regulators, mediators, and adaptors. In the present study, although network hubs were absent from the BS network, several module hubs and connectors were identified (Figure 3e). For the module hubs in the BS network, most keystone taxa were affiliated with Proteobacteria, the most abundant phylum, including the family Sphingomonadaceae, the order SC-I-84, and the genera Lysobacter and Arthrobacter. Similar keystones have been identified in many studies [61,85], and these bacterial hubs are known to be fast growers with the ability to utilize the majority of root-derived carbon substrates. Sphingomonadaceae are sensitive to agricultural land use and play an important role in soil P cycling. Lysobacter are proficient in producing a vast array of extracellular enzymes and bioactive secondary metabolites. Arthrobacter belongs to Actinobacteria, which can rapidly utilize available resources to facilitate growth, and has been reported as a beneficial bacterium owing to its role in promoting plant growth. Interestingly, we observed that two of the four module hubs in the DBS network were the same as those in the BS network (the genus Kaistobacter and the order SC-I-84). Two network hubs and two module hubs were identified in the BCS network, with Acidimicrobiale identified as the module hub in BCS with higher pH values. One possible reason is that members of Acidimicrobiales are sensitive to changes in soil pH [61], and their relative abundance increases with increasing pH values [86].
4.4. Response of Soil Multifunctionality and Properties to the Bacterial Communities in Different Soil Types
Numerous studies have shown that the composition of soil bacterial communities depends on environmental soil parameters [87]. Additionally, many bacterial communities are highly correlated with specific soil factors and can serve as indicators of soil conditions [88]. Moreover, soil type has been widely reported to have a significant impact on bacterial community composition. In the current study, the effects of edaphic factors on soil bacterial communities showed significant differences between the soil types. Therefore, investigating the correlation between microbial community diversity and soil environmental factors in different soil types can enhance our understanding of the variation in bacterial community structures in the NCP and promote the sustainable development of regional agriculture.
Based on the RDA analysis, soil pH was the most important factor influencing the bacterial community structure in BCS, which could be attributed to the higher pH resulting from soil calcification in this soil type. For BS, soil nutrient content plays an important role in shaping the soil bacterial community structure. It is worth noting that BS originally has dark humus-rich surface horizons that contain higher nutrient levels. Soil enzyme activity plays an important role in shaping the community structure, possibly because of the causal relationship between enzyme activity and soil microorganisms, which are important sources of soil enzymes. Soil environmental factors varied considerably between the three soil types (Figure 1, Table S3) and displayed positive or negative relationships with the relative abundance of the most enriched genera in the soil types (Figure 4). Among all the measured soil environmental factors, soil pH, TP, and SOC exerted significant impacts on bacterial community structures in all three soil types.
Previous studies have confirmed the positive correlations between microbial diversity and multifunctionality in natural and agricultural ecosystems [89,90,91,92,93]. However, contrary to these studies, we observed that soil bacterial diversity (Shannon indices) and soil bacterial community composition (network clustering coefficients) were both negatively related to SEMF in the BCS sites only, not in the BS and DBS sites, which was confirmed by regression analysis. However, the use of multifunctionality to address single-ecosystem processes is discontinuous. This is because exploring biodiversity–multifunctionality relationships depends on the number of measured functions and their combinations. In this study, the lack of measurement data for some available nutrients may be the main reason for this bias. The results of the correlation and random forest analyses further confirmed that SEMF was an important predictor significantly influencing soil bacterial diversity in the BCS and was strongly correlated with TM1, Opitutae, and Deinococci enriched at high pH and Ca2+ concentrations.
In our study, although we selected typical zonal farmland soils with similar crop types (corn and soybean rotation) and cultivation methods, we carefully avoided collecting soil samples from the rhizosphere to minimize the direct influence of plant roots on the microbial community composition. However, long-term crop cultivation and rhizosphere activities may still indirectly influence soil microbial communities by altering the physical and chemical properties of soil [94,95,96]. For example, organic acids and other metabolites secreted by roots can influence soil pH and change nutrient availability, thereby shaping the composition and function of microbial communities [97,98,99]. Additionally, the high microbial activity and diversity in the rhizosphere may promote increased soil enzyme activity, which was also observed in our study. Therefore, future research should further explore the long-term impacts of different crops and their rhizospheres on soil microbial communities to reveal the complex interactions among crops, soil, and microbes. This is crucial for developing rational agricultural management practices to improve soil health and the functioning of farmland ecosystems.
5. Conclusions
In summary, we examined the variation in soil physicochemical properties and the associated bacterial community structures across three typical types of zonal soils in the Northeast China Plain. Our findings indicate that different soil types have distinct bacterial community structures, soil multifunctionality, and diverse co-occurrence patterns. Compared with BCS and DBS, BS was characterized by higher levels of soil nutrients and biodiversity and a more complex and stable bacterial co-occurrence network. Keystone species were identified through their network positions, such as network hubs and module hubs, and the most influential and essential members of the soil microbial community in the three soil types were distinguished and predicted. Our results suggest that soil pH and TP are the primary drivers of variation in bacterial community composition in BCS and BS, respectively. In addition, changes in microbiota composition and diversity were strongly related to changes in soil multifunctionality only in BCS, and TM1, Opitutae, and Deinococci abundances were the most important variables for predicting soil ecosystem multifunctionality.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14061297/s1, Table S1. The basic information of soil sample sites; Table S2. Relative abundance of major taxa (top 10) in the bacterial communities of different soil types at the phylum level; Table S3. Details of taxonomy of connectors, module hubs, and network hubs in three types of soil bacterial networks.
Author Contributions
Conceptualization, Y.S. and X.J.; methodology, Y.S. and X.J.; software, M.H.; validation, M.H. and H.Y.; formal analysis, M.H. and X.Z.; investigation, Y.S., H.Y., L.M. and M.H.; resources, Y.W. and Y.C.; data curation, X.Z. and M.H.; writing—original draft preparation, M.H.; writing—review and editing, M.H. and H.Y.; visualization, M.H.; supervision, Y.S. and X.Z.; project administration, Y.W. and Y.C.; funding acquisition, Y.S. and X.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science and Technology Basic Resources Survey Special Project, grant number 2021FY100400, and the National Natural Science Foundation of China, grant number 42077081.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
We sincerely thank Associate Researcher Pan Fengjuan for her revisions and suggestions for the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Banerjee, S.; van der Heijden, M.G.A. Soil Microbiomes and One Health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef]
- Garbeva, P.; Van Veen, J.A.; Van Elsas, J.D. Microbial Diversity in Soil: Selection of Microbial Populations by Plant and Soil Type and Implications for Disease Suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Sun, B.; Wu, L.; Gao, Q.; Wang, F.; Wen, C.; Wang, M.; Liang, Y.; Hale, L.; Zhou, J.; et al. Zonal Soil Type Determines Soil Microbial Responses to Maize Cropping and Fertilization. mSystems 2016, 1, e00075-16. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z. Chinese Soil Taxonomy: Theories, Methods and Practice; Science Press: Beijing, China, 1999; pp. 580–613. (In Chinese) [Google Scholar]
- Gray, J.M.; Humphreys, G.S.; Deckers, J.A. Distribution Patterns of World Reference Base Soil Groups Relative to Soil Forming Factors. Geoderma 2011, 160, 373–383. [Google Scholar] [CrossRef]
- Schad, P. World Reference Base for Soil Resources. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-92-5-108370-3. [Google Scholar]
- Ling, N.; Sun, Y.; Ma, J.; Guo, J.; Zhu, P.; Peng, C.; Yu, G.; Ran, W.; Guo, S.; Shen, Q. Response of the Bacterial Diversity and Soil Enzyme Activity in Particle-Size Fractions of Mollisol after Different Fertilization in a Long-Term Experiment. Biol. Fertil. Soils 2014, 50, 901–911. [Google Scholar] [CrossRef]
- Mikhailova, E.A.; Zurqani, H.A.; Post, C.J.; Schlautman, M.A.; Post, G.C. Soil Diversity (Pedodiversity) and Ecosystem Services. Land 2021, 10, 288. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil Structure and Microbiome Functions in Agroecosystems. Nat. Rev. Earth Environ. 2022, 4, 4–18. [Google Scholar] [CrossRef]
- Nie, M.; Meng, H.; Li, K.; Wan, J.R.; Quan, Z.X.; Fang, C.M.; Chen, J.K.; Li, B. Comparison of Bacterial and Fungal Communities between Natural and Planted Pine Forests in Subtropical China. Curr. Microbiol. 2012, 64, 34–42. [Google Scholar] [CrossRef]
- Tripathi, B.M.; Kim, M.; Singh, D.; Lee-Cruz, L.; Lai-Hoe, A.; Ainuddin, A.N.; Go, R.; Rahim, R.A.; Husni, M.H.A.; Chun, J.; et al. Tropical Soil Bacterial Communities in Malaysia: PH Dominates in the Equatorial Tropics Too. Microb. Ecol. 2012, 64, 474–484. [Google Scholar] [CrossRef]
- Cao, P.; Zhang, L.M.; Shen, J.P.; Zheng, Y.M.; Di, H.J.; He, J.Z. Distribution and Diversity of Archaeal Communities in Selected Chinese Soils. FEMS Microbiol. Ecol. 2012, 80, 146–158. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Tu, Q.; Zhi, X. Functional Molecular Ecological Networks. mBio 2010, 1, e00169-10. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, H.; Dsouza, M.; Lou, J.; He, Y.; Dai, Z.; Brookes, P.C.; Xu, J.; Gilbert, J.A. Geographic Patterns of Co-Occurrence Network Topological Features for Soil Microbiota at Continental Scale in Eastern China. ISME J. 2016, 10, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil Bacterial Networks Are Less Stable under Drought than Fungal Networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural Intensification Reduces Microbial Network Complexity and the Abundance of Keystone Taxa in Roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-Bacterial Diversity and Microbiome Complexity Predict Ecosystem Functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Y.; Hu, C.; Zheng, X.; Zhang, J.; Zhang, H.; Bai, N.; Zhang, H.; Tian, M.; Ban, S.; et al. Stochastic Processes Drive Bacterial and Fungal Community Assembly in Sustainable Intensive Agricultural Soils of Shanghai, China. Sci. Total Environ. 2021, 778, 146021. [Google Scholar] [CrossRef]
- Luo, F.; Zhong, J.; Yang, Y.; Scheuermann, R.H.; Zhou, J. Application of Random Matrix Theory to Biological Networks. Phys. Lett. A 2006, 357, 420–423. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.-H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular Ecological Network Analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Raes, J. Microbial Interactions: From Networks to Models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Karimi, B.; Dequiedt, S.; Terrat, S.; Jolivet, C.; Arrouays, D.; Wincker, P.; Cruaud, C.; Bispo, A.; Prevost-Boure, N.C.; Ranjard, L. Biogeography of Soil Bacterial Networks along a Gradient of Cropping Intensity. Sci. Rep. 2019, 9, 3812. [Google Scholar] [CrossRef]
- Feng, K.; Zhang, Y.; He, Z.; Ning, D.; Deng, Y. Interdomain Ecological Networks between Plants and Microbes. Mol. Ecol. Resour. 2019, 19, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, Y.; Ye, S.; Liu, S.; Stirling, E.; Gilbert, J.A.; Faust, K.; Knight, R.; Jansson, J.K.; Cardona, C.; et al. Earth Microbial Co-Occurrence Network Reveals Interconnection Pattern across Microbiomes. Microbiome 2020, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Zhao, C.; Kirkby, C.A.; Coggins, S.; Zhao, S.; Bissett, A.; van der Heijden, M.G.A.; Kirkegaard, J.A.; Richardson, A.E. Microbial Interkingdom Associations across Soil Depths Reveal Network Connectivity and Keystone Taxa Linked to Soil Fine-Fraction Carbon Content. Agric. Ecosyst. Environ. 2021, 320, 107559. [Google Scholar] [CrossRef]
- Berry, D.; Widder, S. Deciphering Microbial Interactions and Detecting Keystone Species with Co-Occurrence Networks. Front. Microbiol. 2014, 5, 90985. [Google Scholar] [CrossRef] [PubMed]
- Coller, E.; Cestaro, A.; Zanzotti, R.; Bertoldi, D.; Pindo, M.; Larger, S.; Albanese, D.; Mescalchin, E.; Donati, C. Microbiome of Vineyard Soils Is Shaped by Geography and Management. Microbiome 2019, 7, 140. [Google Scholar] [CrossRef]
- Yuan, M.M.; Guo, X.; Wu, L.L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.L.; Yang, Y.; et al. Climate Warming Enhances Microbial Network Complexity and Stability. Nat. Clim. Chang. 2021, 11, 343–348. [Google Scholar] [CrossRef]
- HJ615-2011; Chinese Standards for determination of Soil Organic Carbon. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2011.
- Nannipieri, P.; Ceccanti, B.; Cervelli, S.; Matarese, E. Extraction of Phosphatase, Urease, Proteases, Organic Carbon, and Nitrogen from Soil. Soil Sci. Soc. Am. J. 1980, 44, 1011–1016. [Google Scholar] [CrossRef]
- Schinner, F.; von Mersi, W. Xylanase-, CM-Cellulase- and Invertase Activity in Soil: An Improved Method. Soil Biol. Biochem. 1990, 22, 511–515. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil Analysis; SSSA Book Series; Wiley: Hoboken, NJ, USA, 1994; pp. 775–833. ISBN 9780891188650. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2′s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Yanga, Y. Phylogenetic Molecular Ecological Network of Soil Microbial Communities in Response to Elevated CO2. mBio 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Bastian, M.; Heymann, S.; Jacomy, M. Gephi: An Open Source Software for Exploring and Manipulating Networks. BT—International AAAI Conference on Weblogs and Social. Int. AAAI Conf. Weblogs Soc. Media 2009, 3, 361–362. [Google Scholar]
- Wang, S.; Pi, Y.; Song, Y.; Jiang, Y.; Zhou, L.; Liu, W.; Zhu, G. Hotspot of Dissimilatory Nitrate Reduction to Ammonium (DNRA) Process in Freshwater Sediments of Riparian Zones. Water Res. 2020, 173, 115539. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.A.; Wood, S.A.; Bardgett, R.D.; Black, H.I.J.; Bonkowski, M.; Eggers, T.; Grayston, S.J.; Kandeler, E.; Manning, P.; Setälä, H.; et al. Discontinuity in the Responses of Ecosystem Processes and Multifunctionality to Altered Soil Community Composition. Proc. Natl. Acad. Sci. USA 2014, 111, 14478–14483. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant Species Richness and Ecosystem Multifunctionality in Global Drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Team, R. R Core Team. R A Language and Environment for Statistical Computing 2014; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Son, E.D.; Choi, G.H.; Kim, H.; Lee, B.; Chang, I.S.; Hwang, J.S. Vegan: Community Ecology Package. Biol. Pharm. Bull. 2007, 30, 1395–1399. [Google Scholar] [CrossRef]
- BREIMAN, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Aphalo, P.J. Ggpmisc: Miscellaneous Extensions to “Ggplot2”. Available online: https://cran.r-project.org/web/packages/ggpmisc/ggpmisc.pdf (accessed on 7 May 2024).
- Ge, Y.; He, J.-Z.; Zhu, Y.-G.; Zhang, J.; Zhang, J.; Zhang, J.; Zhang, J.; Xu, Z.; Zhang, L.-M.; Zheng, Y.-M. Differences in Soil Bacterial Diversity: Driven by Contemporary Disturbances or Historical Contingencies? ISME J. 2008, 2, 254–264. [Google Scholar] [CrossRef]
- Levine, U.Y.; Teal, T.K.; Robertson, G.P.; Schmidt, T.M. Agriculture’s Impact on Microbial Diversity and Associated Fluxes of Carbon Dioxide and Methane. ISME J. 2011, 5, 1683–1691. [Google Scholar] [CrossRef]
- Sessitsch, A.; Weilharter, A.; Gerzabek, M.H.; Kirchmann, H.; Kandeler, E. Microbial Population Structures in Soil Particle Size Fractions of a Long-Term Fertilizer Field Experiment. Appl. Environ. Microbiol. 2001, 67, 4215–4224. [Google Scholar] [CrossRef]
- Orozco-Aceves, M.; Tibbett, M.; Standish, R.J. Correlation between Soil Development and Native Plant Growth in Forest Restoration after Surface Mining. Ecol. Eng. 2017, 106, 209–218. [Google Scholar] [CrossRef]
- Huang, L.L.; Kou, W.B.; Wu, L.; Feinstein, L.; Kong, Z.Y.; Ge, G. Microbial Composition and Activity of Natural, Restored, and Reclaimed Wetland Soils: A Case Study of Poyang Lake Basin, China. Wetlands 2019, 39, 113–123. [Google Scholar] [CrossRef]
- Liu, X.; Lee Burras, C.; Kravchenko, Y.S.; Duran, A.; Huffman, T.; Morras, H.; Studdert, G.; Zhang, X.; Cruse, R.M.; Yuan, X. Overview of Mollisols in the World: Distribution, Land Use and Management. Can. J. Soil Sci. 2012, 92, 383–402. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Liu, B.; Zheng, Q.; Yin, J. Characteristics and Factors Controlling the Development of Ephemeral Gullies in Cultivated Catchments of Black Soil Region, Northeast China. Soil Tillage Res. 2007, 96, 28–41. [Google Scholar] [CrossRef]
- Eckmeier, E.; Gerlach, R.; Gehrt, E.; Schmidt, M.W.I. Pedogenesis of Chernozems in Central Europe—A Review. Geoderma 2007, 139, 288–299. [Google Scholar] [CrossRef]
- Burns, R.G.; De Forest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil Enzymes in a Changing Environment: Current Knowledge and Future Directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Stone, M.M.; De Forest, J.L.; Plante, A.F. Changes in Extracellular Enzyme Activity and Microbial Community Structure with Soil Depth at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2014, 75, 237–247. [Google Scholar] [CrossRef]
- Liu, X.-M.; Li, Q.; Liang, W.-J.; Jiang, Y. Distribution of Soil Enzyme Activities and Microbial Biomass Along a Latitudinal Gradient in Farmlands of Songliao Plain, Northeast China. Pedosphere 2008, 18, 431–440. [Google Scholar] [CrossRef]
- Yang, B.; Banerjee, S.; Herzog, C.; Ramírez, A.C.; Dahlin, P.; van der Heijden, M.G.A. Impact of Land Use Type and Organic Farming on the Abundance, Diversity, Community Composition and Functional Properties of Soil Nematode Communities in Vegetable Farming. Agric. Ecosyst. Environ. 2021, 318, 107488. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Yao, Q.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Diversity and Distribution Patterns of Acidobacterial Communities in the Black Soil Zone of Northeast China. Soil Biol. Biochem. 2016, 95, 212–222. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. Organic Amendments: Microbial Community Structure, Activity and Abundance of Agriculturally Relevant Microbes Are Driven by Long-Term Fertilization Strategies. Front. Microbiol. 2016, 7, 215318. [Google Scholar] [CrossRef] [PubMed]
- Wolińska, A.; Kuźniar, A.; Zielenkiewicz, U.; Izak, D.; Szafranek-Nakonieczna, A.; Banach, A.; Błaszczyk, M. Bacteroidetes as a Sensitive Biological Indicator of Agricultural Soil Usage Revealed by a Culture-Independent Approach. Appl. Soil Ecol. 2017, 119, 128–137. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, Y.; Chen, W.; Guo, Y.; Wu, M.; Wang, Y.; Li, H. Soil Type and PH Mediated Arable Soil Bacterial Compositional Variation across Geographic Distance in North China Plain. Appl. Soil Ecol. 2022, 169, 104220. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. High Throughput Sequencing Analysis of Biogeographical Distribution of Bacterial Communities in the Black Soils of Northeast China. Soil Biol. Biochem. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-Based Assessment of Soil PH as a Predictor of Soil Bacterial Community Structure at the Continental Scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil Bacterial and Fungal Communities across a PH Gradient in an Arable Soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Zhao, X.; Wang, Y.; Lv, X.; Chen, Y.; Jiao, X.; Sui, Y. Pedogenesis of Typical Zonal Soil Drives Belowground Bacterial Communities of Arable Land in the Northeast China Plain. Sci. Rep. 2023, 13, 14555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cong, J.; Lu, H.; Li, G.; Qu, Y.; Su, X.; Zhou, J.; Li, D. Community Structure and Elevational Diversity Patterns of Soil Acidobacteria. J. Environ. Sci. 2014, 26, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zhang, R.; Xu, N.; Liu, Y.; Ni, H.; Lei, J.; Li, M. Diversity of Soil Acidobacterial Community of Different Land Use Types in the Sanjiang Plain, Northeast of China. Int. J. Agric. Biol. 2017, 19, 1279–1285. [Google Scholar] [CrossRef]
- Wang, S.; Dong, L.; Luo, Y.; Jia, W.; Qu, Y. Characterization of Rhizosphere Microbial Communities in Continuous Cropping Maca (Lepidium Meyenii) Red Soil, Yunnan, China. Arch. Agron. Soil Sci. 2020, 66, 805–818. [Google Scholar] [CrossRef]
- Lee, S.H.; Ka, J.O.; Cho, J.C. Members of the Phylum Acidobacteria Are Dominant and Metabolically Active in Rhizosphere Soil. FEMS Microbiol. Lett. 2008, 285, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an Ecological Classification of Soil Bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Chu, H.; Chu, H.; Fierer, N.; Lauber, C.L.; Caporaso, J.G.; Caporaso, J.G.; Knight, R.; Grogan, P. Soil Bacterial Diversity in the Arctic Is Not Fundamentally Different from That Found in other Biomes. Environ. Microbiol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Xiong, J.; Zhang, H.; Feng, Y.; Lin, X.; Li, X.; Liang, W.; Chu, H. Soil PH Drives the Spatial Distribution of Bacterial Communities along Elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Hill, T.C.J.; Walsh, K.A.; Harris, J.A.; Moffett, B.F. Using Ecological Diversity Measures with Bacterial Communities. FEMS Microbiol. Ecol. 2003, 43, 1–11. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Y.; Li, T.; Chen, L.; Chen, Y.; Sui, P. Changes in Soil Microbial Biomass, Diversity, and Activity with Crop Rotation in Cropping Systems: A Global Synthesis. Appl. Soil Ecol. 2023, 186, 104815. [Google Scholar] [CrossRef]
- Grządziel, J.; Gałązka, A. Microplot Long-Term Experiment Reveals Strong Soil Type Influence on Bacteria Composition and Its Functional Diversity. Appl. Soil Ecol. 2018, 124, 117–123. [Google Scholar] [CrossRef]
- Yang, Y.; Li, T.; Wang, Y.; Cheng, H.; Chang, S.X.; Liang, C.; An, S. Negative Effects of Multiple Global Change Factors on Soil Microbial Diversity. Soil Biol. Biochem. 2021, 156, 108229. [Google Scholar] [CrossRef]
- Xue, P.; Minasny, B.; McBratney, A.; Wilson, N.L.; Tang, Y.; Luo, Y. Distinctive Role of Soil Type and Land Use in Driving Bacterial Communities and Carbon Cycling Functions down Soil Profiles. Catena 2023, 223, 106903. [Google Scholar] [CrossRef]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using Network Analysis to Explore Co-Occurrence Patterns in Soil Microbial Communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef]
- Wu, L.; Yang, Y.; Chen, S.; Zhao, M.; Zhu, Z.; Yang, S.; Qu, Y.; Ma, Q.; He, Z.; Zhou, J.; et al. Long-Term Successional Dynamics of Microbial Association Networks in Anaerobic Digestion Processes. Water Res. 2016, 104, 1–10. [Google Scholar] [CrossRef]
- Eiler, A.; Heinrich, F.; Bertilsson, S. Coherent Dynamics and Association Networks among Lake Bacterioplankton Taxa. ISME J. 2012, 6, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.J.; Strogatz, S.H. Collective Dynamics of ‘Small-World’ Networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Xiong, W.; Li, R.; Ren, Y.; Liu, C.; Zhao, Q.; Wu, H.; Jousset, A.; Shen, Q. Distinct Roles for Soil Fungal and Bacterial Communities Associated with the Suppression of Vanilla Fusarium Wilt Disease. Soil Biol. Biochem. 2017, 107, 198–207. [Google Scholar] [CrossRef]
- Qi, G.; Ma, G.; Chen, S.; Lin, C.; Zhao, X. Microbial Network and Soil Properties Are Changed in Bacterial Wilt-Susceptible Soil. Appl. Environ. Microbiol. 2019, 85, e00162-19. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.; van der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.C.; Schlaeppi, K. Cropping Practices Manipulate Abundance Patterns of Root and Soil Microbiome Members Paving the Way to Smart Farming. Microbiome 2018, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone Taxa as Drivers of Microbiome Structure and Functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.M.; Pimm, S.L.; Solé, R. V Ecological Networks and Their Fragility. Nature 2006, 442, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going Back to the Roots: The Microbial Ecology of the Rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Zhou, J.; Guan, D.; Zhou, B.; Zhao, B.; Ma, M.; Qin, J.; Jiang, X.; Chen, S.; Cao, F.; Shen, D.; et al. Influence of 34-Years of Fertilization on Bacterial Communities in an Intensively Cultivated Black Soil in Northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Lian, T.; Mu, Y.; Jin, J.; Ma, Q.; Cheng, Y.; Cai, Z.; Nian, H. Impact of Intercropping on the Coupling between Soil Microbial Community Structure, Activity, and Nutrient-Use Efficiencies. PeerJ 2019, 2019, e6412. [Google Scholar] [CrossRef] [PubMed]
- Kuramae, E.E.; Yergeau, E.; Wong, L.C.; Pijl, A.S.; van Veen, J.A.; Kowalchuk, G.A. Soil Characteristics More Strongly Influence Soil Bacterial Communities than Land-Use Type. FEMS Microbiol. Ecol. 2012, 79, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial Diversity Drives Multifunctionality in Terrestrial Ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Delgado-Baquerizo, M.; Wang, J.T.; Hu, H.W.; Cai, Z.J.; Zhu, Y.N.; Singh, B.K. Fungal Richness Contributes to Multifunctionality in Boreal Forest Soil. Soil Biol. Biochem. 2019, 136, 107526. [Google Scholar] [CrossRef]
- Chen, H.; Dai, Z.; Veach, A.M.; Zheng, J.; Xu, J.; Schadt, C.W. Global Meta-Analyses Show That Conservation Tillage Practices Promote Soil Fungal and Bacterial Biomass. Agric. Ecosyst. Environ. 2020, 293, 106841. [Google Scholar] [CrossRef]
- Chen, Q.L.; Ding, J.; Zhu, D.; Hu, H.W.; Delgado-Baquerizo, M.; Ma, Y.B.; He, J.Z.; Zhu, Y.G. Rare Microbial Taxa as the Major Drivers of Ecosystem Multifunctionality in Long-Term Fertilized Soils. Soil Biol. Biochem. 2020, 141, 107686. [Google Scholar] [CrossRef]
- Chen, L.F.; He, Z.B.; Zhao, W.Z.; Liu, J.L.; Zhou, H.; Li, J.; Meng, Y.Y.; Wang, L.S. Soil Structure and Nutrient Supply Drive Changes in Soil Microbial Communities during Conversion of Virgin Desert Soil to Irrigated Cropland. Eur. J. Soil Sci. 2020, 71, 768–781. [Google Scholar] [CrossRef]
- Peng, M.; He, H.; Wang, X.; Wang, Z.; Zhuang, L. Comparison of Network Connectivity and Environmental Driving Factors of Root-Associated Fungal Communities of Desert Ephemeral Plants in Two Habitat Soils. J. Environ. Manag. 2023, 332, 117375. [Google Scholar] [CrossRef]
- Feng, C.; Yi, Z.; Qian, W.; Liu, H.; Jiang, X. Rotations Improve the Diversity of Rhizosphere Soil Bacterial Communities, Enzyme Activities and Tomato Yield. PLoS ONE 2023, 18, e0270944. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, G.; Jin, J.; Liu, J.; Zhang, Q.; Liu, X. Bacterial Communities in Soybean Rhizosphere in Response to Soil Type, Soybean Genotype, and Their Growth Stage. Soil Biol. Biochem. 2009, 41, 919–925. [Google Scholar] [CrossRef]
- Bourceret, A.; Guan, R.; Dorau, K.; Mansfeldt, T.; Omidbakhshfard, A.; Medeiros, D.B.; Fernie, A.R.; Hofmann, J.; Sonnewald, U.; Mayer, J.; et al. Maize Field Study Reveals Covaried Microbiota and Metabolic Changes in Roots over Plant Growth. mBio 2022, 13, e02584-21. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Z.; Chen, Z.; White, J.F.; Malik, K.; Chen, T.; Li, C. Inoculation of Barley (Hordeum vulgare) with the Endophyte Epichloë bromicola Affects Plant Growth, and the Microbial Community in Roots and Rhizosphere Soil. J. Fungi 2022, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tian, L.; Chang, J.; Shi, S.; Zhang, J.; Xie, H.; Cai, Y.; Chen, D.; Kuramae, E.E.; van Veen, J.A.; et al. Rice Domestication Influences the Composition and Function of the Rhizosphere Bacterial Chemotaxis Systems. Plant Soil 2021, 466, 81–99. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).