Nighttime Warming Reduced Copper Concentration and Accumulation in Wheat Grown in Copper-Contaminated Soil by Affecting Physiological Traits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Site, Plant Culture, and Experimental Design
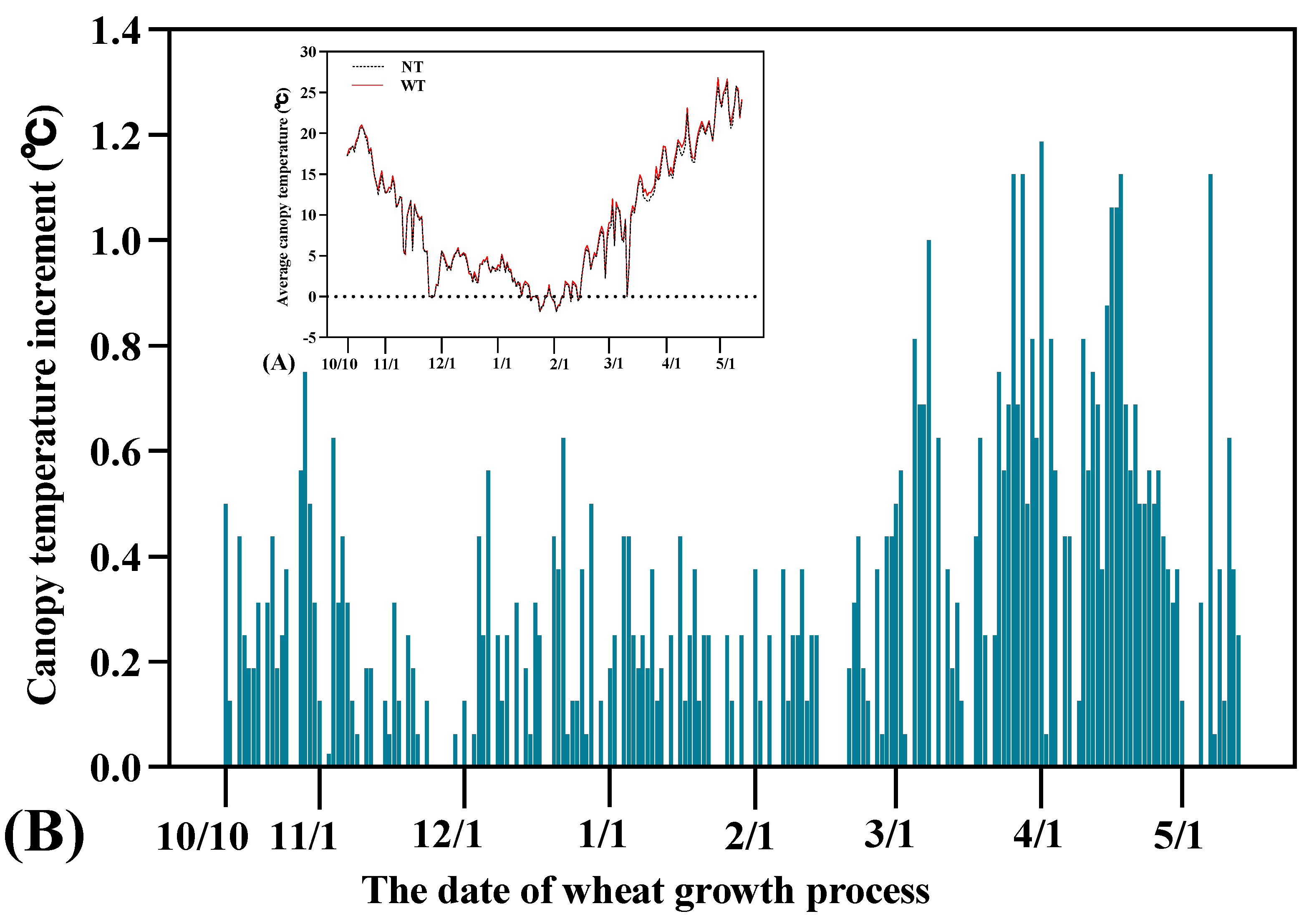
2.2. Determination of the Canopy Temperature and Its Increment
2.3. Determination of Available Cu Concentration in Soil
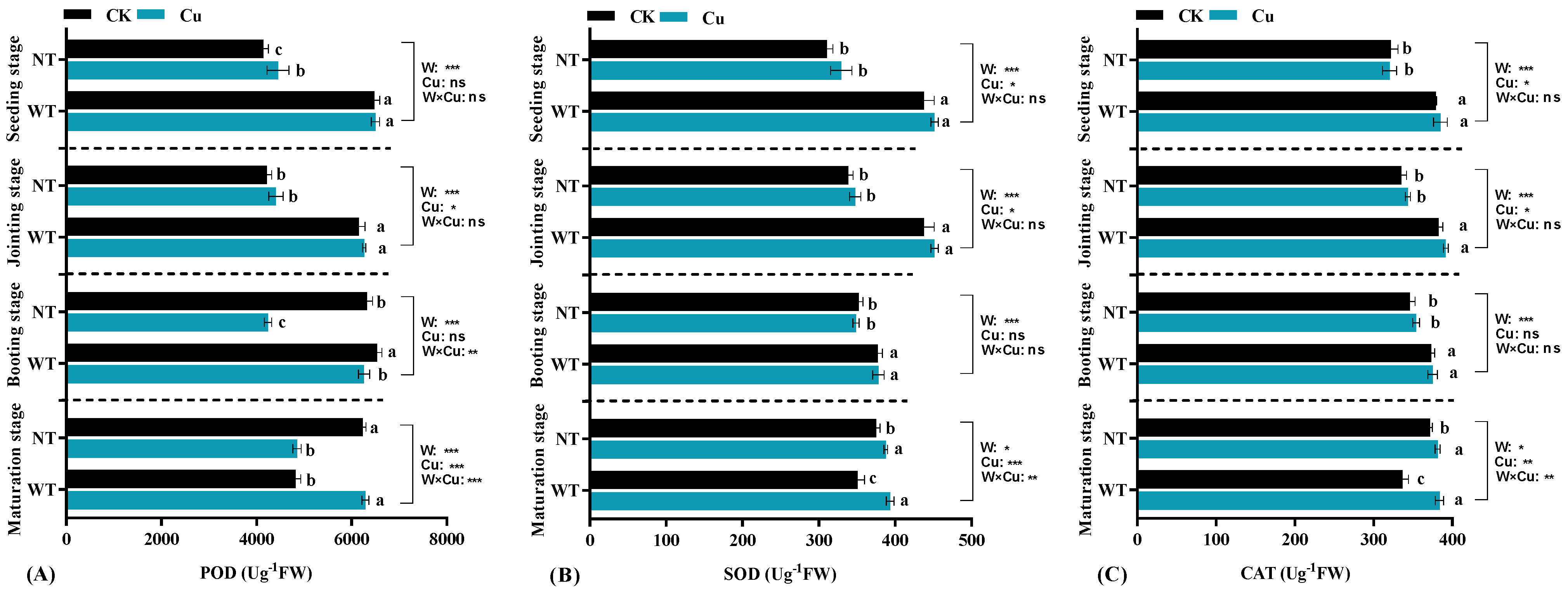
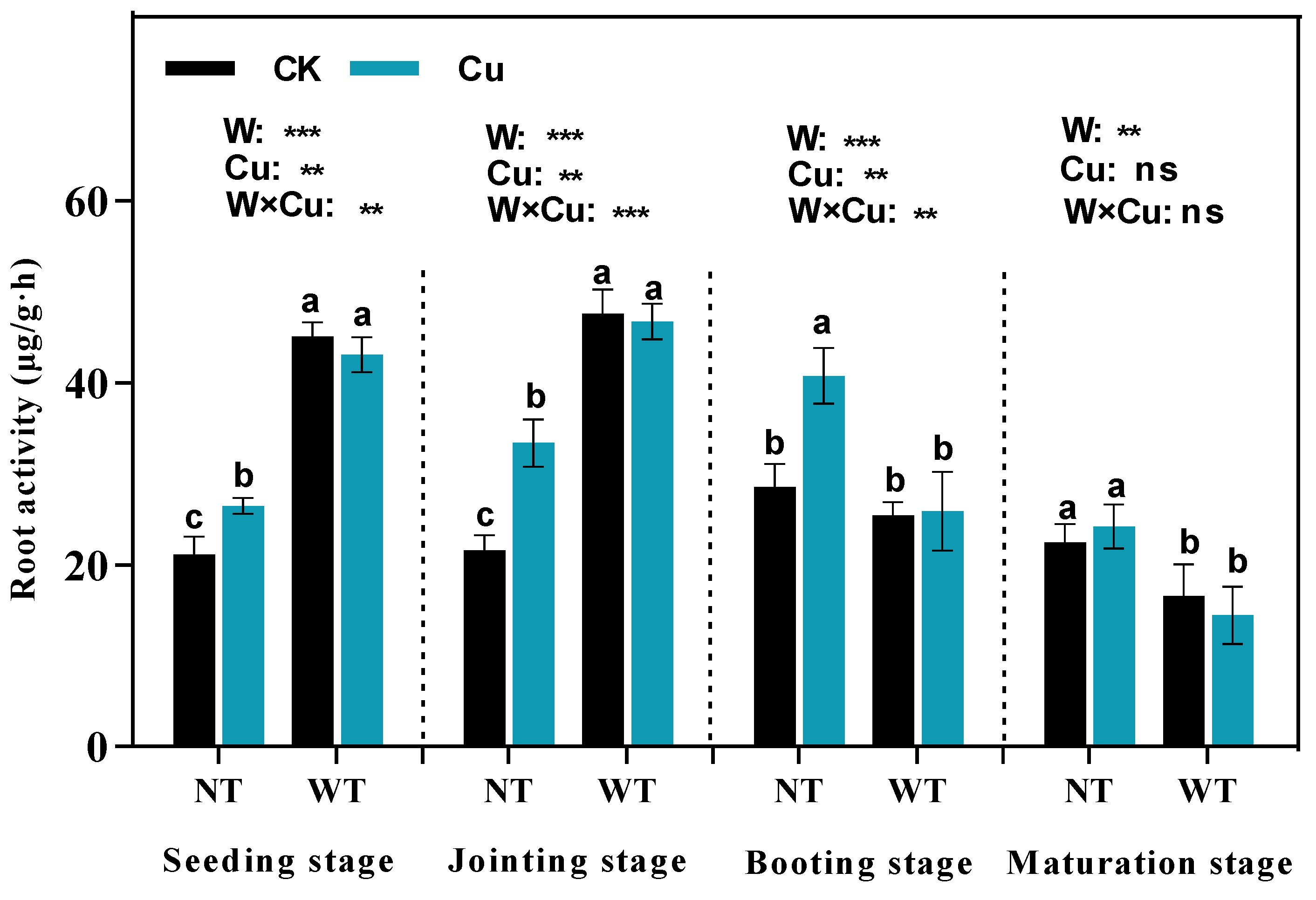
2.4. Determination of Antioxidant Enzyme Activities, Photosynthesis in Leaves, and Activities in Roots
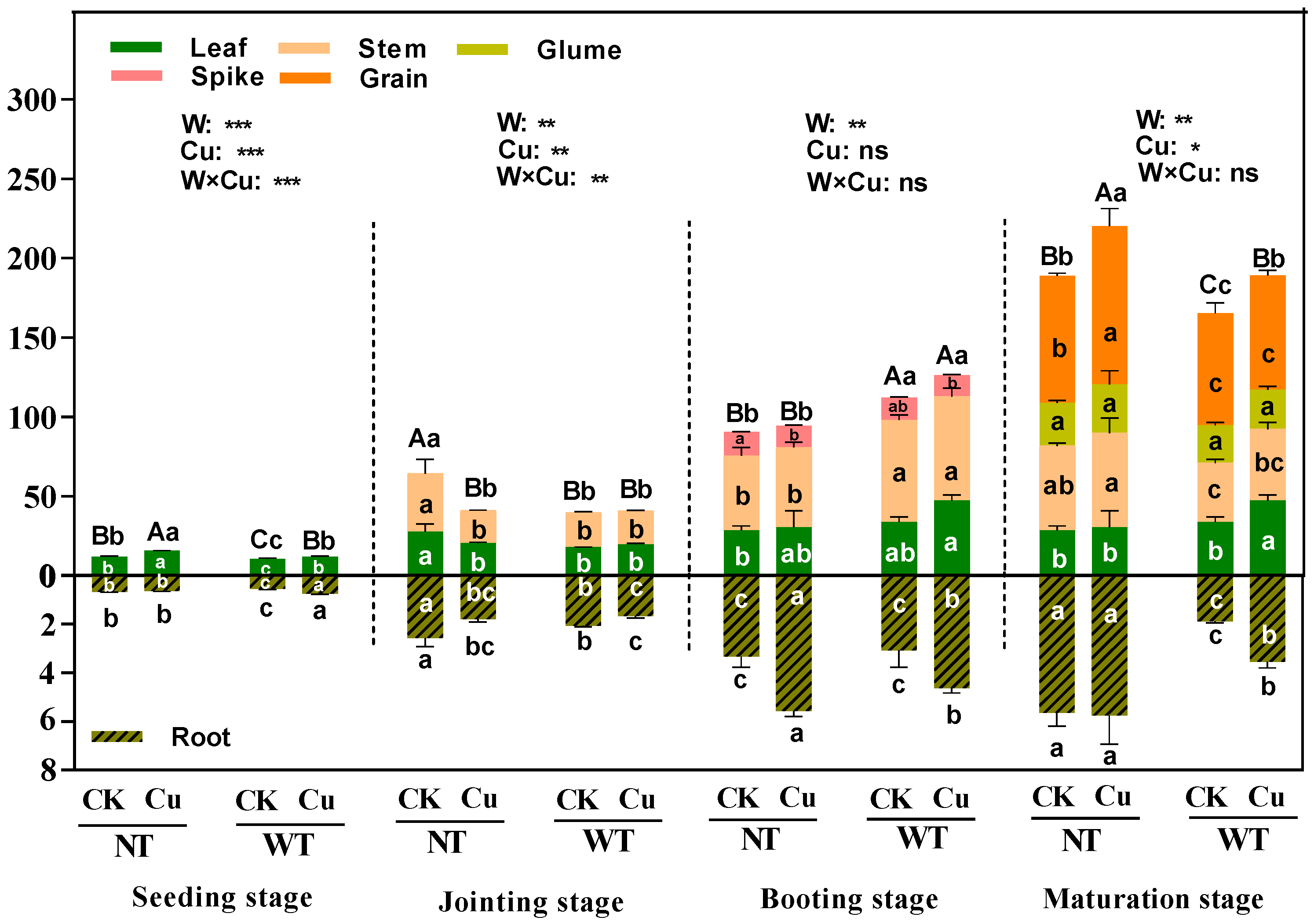
2.5. Determination of Biomass and Yield in Wheat
2.6. Determination of Cu Concentration and Accumulation in Wheat
2.7. Calculation of Cu Translocation Factor in Wheat
2.8. Statistical Analysis
3. Results and Discussion
3.1. Warming Effect of the Night Warming System
3.2. Available Cu Concentration in Soil
3.3. Antioxidant Enzyme Activities of Leaves
3.4. Root Activities, Leaf Transpiration Rate, and Net Photosynthetic Rate
3.5. Biomass and Yield of Different Tissues in Wheat
3.6. Cu Concentration in Different Tissues in Wheat
3.7. Cu Accumulation in Different Tissues in Wheat
3.8. Cu Translocation Factors in Different Tissues in Wheat
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ghori, N.H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. J. Environ. Sci. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Guo, G.; Wu, F.; Xie, F.; Zhang, R. Spatial distribution and pollution assessment of heavy metals in urban soils from southwest China. J. Environ. Sci. 2012, 24, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Koz, B.; Cevik, U.; Akbulut, S. Heavy metal analysis around Murgul (Artvin) copper mining area of Turkey using moss and soil. Ecol. Indic. 2012, 20, 17–23. [Google Scholar] [CrossRef]
- Obiora, S.C.; Chibuike, G.U. Heavy metal polluted soils: Effect on pants and boremediation methods. Appl. Environ. Soil Sci. 2014, 2014, 752708. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Wang, L.; Li, F. Heavy metal occurrence and risk assessment in dairy feeds and manures from the typical intensive dairy farms in China. Environ. Sci. Pollut. Res. 2019, 26, 6348–6358. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Liu, H.; Zhang, Y.; Gu, G.H.; Hu, J.Y. Micro-assessment of heavy metal immobilization within alkali-activated copper tailings-slag geopolymer. Cem. Concr. Comp. 2024, 149, 105510. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Gemin, L.G.; Lara, G.B.D.; Mógor, A.F.; Mazaro, S.M.; Sant’Anna-Santos, B.F.; Mógor, G.; Amatussi, J.D.O.; Cordeiro, E.C.N.; Marques, H.M.C. Polysaccharides combined to copper and magnesium improve tomato growth, yield, anti-oxidant and plant defense enzymes. Sci. Hortic. 2023, 310, 111758. [Google Scholar] [CrossRef]
- Sağlam, A.; Yetişsin, F.; Demiralay, M.; Terzi, R. Copper stress and responses in plants. In Plant Metal Interaction; Elsevier: Amsterdam, The Netherlands, 2016; pp. 21–40. [Google Scholar]
- Lamb, D.T.; Naidu, R.; Ming, H.; Megharaj, M. Copper phytotoxicity in native and agronomical plant species. Ecotox. Environ. Saf. 2012, 85, 23–29. [Google Scholar] [CrossRef]
- Maas, S.; Scheifler, R.; Benslama, M.; Crini, N.; Lucot, E.; Brahmia, Z.; Benyacoub, S.; Giraudoux, P. Spatial distribution of heavy metal concentrations in urban, suburban and agricultural soils in a Mediterranean city of Algeria. Environ. Pollut. 2010, 158, 2294–2301. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Chavas, D.R.; Izaurralde, R.C.; Thomson, A.M.; Gao, X.J. Long-term climate change impacts on agricultural productivity in eastern China. Agric. For. Meteorol. 2009, 149, 1118–1128. [Google Scholar] [CrossRef]
- Davy, R.; Esau, I.; Chernokulsky, A.; Outten, S.; Zilitinkevich, S. Diurnal asymmetry to the observed global warming. J. Climatol. 2017, 37, 79–93. [Google Scholar] [CrossRef]
- Cai, C.; Yin, X.; He, S.; Jiang, W.; Si, C.; Struik, P.C.; Luo, W.H.; Li, G.; Xie, Y.T.; Xiong, Y.; et al. Responses of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments. Glob. Chang. Biol. 2015, 49, 7625–7638. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Kevin, E.; Trenberth, A.D.; Gerard, V.D.S.; Philip, D.J.; Jonathan, B.; Keith, R.B.; Justin, S. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Dorau, K.; Bamminger, C.; Koch, D.; Mansfeldt, T. Evidences of soil warming from long-term trends (1951-2018) in North Rhine-Westphalia, Germany. Clim. Chang. 2022, 170, 9. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef]
- Zheng, C.Y.; Deng, A.X.; Chen, C.Q. Nighttime warming increases winter-sown wheat yield across major Chinese cropping regions. Field Crop. Res. 2017, 214, 202–210. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Xu, L.; Xu, Y.Z.; Xi, M.; Tu, D.B.; Chen, J.H.; Wu, W. A meta-analysis of the effects of global warming on rice and wheat yields in a rice–wheat rotation system. Food Energy Secur. 2021, 10, e316. [Google Scholar] [CrossRef]
- Wan, S.; Xia, J.; Liu, W.; Niu, S. Photosynthetic overcompensation under nocturnal warming enhances grassland carbon sequestration. Ecology 2009, 90, 2700–2710. [Google Scholar] [CrossRef]
- Bhaduri, A.M.; Fulekar, M.H. Antioxidant enzyme responses of plants to heavy metal stress. Rev. Environ. Sci. Biotechnol. 2012, 11, 55–69. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, Y.Y.; Nawaz, M.A.; Chen, C.; Liu, L.; Lu, Z.; Kong, Q.S.; Cheng, F.; Bie, Z.L. Improving magnesium uptake, photosynthesis and antioxidant enzyme activities of watermelon by grafting onto pumpkin rootstock under low magnesium. Plant Soil. 2016, 409, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubian, I.; Ghassemi, S.; Nazari, M.; Raey, Y.; Smith, D.L. Response of physiological traits, antioxidant enzyme and nutrient uptake of soybean to Azotobacter Chroococcum and zinc sulfate under salinity. S. Afr. J. Bot. 2021, 143, 42–51. [Google Scholar] [CrossRef]
- Fan, C.; Hou, M.; Si, P.; Sun, H.; Zhang, K.; Bai, Z.; Wang, G.; Li, C.; Liu, L.; Zhang, Y. Response of root and root hair phenotypes of cotton seedlings under high temperature revealed with RhizoPot. Front. Plant Sci. 2022, 13, 1007145. [Google Scholar] [CrossRef] [PubMed]
- GB 15618-2018; Soil Environmental Quality Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment of People Republic of China: Beijing, China, 2018.
- Tian, Y.L.; Chen, J.; Chen, C.Q.; Deng, A.X.; Song, Z.W.; Zheng, C.Y.; Hoogmoed, W.; Zhang, W. Warming impacts on winter wheat phenophase and grain yield under field conditions in Yangtze Delta Plain, China. Field Crop. Res. 2012, 134, 193–199. [Google Scholar] [CrossRef]
- Liang, J.; Yang, Z.X.; Tang, L.; Zeng, G.M.; Yu, M.; Li, X.D.; Wu, H.P.; Qian, Y.Y.; Li, X.M.; Luo, Y. Changes in heavy metal mobility and availability from contaminated wetland soil remediated with combined biochar-compost. Chemosphere 2017, 181, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Wang, X.; Chen, C.; Peng, B.; Tan, C.; Li, H. Varying effect of biochar on Cd, Pb and As mobility in a multi-metal contaminated paddy soil. Chemosphere 2016, 152, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2008. [Google Scholar]
- Rezvani, M.; Zaefarian, F. Bioaccumulation and translocation factors of cadmium and lead in Aeluropus littoralis. Aus. J. Agric. Eng. 2011, 2, 114–119. [Google Scholar]
- Chen, J.; Chen, C.; Tian, Y.L.; Zhang, X.; Dong, W.; Zhang, B.; Zhang, J.; Zheng, C.; Deng, A.; Song, Z. Differences in the impacts of nighttime warming on crop growth of rice-based cropping systems under field conditions. Eur. J. Agron. 2017, 82, 80–92. [Google Scholar] [CrossRef]
- Beier, C.; Emmett, B.; Gundersen, P.; Albert Tietema, A.; Peñuelas, J.; Estiarte, M.; Gordon, C.; Gorissen, A.; Llorens, L.; Roda, F.; et al. Novel approaches to study climate change effects on terrestrial ecosystems in the field: Drought and passive nighttime warming. Ecosystems 2004, 7, 583–597. [Google Scholar] [CrossRef]
- Bansal, D.; Tiwari, P.; Talukdar, G.; Rawat, G.S. Impacts of passive experimental warming on daytime and night-time respiration in a semi-natural grassland. Biogeosciences 2023. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Wang, W.; Li, T.Q.; He, Z.L.; Yang, X. Current status of agricultural soil pollution by heavy metals in China: A meta-analysis. Sci. Total Environ. 2018, 651, 3034–3042. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, Z.; Huang, J.; Guo, L.; Nie, X.; Wang, Y.; Zhang, Y.; Zeng, G.M. Adsorption characteristics of Cu and Zn onto various size fractions of aggregates from red paddy soil. J. Hazard. Mater. 2014, 264, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.R. Soil Geography, 2nd ed.; China Agriculture Press: Beijing, China, 2016. [Google Scholar]
- Meite, F.; Alvarez-Zaldívar, P.; Crochet, A.; Wiegert, C.; Payraudeau, S.; Imfeld, G. Impact of rainfall patterns and frequency on the export of pesticides and heavy-metals from agricultural soils. Sci. Total Environ. 2018, 616–617, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhang, M. Effect of moisture regime on the redistribution of heavy metals in paddy soil. J. Environ. Sci. 2011, 23, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Lo’ay, A.A.; EL-Khateeb, A.Y. Antioxidant enzyme activities and exogenous ascorbic acid treatment of ‘Williams’ banana during long-term cold storage stress. Sci. Hortic. 2018, 234, 210–219. [Google Scholar] [CrossRef]
- Roach, T.; Neuner, G.; Kranner, I.; Buchner, O. Heat acclimation under drought stress induces antioxidant enzyme activity in the alpine plant primula minima. Antioxidants 2023, 12, 1093. [Google Scholar] [CrossRef]
- Huang, W.D.; He, Y.Z.; Wang, H.H.; Zhu, Y.Z. Leaf physiological responses of three psammophytes to combined effects of warming and precipitation reduction in Horqin Sandy Land, northeast China. Front. Plant Sci. 2022, 12, 785653. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.N.; Classen, A.T.; Zhang, Z.H.; He, J.S. Asymmetric winter warming advanced plant phenology to a greater extent than symmetric warming in an alpine meadow. Funct. Ecol. 2017, 31, 2147–2156. [Google Scholar] [CrossRef]
- Huang, B.R.; Rachmilevitch, S.; Xu, J.C. Root carbon and protein metabolism associated with heat tolerance. J. Exp. Bot. 2012, 63, 3455–3465. [Google Scholar] [CrossRef]
- Zhou, H.R.; Xu, M.; Hou, R.X.; Zheng, Y.P.; Chi, Y.G.; Ouyang, Z. Thermal acclimation of photosynthesis to experimental warming is season dependent for winter wheat (Triticum aestivum L.). Environ. Exp. Bot. 2018, 150, 249–259. [Google Scholar] [CrossRef]
- Fan, Y.H.; Tian, M.Y.; Jing, Q.; Tian, Z.W.; Han, H.M.; Jiang, D.; Cao, W.X.; Dai, T.B. Winter night warming improves pre-anthesis crop growth and post-anthesis photosynthesis involved in grain yield of winter wheat (Triticum aestivum L.). Field Crop. Res. 2015, 178, 100–108. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Hu, W.; Gao, M.; Du, K.; Liu, Y.; Xu, B.J.; Wang, Y.J.; Zhou, Z.G.; Zhao, W.Q. Combined effect of elevated temperature and drought stress on carbohydrate metabolism of cotton (Gossypium hirsutum L.) subtending leaves. Physiol. Plant. 2023, 175, e13866. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, X.; Jing, X.; Zhu, B. A meta-analysis of soil extracellular enzyme activities in response to global change. Soil Biol. Biochem. 2018, 123, 21–32. [Google Scholar] [CrossRef]
- Hu, Y.J.; Yin, J. Wheat Ecological Cultivation; Science Press: Beijing, China, 2014. [Google Scholar]
- Chen, J.; Tian, Y.L.; Zhang, X.; Zheng, C.Y.; Song, Z.W.; Deng, A.X.; Zhang, W.J. Nighttime warming will increase winter wheat yield through improving plant development and grain growth in north China. J. Plant Growth Regul. 2018, 33, 397–407. [Google Scholar] [CrossRef]
- Zhao, C.; Piao, S.; Huang, Y.; Wang, X.H.; Ciais, P.; Huang, M.T.; Zeng, Z.Z.; Peng, S.S. Field warming experiments shed light on the wheat yield response to temperature in China. Nat. Commun. 2016, 7, 13530. [Google Scholar] [CrossRef]
- Qi, L.; Li, C.; Lai, L.; Kou, T.; Deng, Y. Nighttime warming promotes copper translocation from root to shoot of wheat (Triticum aestivum L.) through enlarging root systems. Front. Environ. Sci. 2024, 12, 1284198. [Google Scholar] [CrossRef]
- Turner, D.W.; Lahav, E. Temperature influences nutrient absorption and uptake rates of bananas grown in controlled environments. Sci. Hortic. 1985, 26, 311–322. [Google Scholar] [CrossRef]
- GB 15199-94; Tolerance Limit of Copper in Foods. Ministry of Health of People Republic of China: Beijing, China, 1994.
- Pregitzer, K.S.; King, J.S.; Burton, A.J.; Brown, S.E. Responses of tree fine roots to temperature. New Phytol. 2010, 147, 105–115. [Google Scholar] [CrossRef]



| Translocation Factor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Seeding Stage | Jointing Stage | Booting Stage | Maturation Stage | ||||||||
| Root to Leaf | Root to Stem | Stem to Leaf | Root to Stem | Stem to Leaf | Stem to Spike | Root to Stem | Stem to Leaf | Stem to Glume | Stem to Grain | ||
| NT | CK | 0.02 ± 0.00 c | 0.16 ± 0.04 a | 2.24 ± 0.14 a | 0.11 ± 0.03 b | 3.96 ± 1.02 a | 4.25 ± 1.19 a | 0.19 ± 0.03 a | 0.90 ± 0.10 b | 0.47 ± 0.13 b | 1.42 ± 0.26 a |
| Cu | 0.08 ± 0.01 b | 0.05 ± 0.01 c | 2.20 ± 0.17 a | 0.10 ± 0.01 b | 1.19 ± 0.18 b | 1.52 ± 0.32 b | 0.10 ± 0.02 b | 1.83 ± 0.38 a | 1.07 ± 0.10 a | 1.45 ± 0.15 a | |
| WT | CK | 0.11 ± 0.01 a | 0.11 ± 0.01 b | 2.00 ± 0.06 a | 0.17 ± 0.03 a | 2.37 ± 0.68 b | 1.66 ± 0.53 b | 0.12 ± 0.02 b | 0.73 ± 0.04 b | 1.57 ± 0.42 a | 1.27 ± 0.32 ab |
| Cu | 0.02 ± 0.01 c | 0.05 ± 0.00 c | 1.58 ± 0.32 b | 0.12 ± 0.01 b | 2.03 ± 0.29 b | 2.24 ± 0.51 b | 0.05 ± 0.01 c | 1.76 ± 0.41 a | 1.60 ± 0.37 a | 0.91 ± 0.12 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Liu, F.; Song, P.; Liu, X.; Liu, Q.; Kou, T. Nighttime Warming Reduced Copper Concentration and Accumulation in Wheat Grown in Copper-Contaminated Soil by Affecting Physiological Traits. Agronomy 2024, 14, 1302. https://doi.org/10.3390/agronomy14061302
Cheng X, Liu F, Song P, Liu X, Liu Q, Kou T. Nighttime Warming Reduced Copper Concentration and Accumulation in Wheat Grown in Copper-Contaminated Soil by Affecting Physiological Traits. Agronomy. 2024; 14(6):1302. https://doi.org/10.3390/agronomy14061302
Chicago/Turabian StyleCheng, Xianghan, Feifei Liu, Peng Song, Xiaolei Liu, Qin Liu, and Taiji Kou. 2024. "Nighttime Warming Reduced Copper Concentration and Accumulation in Wheat Grown in Copper-Contaminated Soil by Affecting Physiological Traits" Agronomy 14, no. 6: 1302. https://doi.org/10.3390/agronomy14061302




