Use of Biofertilizers as an Effective Management Strategy to Improve the Photosynthetic Apparatus, Yield, and Tolerance to Drought Stress of Drip-Irrigated Wheat in Semi-Arid Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Experimental Design
2.3. Drip Irrigation System
2.4. Isolation of Microorganisms and Plant Inoculation
2.5. Plant Management
2.6. Morphological Growth Traits and Mycorrhization Rate
2.7. Photosynthetic Efficiency, Gas Exchanges, and Carotenoid Pigment Quantification
2.8. Total Soluble Sugars, Protein Profile, and Free Proline
2.9. Antioxidant Enzymes Determination
2.10. Stress Indicators (Malondialdehyde and Hydrogen Peroxide) Determination
2.11. Soil Quality
2.12. Data Collection
2.13. Normalized Difference Vegetation Index (NDVI)
2.14. Statistical Data Analysis
3. Results
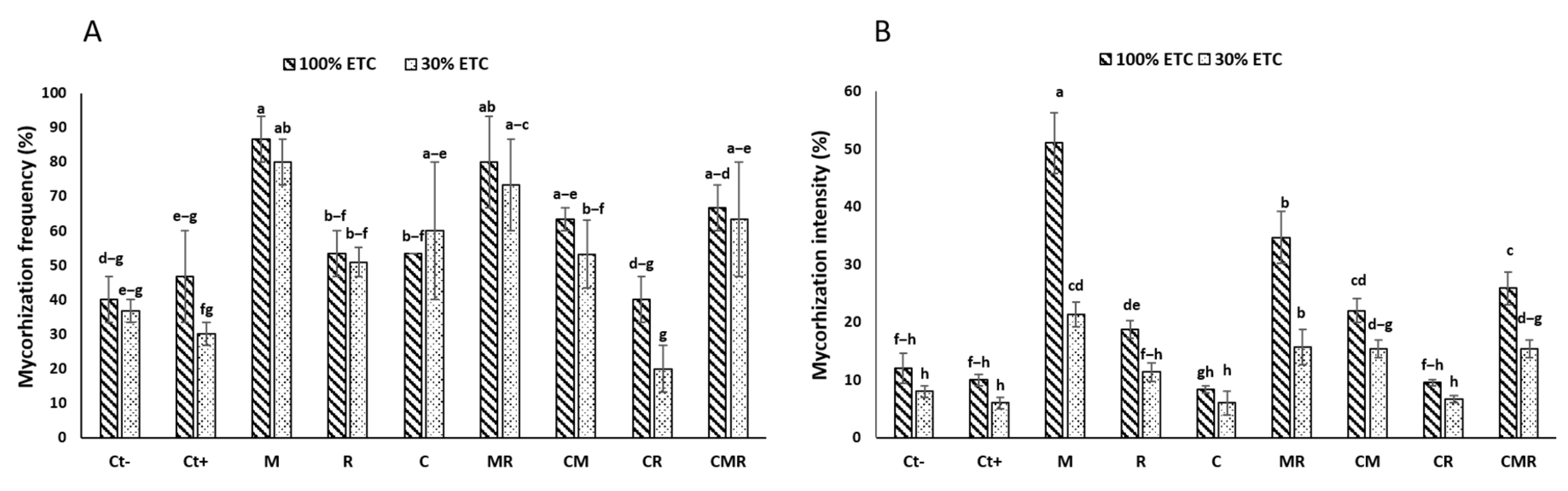
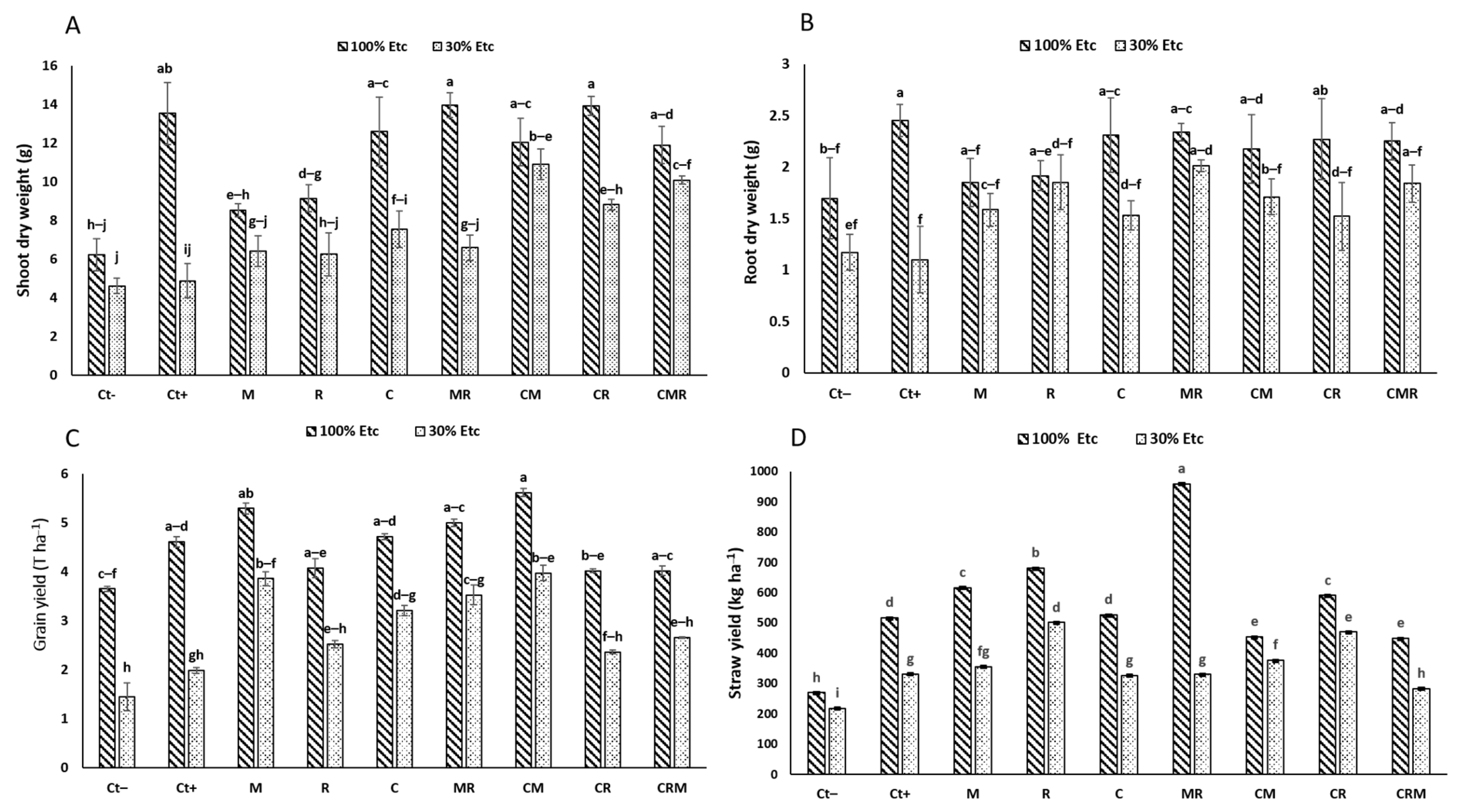
3.1. Growth Measurement and Symbiotic Development
3.1.1. Mycorrhizal Colonization
3.1.2. Growth Assessment
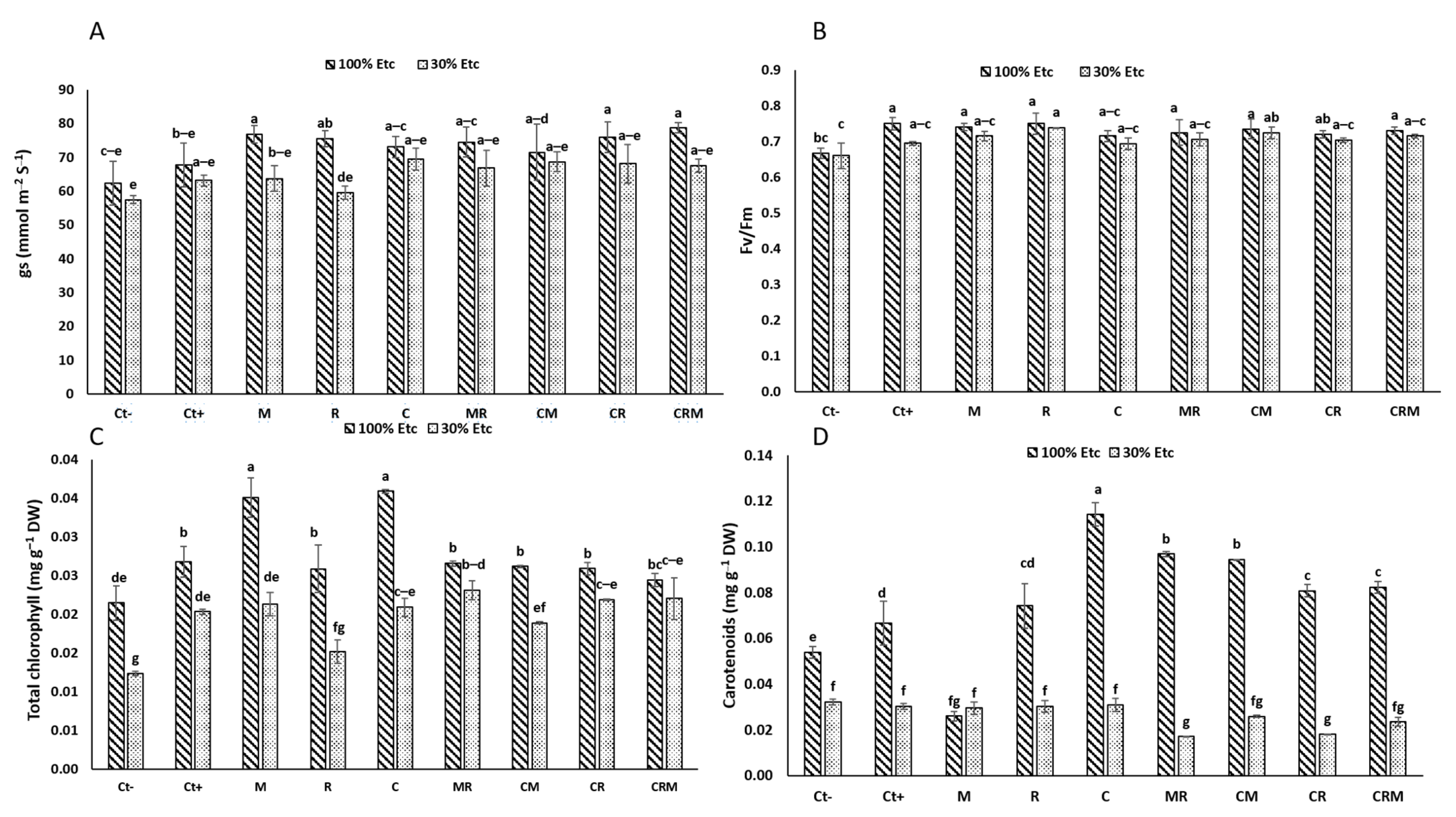
3.2. Effect of Field-Drought Stress and Microbial Biofertilizers on the Efficiency of the Photosynthetic Machinery
3.3. Total Soluble Sugar, Protein Content, and Proline Quantification in Durum Wheat Plants
3.4. Enzymatic Activities in Durum Wheat Plants
3.5. Stress Markers in Durum Wheat Plants
3.6. Soil Parameters
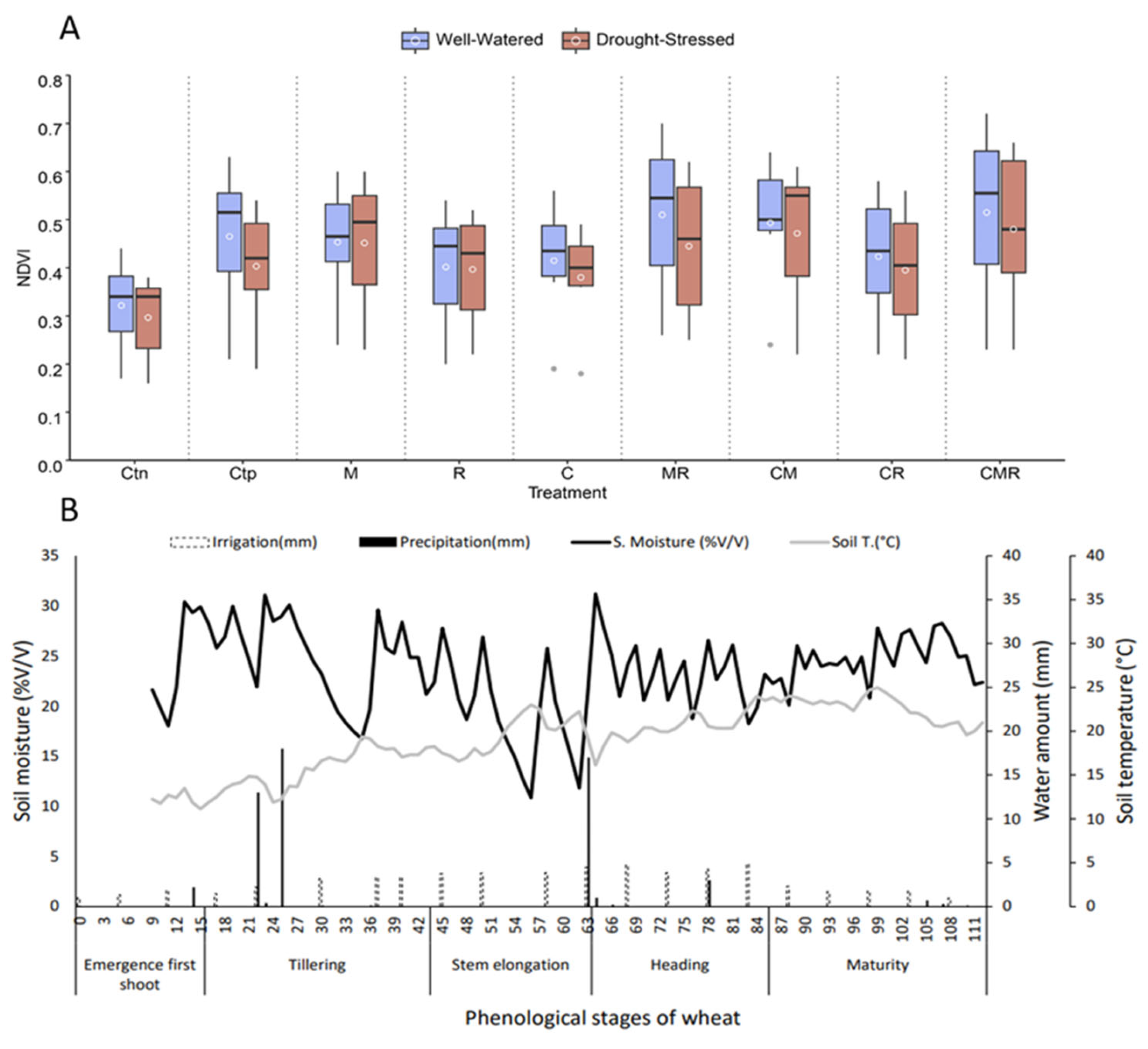
3.7. Collected Data from GreenSeeker and TDR Tools
3.8. Principal Component Analysis
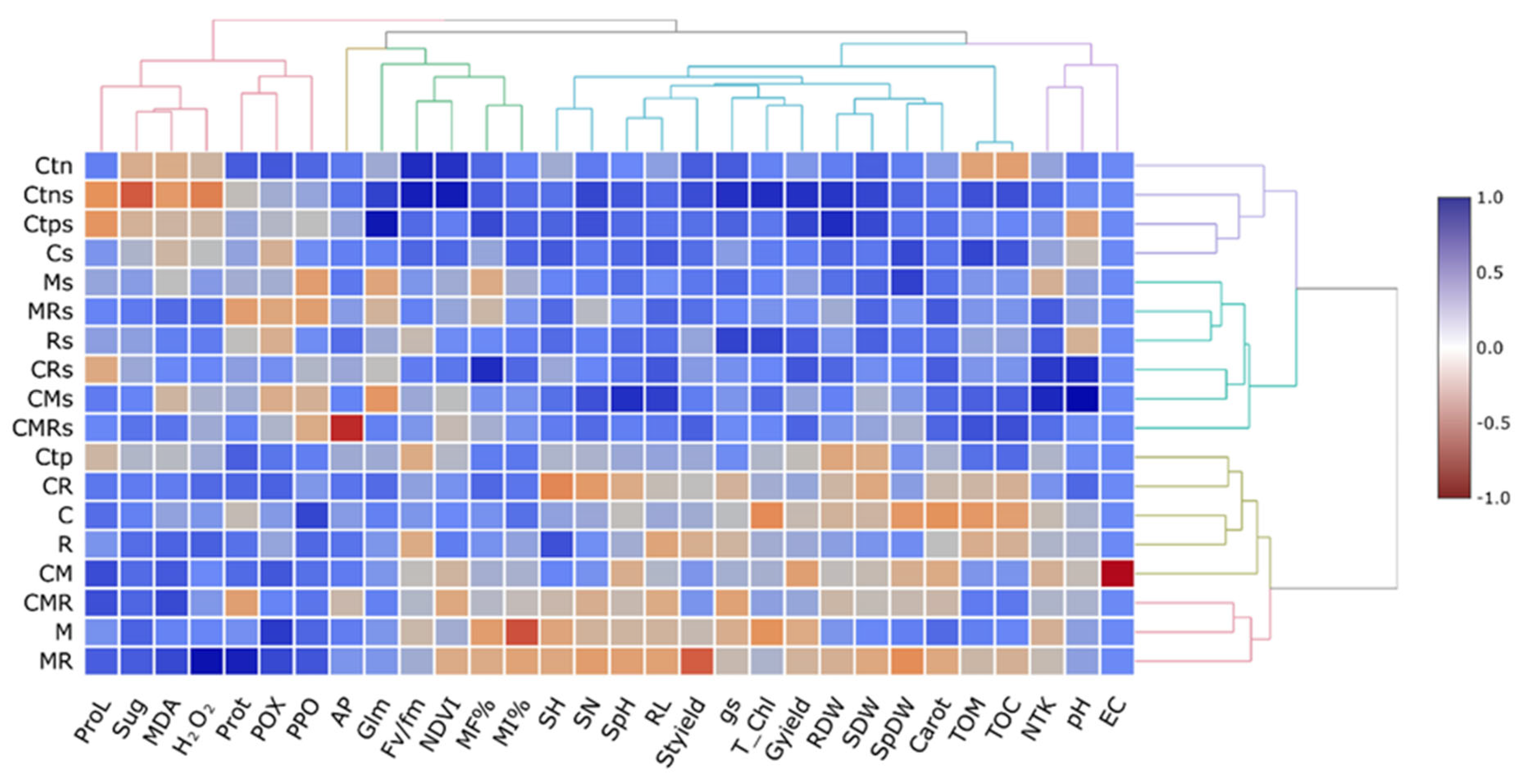
3.9. Cluster Analysis and Dendrograms in a Heat Map Matrix
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chieb, M.; Gachomo, E.W. The Role of Plant Growth Promoting Rhizobacteria in Plant Drought Stress Responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef] [PubMed]
- Hirwa, H.; Peng, Y.; Zhang, Q.; Qiao, Y.; Leng, P.; Tian, C.; Yang, G.; Muhirwa, F.; Diop, S.; Kayiranga, A.; et al. Virtual Water Transfers in Africa: Assessing Topical Condition of Water Scarcity, Water Savings, and Policy Implications. Sci. Total Environ. 2022, 835, 155343. [Google Scholar] [CrossRef] [PubMed]
- Balbaa, M.G.; Osman, H.T.; Kandil, E.E.; Javed, T.; Lamlom, S.F.; Ali, H.M.; Kalaji, H.M.; Wróbel, J.; Telesiñski, A.; Brysiewicz, A.; et al. Determination of Morpho-Physiological and Yield Traits of Maize Inbred Lines (Zea mays L.) under Optimal and Drought Stress Conditions. Front. Plant Sci. 2022, 13, 959203. [Google Scholar] [CrossRef]
- Abd El Mageed, T.A.; Semida, W.; Hemida, K.A.; Gyushi, M.A.H.; Rady, M.M.; Abdelkhalik, A.; Merah, O.; Brestic, M.; Mohamed, H.I.; El Sabagh, A.; et al. Glutathione-Mediated Changes in Productivity, Photosynthetic Efficiency, Osmolytes, and Antioxidant Capacity of Common Beans (Phaseolus vulgaris) Grown under Water Deficit. PeerJ 2023, 11, 15343. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavareddy, P.; Lekshmy, S.V.; Struik, P.C.; Makarla, U.; Yin, X.; Sreeman, S. Production and Scavenging of Reactive Oxygen Species Confer to Differential Sensitivity of Rice and Wheat to Drought Stress. Crop. Environ. 2022, 1, 15–23. [Google Scholar] [CrossRef]
- Rady, M.M.; Boriek, S.H.K.; El-Mageed, T.A.A.; El-Yazal, M.A.S.; Ali, E.F.; Hassan, F.A.S.; Abdelkhalik, A. Exogenous Gibberellic Acid or Dilute Bee Honey Boosts Drought Stress Tolerance in Vicia faba by Rebalancing Osmoprotectants, Antioxidants, Nutrients, and Phytohormones. Plants 2021, 10, 748. [Google Scholar] [CrossRef]
- Snapp, S.; Morrone, V. Perennial Wheat; Montana State University Extension: Bozeman, MT, USA, 2014; pp. 1–3. [Google Scholar]
- Abdollahi, M.R.; Zaefarian, F.; Hunt, H.; Anwar, M.N.; Thomas, D.G.; Ravindran, V. Wheat Particle Size, Insoluble Fibre Sources and Whole Wheat Feeding Influence Gizzard Musculature and Nutrient Utilisation to Different Extents in Broiler Chickens. J. Anim. Physiol. Anim. Nutr. 2019, 103, 146–161. [Google Scholar] [CrossRef]
- Lachuga, Y.; Meskhi, B.; Pakhomov, V.; Semenikhina, Y.; Kambulov, S.; Rudoy, D.; Maltseva, T. Experience in the Cultivation of a New Perennial Cereal Crop Trititrigia in the Conditions of South of the Rostov Region. Agriculture 2023, 13, 605. [Google Scholar] [CrossRef]
- Tadesse, W.; Zegeye, H.; Debele, T.; Kassa, D.; Shiferaw, W.; Solomon, T.; Negash, T.; Geleta, N.; Bishaw, Z.; Assefa, S. Wheat production and breeding in Ethiopia: Retrospect and prospects. Crop Breed. Genet. Genom. 2022, 4, 3. [Google Scholar]
- Faostat Global Cereal Production Forecast Unchanged from Last Month, Utilization and Trade up, and Stocks down But Still Foreseen to Reach an All-Time High. 2023. Available online: https://uga.ua/en/news/global-cereal-production-forecast-unchanged-from-last-month-utilization-and-trade-up-and-stocks-down-but-still-foreseen-to-reach-an-all-time-high/ (accessed on 14 February 2024).
- Yang, D.; Li, S.; Kang, S.; Du, T.; Guo, P.; Mao, X.; Tong, L.; Hao, X.; Ding, R.; Niu, J. Effect of Drip Irrigation on Wheat Evapotranspiration, Soil Evaporation and Transpiration in Northwest China. Agric. Water Manag. 2020, 232, 106001. [Google Scholar] [CrossRef]
- Kavianand, G.; Nivas, V.M.; Kiruthika, R.; Lalitha, S. Smart Drip Irrigation System for Sustainable Agriculture. In Proceedings of the IEEE International Conference on Technological Innovations in ICT for Agriculture and Rural Development, TIAR 2016, Chennai, India, 15–16 July 2016; pp. 19–22. [Google Scholar] [CrossRef]
- Azad, N.; Behmanesh, J.; Rezaverdinejad, V.; Khodaverdiloo, H.; Thompson, S.E.; Mallants, D.; Ramos, T.B.; He, H. CNN Deep Learning Performance in Estimating Nitrate Uptake by Maize and Root Zone Losses under Surface Drip Irrigation. J. Hydrol. 2023, 625, 130148. [Google Scholar] [CrossRef]
- Illés, Á.; Szabó, A.; Mousavi, S.M.N.; Bojtor, C.; Vad, A.; Harsányi, E.; Sinka, L. The Influence of Precision Dripping Irrigation System on the Phenology and Yield Indices of Sweet Maize Hybrids. Water 2022, 14, 2480. [Google Scholar] [CrossRef]
- FAO. Chapitre 6: Drip Irrigation. 2012. Available online: https://www.fao.org/4/s8684e/s8684e07.htm (accessed on 14 February 2024).
- Tahiri, A.; Meddich, A.; Raklami, A.; Alahmad, A.; Bechtaoui, N.; Anli, M.; Göttfert, M.; Heulin, T.; Achouak, W.; Oufdou, K. Assessing the Potential Role of Compost, PGPR, and AMF in Improving Tomato Plant Growth, Yield, Fruit Quality, and Water Stress Tolerance. J. Soil Sci. Plant Nutr. 2021, 22, 743–764. [Google Scholar] [CrossRef]
- Latef, A.A.H.A.; Chaoxing, H. Arbuscular Mycorrhizal Influence on Growth, Photosynthetic Pigments, Osmotic Adjustment and Oxidative Stress in Tomato Plants Subjected to Low Temperature Stress. Acta Physiol. Plant 2011, 33, 1217–1225. [Google Scholar] [CrossRef]
- Boutaj, H.; Meddich, A.; Wahbi, S.; Moukhli, A.; El Alaoui-Talibi, Z.; Douira, A.; Filali-Maltouf, A.; El Modafar, C. Effect of Arbuscular Mycorrhizal Fungi on Verticillium Wilt Development of Olive Trees Caused by Verticillium dahliae. Res. J. Biotechnol. 2019, 14, 79–88. [Google Scholar]
- Zou, Y.N.; Wu, Q.S.; Kuča, K. Unravelling the Role of Arbuscular Mycorrhizal Fungi in Mitigating the Oxidative Burst of Plants under Drought Stress. Plant Biol. 2021, 23, 50–57. [Google Scholar] [CrossRef]
- Benaffari, W.; Boutasknit, A.; Anli, M.; Ait-El-mokhtar, M.; Ait-Rahou, Y.; Ben-Laouane, R.; Ahmed, H.; Mitsui, T.; Baslam, M.; Meddich, A. The Native Arbuscular Mycorrhizal Fungi and Vermicompost-Based Organic Amendments Enhance Soil Fertility, Growth Performance, and the Drought Stress Tolerance of Quinoa. Plants 2022, 11, 393. [Google Scholar] [CrossRef]
- Raklami, A.; Meddich, A.; Pajuelo, E.; Marschner, B.; Heinze, S.; Oufdou, K. Combined Application of Marble Waste and Beneficial Microorganisms: Toward a Cost Effective Approach for Restoration of Heavy Metals Contaminated Sites. Envirion. Sci. Pollut. Res. 2022, 29, 45683–45697. [Google Scholar] [CrossRef]
- Singh, V.K.; Malhi, G.S.; Kaur, M.; Singh, G.; Jatav, H.S. Use of Organic Soil Amendments for Improving Soil Ecosystem Health and Crop Productivity. In Ecosystem Services: Types, Management and Benefits; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2022. [Google Scholar]
- Ayangbenro, A.S.; Chukwuneme, C.F.; Ayilara, M.S.; Kutu, F.R.; Khantsi, M.; Adeleke, B.S.; Glick, B.R.; Babalola, O.O. Harnessing the Rhizosphere Soil Microbiome of Organically Amended Soil for Plant Productivity. Agronomy 2022, 12, 3179. [Google Scholar] [CrossRef]
- Meddich, A.; Oufdou, K.; Boutasknit, A.; Raklami, A.; Tahiri, A.; Ben-Laouane, R.; Ait-El-Mokhtar, M.; Anli, M.; Mitsui, T.; Wahbi, S.; et al. Use of Organic and Biological Fertilizers as Strategies to Improve Crop Biomass, Yields and Physicochemical Parameters of Soil. In Nutrient Dynamics for Sustainable Crop Production; Springer: Singapore, 2019; pp. 247–288. ISBN 9789811386602. [Google Scholar]
- Sawicka, B.; Pszczotkowski, P.; Barbas, P.; Skiba, D.; Bienia, B. Biotechnology: Role in Ecological Sustainability and Research; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Ikan, C.; Ben-Laouane, R.; Ouhaddou, R.; Ghoulam, C.; Meddich, A. Co-Inoculation of Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Can Mitigate the Effects of Drought in Wheat Plants (Triticum durum). Plant Biosyst. 2023, 157, 907–919. [Google Scholar]
- Arora, S.; Jha, P.N. Drought-Tolerant Enterobacter Bugandensis WRS7 Induces Systemic Tolerance in Triticum aestivum L. (Wheat) under Drought Conditions. J. Plant Growth Regul. 2023, 42, 7715–7730. [Google Scholar] [CrossRef]
- Mushtaq, Z. PGPR: Present Role, Mechanism of Action and Future Prospects along Bottlenecks in Commercialization. EQA-Int. J. Environ. Qual. 2020, 41, 9–15. [Google Scholar] [CrossRef]
- Chandwani, S.; Amaresan, N. Role of ACC Deaminase Producing Bacteria for Abiotic Stress Management and Sustainable Agriculture Production. Envirion. Sci. Pollut. Res. 2022, 29, 22843–22859. [Google Scholar] [CrossRef]
- Camaille, M.; Fabre, N.; Clément, C.; Barka, E.A. Advances in Wheat Physiology in Response to Drought and the Role of Plant Growth Promoting Rhizobacteria to Trigger Drought Tolerance. Microorganism 2021, 9, 687. [Google Scholar] [CrossRef]
- Allen, R.; Pereira, L.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements-FAO Irrigation and Drainage Paper 56; FAO: Rome, Italy, 1998. [Google Scholar]
- Anli, M.; Baslam, M.; Tahiri, A.; Raklami, A.; Symanczik, S.; Boutasknit, A.; Ait-El-Mokhtar, M.; Ben-Laouane, R.; Toubali, S.; Ait Rahou, Y.; et al. Biofertilizers as Strategies to Improve Photosynthetic Apparatus, Growth, and Drought Stress Tolerance in the Date Palm. Front. Plant Sci. 2020, 11, 516818. [Google Scholar] [CrossRef]
- Meddich, A.; Elouaqoudi, F.; Khadra, A.; Bourzik, W. Valorisation Des Déchets d’origine Végétale et Industrielle Par Compostage. Rev. Compos. Matériaux Avancées 2016, 26, 451–469. [Google Scholar] [CrossRef]
- Rouzbeh, R.; Daneshian, J.; Farahani, H.A. Super Nitro plus Influence on Yield and Yield Components of Two Wheat Cultivars under NPK Fertilizer Application. J. Plant Breed. Crop Sci. 2009, 1, 293–297. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN18. [Google Scholar] [CrossRef]
- Trouvelot, A. Measure du taux de mycorrhization d’un systeme radiculaire. Recherche de methods d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae, Proceedings of the 1st European Symposium on Mycorrhizae, Dijon, France, 1–5 July 1985; INRA: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Harley, P.C.; Loreto, F.; Di Marco, G.; Sharkey, T.D. Theoretical Considerations When Estimating the Mesophyll Conductance to CO2 Flux by Analysis of the Response of Photosynthesis to CO2. Plant Physiol. 1992, 98, 1429–1436. [Google Scholar] [CrossRef]
- Lichtenthaler, H. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding MARION. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Tejera García, A.; Olivera, M.; Iribarne, C.; Lluch, C. Partial Purification and Characterization of a Non-Specific Acid Phosphatase in Leaves and Root Nodules of Phaseolus vulgaris. Plant Physiol. Biochem. 2004, 42, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Polle, A.; Otter, T.; Seifert, F. Apoplastic Peroxidases and Lignification in Needles of Norway Spruce (Picea abies L.). Plant Physiol. 1994, 106, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P. Nitrogen Metabolism in Durum Wheat under Salinity: Accumulation of Proline and Glycine Betaine. Funct. Plant Biol. 2008, 35, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Wada, A.; Shibuta, T. Changes in Phenoloxidase Activities of the Galls on Leaves of Ulmus Davidana Formed by Tetraneura fusiformis (Homoptera: Eriosomatidae). Appl. Entomol. Zool. 1997, 32, 365–371. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Matowe, W. Drought Tolerance in Two Mosses: Correlated with Enzymatic Defence Against Lipid Peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants Protective Role of Exogenous Polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Aubert, G. Méthodes D’analyses des Sols, 2nd ed.; Methods of Soil Analysis; Centre Régional de Documentation Pédagogique: Marseille, France, 1978; p. 191. [Google Scholar]
- Bremner, J.M. Determination of Nitrogen in Soil by the Kjeldahl Method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Cornejo, P.; Meier, S.; Borie, G.; Rillig, M.C.; Borie, F. Glomalin-Related Soil Protein in a Mediterranean Ecosystem Affected by a Copper Smelter and Its Contribution to Cu and Zn Sequestration. Sci. Total Environ. 2008, 406, 154–160. [Google Scholar] [CrossRef]
- Kour, D.; Yadav, A.N. Bacterial Mitigation of Drought Stress in Plants: Current Perspectives and Future Challenges. Curr. Microbiol. 2022, 79, 248. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Lu, X.; Gu, S.; Guo, X. Improving Nutrient and Water Use Efficiencies Using Water-Drip Irrigation and Fertilization Technology in Northeast China. Agric. Water Manag. 2020, 241, 106352. [Google Scholar] [CrossRef]
- Yang, D.; Li, S.; Wu, M.; Yang, H.; Zhang, W.; Chen, J.; Wang, C.; Huang, S.; Zhang, R.; Zhang, Y. Drip Irrigation Improves Spring Wheat Water Productivity by Reducing Leaf Area While Increasing Yield. Eur. J. Agron. 2023, 143, 126710. [Google Scholar] [CrossRef]
- Ben-Laouane, R.; Baslam, M.; Ait-El-mokhtar, M.; Anli, M.; Boutasknit, A.; Ait-Rahou, Y.; Toubali, S.; Mitsui, T.; Oufdou, K.; Wahbi, S.; et al. Potential of Native Arbuscular Mycorrhizal Fungi, Rhizobia, and/or Green Compost as Alfalfa (Medicago sativa) Enhancers under Salinity. Microorganisms 2020, 8, 1695. [Google Scholar] [CrossRef]
- Boutasknit, A.; Baslam, M.; Ait-El-mokhtar, M.; Anli, M.; Ben-Laouane, R.; Douira, A.; El Modafar, C.; Mitsui, T.; Wahbi, S.; Meddich, A. Arbuscular Mycorrhizal Fungi Mediate Drought Tolerance and Recovery in Two Contrasting Carob (Ceratonia siliqua L.) Ecotypes by Regulating Stomatal, Water Relations, and (in)Organic Adjustments. Plants 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Hu, Y.; Zhang, K.; Tian, C.; Guo, J. Arbuscular Mycorrhizal Fungi Improve Plant Growth of Ricinus communis by Altering Photosynthetic Properties and Increasing Pigments under Drought and Salt Stress. Ind. Crops Prod. 2018, 117, 13–19. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Arbuscular Mycorrhizas Modulate Root Polyamine Metabolism to Enhance Drought Tolerance of Trifoliate Orange. Environ. Exp. Bot. 2020, 171, 103926. [Google Scholar] [CrossRef]
- He, J.D.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhizas Enhance Drought Tolerance of Trifoliate Orange by Enhancing Activities and Gene Expression of Antioxidant Enzymes. Sci. Hortic. 2020, 262, 108745. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-lozano, J.M. Enhanced Drought Stress Tolerance by the Arbuscular Mycorrhizal Symbiosis in a Drought-Sensitive Maize Cultivar Is Related to a Broader and Differential Regulation of Host Plant Aquaporins than in a Drought-Tolerant Cultivar. Front. Plant Sci. 2017, 8, 268043. [Google Scholar] [CrossRef]
- Shah, Z.H.; Rehman, H.M.; Akhtar, T.; Daur, I.; Nawaz, M.A.; Ahmad, M.Q.; Rana, I.A.; Atif, R.M. Redox and Ionic Homeostasis Regulations against Oxidative, Salinity and Drought Stress in Wheat (A Systems Biology Approach). Front. Genet. 2017, 8, 141. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Singh, A.K.; Ansari, M.I. Effect of Drought Stress on Crop Production. In New Frontiers in Stress Management for Durable Agriculture; Springer: Singapore, 2020; pp. 35–47. [Google Scholar]
- Farooq, M.; Aziz, T.; Wahid, A.; Lee, D.J.; Siddique, K.H. Chilling Tolerance in Maize: Agronomic and Physiological Approaches. Crop. Pasture Sci. 2009, 60, 501–516. [Google Scholar] [CrossRef]
- Sakran, R.; Ghazy, M.; Rehan, M.; Plants, A.A. Molecular Genetic Diversity and Combining Ability for Some Physiological and Agronomic Traits in Rice under Well-Watered and Water-Deficit Conditions. Plants 2022, 11, 702. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh, G.; Khanna, V. Growth of Lentil (Lens Culinaris Medikus) as Influenced by Phosphorus, Rhizobium and Plant Growth Promoting Rhizobacteria. Indian J. Agric. Res. 2016, 50, 567–572. [Google Scholar] [CrossRef]
- Darakeh, S.A.S.S.; Weisany, W.; Tahir, N.A.R.; Schenk, P.M. Physiological and Biochemical Responses of Black Cumin to Vermicompost and Plant Biostimulants: Arbuscular Mycorrhizal and Plant Growth-Promoting Rhizobacteria. Ind. Crops Prod. 2022, 188, 115557. [Google Scholar] [CrossRef]
- Niaz, H.; Mushtaq, Z.; Jaffar, M.; Zhang, J.; Asghar, H. Mycorrhizae-Rhizobacterial Interaction to Enhance the Growth, Development, Nutrient Content, and Physiological Attributes of Okra in Compost Amended Soil. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Bhardwaj, D.; Ansari, M.W.; Sahoo, R.K.; Tuteja, N. Biofertilizers Function as Key Player in Sustainable Agriculture by Improving Soil Fertility, Plant Tolerance and Crop Productivity. Microb. Cell Fact. 2014, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Azemi-Ardakani, M.; Dehestani-Ardakani, M.; Zarei, A.; Soltani-Gerdfaramarzi, S. Influence of Different Soil Amendments on Drought Stress Tolerance of Maclura Pomifera. Plant Physiol. Rep. 2020, 25, 405–417. [Google Scholar] [CrossRef]
- Furlan, A.; Bianucci, E.; Sequeira, M.; Álvarez, L.; Peralta, J.M.; Valente, C.; Guarnieri, V.; Castro, S. Combined Application of Microbial and Non-Microbial Biostimulants to Improve Growth of Peanut Plants Exposed to Abiotic Stresses. In Microbial Probiotics for Agricultural Systems; Springer: Cham, Switzerland, 2019; pp. 239–256. [Google Scholar] [CrossRef]
- Radzikowska-Kujawska, D.; John, P.; Piechota, T.; Nowicki, M.; Kowalczewski, P.Ł. Response of Winter Wheat (Triticum aestivum L.) to Selected Biostimulants under Drought Conditions. Agriculture 2023, 13, 121. [Google Scholar] [CrossRef]
- Raklami, A.; Tahiri, A.; Bechtaoui, N.; Abdelhay, E.G.; Pajuelo, E.; Baslam, M.; Meddich, A.; Oufdou, K. Restoring the Plant Productivity of Heavy Metal-Contaminated Soil Using Phosphate Sludge, Marble Waste, and Beneficial Microorganisms. J. Envirion. Sci. 2021, 99, 210–221. [Google Scholar] [CrossRef]
- Yang, Y.; Han, X.; Liang, Y.; Ghosh, A.; Chen, J.; Tang, M. The Combined Effects of Arbuscular Mycorrhizal Fungi (AMF) and Lead (Pb) Stress on Pb Accumulation, Plant Growth Parameters, Photosynthesis, and Antioxidant Enzymes in Robinia Pseudoacacia L. PLoS ONE 2015, 10, e0145726. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, F.; Zhang, D.; Srivastava, A.K.; Wu, Q. Mycorrhiza Stimulates Root-Hair Growth and IAA Synthesis and Transport in Trifoliate Orange under Drought Stress. Sci. Rep. 2018, 8, 1978. [Google Scholar] [CrossRef]
- Le Pioufle, O.; Ganoudi, M.; Calonne-salmon, M.; Dhaou, F. Ben Rhizophagus irregularis MUCL 41833 Improves Phosphorus Uptake and Water Use Efficiency in Maize Plants During Recovery from Drought Stress. Front. Plant. Sci. 2019, 10, 459006. [Google Scholar] [CrossRef]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q. Mechanistic Insights into Arbuscular Mycorrhizal Fungi-Mediated Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef]
- Bashir, S.S.; Hussain, A.; Hussain, S.J.; Ali, O.; Nabi, S.Z.; Dar, N.A.; Baloch, F.S. Plant Drought Stress Tolerance: Understanding Its Physiological, Biochemical and Molecular Mechanisms. Biotechnol. Biotechnol. Equip. 2022, 35, 1912–1925. [Google Scholar] [CrossRef]
- Baccari, S.; Elloumi, O.; Chaari-Rkhis, A.; Fenollosa, E.; Morales, M.; Drira, N.; Ben Abdallah, F.; Fki, L.; Munné-Bosch, S. Linking Leaf Water Potential, Photosynthesis and Chlorophyll Loss with Mechanisms of Photo- and Antioxidant Protection in Juvenile Olive Trees Subjected to Severe Drought. Front. Plant Sci. 2020, 11, 614144. [Google Scholar] [CrossRef] [PubMed]
- Raheleh, K.; Ghassemi, S.; Asghari, B. Bio-Fertilizer Improves Physio-Biochemical Characteristics and Grain Yield of Safflower (Carthamus tinctorius L.) under Drought Stress. Russ. Agric. Sci. 2019, 45, 458–463. [Google Scholar] [CrossRef]
- Baslam, M.; Mitsui, T.; Hodges, M.; Priesack, E.; Herritt, M.T.; Aranjuelo, I.; Sanz-Sáez, Á. Photosynthesis in a Changing Global Climate: Scaling Up and Scaling Down in Crops. Front. Plant Sci. 2020, 11, 00882. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Gaju, O.; Bowerman, A.F.; Buck, S.A.; Evans, J.R.; Furbank, R.T.; Gilliham, M.; Millar, A.H.; Pogson, B.J.; Reynolds, M.P.; et al. Enhancing crop yields through improvements in the efficiency of photosynthesis and respiration. New Phytol. 2023, 237, 60–77. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, Á.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular Mycorrhizal Symbiosis Induces Strigolactone Biosynthesis under Drought and Improves Drought Tolerance in Lettuce and Tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular Mycorrhizal Symbiosis Alters Stomatal Conductance of Host Plants More under Drought than under Amply Watered Conditions: A Meta-Analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef]
- Boutasknit, A.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Ben-Laouane, R.; Ait-Rahou, Y.; Mitsui, T.; Douira, A.; El Modafar, C.; Wahbi, S.; et al. Assemblage of Indigenous Arbuscular Mycorrhizal Fungi and Green Waste Compost Enhance Drought Stress Tolerance in Carob (Ceratonia siliqua L.) Trees. Sci. Rep. 2021, 11, 22835. [Google Scholar] [CrossRef]
- Meddich, A.; Jaiti, F.; Bourzik, W.; El Asli, A.; Hafidi, M. Use of Mycorrhizal Fungi as a Strategy for Improving the Drought Tolerance in Date Palm (Phoenix dactylifera). Sci. Hortic. 2015, 192, 468–474. [Google Scholar] [CrossRef]
- Miransari, M.; Abrishamchi, A.; Khoshbakht, K.; Niknam, V. Plant Hormones as Signals in Arbuscular Mycorrhizal Symbiosis. Crit. Rev. Biotechnol. 2012, 34, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the Impact of Arbuscular Mycorrhizal Symbiosis on Tomato Tolerance to Water Stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef]
- Ouledali, S.; Ennajeh, M.; Ferrandino, A.; Khemira, H.; Schubert, A.; Secchi, F. Influence of Arbuscular Mycorrhizal Fungi Inoculation on the Control of Stomata Functioning by Abscisic Acid (ABA) in Drought-Stressed Olive Plants. S. Afr. J. Bot. 2019, 121, 152–158. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of Arbuscular Mycorrhizae on Photosynthesis and Water Status of Maize Plants under Salt Stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Ferrández, T.; Morales, M.A.; Morte, A.; Alarcón, J.J. Variations in Water Status, Gas Exchange, and Growth in Rosmarinus Officinalis Plants Infected with Glomus Deserticola under Drought Conditions. J. Plant Physiol. 2004, 161, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Yooyongwech, S.; Samphumphuang, T.; Tisarum, R.; Theerawitaya, C.; Cha-Um, S. Arbuscular Mycorrhizal Fungi (AMF) Improved Water Deficit Tolerance in Two Different Sweet Potato Genotypes Involves Osmotic Adjustments via Soluble Sugar and Free Proline. Sci. Hortic. 2016, 198, 107–117. [Google Scholar] [CrossRef]
- Rahbarian, R.; Khavari-Nejad, R.; Ganjeali, A.; Bagheri, A.; Najafi, F. Drought Stress Effects on Photosynthesis, Chlorophyll Fluorescence and Water Relations in Tolerant and Susceptible Chickpea (Cicer arietinum L.) Genotypes. Acta Biol. Cracoviensia Ser. Bot. 2011, 53, 47–56. [Google Scholar] [CrossRef]
- Meddich, A.; Ait Rahou, Y.; Boutasknit, A.; Ait-El-Mokhtar, M.; Fakhech, A.; Lahbouki, S.; Benaffari, W.; Ben-Laouane, R.; Wahbi, S. Role of Mycorrhizal Fungi in Improving the Tolerance of Melon (Cucumus melo) under Two Water Deficit Partial Root Drying and Regulated Deficit Irrigation. Plant Biosyst. 2022, 156, 469–479. [Google Scholar] [CrossRef]
- Saxena, R.; Kumar, M.; Tomar, R.S. Plant Responses and Resilience towards Drought and Salinity Stress. Plant Arch. 2019, 19, 50–58. [Google Scholar]
- Ahanger, M.A.; Hashem, A.; Abd-Allah, E.F.; Ahmad, P. Arbuscular Mycorrhiza in Crop Improvement under Environmental Stress. Emerg. Technol. Manag. Crop Stress Toler. 2014, 2, 69–95. [Google Scholar] [CrossRef]
- Pasbani, B.; Salimi, A.; Aliasgharzad, N.; Hajiboland, R. Scientia Horticulturae Colonization with Arbuscular Mycorrhizal Fungi Mitigates Cold Stress through Improvement of Antioxidant Defense and Accumulation of Protecting Molecules in Eggplants. Sci. Hortic. 2020, 272, 109575. [Google Scholar] [CrossRef]
- Begum, N.; Wang, L.; Ahmad, H.; Akhtar, K.; Roy, R.; Khan, M.I.; Zhao, T. Co-Inoculation of Arbuscular Mycorrhizal Fungi and the Plant Growth-Promoting Rhizobacteria Improve Growth and Photosynthesis in Tobacco Under Drought Stress by Up-Regulating Antioxidant and Mineral Nutrition Metabolism. Microb. Ecol. 2022, 83, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Ma, X.; Shah, S.; Wu, X.; Shaheen, A.; Xiao, L.; Wu, Y.; Wang, S. Drought-Hardening Improves Drought Tolerance in Nicotiana Tabacum at Physiological, Biochemical, and Molecular Levels. BMC Plant Biol. 2020, 20, 486. [Google Scholar] [CrossRef] [PubMed]
- Abobatta, W.F. Plant Responses and Tolerance to Combined Salt and Drought Stress. In Salt and Drought Stress Tolerance in Plants: Signaling Networks and Adaptive Mechanisms; Springer: Cham, Switzerland, 2020; pp. 17–52. [Google Scholar] [CrossRef]
- Ali, N.; Akmal, M. Wheat Growth, Yield, and Quality under Water Deficit and Reduced Nitrogen Supply. A Review. Gesunde Pflanz. 2022, 74, 371–383. [Google Scholar] [CrossRef]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Rizwan, M.S.; Hussain, M.; Jabran, K.; Cheema, M.A. Terminal Drought and Heat Stress Alter Physiological and Biochemical Attributes in Flag Leaf of Bread Wheat. PLoS ONE 2020, 15, e0232974. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Song, F.; Liu, S.; Liu, F. Role of Arbuscular Mycorrhiza in Alleviating Salinity Stress in Wheat (Triticum aestivum L.) Grown under Ambient and Elevated CO2. J. Agron. Crop. Sci. 2016, 202, 486–496. [Google Scholar] [CrossRef]
- Ouhaddou, R.; Ben-Laouane, R.; Lahlali, R.; Anli, M.; Ikan, C.; Boutasknit, A.; Slimani, A.; Oufdou, K.; Baslam, M.; Ait Barka, E.; et al. Application of Indigenous Rhizospheric Microorganisms and Local Compost as Enhancers of Lettuce Growth, Development, and Salt Stress Tolerance. Microorganisms 2022, 10, 1625. [Google Scholar] [CrossRef]
- Santander, C.; Aroca, R.; Cartes, P.; Vidal, G.; Cornejo, P. Aquaporins and Cation Transporters Are Differentially Regulated by Two Arbuscular Mycorrhizal Fungi Strains in Lettuce Cultivars Growing under Salinity Conditions. Plant Physiol. Biochem. 2021, 158, 396–409. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.; Botany, L. AMF Inoculation and Phosphorus Supplementation Alleviates Drought Induced Growth and Photosynthetic Decline in Nicotiana Tabacum by Up-Regulating antioxidant metabolism and osmolyte accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Wang, H.; Tang, X.; Wang, H.; Shao, H.-B. Proline Accumulation and Metabolism-Related Genes Expression Profiles in Kosteletzkya Virginica Seedlings under Salt Stress. Front. Plant Sci. 2015, 6, 792. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Asthir, B. Proline: A Key Player in Plant Abiotic Stress Tolerance. Biol. Plant 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Ullah, A.; Farooq, M. The Challenge of Drought Stress for Grain Legumes and Options for Improvement. Arch. Agron. Soil Sci. 2022, 68, 1601–1618. [Google Scholar] [CrossRef]
- Ojuederie, O.; Olanrewaju, O.; Babalola, O. Plant Growth Promoting Rhizobacterial Mitigation of Drought Stress in Crop Plants: Implications for Sustainable Agriculture. Agronomy 2019, 9, 712. [Google Scholar] [CrossRef]
- Gupta, A.; Bano, A.; Rai, S.; Mishra, R.; Singh, M.; Stress, S.S.-P. Mechanistic Insights of Plant-Microbe Interaction towards Drought and Salinity Stress in Plants for Enhancing the Agriculture Productivity. Plant Stress 2022, 4, 100073. [Google Scholar] [CrossRef]
- Ali, S.; Moon, Y.; Hamayun, M.; Khan, M.A.; Bibi, K.; Lee, I.; Ali, S.; Moon, Y.; Hamayun, M.; Aaqil, M. Pragmatic Role of Microbial Plant Biostimulants in Abiotic Stress Relief in Crop Plants. J. Plant Interact. 2022, 17, 705–718. [Google Scholar] [CrossRef]
- Das, D.; Bisht, K.; Chauhan, A.; Gautam, S.; Jaiswal, J.P.; Salvi, P.; Lohani, P. Morpho-Physiological and Biochemical Responses in Wheat Foliar Sprayed with Zinc-Chitosan-Salicylic Acid Nanoparticles during Drought Stress. Plant Nano Biol. 2023, 4, 100034. [Google Scholar] [CrossRef]
- Gil-Ortiz, R.; Naranjo, M.Á.; Atares, S.; Vicente, O. Antioxidant Responses of Water-Stressed Cherry Tomato Plants to Natural Biostimulants. Agronomy 2023, 13, 2314. [Google Scholar] [CrossRef]
- Habib, N.; Ali, Q.; Ali, S.; Javed, M.T.; Haider, M.Z.; Perveen, R.; Shahid, M.R.; Rizwan, M.; Abdel-daim, M.M.; Elkelish, A. Use of Nitric Oxide and Hydrogen Peroxide for Better yield of wheat (Triticum aestivum L.) under water deficit conditions: Growth, osmoregulation, and antioxidative defense mechanism. Plants 2020, 9, 285. [Google Scholar] [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef] [PubMed]
- Ouhaddou, R.; Ech-chatir, L.; Anli, M.; Ben-Laouane, R.; Boutasknit, A.; Meddich, A. Secondary Metabolites, Osmolytes and Antioxidant Activity as the Main Attributes Enhanced by Biostimulants for Growth and Resilience of Lettuce to Drought Stress. Gesunde Pflanz. 2023, 75, 1737–1753. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I. Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S. Impact of PGPR Inoculation on Growth and Antioxidant Status of Wheat under Saline Conditions. Plant Biol. 2012, 14, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Yasmeen, T.; Ali, Q.; Ali, S.; Saleem, M.; Hussain, S.; Rizvi, H. Ecotoxicology and Environmental Safety In Fl Uence of Pseudomonas Aeruginosa as PGPR on Oxidative Stress Tolerance in Wheat under Zn Stress. Ecotoxicol. Environ. Saf. 2014, 104, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Kong, C.; Yan, K.; Xie, Z. Transcriptome Analysis Reveals the Impact of Arbuscular Mycorrhizal Symbiosis on Sesbania Cannabina Expose to High Salinity. Sci. Rep. 2019, 9, 2780. [Google Scholar] [CrossRef]
- Sun, D.; Li, K.; Bi, Q.; Zhu, J.; Zhang, Q.; Jin, C.; Lingli, L.; Xianyong, L. Effects of Organic Amendment on Soil Aggregation and Microbial Community Composition during Drying-Rewetting Alternation. Sci. Total Environ. 2017, 574, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Gaiotti, F.; Marcuzzo, P.; Belfiore, N.; Lovat, L.; Fornasier, F.; Tomasi, D. Influence of Compost Addition on Soil Properties, Root Growth and Vine Performances of Vitis vinifera Cv Cabernet Sauvignon. Sci. Hortic. 2017, 225, 88–95. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of Arbuscular Mycorrhizal Fungi, Phosphate–Solubilizing Bacteria, and Silicon to P Uptake by Plant. Front. Plant Sci. 2021, 12, 699618. [Google Scholar] [CrossRef]
- Iftikhar, A.; Farooq, R.; Akhtar, M.; Khalid, H.; Hussain, N.; Ali, Q. Ecological and Sustainable Implications of Phosphorous—Solubilizing Microorganisms in Soil. Discov. Appl. Sci. 2024, 6, 33. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Zhang, Q.; Li, S.; Sun, Y.; Lu, W.; Ma, C. Response of Alfalfa Growth to Arbuscular Mycorrhizal Fungi and Phosphate-Solubilizing Bacteria under Different Phosphorus Application Levels. AMB Express 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Charagh, S.; Abbas, S.; Hassan, M.U.; Saeed, F.; Haider, S.; Sharif, R.; Anand, A.; Corpas, F.J.; Jin, W.; et al. Assessment of Proline Function in Higher Plants under Extreme Temperatures. Plant Biol. 2023, 25, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Q.; Wang, H.; Nie, S.; Liang, Z. Effects of Soil Salinity on the Content, Composition, and Ion Binding Capacity of Glomalin-Related Soil Protein (GRSP). Sci. Total Environ. 2017, 581–582, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiong, J.; Fang, L.; Han, F.; Zhao, X.; Fan, Q.; Tan, W. Sequestration of Heavy Metals in Soil Aggregates Induced by Glomalin-Related Soil Protein: A Five-Year Phytoremediation Field Study. J. Hazard. Mater. 2022, 437, 129445. [Google Scholar] [CrossRef]
- González-Chávez, M.C.; Carrillo-González, R.; Wright, S.F.; Nichols, K.A. The Role of Glomalin, a Protein Produced by Arbuscular Mycorrhizal Fungi, in Sequestering Potentially Toxic Elements. Environ. Pollut. 2004, 130, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Kohler, J.; Caravaca, F.; Roldán, A. An AM Fungus and a PGPR Intensify the Adverse Effects of Salinity on the Stability of Rhizosphere Soil Aggregates of Lactuca Sativa. Soil Biol. Biochem. 2010, 42, 429–434. [Google Scholar] [CrossRef]
- Morris, E.K.; Morris, D.J.P.; Vogt, S.; Gleber, S.C.; Bigalke, M.; Wilcke, W.; Rillig, M.C. Visualizing the Dynamics of Soil Aggregation as Affected by Arbuscular Mycorrhizal Fungi. ISME J. 2019, 13, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.A.; Joo, J.H. Plant Growth-Promoting Rhizobacteria Improved Salinity Tolerance of Lactuca sativa and Raphanus sativus. J. Microbiol. Biotechnol. 2018, 28, 938–945. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of Salinity Stress on Plants and Its Tolerance Strategies: A Review. Environ. Pollut. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Song, Q.; Song, X.; Deng, X.; Luo, J.; Wang, J.; Min, K.; Song, R. Effects of Plant Growth Promoting Rhizobacteria Microbial on the Growth, Rhizosphere Soil Properties, and Bacterial Community of Pinus sylvestris Var. Mongolica Seedlings. Scand. J. For. Res. 2021, 36, 249–262. [Google Scholar] [CrossRef]
- Cabrera-Bosquet, L.; Molero, G.; Stellacci, A.; Bort, J.; Nogués, S.; Araus, J. NDVI as a Potential Tool for Predicting Biomass, Plant Nitrogen Content and Growth in Wheat Genotypes Subjected to Different Water and Nitrogen Conditions. Cereal Res. Commun. 2011, 39, 147–159. [Google Scholar] [CrossRef]
- Ahmad, N.; Javed, A.; Gohar, S.; Ahmed, J.; Sher, A.; Abdullah, M.; Asghar, S.; Javed, K.; Iqbal, J.; Kumar, S.; et al. Estimation of Drought Effects on Different Bread Wheat Genotypes Using Morpho-Physiological Traits. Biochem. Syst. Ecol. 2022, 104, 104483. [Google Scholar] [CrossRef]
- Krupa, M.; Witkowicz, R. Biostimulants as a Response to the Negative Impact of Agricultural Chemicals on Vegetation Indices and Yield of Common Buckwheat (Fagopyrum esculentum Moench). Agriculture 2023, 13, 825. [Google Scholar] [CrossRef]
- Chatterjee, S.; Desai, A.R.; Zhu, J.; Townsend, P.A.; Huang, J. Soil Moisture as an Essential Component for Delineating and Forecasting Agricultural Rather than Meteorological Drought. Remote Sens. Environ. 2022, 269, 112833. [Google Scholar] [CrossRef]
- Bhatt, R.; Kukal, S.S. Soil Moisture Dynamics during Intervening Period in Rice-Wheat Sequence as Affected by Different Tillage Methods at Ludhiana, Punjab, India. Soil Environ. 2015, 34, 82–88. [Google Scholar]
- Li, Q.; Dong, B.; Qiao, Y.; Liu, M.; Zhang, J. Root Growth, Available Soil Water, and Water-Use Efficiency of Winter Wheat under Different Irrigation Regimes Applied at Different Growth Stages in North China. Agric. Water Manag. 2010, 97, 1676–1682. [Google Scholar] [CrossRef]



| Water Regime | Treatments | Spikelet Height (cm) | Shoot Height (cm) | Root Length (cm) | Seeds Number | Spikelet Dry Weight (g) |
|---|---|---|---|---|---|---|
| 100% ETc | Ct− | 13.50 ± 0.50 c–g | 63.17 ±4.36 a–d | 16.50 ± 0.50 a–e | 17.00 ± 1.00 d–g | 1.32 ± 0.07 d–g |
| Ct+ | 14.83 ± 0.76 b–f | 64.33 ± 6.06 a–d | 16.67 ± 1.04 a–e | 22.00 ± 4.36 a–c | 1.40 ± 0.04 c–f | |
| M | 16.50 ± 0.50 ab | 71.00 ± 9.57 ab | 18.33 ± 1.53 a–c | 25.00 ± 4.00 a–c | 1.33 ± 0.12 d–g | |
| R | 15.00 ± 1.00 e–h | 51.67 ± 7.06 a | 19.17 ± 0.76 a–e | 18.67 ± 2.31 c–g | 1.38 ± 0.01 c–g | |
| C | 15.83 ± 0.76 a–d | 61.83 ± 5.53 a–d | 16.83 ± 1.04 ab | 21.00 ± 2.65 b–d | 1.81 ± 0.25 ab | |
| MR | 17.83 ± 0.76 a | 70.50 ± 6.50 a–c | 19.33 ± 1.53 a | 28.00 ± 2.65 a | 1.86 ± 0.21 a | |
| CR | 17.00 ± 1.00 ab | 58.33 ±10.65 b–d | 17.33 ± 1.26 a–d | 19.00 ± 1.00 c–f | 1.68 ± 0.05 a–c | |
| CM | 17.17 ± 0.29 ab | 75.67 ±7.89 a | 17.83 ± 0.76 a–d | 28.33 ± 0.58 a | 1.44 ± 0.02 c–e | |
| CMR | 16.17 ± 1.26 a–c | 67.17 ±6.24 a–d | 18.83 ± 0.76 ab | 25.67 ± 2.52 ab | 1.61 ± 0.11 a–d | |
| 30% ETc | Ct− | 11.50 ± 0.50 e | 55.83 ± 7.17 b–d | 14.67 ± 2.08 c–e | 12.33 ± 0.58 g | 1.23 ± 0.04 e–g |
| Ct+ | 12.33 ± 0.58 gh | 54.17 ± 6.77 cd | 15.00 ± 1.32 b–e | 13.33 ± 0.58 fg | 1.28 ± 0.08 e–g | |
| M | 12.50 ± 1.32 e–g | 58.17 ± 7.91 b–d | 15.83 ± 1.61 a–e | 17.33 ± 1.53 d–g | 1.09 ± 0.04 g | |
| R | 12.33 ± 0.58 cd | 55.17 ± 6.65 d | 14.83 ± 0.76 c–e | 17.33 ± 2.52 d–g | 1.29 ± 0.07 e–g | |
| C | 12.17 ± 1.04 f–h | 53.00 ± 7.27 d | 14.17 ± 1.04 d–e | 16.67 ± 0.58 d–g | 1.12 ± 0.09 fg | |
| MR | 13.67 ± 1.53 c–g | 55.00 ±6.54 b–d | 14.50 ± 1.32 c–e | 22.67 ± 1.53 a–d | 1.39 ± 0.01 c–f | |
| CR | 9.83 ± 0.29 h | 55.67 ± 5.43 b–d | 13.17 ± 1.26 e | 13.33 ± 0.58 fg | 1.42 ± 0.04 c–e | |
| CM | 12.67 ± 1.15 e–g | 62.67 ± 5.54 a–d | 14.00 ± 2.00 de | 18.00 ± 1.73 d–g | 1.36 ± 0.03 d–g | |
| CMR | 13.17 ± 0.76 d–g | 57.17 ± 16.80 b–d | 15.17 ± 0.76 b–e | 15.67 ± 1.53 e–g | 1.52 ± 0.04 b–e |
| Regime of Water | Treatment Type | Protein Content (mg·g−1 FM) | Total Soluble Sugars (mg·g−1 FM) | Proline Content (µmol·g−1 FM) | PPO (µmol·min−1·mg−1 Protein) | POX (µmol·min−1·mg−1·Protein) |
|---|---|---|---|---|---|---|
| 100% ETc | Ct− | 65.6 ± 0.13 de | 206.4 ± 18.39 a | 30.5 ± 1.94 d–f | 73.6 ± 13.41 de | 23.1 ± 1.35 c |
| Ct+ | 66.9 ± 17.09 c–e | 168.64 ± 2.18 c | 57.0 ± 2.68 bc | 79.2 ± 13.11 b–e | 26.7 ± 6.43 a–c | |
| M | 87.3 ± 18.61 b–d | 88.89 ± 3.40 fg | 36.2 ± 4.02 de | 73.2 ± 2.07 de | 19.4 ± 0.34 c | |
| R | 74.3 ± 1.93 b–e | 98.4 ± 4.65 d–f | 37.2 ± 3.97 de | 73.7 ± 7.07 de | 32.5 ± 5.94 a–c | |
| C | 110.2 ± 7.58 ab | 117.7 ± 14.66 e–g | 24.8 ± 4.63 e–h | 65.3 ± 1.70 e | 31.2 ± 6.87 a–c | |
| MR | 40.3 ± 4.75 e | 81.7 ± 5.38 e–g | 19.4 ± 2.50 f–h | 69.2 ± 6.49 de | 21.2 ± 1.91 c | |
| CR | 71.4 ± 11.03 b–e | 97.9 ± 2.71 g | 13.6 ± 1.35 h | 75.7 ± 11.91 ce | 22.9 ± 6.94 c | |
| CM | 70.1 ± 6.89 b–e | 111.9 ± 4.66 e–g | 27.9 ± 2.81 d–h | 85.0 ± 7.04 a–e | 24.3 ± 1.9 bc | |
| CMR | 129.5 ± 8.29 a | 91.8 ± 3.34 e–g | 14.9 ± 0.75 gh | 77.2 ± 5.02 c–e | 28.5 ± 4.52 a–c | |
| 30% ETc | Ct− | 109.2 ± 6.38 ab | 289.8 ± 13.37 a | 74.8 ± 16.87 a | 88.4 ± 10.68 a–e | 33.5 ± 6.80 a–c |
| Ct+ | 97.0 ± 6.38 a–d | 200.6 ± 16.66 b | 73.1 ± 0.57 bc | 94.7 ± 1.95 a–d | 34.9 ± 1.27 a–c | |
| M | 99.9 ± 4.40 a–d | 144.1 ± 1.28 cd | 42.6 ± 2.00 cd | 107.9 ± 10.20 a | 33.6 ± 4.74 ab | |
| R | 107.8 ± 9.78 a–c | 147.2 ± 2.31 cd | 40.7 ± 0.47 d | 82.6 ± 6.90 cd | 39.4 ± 1.14 ab | |
| C | 95.5 ± 19.05 a–d | 167.1 ± 9.76 c | 37.4 ± 1.88 de | 82.6 ± 11.95 a | 38.9 ± 3.93 a–c | |
| MR | 130.5 ± 9.03 a | 110.9 ± 3.60 c | 32.0 ± 8.18 d–f | 107.3 ± 4.47 de | 41.0 ± 1.05 a | |
| CR | 99.6 ± 9.64 a–d | 120.5 ± 14.51 e–g | 29.0 ± 0.88 d–g | 100.9 ± 6.36 a–c | 39.7 ±3.92 ab | |
| CM | 93.8 ± 11.30 a–d | 155.6 ± 16.12 de | 63.9 ± 3.05 ab | 92.8 ± 4.47 a–d | 29.9 ± 1.38 a–c | |
| CMR | 81.3 ± 9.69 b–e | 105.3 ± 7.06 e–g | 33.2 ± 0.43 d–f | 103.0 ± 9.47 ab | 34.6 ± 2.67 a–c |
| Water Regime | Treatments | AP (mg·kg−1) | NTK (%) | EC (mS·cm−1) | pH | TOM (%) | TOC (%) | T-GRSP (mg/g) |
|---|---|---|---|---|---|---|---|---|
| 100% ETc | Ct− | 5.42 ± 0.96 fg | 0.16 ± 0.01 ab | 0.2 ± 0.01 f | 8.1 ± 0.54 a–c | 1.5 ± 0.25 ab | 0.9 ± 0.15 ab | 1.20 ± 0.02 b–d |
| Ct+ | 17.59 ± 2.97 bc | 0.17 ± 0.04 ab | 0.2 ± 0.01 c–f | 8.2 ± 0.44 a–c | 0.7 ± 0.51 ab | 0.4 ± 0.3 ab | 1.2 0± 0.17 b–d | |
| M | 6.45 ± 0.15 e–g | 0.19 ± 0.06 a | 0.2 ± 0.01 b–d | 8.3 ± 0.24 ab | 0.8 ± 0.08 a–e | 0.5 ± 0.05 a–e | 1.10 ± 0.12 b–d | |
| R | 4.08 ± 0.20 fg | 0.17 ± 0.01 ab | 0.2 ± 0.01 b | 8.4 ± 0.20 ab | 1.4 ± 0.34 a–c | 0.8 ± 0.2 a–c | 1.10 ± 0.23 b–d | |
| C | 13.67 ± 7.82 c–f | 0.18 ± 0.03 ab | 0.2 ± 0.00 d–f | 8.4 ± 0.03 ab | 1.6 ± 0.17 a | 0.9 ± 0.1 a | 1.00 ± 0.02 c–e | |
| MR | 12.20 ± 2.92 c–g | 0.18 ± 0.05 ab | 0.2 ± 0.01 d–f | 8.3 ± 0.04 ab | 1.3 ± 0.08 a–d | 0.8 ± 0.05 a–d | 1.10 ± 0.10 b–e | |
| CR | 5.92 ± 0.53 e–g | 0.19 ± 0.01 a | 0.3 ± 0.01 a | 8.5 ± 0.18 ab | 0.94 ± 0.43 a–e | 0.55 ± 0.25 a–e | 1.10 ± 0.16 b–e | |
| CM | 4.29 ± 0.73 fg | 0.15 ± 0.03 ab | 0.2 ± 0.01 b–d | 8.0 ± 0.10 a–c | 1.31 ± 0.25 a–d | 0.8 ± 0.15 a–d | 0.90 ± 0.05 d–f | |
| CMR | 25.43 ± 8.40 b | 0.17 ± 0.02 ab | 0.2 ± 0.01 c–f | 8.4 ± 0.02 ab | 0.77 ± 0.25 ab | 0.45 ± 0.15 ab | 1.00 ± 0.23 c–e | |
| 30% ETc | Ct− | 4.07 ± 0.57 fg | 0.13 ± 0.02 ab | 0.2 ± 0.01 ef | 8.2 ± 0.50 a–c | 0.5± 0.17 d–e | 0.3 ± 0.10 de | 0.70 ± 0.13 ef |
| Ct+ | 15.92 ± 5.67 b–e | 0.15 ± 0.02 ab | 0.2 ± 0.01 d–f | 8.7 ± 0.09 a | 0.9 ± 0.34 b–e | 0.5 ± 0.20 b–e | 0.50 ± 0.13 f | |
| M | 5.31 ± 0.24 fg | 0.19 ± 0.05 a | 0.2 ± 0.01 f | 8.3 ± 0.35 a | 0.94 ± 0.51 a–e | 0.55 ± 0.15 a–e | 1.50 ± 0.01 a | |
| R | 3.05 ± 0.08 g | 0.12 ± 0.03 ab | 0.2 ± 0.01 f | 8.6 ± 0.05 a | 1.03 ± 0.10 a–e | 0.6 ± 0.30 a–e | 1.20 ± 0.10 a–c | |
| C | 6.98 ± 2.29 d–g | 0.16 ± 0.01 ab | 0.2 ± 0.00 bc | 8.5 ± 0.02 a | 0.43 ± 0.08 e | 0.33 ± 0.05 e | 1.00 ± 0.04 c–e | |
| MR | 13.77 ± 1.00 c–f | 0.12 ± 0.03 ab | 0.2 ± 0.01 c–e | 8.3 ± 0.09 ab | 0.94 ± 0.08 a–e | 0.55 ± 0.05 a–e | 1.40 ± 0.13 ab | |
| CR | 8.19 ± 0.55 c–g | 0.09 ± 0.04 b | 0.2 ± 0.02 d–f | 7.5 ± 0.13 c | 0.60 ± 0.08 c–e | 0.35 ± 0.05 c–e | 1.60 ± 0.03 a | |
| CM | 16.82 ± 0.57 b–d | 0.10 ± 0.02 ab | 0.2± 0.01 d–f | 7.7 ± 0.09 bc | 0.94 ± 0.25 a–e | 0.55 ± 0.15 a–e | 1.30 ± 0.06 ac | |
| CMR | 60.39 ± 2.04 a | 0.13 ± 0.02 ab | 0.2 ± 0.00 f | 8.2 ± 0.02 a–c | 0.51 ± 0.17 ab | 0.30 ± 0.10 ab | 1.00 ±0.03 b–e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikan, C.; Soussani, F.-E.; Ouhaddou, R.; Ech-Chatir, L.; Errouh, F.; Boutasknit, A.; Assouguem, A.; Ali, E.A.; Ullah, R.; Ait Barka, E.; et al. Use of Biofertilizers as an Effective Management Strategy to Improve the Photosynthetic Apparatus, Yield, and Tolerance to Drought Stress of Drip-Irrigated Wheat in Semi-Arid Environments. Agronomy 2024, 14, 1316. https://doi.org/10.3390/agronomy14061316
Ikan C, Soussani F-E, Ouhaddou R, Ech-Chatir L, Errouh F, Boutasknit A, Assouguem A, Ali EA, Ullah R, Ait Barka E, et al. Use of Biofertilizers as an Effective Management Strategy to Improve the Photosynthetic Apparatus, Yield, and Tolerance to Drought Stress of Drip-Irrigated Wheat in Semi-Arid Environments. Agronomy. 2024; 14(6):1316. https://doi.org/10.3390/agronomy14061316
Chicago/Turabian StyleIkan, Chayma, Fatima-Ezzahra Soussani, Redouane Ouhaddou, Lahoucine Ech-Chatir, Farid Errouh, Abderrahim Boutasknit, Amine Assouguem, Essam A. Ali, Riaz Ullah, Essaid Ait Barka, and et al. 2024. "Use of Biofertilizers as an Effective Management Strategy to Improve the Photosynthetic Apparatus, Yield, and Tolerance to Drought Stress of Drip-Irrigated Wheat in Semi-Arid Environments" Agronomy 14, no. 6: 1316. https://doi.org/10.3390/agronomy14061316
APA StyleIkan, C., Soussani, F.-E., Ouhaddou, R., Ech-Chatir, L., Errouh, F., Boutasknit, A., Assouguem, A., Ali, E. A., Ullah, R., Ait Barka, E., Lahlali, R., & Meddich, A. (2024). Use of Biofertilizers as an Effective Management Strategy to Improve the Photosynthetic Apparatus, Yield, and Tolerance to Drought Stress of Drip-Irrigated Wheat in Semi-Arid Environments. Agronomy, 14(6), 1316. https://doi.org/10.3390/agronomy14061316












