Optimization of Hairy Root Transformation and Application of RUBY as a Reporter in Lotus corniculatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Construction of the Binary Vector
2.3. A. rhizogenes-Mediated Hairy Root Transformation
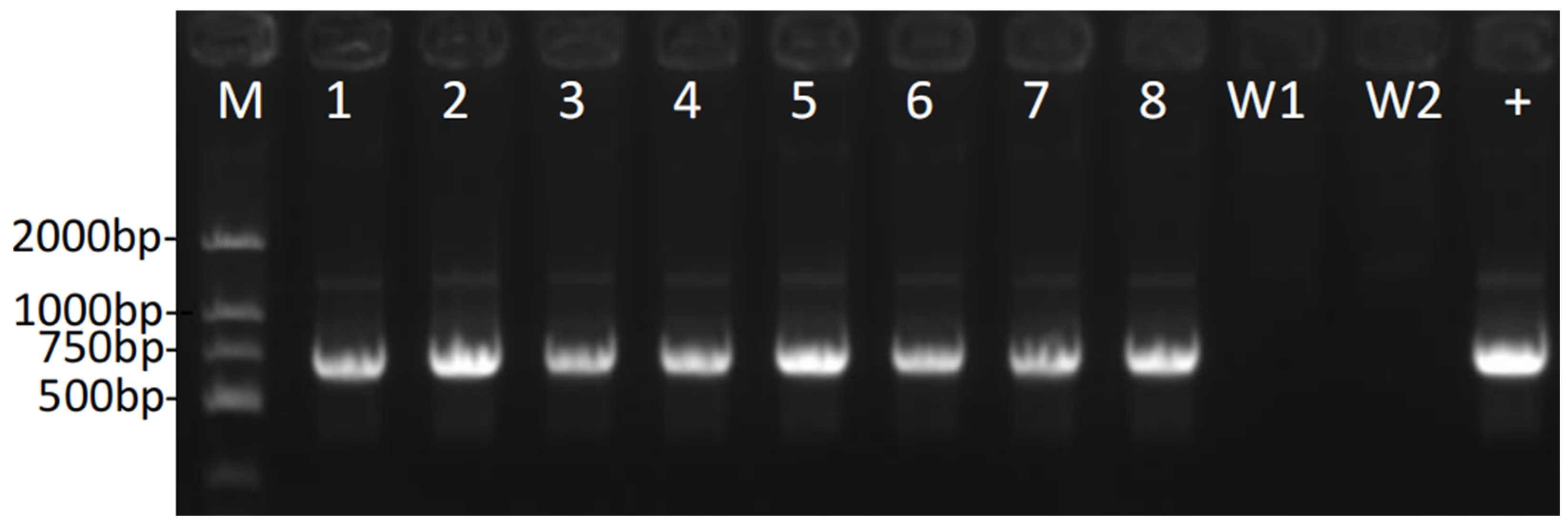
2.4. Genetically Modified Identification with PCR Amplification
2.5. Statistical Analysis of Hairy Root Transformation Frequency
2.6. Nodulation Assay
3. Results
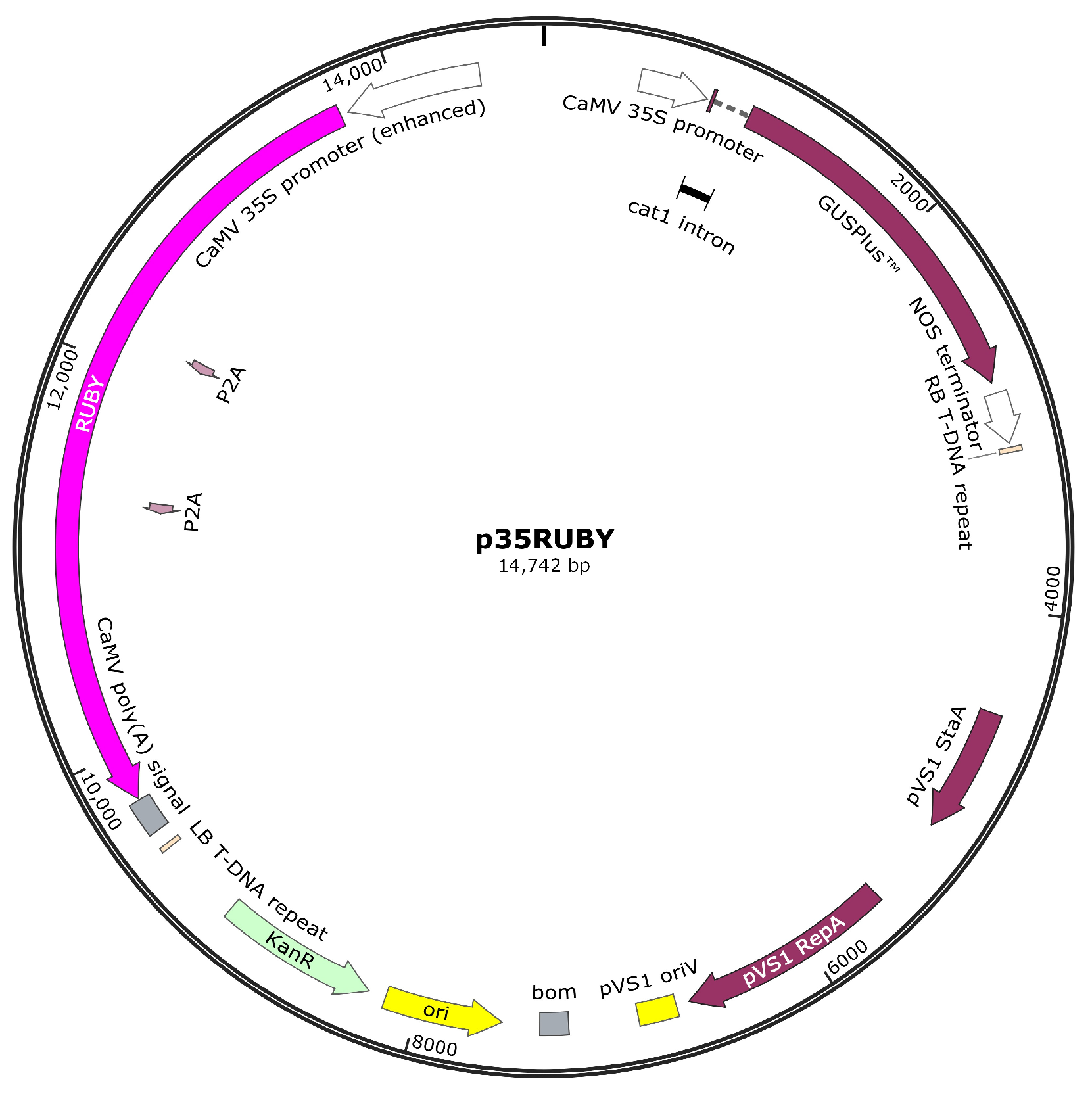
3.1. Binary Vector with RUBY as a Reporter System
3.2. Vividly Red Hairy Roots Were Produced from Hypocotyl Incisions
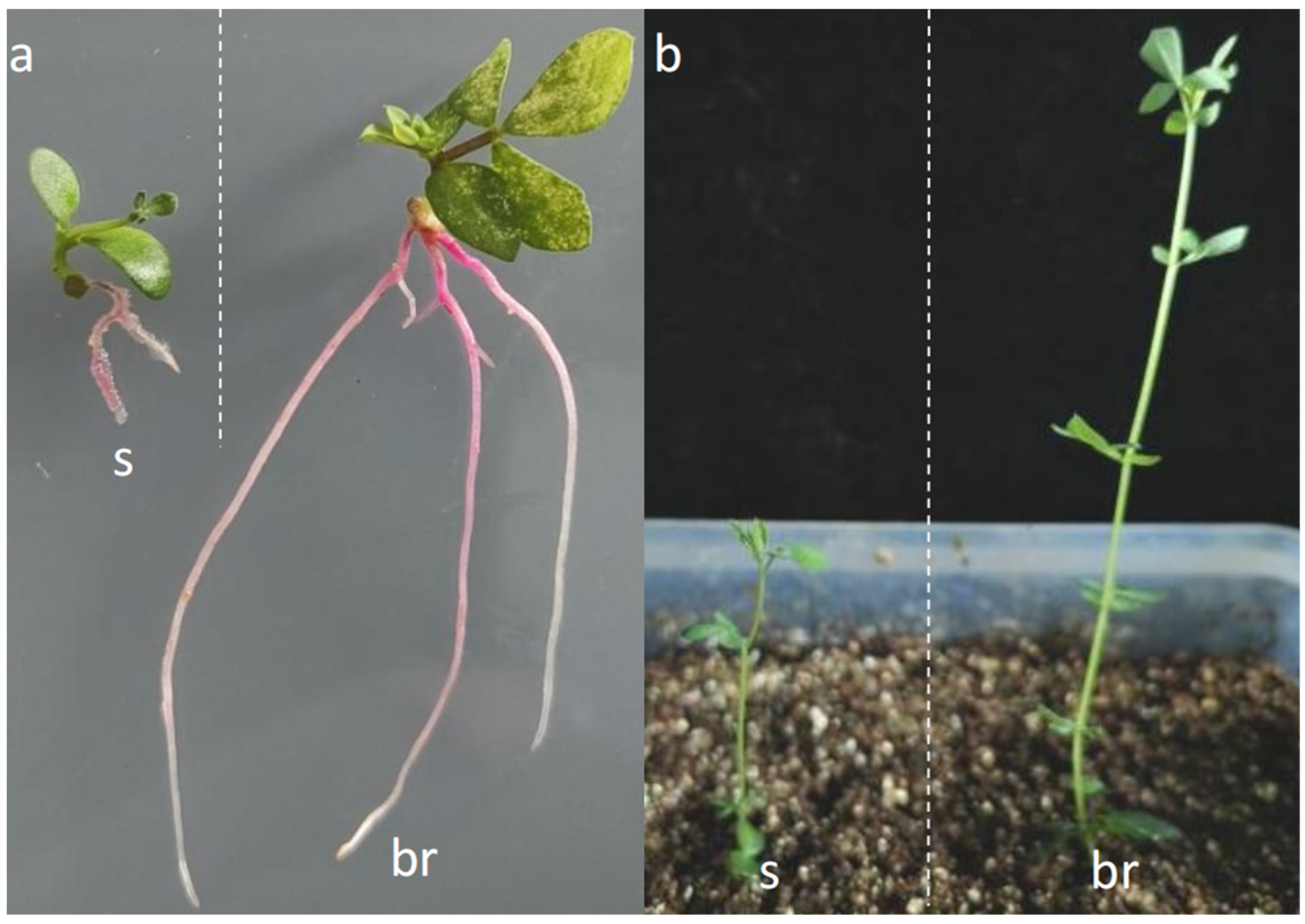
3.3. Young Branches as Explants Are Better Than Those as Seedlings
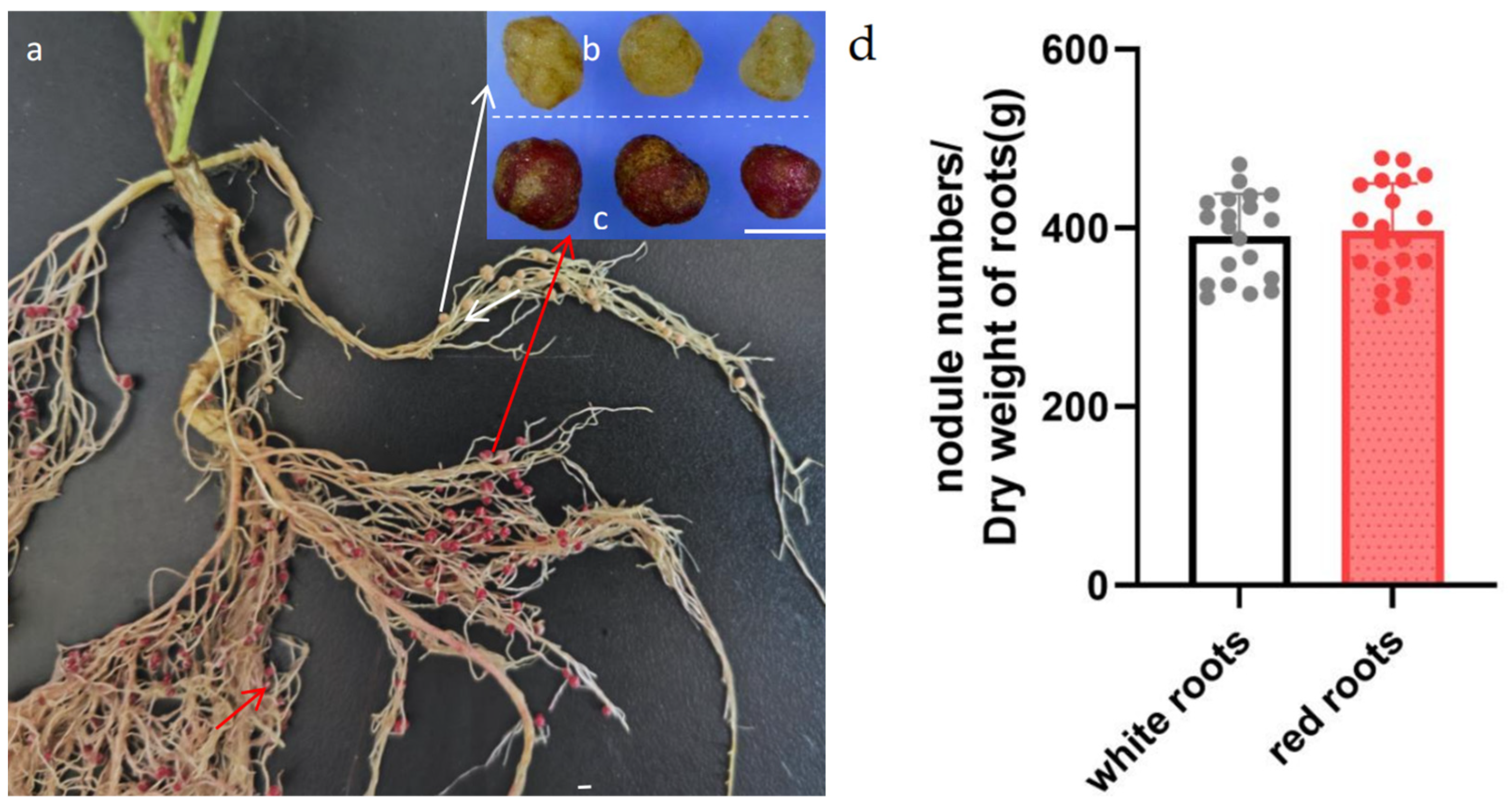
3.4. Evaluating the Reliability of RUBY as a Reporter in the Study of Rhizobia–Legume Symbiosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beyaz, R.; MacAdam, J.W. X-Radiation of Lotus corniculatus L. Seeds Improves Germination and Initial Seedling Growth. Int. J. Radiat. Biol. 2023, 99, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Montiel, J.; Reid, D.; Grønbæk, T.H.; Benfeldt, C.M.; James, E.K.; Ott, T.; Ditengou, F.A.; Nadzieja, M.; Kelly, S.; Stougaard, J. Distinct Signaling Routes Mediate Intercellular and Intracellular Rhizobial Infection in Lotus japonicus. Plant Physiol. 2021, 185, 1131–1147. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Nakamura, Y.; Kaneko, T.; Asamizu, E.; Kato, T.; Nakao, M.; Sasamoto, S.; Watanabe, A.; Ono, A.; Kawashima, K.; et al. Genome Structure of the Legume, Lotus japonicus. DNA Res. 2008, 15, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, X.; Li, H.; Liu, S.; Jin, L.; Lyu, Y.; Shi, M.; Liu, S.; Yang, X.; Lyu, S. Anthocyanin, a Novel and User-Friendly Reporter for Convenient, Non-Destructive, Low Cost, Directly Visual Selection of Transgenic Hairy Roots in the Study of Rhizobia-Legume Symbiosis. Plant Methods 2020, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, X.; Zhong, L.; Wang, X.; Jin, L.; Lyu, S. One-Step Generation of Composite Soybean Plants with Transgenic Roots by Agrobacterium rhizogenes-Mediated Transformation. BMC Plant Biol. 2020, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Mugnier, J. Establishment of New Axenic Hairy Root Lines by Inoculation with Agrobacterium rhizogenes. Plant Cell Rep. 1988, 7, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Boisson-Dernier, A.; Chabaud, M.; Garcia, F.; Bécard, G.; Rosenberg, C.; Barker, D.G. Agrobacterium rhizogenes-Transformed Roots of Medicago truncatula for the Study of Nitrogen-Fixing and Endomycorrhizal Symbiotic Associations. MPMI 2001, 14, 695–700. [Google Scholar] [CrossRef]
- Stiller, J.; Martirani, L.; Tuppale, S.; Chian, R.-J.; Chiurazzi, M.; Gresshoff, P.M. High Frequency Transformation and Regeneration of Transgenic Plants in the Model Legume Lotus japonicus. J. Exp. Bot. 1997, 48, 1357–1365. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, J.; Yuan, C.; Guo, Y.; Yu, H.; Li, Y.; Huang, C. Cas9-PF, an Early Flowering and Visual Selection Marker System, Enhances the Frequency of Editing Event Occurrence and Expedites the Isolation of Genome-edited and Transgene-free Plants. Plant Biotechnol. J. 2019, 17, 1191–1193. [Google Scholar] [CrossRef]
- Lin, X.; Jia, Y.; Heal, R.; Prokchorchik, M.; Sindalovskaya, M.; Olave-Achury, A.; Makechemu, M.; Fairhead, S.; Noureen, A.; Heo, J.; et al. Solanum Americanum Genome-Assisted Discovery of Immune Receptors That Detect Potato Late Blight Pathogen Effectors. Nat. Genet. 2023, 55, 1579–1588. [Google Scholar] [CrossRef]
- Timoneda, A.; Yunusov, T.; Quan, C.; Gavrin, A.; Brockington, S.F.; Schornack, S. MycoRed: Betalain Pigments Enable In Vivo Real-Time Visualisation of Arbuscular Mycorrhizal Colonisation. PLoS Biol. 2021, 19, e3001326. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Sun, H.; Zhan, H.; Zhao, Y. A Reporter for Noninvasively Monitoring Gene Expression and Plant Transformation. Hortic. Res. 2020, 7, 152. [Google Scholar] [CrossRef] [PubMed]
- Polturak, G.; Aharoni, A. Advances and Future Directions in Betalain Metabolic Engineering. New Phytol. 2019, 224, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cai, Y.; Liu, X.; Yao, W.; Wu, S.; Hou, W. The RUBY Reporter for Visual Selection in Soybean Genome Editing. aBIOTECH 2024, 1–5. [Google Scholar] [CrossRef]
- Ajdanian, L.; Niazian, M.; Torkamaneh, D. Optimizing Ex Vitro One-step RUBY-equipped Hairy Root Transformation in Drug- and Hemp-type Cannabis. Plant Biotechnol. J. 2024, 22, 1957–1959. [Google Scholar] [CrossRef] [PubMed]
- Niazian, M.; Belzile, F.; Curtin, S.J.; De Ronne, M.; Torkamaneh, D. Optimization of In Vitro and Ex Vitro Agrobacterium rhizogenes-Mediated Hairy Root Transformation of Soybean for Visual Screening of Transformants Using RUBY. Front. Plant Sci. 2023, 14, 1207762. [Google Scholar] [CrossRef]
- Wang, X.; Teng, C.; Wei, H.; Liu, S.; Xuan, H.; Peng, W.; Li, Q.; Hao, H.; Lyu, Q.; Lyu, S.; et al. Development of a Set of Novel Binary Expression Vectors for Plant Gene Function Analysis and Genetic Transformation. Front. Plant Sci. 2023, 13, 1104905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kondorosi, É.; Kereszt, A. An Anthocyanin Marker for Direct Visualization of Plant Transformation and Its Use to Study Nitrogen-Fixing Nodule Development. J. Plant Res. 2019, 132, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhong, Y.; Feng, B.; Qi, X.; Yan, T.; Liu, J.; Guo, S.; Wang, Y.; Liu, Z.; Cheng, D.; et al. The RUBY Reporter Enables Efficient Haploid Identification in Maize and Tomato. Plant Biotechnol. J. 2023, 21, 1707–1715. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Biological Properties and Applications of Betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef]
- Vogt, T.; Jones, P. Glycosyltransferases in Plant Natural Product Synthesis: Characterization of a Supergene Family. Trends Plant Sci. 2000, 5, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Wagner, A.E.; Schini-Kerth, V.B.; Rimbach, G. Betanin—A Food Colorant with Biological Activity. Mol. Nutr. Food Res. 2015, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Biological Activities of Plant Pigments Betalains. Crit. Rev. Food Sci. Nutr. 2016, 56, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Nishihara, M.; Kohakura, M.; Kimura, K.; Yashiro, T.; Takasawa, S.; Arimura, G. Metabolic Engineering of Betacyanin in Vegetables for Anti-inflammatory Therapy. Biotech. Bioeng. 2023, 120, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Grützner, R.; Schubert, R.; Horn, C.; Yang, C.; Vogt, T.; Marillonnet, S. Engineering Betalain Biosynthesis in Tomato for High Level Betanin Production in Fruits. Front. Plant Sci. 2021, 12, 682443. [Google Scholar] [CrossRef]
- Lu, J.; Li, S.; Deng, S.; Wang, M.; Wu, Y.; Li, M.; Dong, J.; Lu, S.; Su, C.; Li, G.; et al. A Method of Genetic Transformation and Gene Editing of Succulents without Tissue Culture. Plant Biotechnol. J. 2024, 22, 1981–1988. [Google Scholar] [CrossRef]
| Explants | Numbers of Explants | Numbers of Composite Plants | Transformation Frequency | Average of Total Roots in Transgenic Composite Plant | Average of Transgenic Hairy Roots in Transgenic Composite Plant |
|---|---|---|---|---|---|
| seedling | 30 | 20 | 62.2% | 3.00 ± 0.56 B | 1.65 ± 0.49 B |
| 30 | 17 | 2.53 ± 0.72 B | 1.59 ± 0.51 B | ||
| 30 | 18 | 2.56 ± 0.74 B | 1.56 ± 0.62 B | ||
| young branch | 30 | 26 | 81.1% | 4.15 ± 0.88 A | 2.27 ± 0.72 A |
| 30 | 24 | 3.96 ± 1.08 A | 2.17 ± 0.82 A | ||
| 30 | 23 | 3.82 ± 0.89 A | 2.13 ± 0.76 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, K.; Zhang, X.; Yu, W.; Lyu, S.; Fan, Y. Optimization of Hairy Root Transformation and Application of RUBY as a Reporter in Lotus corniculatus. Agronomy 2024, 14, 1335. https://doi.org/10.3390/agronomy14061335
Lyu K, Zhang X, Yu W, Lyu S, Fan Y. Optimization of Hairy Root Transformation and Application of RUBY as a Reporter in Lotus corniculatus. Agronomy. 2024; 14(6):1335. https://doi.org/10.3390/agronomy14061335
Chicago/Turabian StyleLyu, Kaidi, Xingli Zhang, Wenjie Yu, Shanhua Lyu, and Yinglun Fan. 2024. "Optimization of Hairy Root Transformation and Application of RUBY as a Reporter in Lotus corniculatus" Agronomy 14, no. 6: 1335. https://doi.org/10.3390/agronomy14061335
APA StyleLyu, K., Zhang, X., Yu, W., Lyu, S., & Fan, Y. (2024). Optimization of Hairy Root Transformation and Application of RUBY as a Reporter in Lotus corniculatus. Agronomy, 14(6), 1335. https://doi.org/10.3390/agronomy14061335






