Abstract
The obstacle associated with continuous cropping is an important problem in the production of muskmelon (Cucumis melo L.). The allelochemicals from root exudates play an active role in root–microbe communication. The primary objective of this study was to delve into the impact of root exudates and the continuous cultivation of muskmelon on the growth and colonization patterns of Trichoderma viride T23. It was observed that the root exudates of muskmelon significantly promoted mycelial growth and the sporulation of Trichoderma viride T23 at concentrations of 0.05, 0.1 g·mL−1, while at a concentration of 0.05 g·mL−1, the enzyme activities of β-glucosidase, chitinase and cellulase were 12.34, 13.23, and 17.85 U·mL−1, respectively, which were higher than those of the control. With increasing concentrations of root exudates, the hyphal growth, spore germination, and the three enzyme activities of Trichoderma viride T23 were decreased. The findings from the pot experiments revealed that the total phenolic acid content in the soil of replanted muskmelon demonstrated a trend of escalating over the course of the first growth cycle of continuous cropping to the fourth growth cycle of continuous cropping. The population density in the rhizosphere soil of Trichoderma viride T23 in the first growth cycle and the second growth cycle of continuous cropping shows a significant difference compared with other treatments, which led to statistically significant increments of stem diameter, leaf area, fresh weight, dry weight and SPAD index. It is necessary to increase the dose of the beneficial microorganism or degrade the phenolics in the rhizosphere soil to promote effectiveness while increasing the growth cycles of continuous cropping.
1. Introduction
The muskmelon (Cucumis melo L.), which is a species of melon, has been bred into several cultivated varieties worldwide [1,2]. In China, the cultivation of muskmelons has been on a steady rise, driven by ever-growing market demand. In some regions, due to limited arable land resources and insufficient land for crop rotation, farmers may be compelled to continuously crop melons on the same plot. Since melons possess high economic value, farmers are often tempted to capitalize on this when market demand is high and prices are favorable, thereby seeking to maximize their economic benefits. However, this practice of continuous cropping can have long-term implications on soil fertility and crop health, necessitating a careful balance between immediate profits and sustainable agricultural practices [3]. The incidence and severity of diseases caused by plant pathogenic microorganisms in soils used for the continuous cropping of muskmelons have shown a marked increase prior to harvest, posing a significant threat to crop yield and quality [4,5]. Moreover, autotoxicity is also a major problem in the continuous cropping of muskmelon, and the plant species releases phenolic acids that severely inhibit seed germination and/or seedling development of the same species and adjust the microbial composition and quantity in the soil [6,7]. Studies have shown that the continuous accumulation of root exudates, including phenolic acids, in addition to the imbalance of soil microbial composition, can also inhibit beneficial microorganisms and accelerate the growth of pathogenic microorganisms [8,9]. Previous studies have conclusively demonstrated that phenolic acids, such as ferulic acid, benzoic acid, and cinnamic acid, isolated and identified from the root exudates of muskmelon, exert significant impacts on the soil microbial community. Notably, these acids tend to accelerate the progression of Fusarium wilt, a disease caused by Fusarium oxysporum f. sp. melonis, thereby posing a serious threat to the health and productivity of muskmelon plants [3,10].
Trichoderma species, which are found in soil and play the role of biological control in agriculture, have been used to ameliorate plant development and control fungal diseases on many crops through various disease resistance mechanisms, such as through multiple enzymes, mycoparasitism and nutrient competition [11,12]. Numerous studies have reported the function of Trichoderma species in increasing the effectiveness of some nutrient elements in the soil and further promoting the growth of the root tips and above-ground plant parts [11,13,14]. Unfortunately, the control efficiency of soil beneficial fungi in disease prevention or root development promotion is often slow and unstable. This limitation can be attributed to various factors, including the complexity of soil microbial communities, environmental conditions, and the interaction between the fungi and the host plant [15,16]. The colonization and capacity of Trichoderma species, as exotic microorganisms, are indeed influenced by the physicochemical properties of soil and the existing microbial populations, which can significantly affect the establishment and effectiveness of Trichoderma in the soil environment, leading to variable and sometimes unpredictable disease control and plant growth promotion outcomes [17,18,19]. At present, there is research available focused on the growth of Trichoderma strains and the formation of chlamydospores in soil and/or on many cultivated plant species under various abiotic stress conditions of temperature, humidity, pH and so on [20,21]. Absolutely, the colonization and growth capabilities of diverse Trichoderma species across a broad range of soil environments remain a crucial area of research. Understanding how these fungi adapt and thrive in diverse soil conditions is essential for maximizing their beneficial effects in agricultural systems. This knowledge can lead to the development of more effective and targeted strategies for disease control and plant growth promotion, ultimately enhancing crop yields and sustainability [22,23].
Our previous research found that Trichoderma viride T23 is a strong antagonist against F. oxysporum due to its capacity to compete for nutrients and space, produce antifungal metabolites, induce the defense response, and increase plant growth [24]. In this study, we aimed to enhance the biocontrol agent’s effectiveness by investigating the growth of T. viride T23 in the root exudates of muskmelon seedlings and its colonization in the soil under the continuous cropping of muskmelon.
2. Materials and Methods
2.1. Detection of the Effect of Muskmelon Root Exudates on the Growth of T. viride T23
Root exudates of muskmelon seedlings (variety: ‘Sweet king’) were collected by the root soaking method [3]. Muskmelon seedlings (8–15 cm tall) were uprooted from soil, and the roots were washed to remove soil. The roots of thirty seedlings were soaked in 300 mL sterile distilled water for 24 h (16 h light/8 h dark) at 28–32 °C, which was filtered using glass syringes fitted with a Gelman Acrodisc CR-PTFE 0.2 μm filter (Baili Biotechnology Co., Ltd., Shanghai, China) and evaporated using a rotary evaporator (Yarong Model RE-52A, Shanghai, China) at 40 °C to a volume of 50 mL.
Root exudates of muskmelon were selected for bioassays at 0, 0.05, 0.1, 0.25, 0.5 and 1.0 g·mL−1 (1 g·mL−1 means that 1 mL distilled water contains the exudates obtained from 1 g fresh root). Root exudates of muskmelon were added to the medium (NaNO3 0.2 g, KCl 0.05 g, KHPO4 0.1 g, sucrose 3.0 g, distilled water 100 mL) in a 1:20 ratio, respectively. A 1 mL spore suspension (1.0 × 108 CFUml−1) of T. viride T23 provided by the Institute of Plant Immunology Lab of Shenyang Agricultural University was then inoculated into the medium and shaken for 7 days at 25 °C and 100 rpm·min−1. The control group consisted of sterile water without any added root exudates, maintaining the concentration of 0 g·mL−1, and each treatment was composed of three replicates.
The fermentation liquid of each treatment (three repeats) with the root exudates of muskmelon was treated by vacuum filtration, respectively. The residual mycelia were cleaned with sterile water thrice and dried with filter paper and weighed, and the number of spores in the fermentation was estimated by the hemocytometer counting chamber. The intensity of the allelopathic effect was expressed in RI (response index) values calculated according to the following formula: RI = 1 − C/T (T ≥ C) or RI = T/C − 1 (T < C), where C is the control data and T is the treatment data. RI values range from +1 to −1. RI > 0 means stimulation, while RI < 0 means inhibition [25].
2.2. Detection of the Effect of Muskmelon Root Exudates on Enzymatic Activity of T. viride T23
The muskmelon root extract was obtained using the method described in Section 2.1. Similarly, the addition of this extract to the fermentation medium of T. viride T23 was carried out in accordance with the same procedure outlined in Section 2.1.
The liquid culture of each treatment (three repeats) with root exudates of muskmelon was filtrated through sterile gauze, respectively. The filtrate was employed for detecting the extracellular enzymatic activity of three enzymes, which were measured using, respectively, an β-glucosidase (β-GC) Assay Kit, a Chitinase Assay Kit, and a Cellulase (CL) Assay Kit (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China).
β-glucosidase (β-GC) assay: Take 0.2 mL of the treated sample filtrate and add 1 mL of extract to the sample filtrate. Centrifuge the solution at 15,000× g (gravitational force) for 20 min at 4 °C. Prepare a standard solution of p-nitrophenol according to the assay kit instructions. Adjust the spectrophotometer wavelength to 400 nm. Measure the absorbance of the samples and the standard solutions at this wavelength. Enzyme activity for β-glucosidase is defined as the amount of enzyme that catalyzes the release of 1 nmol of p-nitrophenol per milliliter per hour under standard conditions [26].
Chitinase assay: Take 0.1 mL of the treated sample filtrate and add 1 mL of extract. Centrifuge the mixture at 10,000× g at 4 °C for 20 min. Collect the supernatant, which contains the chitinase enzyme. Prepare a standard solution of N-acetylglucosamine according to the assay kit’s instructions. Adjust the spectrophotometer wavelength to 585 nm. one unit of chitinase activity is defined as the amount of enzyme that catalyzes the release of 1 μg of N-acetylglucosamine per milliliter per hour at 37 °C [27].
Cellulase assay: Take 0.1 mL of the treated sample filtrate and add 1 mL of extract. Place the mixture in a centrifuge and spin at 8000× g for 10 min at 4 °C. Prepare a standard solution of glucose according to the provided instructions. Measure the absorbance of the samples and the standard solutions at 540 nm. The unit of enzyme activity is defined as the amount of enzyme that catalyzes the production of 1 μg of glucose per minute in the reaction system [28].
2.3. Effect of Muskmelon Continuous Cropping on Phenolic Acid Content in Soil
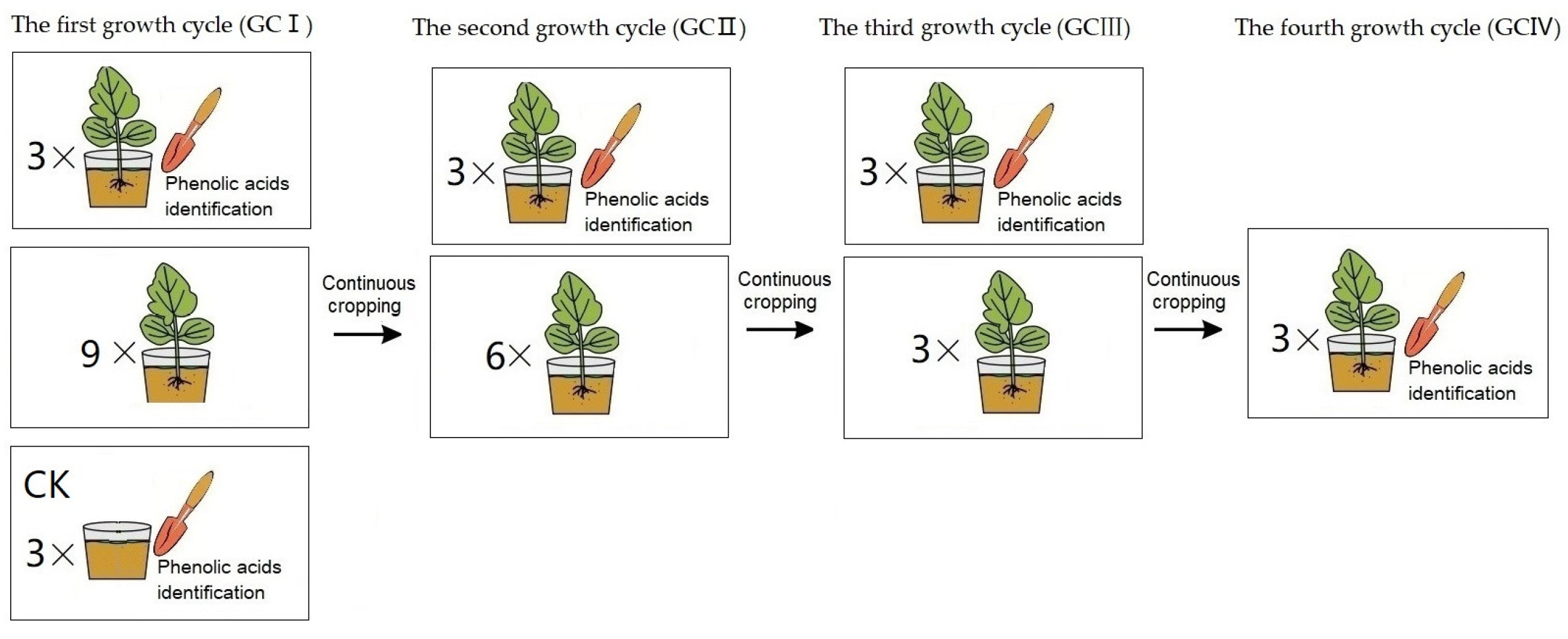
The greenhouse pot experiment was conducted at the Institute of Plant Immunology Lab of Shenyang Agricultural University (located at north latitude 41°82′ and east longitude 123°56′) to investigate phenolic acids of T. viride T23 in soil under continuous cropping conditions (presented in Figure 1). Two seedlings of muskmelon variety ‘Sweet king’ were sown in pots filled with 5 L soil—we used clay loam with 32.3% clay, 33.4% silt, and 33.9% sand. The pot experiment was designed for muskmelon cropping for the first growth cycle (GCI), continuous cropping for the second growth cycle (GCII), a third growth cycle (GCIII), and a fourth growth cycle (GCIV). The potted plants were grown in an insect-proof glasshouse at 25 ± 2 °C for a 14/10 h photoperiod under 50–70% relative humidity. The rhizosphere soils were separately collected during each harvest period, spanning from growth cycle I to growth cycle IV. Additionally, pre-planting soil from growth cycle I was utilized as the control for comparison.
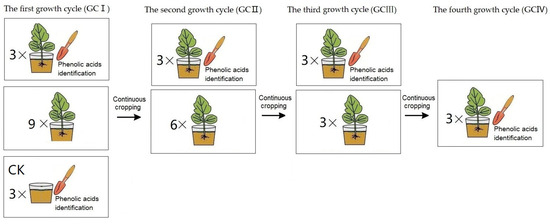
Figure 1.
The treatment procedure of muskmelon seedlings for phenolic acids identification: In GCI, a pot seedling treatment (three replicates) and the control (CK, three replicates) were performed for soil phenolic acid determination, while nine pot seedlings were continuously cropped for the second growth cycle. In GCII, a pot seedling treatment (three replicates) was performed for soil phenolic acid determination, while six pot seedlings were continuously cropped for the third growth cycle. In GCIII, a pot seedling treatment (three replicates) was performed for soil phenolic acid determination, while three pot seedlings were continuously cropped for the fourth growth cycle. In GCIV, a pot seedling treatment (three replicates) performed for soil phenolic acid determination.
The soil samples were dried, and about 25 g soil was used for analysis. Then, 25 mL of 1 M sodium hydroxide was added to the sample and mixed well in a beaker for 24 h. The well-mixed sample was placed in ultrasonic oscillator for 30 min and centrifuged. The supernatant was adjusted at pH 2.5 using hydrochloric acid (12 mol·L−1) for 2 h and centrifuged to remove humeric acid. The solution was passed through a 0.22 μm organic membrane filter, and analysis was performed in an HPLC system (Agilent LC 1100, Santa Clara, CA, USA) with an SB-C18 column (4.6 mm × 250 mm). The mobile phase consisted of pH = 2.8 acetic acid (A) and methanol (B) with a gradient elution. Each supernatant and standard compound were used in the following gradient system: (1) from 0.0 to 15.0 min, 70% A plus 30% B; (2) from 16.0 to 20.0 min, 50% A plus 50% B; and (3) from 21.0 to 30.0 min, 30% A plus 70% B. The strongest absorption peak of each standard appeared between 240 nm and 320 nm. After careful consideration, it was deemed suitable to select 280 nm as the measurement wavelength [10]. The injection volume was 20 μL, and the column temperature was maintained at 25 °C. The phenolic acids of each supernatant were identified by their retention times and quantified with reference to the peak area.
2.4. Inoculation Assay
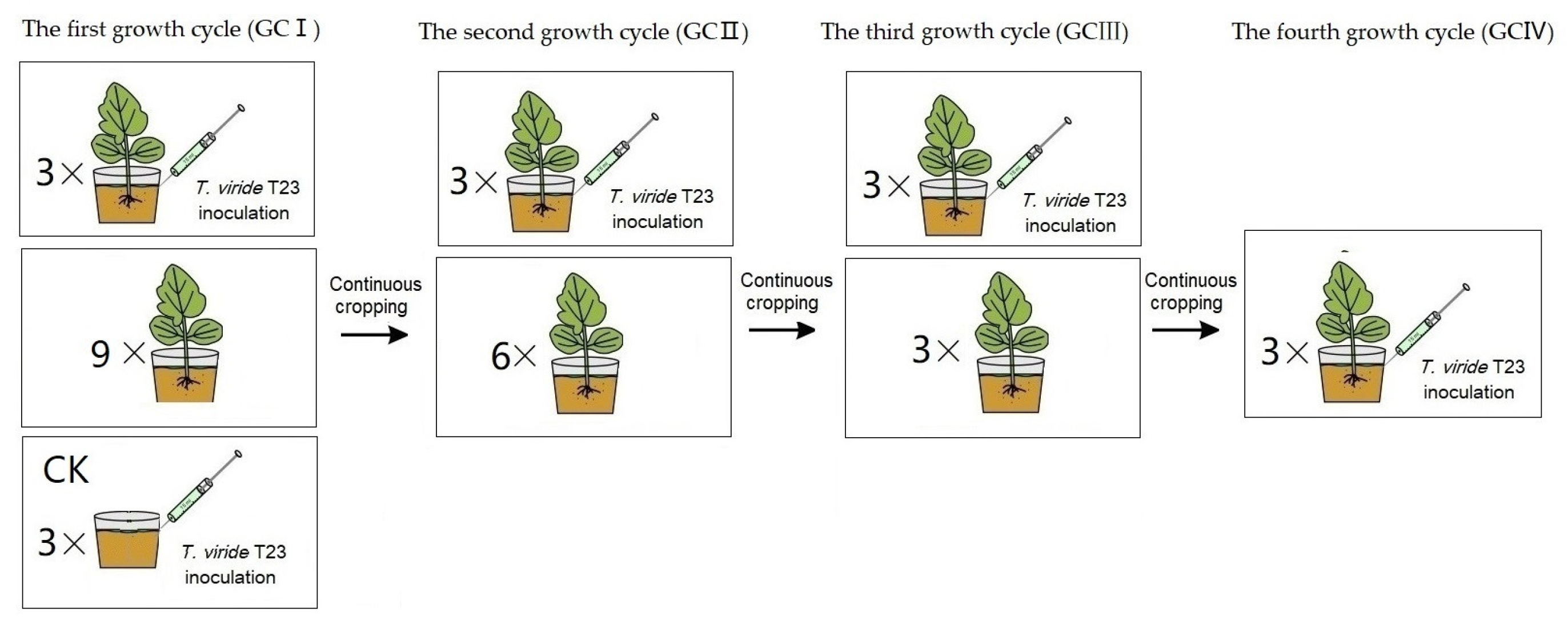
Under continuous cropping, the treatments of T. viride T23 inoculation were established, respectively. The soil composition and muskmelon cultivation methods employed are identical to those described in Section 2.3. Uniformly grown seedlings at the four-leaf stage were carefully selected, and 15 mL of T. viride T23 suspension (3.0 × 107 CFU·mL−1) was evenly injected into the rhizosphere at eight points, each point located 2 cm away from the stem base. As a control, soil was inoculated with Trichoderma without planting melon seedlings (presented in Figure 2). All experimental pots were kept in a growth chamber in a 14 h/10 h light/dark photoperiod at 27 °C–22 °C (day/night) under 60% mean relative humidity.
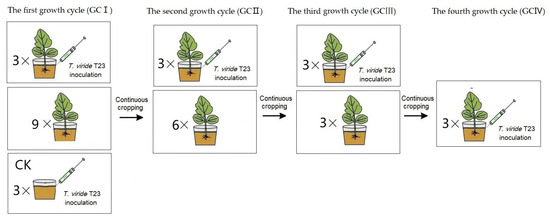
Figure 2.
The treatment procedure of muskmelon seedlings for the inoculation of T. viride T23. In GCI, a pot seedling treatment (three replicates) and the control (CK, three replicates) were performed for the inoculation assay, while nine pot seedlings were continuously cropped. In GCII, a pot seedling treatment (three replicates) was performed for the inoculation assay, while six pot seedlings were continuously cropped. In GCIII, a pot seedling treatment (three replicates) was performed for the inoculation assay, while three pot seedlings were continuously cropped. In GCIV, a pot seedling treatment (three replicates) were performed for the inoculation assay.
At 0 d, 7 d, 14 d, 21 d, 28 d, 35 d, 42 d, and 49 d after inoculation, the rhizosphere soils of each treatment were collected and subjected to RNA extraction by TriZol reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The specific primer pairs for the gene expression determinations of T. viride T23 with reverse transcription quantitative polymerase chain reaction were TEF1-2-F: 5′-AGT ACG CTT GGG TTC TTG AC-3′ and TEF1-2-R: 5′-TCC TGG TTA GCA CTG GTT TG-3′ [29]. The specific fragment was amplified using the primers and then recovered and purified with a gel extraction kit. The purified DNA fragments were subsequently ligated into the pGEM-T Easy Vector and transformed into E. coli DH5α competent cells. After verification, the plasmid was extracted using a plasmid miniprep kit (Thermo Fisher Scientific), and the standard curves were drawn using the qPCR reaction of the diluted recombinant plasmid standard. The copy number was calculated based on the plasmid concentration:
copy number = [plasmid concentration (ng·μg−1) × Plasmid volume (μL) × 6.02 × 1023 (copies·mol−1)] ÷ {660(g·mol−1) × [carrier length (bp) + Fragment length (bp)]}
qRT-PCR was performed using the CFX96 Real-time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). PCR reactions were performed in a 20 μL total volume containing 10 μL of 2 × SuperReal PreMix Plus, 0.6 μL of forward and reverse primer (10 μM), and 1 μL of RNA (100 ng/μg). Each treatment was replicated three times. The qRT-PCR conditions were 95 °C 15 s, 95 °C 10 s, and 57 °C 20 s, with a final extension at 72 °C for 25 s. In total, 40 cycles were performed.
The standard curve of T. viride T23 boasted a slope of −2.3208 and a remarkable correlation coefficient of 0.9982, and it adheres to the following regression equation: CT = −2.3208 Log(copy number) + 24.112.
2.5. Effect of T. viride T23 on Continuous Cropping Muskmelon
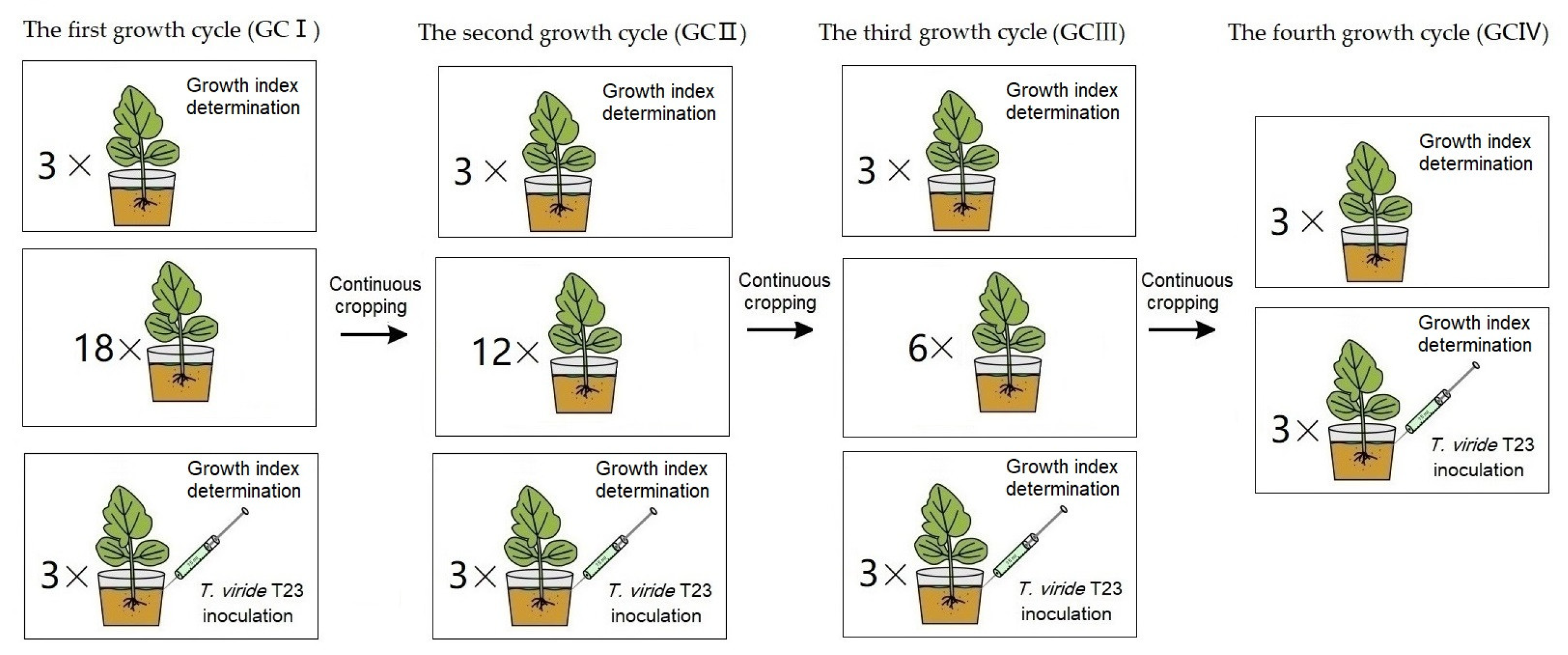
The soil composition and muskmelon cultivation methods employed are identical to those described in Section 2.3, and the T. viride T23 inoculation methods employed are identical to those described in Section 2.4. Measurements—including stem diameter, leaf area, fresh weight, dry weight and chlorophyll content per plant of ‘Sweet king’ plants—were conducted during the fruiting period (Figure 3). The leaf SPAD index was measured by using SPAD-502PLUS (Konica Minolta, Tokyo, Japan) [30].
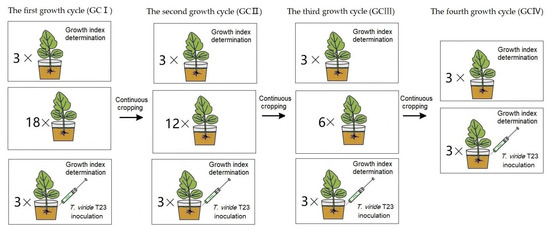
Figure 3.
The treatment procedure of muskmelon seedlings for the effect of T. viride T23. In GCI, pot seedling treatments with T. viride T23 (three replicates) and without T. viride T23 (three replicates) were performed for growth index determination, while eighteen pot seedlings were continuously cropped for the second growth cycle. In GCII, pot seedling treatments with T. viride T23 (three replicates) and without T. viride T23 (three replicates) were performed for growth index determination, while twelve pot seedlings were continuously cropped for the third growth cycle. In GCIII, pot seedling treatments with T. viride T23 (three replicates) and without T. viride T23 (three replicates) were performed for growth index determination, while six pot seedlings were continuously cropped for the fourth growth cycle. In GCIV, pot seedling treatments with T. viride T23 (three replicates) and without T. viride T23 (three replicates) were performed for growth index determination.
2.6. Data Analysis
Statistical analysis was conducted using Statistical Package for the Social Sciences (SPSS) software V25.0 (SPSS Inc., Beijing, China). Significant differences (p ≤ 0.05) between treatments were determined using Duncan’s multiple range test.
3. Results
3.1. Effect of Muskmelon Root Exudates on the Growth of T. viride T23
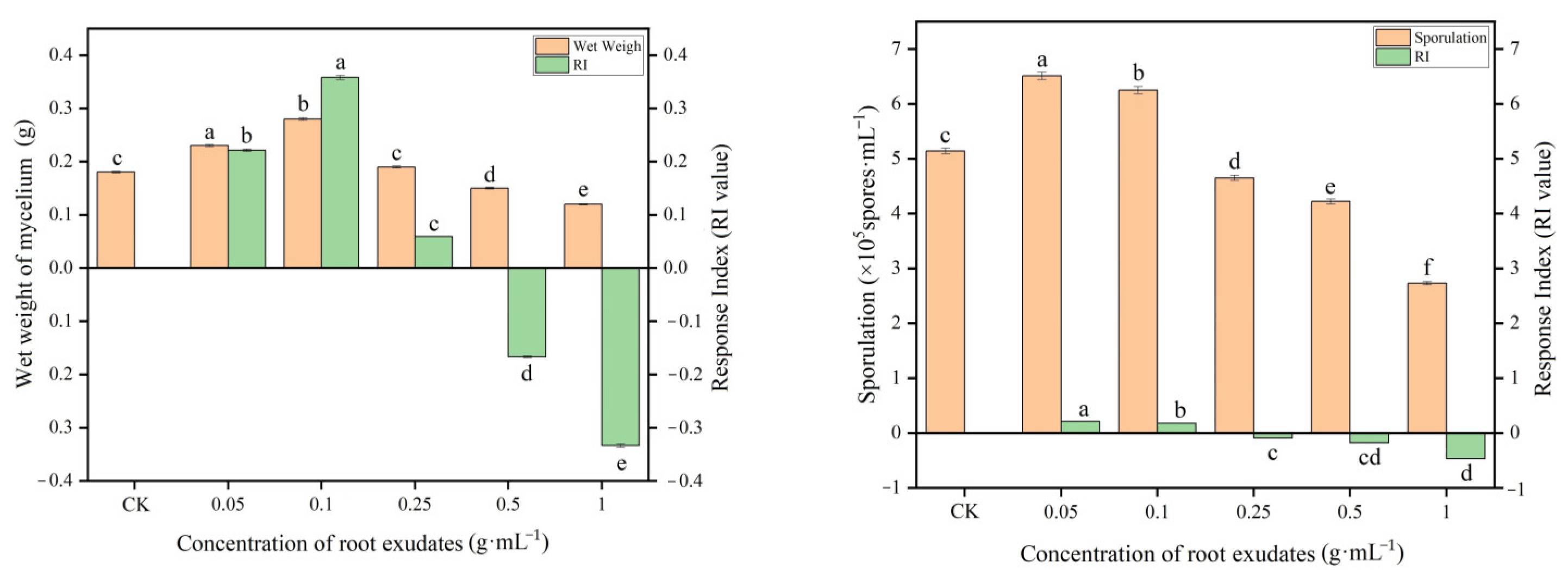
It was revealed from the results (Figure 4) that the root exudates from muskmelon had a significant effect on the mycelium growth and sporulation of T. viride T23. The root exudates of muskmelon were stimulatory to mycelium growth and the sporulation of T. viride T23 at concentrations of 0.05 and 0.1 g·mL−1, as compared with the control (0 g·mL−1). At 0.25 g·mL−1 concentration, the stimulatory effects on mycelium growth were minimal. The significant inhibitory effects on the growth and sporulation were found at 0.5 g·mL−1 and 1.0 g·mL−1.
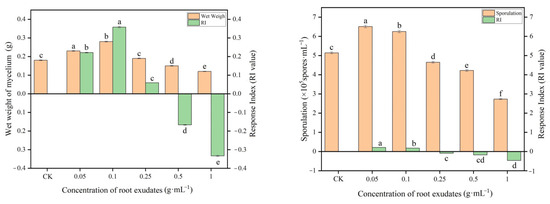
Figure 4.
Effects of muskmelon root exudates on mycelium growth and sporulation of T. viride T23. Note: Different lowercase letters indicate significant differences in mycelium growth and the sporulation of T. viride T23 at distinct concentrations of root exudates.
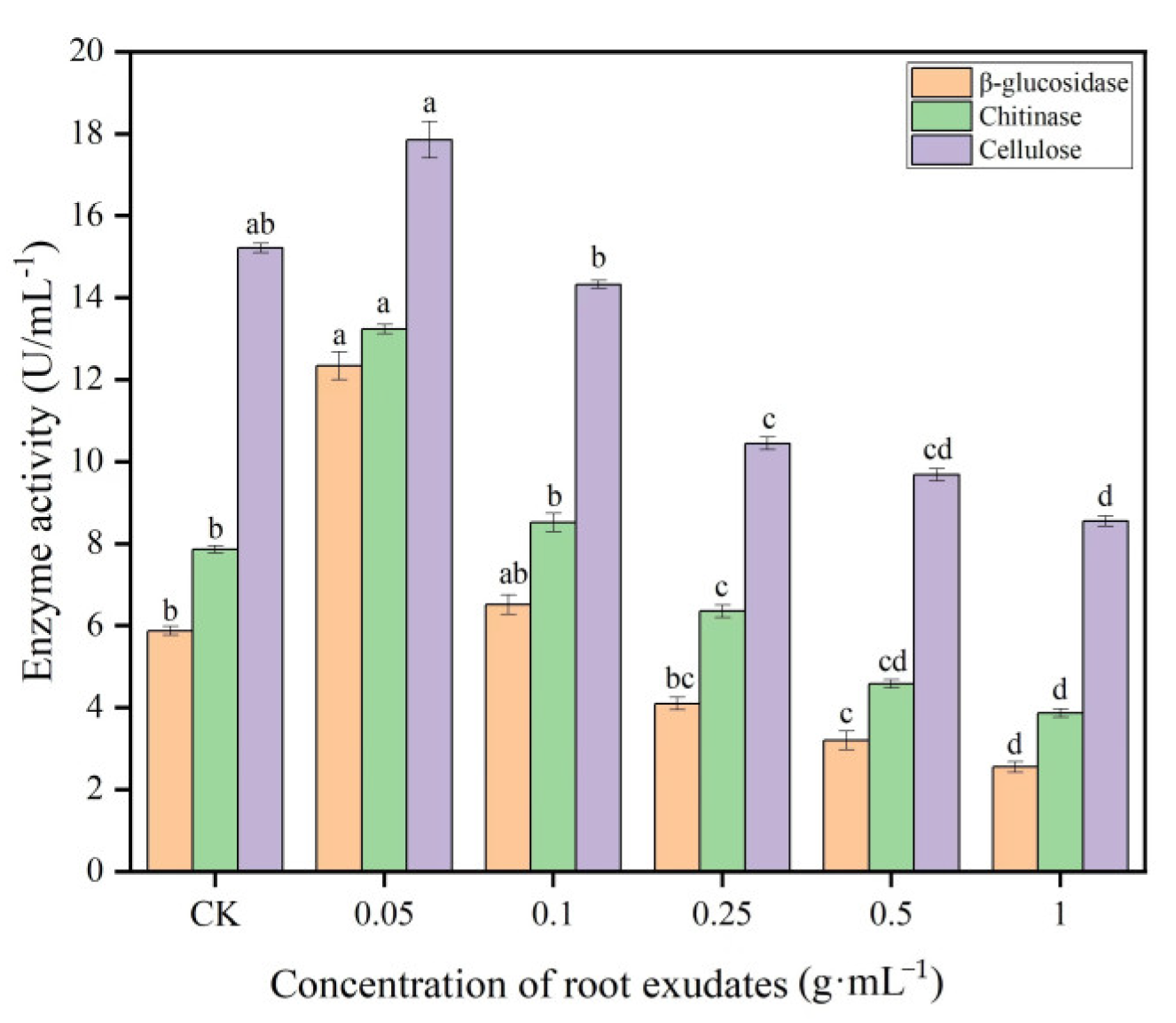
3.2. Effect of Muskmelon Root Exudates on Enzymatic Activity of T. viride T23
The enzyme biosynthesis of Trichoderma is influenced by cultural and environmental conditions, which are commercially available for agricultural use. The results of the effect of muskmelon root exudates on the enzymatic activity of T. viride T23 are summarized in Figure 5. The results showed that the root exudates of muskmelon had a significant effect on the enzymatic activity of T. viride T23, and high activities of β-glucosidase, chitinase and cellulase were observed at concentration of 0.05 g·mL−1 as compared with the control (0 g·mL−1), while there was no significant effect on the enzymatic activity of T. viride T23 at 0.25 g·mL−1 concentration. With the increasing concentration of muskmelon root exudates, the enzymatic activity of T. viride T23 showed the trend of decreasing. The lowest activities of β-glucosidase, chitinase and cellulase were observed at concentration of 1.0 g·mL−1 (2.55 U·mL−1, 38.67 U·mL−1, and 8.55 U·mL−1, respectively).
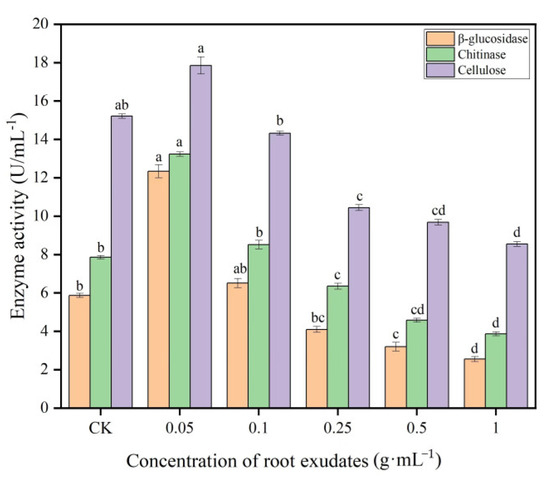
Figure 5.
Effects of muskmelon root exudates on enzymatic activity of T. viride T23. Note: Different lowercase letters indicate significant differences in enzymatic activity of T. viride T23 at distinct concentrations of root exudates.
3.3. Changes of the Phenolic Acid Concentration of the Replanted Muskmelon Soil
Due to the consistency in soil preparation, crop cultivation, and agricultural methods, the phenolic acids detected in the soil during four consecutive muskmelon growth cycles—including ρ-hydroxybenzoic acid, vanillic acid, syringic acid, vanillin, coumaric acid, ferulic acid, benzoic acid, and cinnamic acid—exhibited remarkable similarity, distinct from those found in unplanted soil (CK). The linear regression equations, limit of detection (LOD) and limit of quantitation (LOQ) for these nine phenolic acid standards are presented in Table 1.

Table 1.
Linear regression equations, LOD and LOQ of nine phenolic acids.
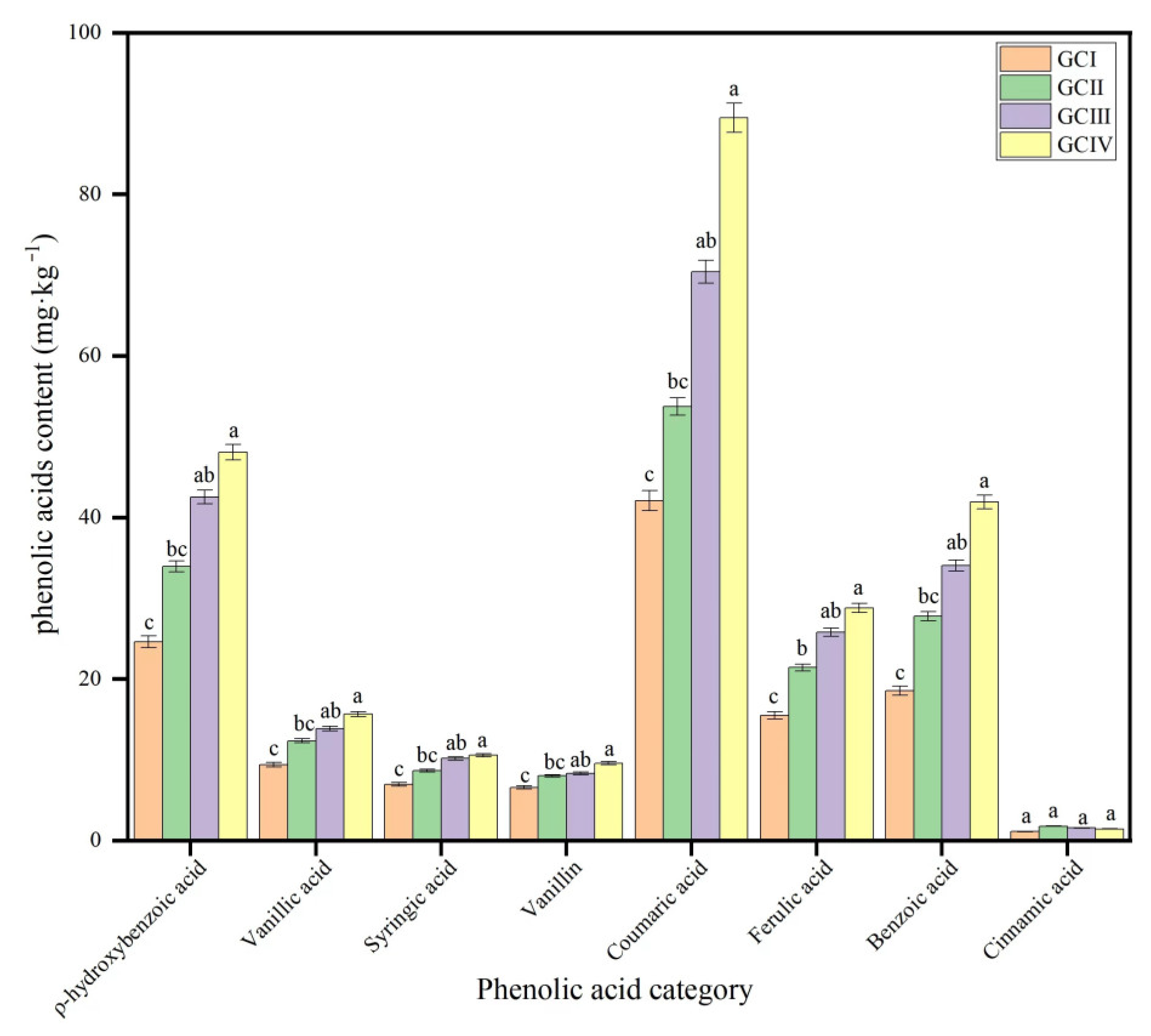
The concentration of the phenolic acid was different in the soil under different growth cycles, and coumaric acid, ρ-hydroxybenzoic acid, benzoic acid and ferulic acid were more abundant than vanillic acid, syringic acid, vanillin and cinnamic acid (Table 2, Figure 6 and Figure 7). With repeated cropping, the phenolic acid concentration in the rhizosphere soil of muskmelon gradually rose, with cinnamic acid being the exception, exhibiting a pattern of an initial increase followed by a subsequent decrease. Through the statistics, the statistical analysis revealed that the overall phenolic acid concentration in dry soil varied significantly, ranging from 124.68 mg·kg−1 to 257.94 mg·kg−1. This variation underscores the biochemical differences in rhizosphere soil between the initial and subsequent cropping cycles.

Table 2.
Contents of phenolic acids of continuous cropping soils in muskmelon (mg·kg−1 dry soil).
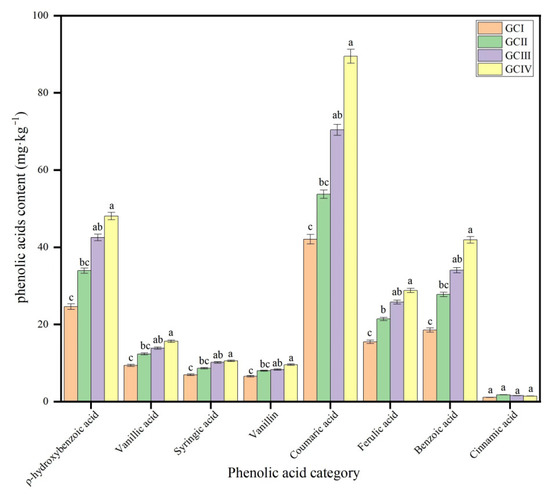
Figure 6.
Contents of phenolic acids of continuous cropping soils in muskmelon (mg·kg−1 dry soil). GCI: the first growth cycle, GCII: the second growth cycle, GCIII: the third growth cycle, GCIV: the fourth growth cycle of continuous cropping. Note: Different lowercase letters indicate significant differences in concentration of the phenolic acid in different growth cycles.
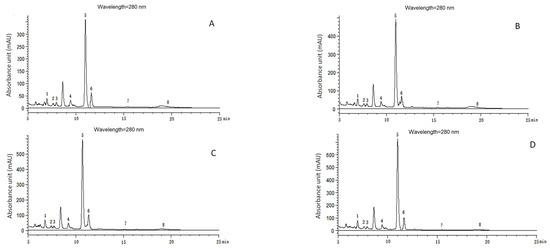
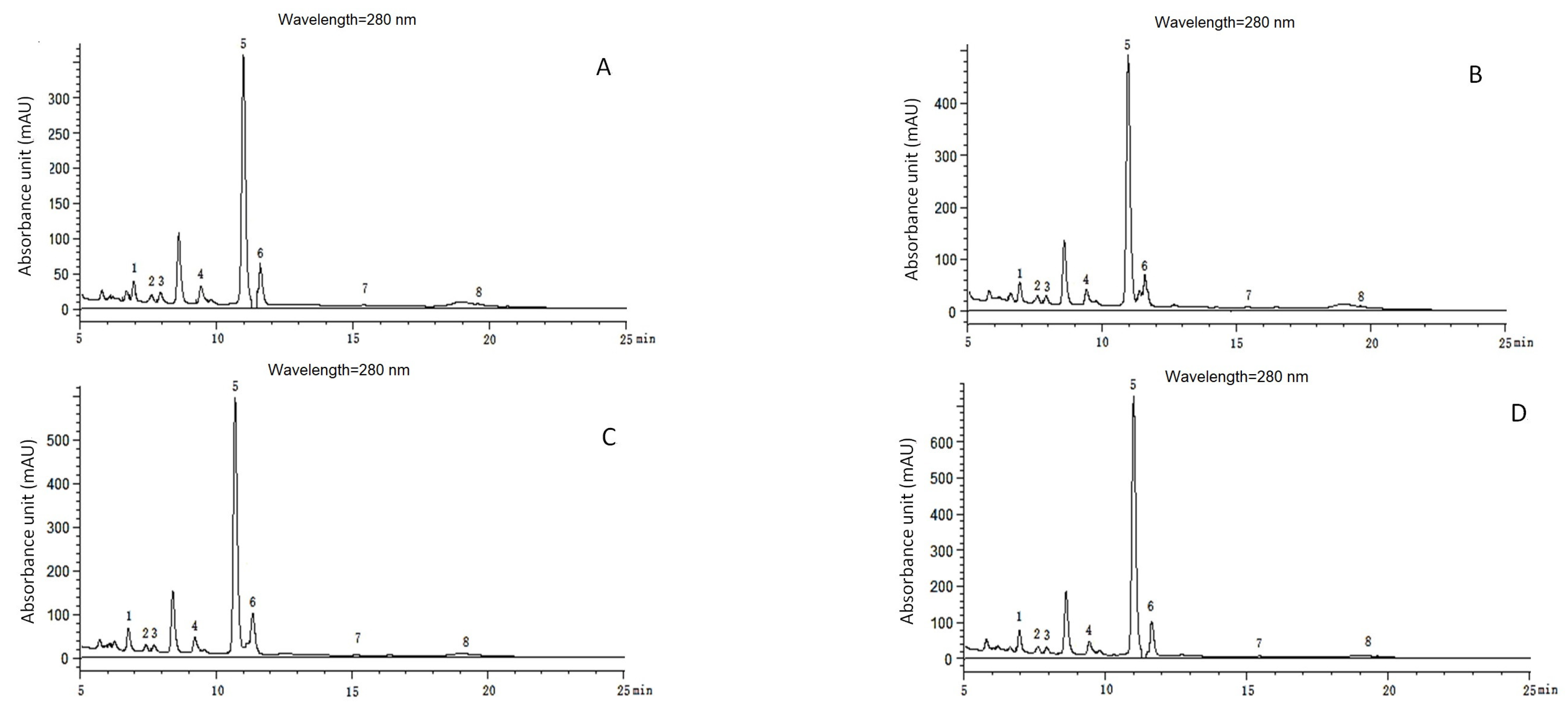
Figure 7.
Chromatograms of phenolic acids detected with HPLC in continuous growth cycles in soil of muskmelon. (A–D): Chromatograms of phenolic acid detected with HPLC in the soil from the first growth cycle to the fourth growth cycle. Phenolic acids: 1. ρ-hydroxybenzoic acid (6.833 min); 2. Vanillic acid (7.479 min); 3. Syringic acid (7.971 min); 4. Vanillin (9.475 min); 5. Coumaric acid (11.037 min); 6. Ferulic acid (11.612 min); 7. Benzoic acid (15.236 min); 8. Cinnamic acid (19.606 min).
3.4. Effect of Continuous Cropping on the Colonization of T. viride T23
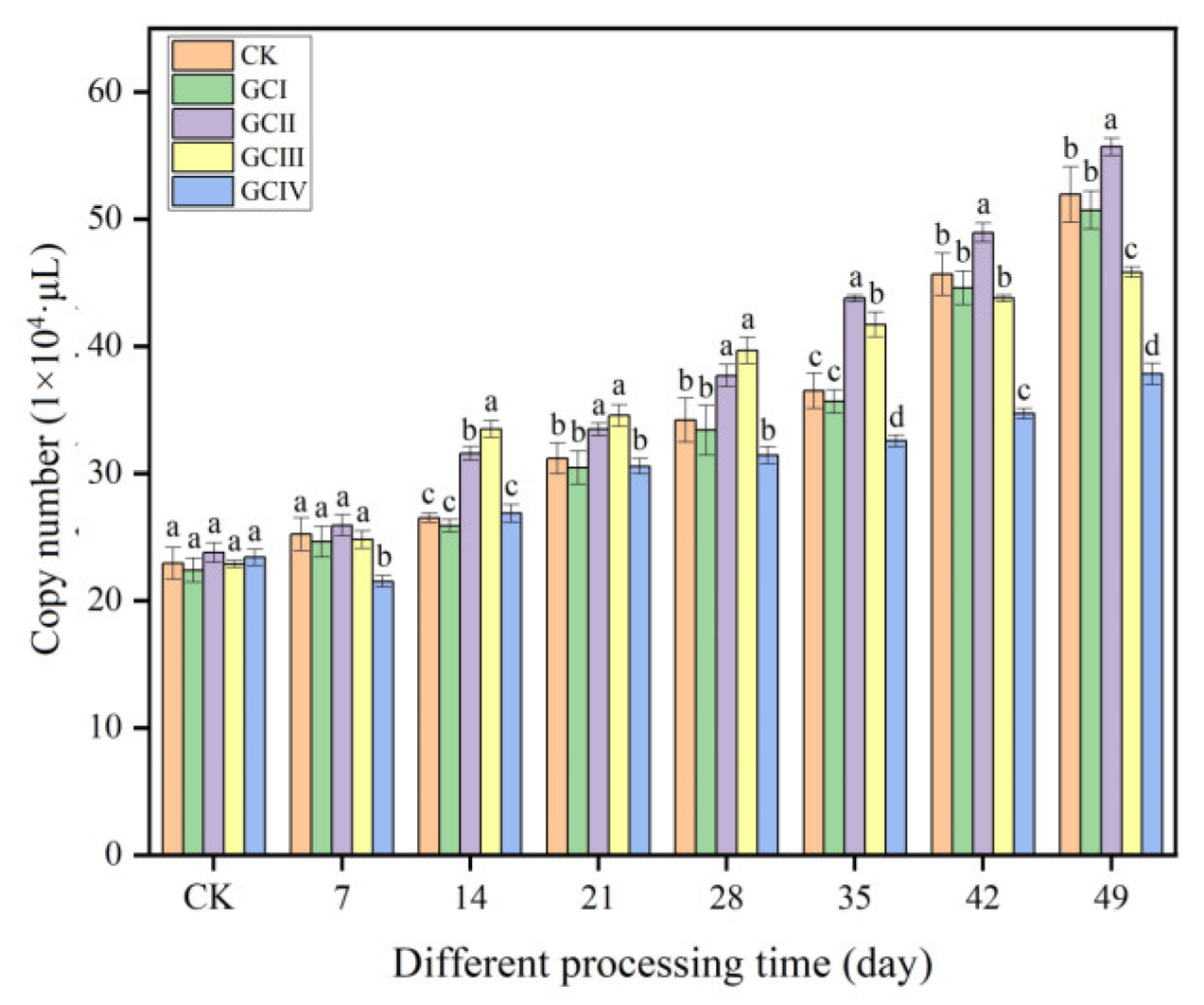
Within a period of 7 weeks, the quantities of Trichoderma in the soil of muskmelon, across various continuous growth cycles, demonstrated a clear trend of increasing, as illustrated in Figure 8. Similar to the ungrown muskmelon control, T. viride T23 effectively colonized the rhizosphere soil of muskmelon during both the first and second growth cycles. Specifically, at 49 days after inoculation, the population density reached 5.07 × 105 copies/μL in the first cycle (GCI) and 5.56 × 105 copies/μL in the second cycle (GCII). The colonization of T. viride T23 in the third growth cycle (GCIII) of continuously cropped soil exceeded that observed in GCI and GCII during the initial 14 to 28 days after inoculation. However, this colonization rate slowed down significantly from 35 to 49 days post-inoculation. In the fourth growth cycle (GCIV), the population density of T. viride T23 in the continuously cropped soil was significantly lower compared to that in GCI, GCII, and GCIII. Specifically, at 49 days after inoculation, the population density was 3.78 × 105 copies/μL and 3.58 × 105 copies/μL, respectively. This observation suggests that continuous cropping exerted a notable influence on the colonization pattern of T. viride T23 within the rhizosphere soil of muskmelon.
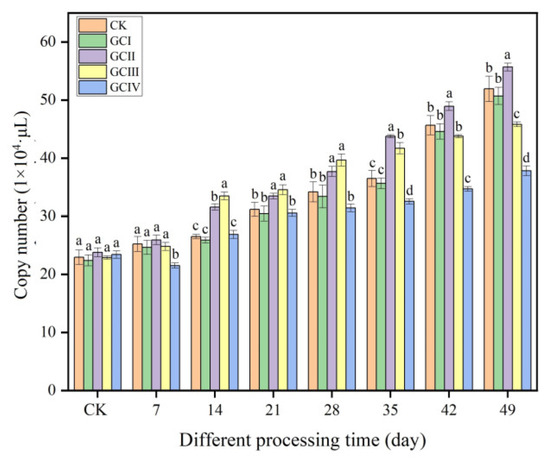
Figure 8.
Colonization of T. viride T23 strain in different continuous cropping soils of muskmelon. Note: Different lowercase letters indicate significant differences in colonization of T. viride T23 in different growth cycles.
3.5. Effect of Continuous Cropping on Promotional Role of T. viride T23
During the fruiting period of muskmelon plants, a significant difference was observed in the morphological characteristics between the treatments without Trichoderma and those with Trichoderma under continuous cropping conditions (Table 3). The presence of T. viride T23 led to a statistically significant increment of stem diameter, leaf area, fresh weight, dry weight and SPAD in the first cycle (GCI) and the second cycle (GCII) of continuous cropping, and we observed the improvement of the growth traits of the muskmelon plant. However, the growth-promoting effect of T. viride T23 on muskmelon was diminished during the third and fourth successive planting growth cycles.

Table 3.
Mean values of stem diameter, leaf area, fresh weight, dry weight and SPAD measured in the plants per treatment, from the first growth cycle to the fourth growth cycle of continuous cropping without T. viride T23 (GCI–GCIV), and from the first growth cycle to the fourth growth cycle of continuous cropping with T. viride T23 (GCI + T23–GCIV + T23).
4. Discussion
It has been demonstrated that root exudates play a crucial role in altering the microbial community structure of soil, potentially leading to imbalances and the exacerbation of soil sickness in continuously cropped fields [31,32]. It has also been reported that root exudates produced by muskmelon plants susceptible to F. oxysporum f. sp. melonis can have phytotoxic effects on muskmelon seed germination and seedling growth [3]. However, few studies showed that the effect of root exudates on the growth of the Trichoderma genus, which can promote root growth through the release of secondary metabolites and interaction after enrichment, which is essential for the survival and development of plants, allowing them to thrive in diverse environments [10,33]. In practical agricultural production, promoting the growth and controlling wilt disease in continuously cropped melon fields through the use of Trichoderma represents an effective biological control method [2]. Therefore, it is crucial to investigate the impact of continuous cropping on the growth, enzyme activity, and colonization patterns of Trichoderma, which is beneficial for developing a comprehensive and systematic approach to the application of Trichoderma in melon production.
As a locally isolated fungal strain, T. viride T23 exhibits significant effectiveness in promoting the growth of muskmelon and controlling Fusarium wilt, which is attributed to its antagonistic mechanisms, including mycoparasitism, competition for space, antibiosis, and the production of cell wall-degrading enzymes (CWDEs) [17,34]. The findings of this study revealed that, within a certain dosage range, root exudates from muskmelon significantly stimulate the mycelium growth, sporulation, and activity of cell wall-degrading enzymes (β-glucosidase, chitinase, and cellulase) in T. viride T23. However, at higher dosages, these exudates exert an inhibitory effect. In a previous study, the application of root exudates from muskmelon exhibited stimulatory effects on the mycelium growth of F. oxysporum f. sp. melonis, even at a comparable dosage range [3]. This observation suggests that interacting fungal species may respond in a similar manner to identical root exudates, thereby emphasizing the intricate nature of fungal interactions within soil ecosystems. These findings are consistent with previous reports indicating that phenolic acids present in root exudates can disrupt the uniformity of beneficial fungi and alter the soil microbial community, ultimately leading to the exacerbation of soil sickness. This suggests that the composition and concentration of root exudates play a crucial role in shaping the soil microbial environment and its impact on plant health [35,36]. Considering the pivotal role of root exudates’ composition and concentration in influencing the soil microbial environment and subsequently plant health, further investigation into the enzymes involved in soil—Trichoderma—pathogen interaction within the rhizosphere is warranted.
The widespread practice of continuously cropping melons in China, aimed at maximizing the productivity per unit area of cultivated land, has unfortunately led to the deterioration of the soil micro-ecological environment. This degradation can have negative impacts on soil fertility, microbial diversity, and ultimately, crop health and yield. It is crucial to address this issue to ensure sustainable agricultural production and maintain the integrity of the soil ecosystem [1,4,37]. There is substantial evidence that the accumulation of phenolic acids breaks the ecological balance of the soil microbial community in rhizospheric soil, which is important for maintaining soil quality, regulating the biochemical cycle, and the formation of soil structure [38,39]. Previous studies have indeed demonstrated the presence of phenolic acids, such as cinnamic acid, benzoic acid, and ferulic acid, in the root exudates of muskmelon, which have been found to exert inhibitory effects on crop growth and contribute to the increased incidence of Fusarium wilt. Understanding the role of these phenolic acids in shaping soil microbial communities and their impact on crop health is crucial for developing sustainable agricultural practices and mitigating soil sickness [3,10]. In this study, in addition to the above three phenolic acids, ρ-hydroxybenzoic acid, vanillic acid, syringic acid, vanillin and coumaric acid were found to increase significantly following four growth cycles of continuous cropping. The expression analysis of Trichoderma in the rhizosphere soil of continuously cropped muskmelon crops indicates that the accumulation of phenolic acids results in alterations in the colonization pattern of T. viride T23. This finding suggests that phenolic acids play a significant role in influencing the interaction between Trichoderma and the soil microbial community, potentially affecting the biocontrol capabilities of Trichoderma against soil-borne diseases. Under the second growth cycle of continuous cropping, the accumulation of phenolic acids initially led to an increase in the amount of T. viride T23 colonizing the rhizosphere soil. However, during the third and fourth growth cycles, it was observed that despite the continued accumulation of phenolic acids, the amount of T. viride T23 colonization significantly decreased compared to the previous two cycles. This suggests that while phenolic acids may initially stimulate the growth of T. viride T23, their continuous accumulation over multiple growth cycles may have a negative impact on its colonization, potentially due to the disruption of the soil microbial balance or the inhibitory effects of certain phenolic acids on Trichoderma. This might be because the accumulation of phenolic acids can alter or modify the soil microbiota to form host-specific microbial communities, and managing the levels of phenolic acids in the rhizosphere may be crucial for optimizing the use of Trichoderma as a biocontrol agent in sustainable agriculture [40,41].
Indeed, studies have demonstrated that the biocontrol effectiveness of Trichoderma species is significantly influenced by soil physical and chemical properties. These properties, which are vital to the overall functioning of agricultural systems, play a crucial role in determining the colonization and activity of Trichoderma in the rhizosphere soil. When the biocontrol effect is relatively poor, it is often associated with the failure of the inoculated Trichoderma species to successfully colonize the rhizosphere soil [42,43]. This failure can be attributed to various factors, including unfavorable soil conditions such as high salinity, low organic matter content, or pH extremes. Such conditions can hamper the growth and colonization of Trichoderma, thereby reducing its biocontrol capabilities. Therefore, it is essential to carefully assess and manage soil physical and chemical properties to ensure optimal conditions for Trichoderma colonization and activity [44,45]. Indeed, there is limited information available on the specific effects of allelochemicals or phenolic acids on the function of Trichoderma species in vivo [10,46]. Allelochemicals, including phenolic acids, are naturally occurring compounds produced by plants and microorganisms that can have diverse biological activities in the soil environment. While these compounds have been shown to affect microbial populations and processes in general, their specific impacts on Trichoderma species and their biocontrol capabilities remain largely unexplored. Understanding how allelochemicals interact with Trichoderma in vivo is crucial for predicting and optimizing the performance of these biocontrol agents in agricultural systems [47,48].
In practical agricultural production, Trichoderma species maintain a crucial relationship with soil type, cultivated crops, and fertilization practices. The use of Trichoderma may sometimes fail to improve crop yield and produce timely disease resistance. This could be due to several reasons related to the period of inoculation and the application amount, which may not support colonization survival or adaptation to soil conditions [49,50]. The experimental setup of our study encompassed four consecutive planting cycles without crop rotation or soil resting periods in between, which is not a common production mode. Nonetheless, understanding the impact of continuous cropping on beneficial microorganisms like Trichoderma can inform principles and methods for their effective utilization in real-world agricultural scenarios. By summarizing these effects, we can better guide the application of Trichoderma in actual production settings [48]. Future research endeavors should strive to pinpoint the precise mechanisms driving this decline and investigate measures to alleviate its consequences. Potential avenues of exploration could encompass studying the interactions between Trichoderma and other soil microorganisms, evaluating the effect of soil amendments or crop rotation on Trichoderma’s efficacy, or cultivating novel Trichoderma strains that exhibit greater resilience within continuous cropping systems. Through comprehending and tackling these obstacles, we can guarantee the enduring and efficient utilization of Trichoderma and other biocontrol agents in enhancing plant vitality and productivity within agricultural frameworks. In this study, to ascertain whether significant differences existed between treatment groups, the Duncan range test was employed in SPSS software subsequent to conducting a one-way analysis of variance (ANOVA). The Duncan test serves as a post hoc comparison method tailored to comparing means, making it particularly apt for our modest sample dataset. Additionally, it offers an intuitive letter-marking system that represents noteworthy disparities between groups. Adhering to the standard SPSS operational protocol, we established a significance level of 0.05 to ascertain substantial differences in mean values among the groups. Leveraging the outcomes of the Duncan test, we pinpointed the groups exhibiting significant disparities and elaborated on their significance in relation to our study hypothesis in the Discussion section. For our analysis, we utilized SPSS version 25.0. While the Duncan test proved suitable for this investigation, we acknowledge its potential limitations in handling all types of data distributions. Consequently, we are considering incorporating additional complementary analytical techniques in future endeavors to bolster the robustness of our findings.
5. Conclusions
The current study has revealed that when T. viride T23 is applied under conditions of continuous cropping, it effectively enhances plant development by increasing plant height, fresh weight, and dry weight, among other parameters. However, it is noteworthy that the beneficial effects of this biocontrol agent gradually diminish as the number of consecutive cropping growth cycles increases. This observation suggests that the soil environment and its associated microbial communities undergo changes over time under continuous cropping, potentially affecting the performance of T. viride T23. Changes in soil physical and chemical properties, the accumulation of allelochemicals or phenolic acids, and alterations in the soil microbial community structure are among the factors that could contribute to the decline in Trichoderma’s biocontrol effectiveness.
Author Contributions
Conceptualization, R.Y. and J.W.; methodology, R.Y. and L.Z.; software, R.Y. and B.L.; validation, R.Y., A.T. and Z.Y.; investigation, R.Y., H.W. and J.L.; formal analysis, R.Y. and A.T.; writing-original draft preparation, J.W.; visualization, R.Y.; supervision, R.Y.; project administration, R.Y.; funding acquisition, R.Y. and Y.X. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Liaoning Provincial Natural Science Foundation—Innovation Capacity Improvement Joint Fund Project, grant number 2022-NLTS-17-03, and the Liaoning Provincial Joint Fund Project General funding plan project, grant number 2023-MSLH-212.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
We sincerely express our heartfelt gratitude to all the students and staff who generously contributed their insights and perspectives to this study. Additionally, we would like to extend our deepest thanks to the anonymous reviewers who offered valuable and constructive feedback on the manuscript, greatly enhancing its quality and impact. Their dedication and expertise have been invaluable in shaping this work, and we are deeply appreciative of their contributions.
Conflicts of Interest
The authors declare that there are no conflicts of interest associated with this study. The funding sources did not influence any aspect of the research process, including the study design, data collection, analysis, interpretation, manuscript preparation, or the decision to publish the results. The authors maintain complete independence in conducting and reporting their findings.
References
- Wonglom, P.; Sunpapao, A. Fusarium incarnatum is associated with postharvest fruit rot of muskmelon (Cucumis melo). J. Phytopath. 2020, 168, 204–210. [Google Scholar] [CrossRef]
- Gava, C.A.T.; Pinto, J.M. Biocontrol of melon wilt caused by Fusarium oxysporum Schlect f. sp. melonis using seed treatment with Trichoderma spp. and liquid compost. Biol. Control 2016, 97, 13–20. [Google Scholar]
- Yang, R.X.; Gao, Z.G.; Liu, X.; Yao, Y.; Cheng, Y. Root exudates from muskmelon (Cucumis melon. L) induce autotoxicity and promote growth of Fusarium oxysporum f. sp. melonis. Allelopath. J. 2014, 33, 175–188. [Google Scholar]
- Wipornpan, N.; Worawoot, A.; Jaturong, K.; Saisamorn, L.; Nakarin, S. Evaluation of a newly identified endophytic fungus, Trichoderma phayaoense for plant growth promotion and biological control of gummy stem blight and wilt of muskmelon. Front. Microbiol. 2021, 21, 634772. [Google Scholar]
- Gauri, S.S.; Mandal, S.M.; Dey, S.; Pati, B.R. Biotransformation of p-coumaric acid and 2,4-dichlorophenoxy acetic acid by Azotobacter sp. strain SSB81. Bioresour. Technol. 2012, 126, 350–353. [Google Scholar] [CrossRef]
- Wu, H.; Lin, W. A commentary and development perspective on the consecutive monoculture problems of medicinal plants. Chin. J. Eco-Agric. 2020, 28, 775–793. [Google Scholar]
- Siles, J.A.; Margesin, R. Abundance and diversity of bacterial, archaeal, and fungal communities along an altitudinal gradient inalpine forest soils: What are the driving factors? Microb. Ecol. 2016, 72, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, J.; Huang, W.; Wu, H.; Chen, J.; Yang, Y. Plant-microbe rhizosphere interactions mediated by Rehmannia glutinosa root exudates under consecutive monoculture. Sci. Rep. 2015, 5, 15871. [Google Scholar] [CrossRef]
- Raklami, A.; Bechtaoui, N.; Tahiri, A.; Anli, M.; Meddich, A.; Oufdou, K. Use of rhizobacteria and mycorrhizae consortium in the open field as a strategy for improving crop nutrition, productivity and soil fertility. Front. Microbiol. 2019, 10, 1106. [Google Scholar] [CrossRef]
- Wang, S.N.; Yang, R.X.; Liu, X.; Yao, Y.; Gao, Z.G. Overcoming the autotoxicity of muskmelon by Trichoderma spp. Allelopath. J. 2016, 39, 29–42. [Google Scholar]
- Ioanna, K.; Alexandros, T.; Antonios, M.; Angeliki, K.; Stella, K.; Charikleia, Z.; Varvara, K.; Artemis, K.; Antigolena, F.; Aristidis, K. Effect of colonization of Trichoderma harzianum on growth development and CBD content of hemp (Cannabis sativa L.). Microorganisms 2021, 9, 518. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Wang, W.W.; Xue, M.; Liu, Z.; Zhang, Q.M.; Hou, J.M.; Xing, M.Y.; Wang, R.; Liu, T. The combination of a biocontrol agent Trichoderma asperellum SC012 and hymexazol reduces the effective fungicide dose to control fusarium wilt in cowpea. J. Fungi 2021, 7, 685. [Google Scholar] [CrossRef] [PubMed]
- Hewedy, O.A.; Abdel-lateif, K.S.; Bakr, R.A. Genetic diversity and biocontrol efficacy of indigenous Trichoderma isolates against Fusarium wilt of pepper. J. Basic Microb. 2019, 60, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Metwally, R.A.; Al-Amri, S.M. Individual and interactive role of Trichoderma viride and arbuscular mycorrhizal fungi on growth and pigment content of onion plants. Lett. Appl. Microbiol. 2020, 70, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Bunbury-Blanchette, A.L.; Walker, A.K. Trichoderma species show biocontrol potential in dual culture and greenhouse bioassays against Fusarium basal rot of onion. Biol. Control 2019, 130, 127–135. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J. Integr. Agric. 2015, 14, 1588–1597. [Google Scholar] [CrossRef]
- Tang, B.; Dong, Y.; He, M.; Liu, J.; Wu, K.; Guan, H. Effects of different planting years of healthy Panax notoginseng on the rhizosphere microbial community in Wenshan of Yunnan province. J. Microbiol. 2020, 9, 2857–2866. [Google Scholar]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Beltrán-Peña, E.; Herrera-Estrella, A.; López-Bucio, J. Trichoderma-induced plant immunity likely involves both hormonal- and camalexin-dependent mechanisms in Arabidopsis thaliana and confers resistance against necrotrophic fungi Botrytis cinerea. Plant Signal. Behav. 2011, 6, 1554–1563. [Google Scholar] [CrossRef]
- Bastakoti, S.; Belbase, S.; Manandhar, S.; Arjyal, C. Trichoderma species as biocontrol agent against soil borne fungal pathogen. Nepal J. Biotechnol. 2017, 5, 39–45. [Google Scholar] [CrossRef]
- Yariv, B.; Udi, L.; Alvaro, C.I.; Tohge, T.; Alisdair, R.F.; Chet, A.V.; Lothar, W. Trichoderma-Plant Root Colonization: Escaping EarlyPlant Defense Responses and Activation of the Antioxidant Machinery for Saline Stress Tolerance. PLoS Pathog. 2013, 9, e1003221. [Google Scholar]
- Montaser, F.A. Improvement of biocontrol of damping-off and root rot/wilt of faba bean by salicylic acid and hydrogen peroxide. Mycobiology 2013, 41, 47–55. [Google Scholar]
- Asad, S.A.; Ali, N.; Hameed, A.; Khan, S.A.; Ahmad, R.; Bilal, M.; Shahzad, M.; Tabassum, A. Biocontrol efficacy of different isolates of Trichoderma against soil borne pathogen Rhizoctonia solani. Pol. J. Microbiol. 2014, 63, 95–103. [Google Scholar] [CrossRef]
- Mona, S.A.; Hashem, A.; Abd-Allahm, E.F.; Alqarawi, A.A.; Soliman, D.W.K.; Wirth, S. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 2017, 16, 1751–1757. [Google Scholar] [CrossRef]
- Tang, L.; Gao, Z.G.; Zhuagn, J.A.; Zhao, H. Effect of microelements on genetic variation of biocontrol agent Trichoderma T23. J. Shenyang Agric. Univ. 2008, 39, 423–426. (In Chinese) [Google Scholar]
- Willamson, G.B.; Richardson, D. Bioassays for Allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol. 1988, 14, 181–187. [Google Scholar] [CrossRef]
- Saleh, R.M.; Kabli1, S.A.; Al-Garni1, S.M.; Al-Ghamdi, M.A.; Abdel-Aty, A.M.; Mohamed, S.A. Solid-state fermentation by Trichoderma viride for enhancing phenolic content, antioxidant and antimicrobialactivities in ginger. Microbiology 2018, 67, 161–167. [Google Scholar]
- Abu-Tahon, M.A.; Isaac, G.S. Anticancer and antifungal efficiencies of purified chitinase produced from Trichoderma viride under submerged fermentation. J. Gen. Appl. Microbiol. 2020, 66, 32–40. [Google Scholar] [CrossRef]
- Faria, M.L.; Kolling, D.; Camassola, M. Comparison of Pennicillium echinulatum and Trichoderma reesei cellulases in relation to their activity against various cellulosic substrates. Biores. Technol. 2008, 99, 1417–1424. [Google Scholar]
- Wang, Y.Y.; Du, J.; Gao, K.X. Screening and identification of biocontrol Trichoderma to wilt of bitter gourd and detection of its colonization by qPCR. Shandong Agric. Sci. 2018, 50, 110–115. (In Chinese) [Google Scholar]
- Cai, H.G.; Mi, G.H.; Chen, F.J. Genotypic variation of leaf SPAD value, nitrogen and nitrate content in maize. Plant Nutr. Fertil. Sci. 2010, 16, 866–873. [Google Scholar]
- Jin, X.; Wu, F.; Zhou, X. Different toxic effects of ferulic and p-hydroxybenzoic acids on cucumber seedling growth were related to their different influences on rhizosphere microbial composition. Biol. Fertil. Soils 2020, 56, 125–136. [Google Scholar] [CrossRef]
- Phoka, N.; Suwannarach, N.; Lumyong, S.; Matsui, K.; Arikit, S.; Sunpapao, A. Role of Volatiles from the Endophytic Fungus Trichoderma asperelloides PSU-P1 in Biocontrol Potential and in Promoting the Plant Growth of Arabidopsis thaliana. J. Fungi 2020, 6, 341. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E. Trichoderma-Not just for biocontrol anymore. Phytoparasitica 2011, 39, 103–108. [Google Scholar] [CrossRef]
- Troian, R.F.; Steindorff, A.S.; Ramada, M.H.; Arruda, W.; Ulhoa, C.J. Mycoparasitism studies of Trichoderma harzianum against Sclerotinia sclerotiorum: Evaluation of antagonism and expression of cell wall-degrading enzymes genes. Biotechnol. Lett. 2014, 36, 2095–2101. [Google Scholar] [CrossRef]
- Chen, S.L.; Zhou, B.L.; Lin, S.S.; Li, X.; Xi, H.J.; Yin, Y.L.; Ye, X.L. Allelopathic effects of cinnamic acid and vanillin on soil microbes, soil enzymes activities and growth of grafted eggplants. Allelopath. J. 2011, 28, 29–40. [Google Scholar]
- Martínez-Medina, A.; Del Mar Alguacil, M.; Pascual, J.A.; Van Wees, S.C. Phytohormone profiles induced by Trichoderma isolates correspond with their biocontrol and plant growth-promoting activity on melon plants. J. Chem. Ecol. 2014, 40, 804–815. [Google Scholar] [CrossRef]
- Yin, C.M.; Wang, G.S.; Li, Y.Y.; Che, J.S.; Shen, X.; Chen, X.S. A new method for analysis of phenolic acids in the soil-soil from replanted apple orchards was investigated. China Agric. Sci. 2013, 46, 4612–4619. (In Chinese) [Google Scholar]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Miki, T.; Doi, H. Leaf phenological shifts and plant-microbe-soil interactions can determine forest productivity and nutrient cycling under climate change in an ecosystem model. Ecol. Res. 2016, 31, 263–274. [Google Scholar] [CrossRef]
- Luo, L.; Yang, L.; Yan, Z.; Jiang, B.; Li, S.; Huang, H. Ginsenosides in root exudates of Panax notoginseng drive the change of soil microbiota through carbon source different utilization. Plant Soil. 2020, 455, 139–153. [Google Scholar] [CrossRef]
- Benizri, E.; Baudoin, E.; Guckert, A. Root Colonization by Inoculated Plant Growth-Promoting Rhizobacteria. Biocontrol Sci. Technol. 2001, 11, 557–574. [Google Scholar] [CrossRef]
- Ruangwong, O.U.; Pornsuriya, C.; Pitija, K.; Sunpapao, A. Biocontrol mechanisms of Trichoderma koningiopsis PSU3-2 against postharvest anthracnose of chili pepper. J. Fungi 2021, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- Ru, Z.; Di, W. Trichoderma spp. from rhizosphere soil and their antagonism against Fusarium sambucinum. Afr. J. Biotechnol. 2012, 11, 4180–4186. [Google Scholar]
- Intana, W.; Kheawleng, S.; Sunpapao, A. Trichoderma asperellum T76-14 released volatile organic compounds against postharvest fruit rot in muskmelons (Cucumis melo) caused by Fusarium incarnatum. J. Fungi 2021, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.; Pozo, M.J. Mycorrhiza-Induced Resistance and Priming of Plant Defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Bai, Y.X.; Wang, G.; Cheng, Y.D.; Shi, P.Y.; Yang, C.C.; Yang, H.W.; Xu, Z.L. Soil acidification in continuous lycropped tobacco alters bacterial community structure and diversity via the accumulation of phenolic acids. Sci. Rep. 2019, 9, 12499. [Google Scholar] [CrossRef] [PubMed]
- Blum, U.; Gerig, T.M. Relationships between phenolic acid concentrations, transpiration, water utilization, leaf area expansion, and uptake of phenolic acids: Nutrient culture studies. J. Chem. Ecol. 2005, 31, 1907–1932. [Google Scholar] [CrossRef] [PubMed]
- Kefeli, V.I.; Kalevitch, M.V.; Borsari, B. Phenolic cycle in plants and environment. J. Cell Mol. Biol. 2003, 2, 13–18. [Google Scholar]
- Wu, J.J.; Zhu, J.H.; Zhang, D.Q.; Cheng, H.Y.; Hao, B.Q.; Cao, A.C.; Yan, D.D.; Wang, Q.X.; Li, Y. Beneficial effect on the soil microenvironment of Trichoderma applied after fumigation for cucumber production. PLoS ONE 2022, 17, e0266347. [Google Scholar] [CrossRef]
- James, T.T.; Harting, R.; Samer, S.; Charles, M.K.; Gerhard, H.B.; Benjamin, A.H. Adhesion as a Focus in Trichoderma–Root Interactions. J. Fungi 2022, 8, 372. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).