Effects of Foliar Ca and Mg Nutrients on the Respiration of ‘Feizixiao’ Litchi Pulp and Identification of Differential Expression Genes Associated with Respiration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Treatment
2.2. Procedures of Sample Collection
2.3. Determination of Total Sugar, Total Acids, and Sugar–Acid Ratio
2.4. Measurement of Total Respiration Rate and Rates of TCA, PPP, and EMP
2.5. Detection of Succinate Dehydrogenase (SDH) and Pyruvate Dehydrogenase Complex (PDC)
2.6. Detection of Glucose Phosphate Isomerase (GPI), Pyruvate Kinase (PK)
2.7. Detection of Malate Dehydrogenase (NAD-MDH) Activity
2.8. Detection of Cytochrome Oxidase (CCO)
2.9. Analysis of Differential Gene Screening and Pathway Selection
2.10. Validation of qRT-PCR
2.11. Data Analysis
3. Results
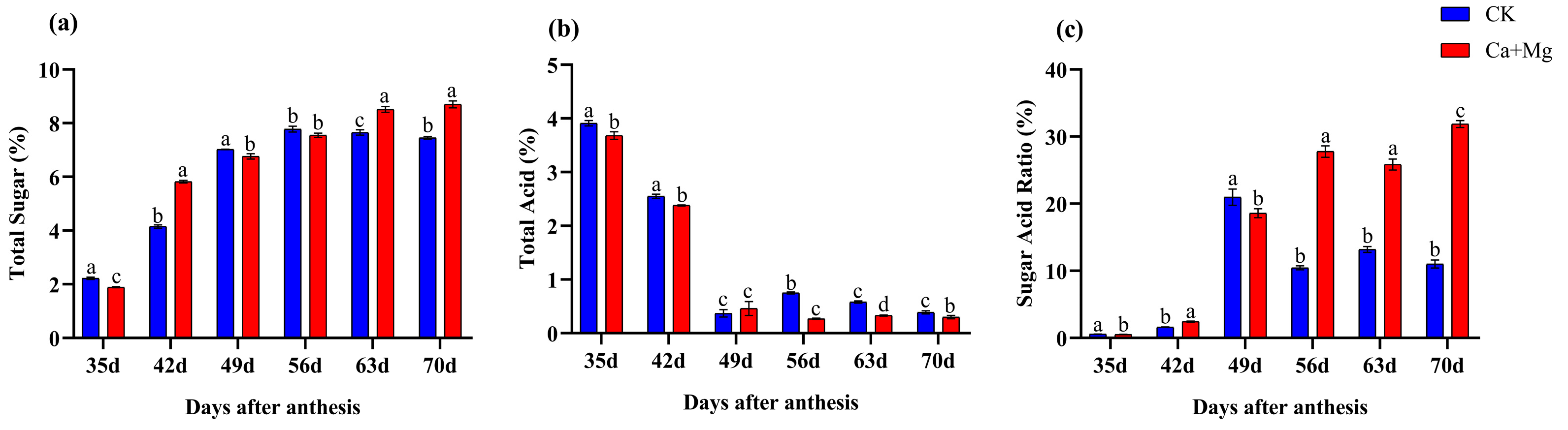
3.1. Changes in Sugar and Acid Contents in Pulp
3.2. Different Changes in Respiration Rate in Pulp
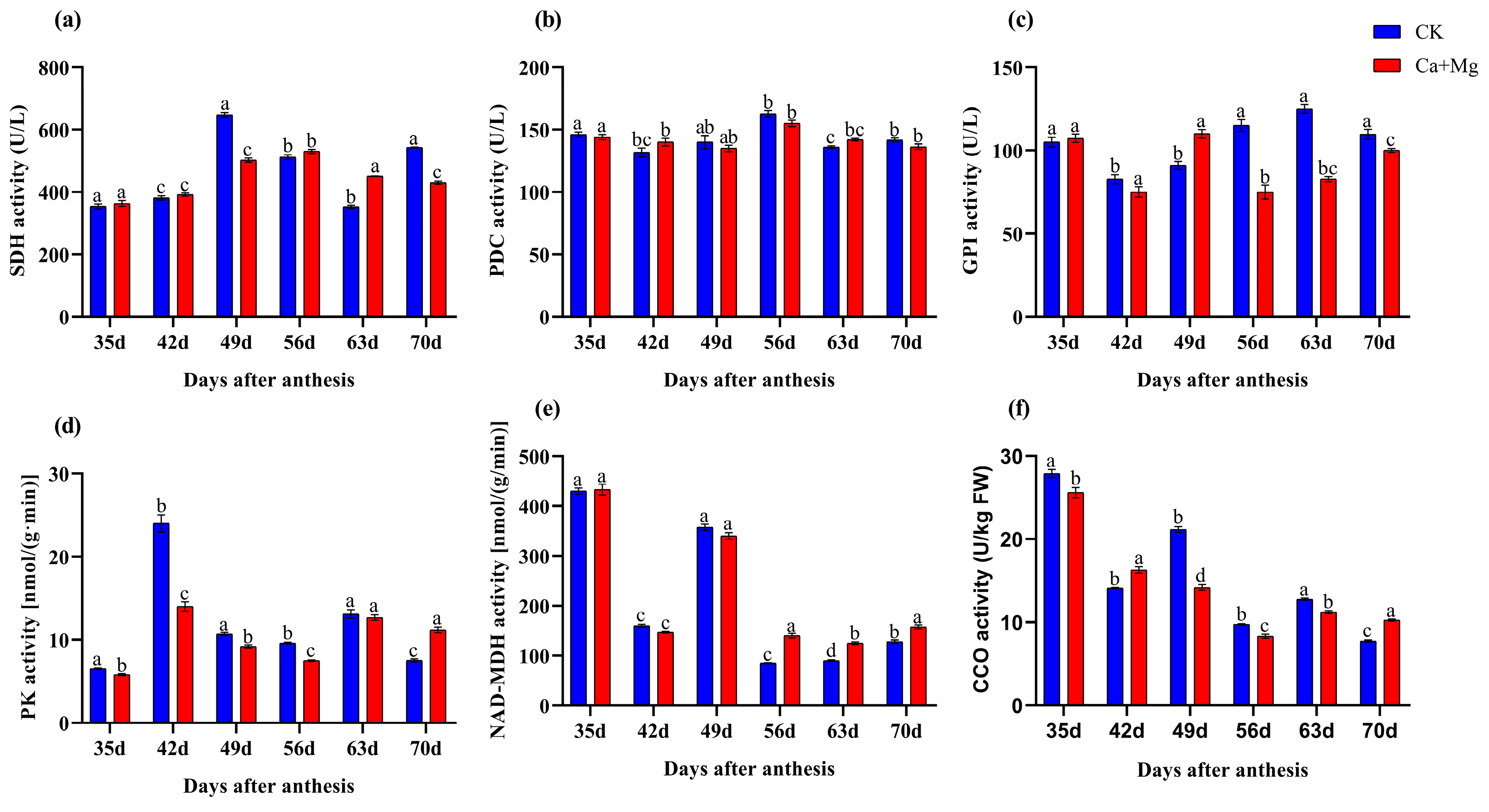
3.3. Changes in Enzyme Activities in Pulp
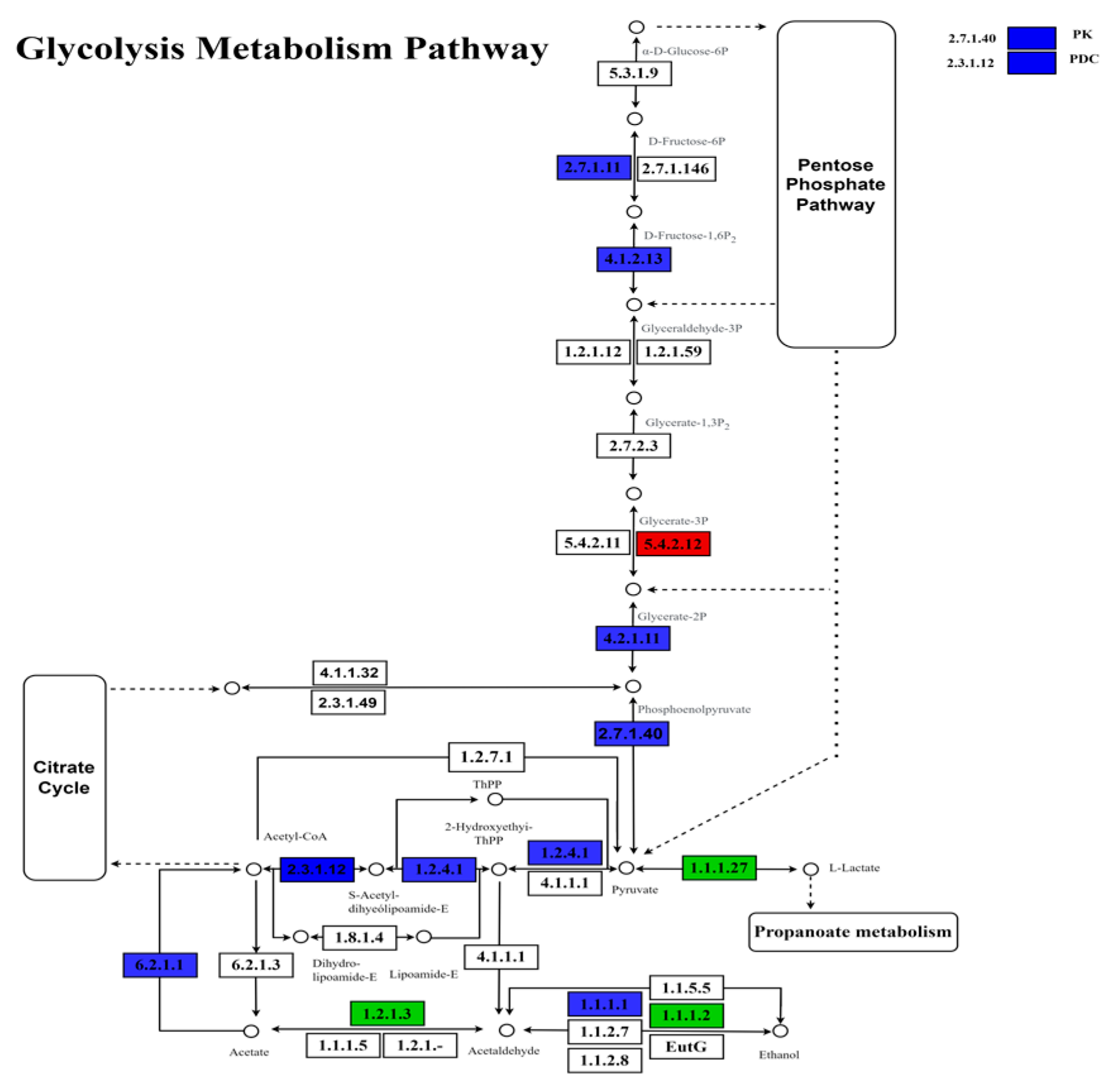
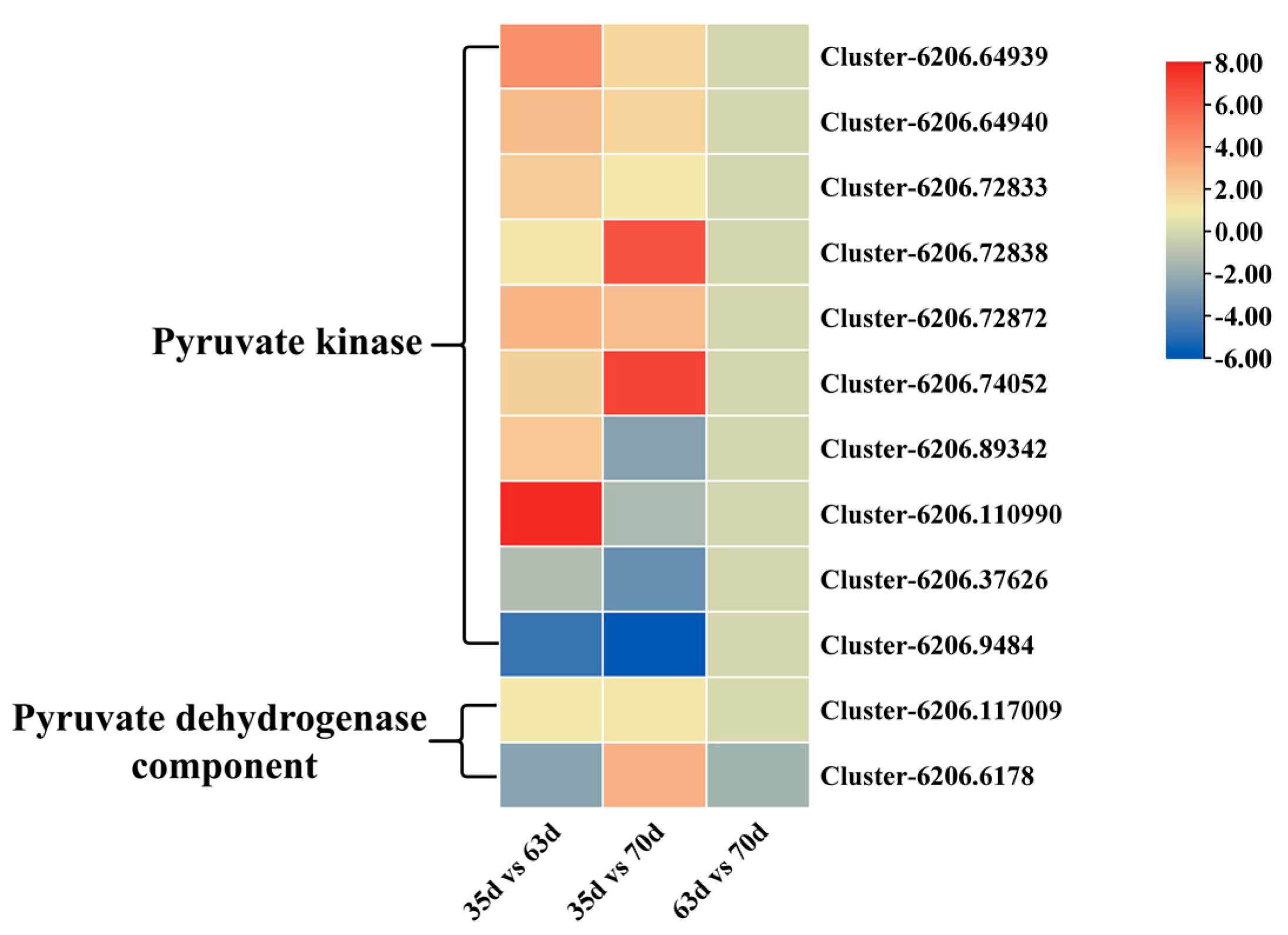
3.4. Differential Gene Screening and Role of EMP, PPP, and TCA
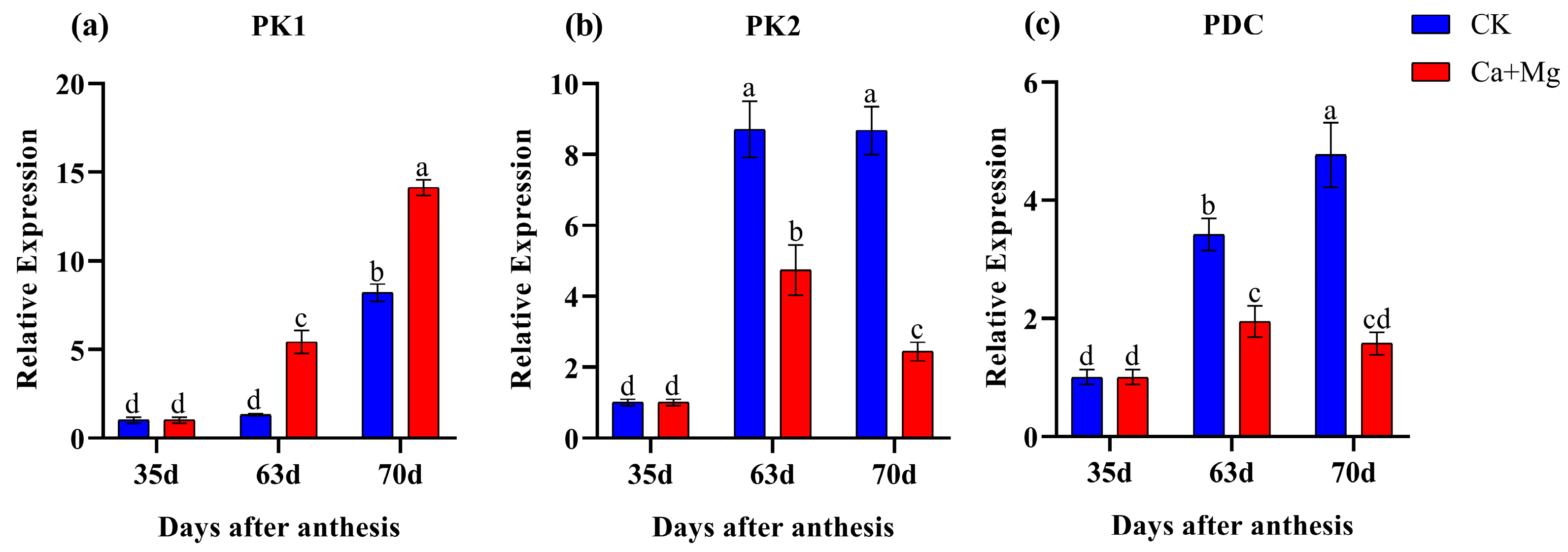
3.5. Quantitative RT-PCR
4. Discussion
4.1. Impact of Ca+Mg on Sugar Content and Fruit Quality
4.2. Influence of Ca+Mg on Respiration and Metabolic Pathways
4.3. Effect of Ca+Mg Treatment on Enzymatic Activity and Metabolic Processes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Menzel, C. The physiology of growth and cropping in lychee. Acta Hortic. 2000, 558, 175–184. [Google Scholar] [CrossRef]
- Sarkar, T.; Nayak, P.; Chakraborty, R. Litchi (Litchi chinensis Sonn.) products and processing technologies: An Update. Ambient Sci. 2018, 5, 11–16. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, N.; Ma, Z.; Che, F.; Mao, J.; Chen, B. The changes in color, soluble sugars, organic acids, anthocyanins and aroma components in “Starkrimson” during the ripening period in China. Molecules 2016, 21, 812. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Song, L.; Liu, H.; Lichter, A.; Kerdchoechuen, O.; Joyce, D.; Shi, J. Postharvest characteristics and handling of litchi fruit—An overview. Aust. J. Exp. Agric. 2006, 46, 1541–1556. [Google Scholar] [CrossRef]
- Prusky, D.B.; Wilson, R.A. Does increased nutritional carbon availability in fruit and foliar hosts contribute to modulation of pathogen colonization? Postharvest Biol. Technol. 2018, 145, 27–32. [Google Scholar] [CrossRef]
- Lazar, T.; Taiz, L.; Zeiger, E. Plant Physiol, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2003. [Google Scholar]
- Vanlerberghe, G.C. Alternative oxidase: A mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Sun, W.; Zhang, J.; Li, H.; Zhang, Y. Effect of exogenous salicylic acid treatment on respiratory chain of Ya-li pears stored at a low Temperature. Acta Agric. Boreali-Sin. 2012, 27, 191–195. [Google Scholar]
- Yang, Z.; Cao, S.; Su, X.; Jiang, Y. Respiratory activity and mitochondrial membrane associated with fruit senescence in postharvest peaches in response to UV-C treatment. Food Chem. 2014, 161, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, H.; Chen, Y.; Wang, H.; Lin, M.; Ritenour, M.A.; Lin, Y. The role of ROS-induced change of respiratory metabolism in pulp breakdown development of longan fruit during storage. Food Chem. 2020, 305, 125439. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Q.; Li, D.; Li, W.; Li, L.; Aalim, H.; Luo, Z. Moderation of respiratory cascades and energy metabolism of fresh-cut pear fruit in response to high CO2 controlled atmosphere. Postharvest Biol. Technol. 2021, 172, 111379. [Google Scholar] [CrossRef]
- Castiglia, D.; Cardi, M.; Landi, S.; Cafasso, D.; Esposito, S. Expression and characterization of a cytosolic glucose 6 phosphate dehydrogenase isoform from barley (Hordeum vulgare) roots. Protein Expr. Purif. 2015, 112, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, L.; Liu, H.; Liu, W.; Pan, B.; Wang, X.; Deng, J.; Wang, C.; Zhang, D.; Li, Z. Molecular mechanisms of furanone production through the EMP and PP pathways in Zygosaccharomyces rouxii with D-fructose addition. Food Res. Int. 2020, 133, 109137. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Martinez, J.I. The regulation of the pentose phosphate pathway: Remember Krebs. Arch. Biochem. Biophys. 2017, 614, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Yuxi, Z.; Dan, Y.; Chunying, L.; Shupeng, G. Dynamic of carbohydrate metabolism and the related genes highlights PPP pathway activation during chilling induced bud dormancy release in tree peony (Paeonia suffruticosa). Sci. Hortic. 2018, 242, 36–43. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Graham, F.D.; Erlemann, K.-R.; Gravel, S.; Rokach, J.; Powell, W.S. Oxidative stress-induced changes in pyridine nucleotides and chemoattractant 5-lipoxygenase products in aging neutrophils. Free Radic. Biol. Med. 2009, 47, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Galeazzi, L.; Bocci, P.; Amici, A.; Brunetti, L.; Ruggieri, S.; Romine, M.; Reed, S.; Osterman, A.L.; Rodionov, D.A.; Sorci, L. Identification of nicotinamide mononucleotide deamidase of the bacterial pyridine nucleotide cycle reveals a novel broadly conserved amidohydrolase family. J. Biol. Chem. 2011, 286, 40365–40375. [Google Scholar] [CrossRef] [PubMed]
- Soto, I.C.; Fontanesi, F.; Liu, J.; Barrientos, A. Biogenesis and assembly of eukaryotic cytochrome c oxidase catalytic core. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Kitazawa, H.; Wang, X.; Sun, H. Regulation of respiratory pathway and electron transport chain in relation to senescence of postharvest white mushroom (Agaricus bisporus) under high O2/CO2 controlled atmospheres. J. Agric. Food Chem. 2017, 65, 3351–3359. [Google Scholar] [CrossRef]
- Recasens, I.; Benavides, A.; Puy, J.; Casero, T. Pre-harvest calcium treatments in relation to the respiration rate and ethylene production of ‘Golden Smoothee’ apples. J. Sci. Food Agric. 2004, 84, 765–771. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, X.; Long, L.E. The effect of postharvest calcium application in hydro-cooling water on tissue calcium content, biochemical changes, and quality attributes of sweet cherry fruit. Food Chem. 2014, 160, 22–30. [Google Scholar] [CrossRef]
- Pathak, J.; Ahmed, H.; Kumari, N.; Pandey, A.; Rajneesh; Sinha, R.P. Role of calcium and potassium in amelioration of environmental stress in plants. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 535–562. [Google Scholar]
- Verma, G.; Srivastava, D.; Tiwari, P.; Chakrabarty, D. ROS modulation in crop plants under drought stress. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 311–336. [Google Scholar]
- Massironi, S.; Rossi, R.E.; Cavalcoli, F.A.; Della Valle, S.; Fraquelli, M.; Conte, D. Nutritional deficiencies in inflammatory bowel disease: Therapeutic approaches. Clin. Nutr. 2013, 32, 904–910. [Google Scholar] [CrossRef]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Sinha, A.; Jawandha, S.; Gill, P.; Singh, H. Influence of pre-harvest sprays of calcium nitrate on storability and quality attributes of plum fruits. J. Food Sci. Technol. 2019, 56, 1427–1437. [Google Scholar] [CrossRef]
- Gao, Q.; Xiong, T.; Li, X.; Chen, W.; Zhu, X. Calcium and calcium sensors in fruit development and ripening. Sci. Hortic. 2019, 253, 412–421. [Google Scholar] [CrossRef]
- Zhou, X.; Su, Y.; Zhang, R.; Zhou, K. Effects of K, Ca and Mg applied in foliar nutrients on pericarp’s coloring of Litchi chinensis Sonn. cv. Feizixiao. Southwest China J. Agric. Sci. 2015, 28, 1713–1718. [Google Scholar]
- Wang, G.; Wang, J.; Han, X.; Chen, R.; Xue, X. Effects of spraying calcium fertilizer on photosynthesis, mineral content, sugar–acid metabolism and fruit quality of Fuji apples. Agronomy 2022, 12, 2563. [Google Scholar] [CrossRef]
- Liao, H.-Z.; Yang, C.K.; Lin, X.K.; Yue, X.Q.; Zhou, K.-B. Effects of spraying foliar calcium and magnesium fertilizer on fruit quality of Feizixiao litchi. J. South. Agric. 2021, 52, 1843–1850. [Google Scholar]
- Jiang, S.-Y.; Xu, H.-Y.; Wang, H.-C.; Hu, G.-B.; Li, J.-G.; Chen, H.-B.; Huang, X.-M. A comparison of the costs of flowering in ‘Feizixiao’and ‘Baitangying’ litchi. Sci. Hortic. 2012, 148, 118–125. [Google Scholar] [CrossRef]
- Shi, S.; Du, J.; Peng, J.; Zhou, K.; Ma, W. The Effects of Mixed Foliar Nutrients of Calcium and Magnesium on the Major Bypass Respiratory Pathways in the Pulp of ‘Feizixiao’ Litchi. Horticulturae 2024, 10, 248. [Google Scholar] [CrossRef]
- Shui, X.; Wang, W.; Ma, W.; Yang, C.; Zhou, K. Mechanism by which high foliar calcium contents inhibit sugar accumulation in feizixiao lychee pulp. Horticulturae 2022, 8, 1044. [Google Scholar] [CrossRef]
- Kour, D.; Sharma, A.; Wali, V.; Bakshi, P.; Bandhari, A. Effect of Regulated Irrigation at Delayed Intervals and Calcium Sprays on Yield and Quality of Litchi (Litchi chinensis Sonn.) Cv. Dehradun. Indian J. Ecol. 2016, 43, 719–723. [Google Scholar]
- Tian, S.; Zhou, X.; Gong, H.; Ma, X.; Zhang, F. Orthogonal test design for optimization of the extraction of polysaccharide from Paeonia sinjiangensis KY Pan. Pharmacogn. Mag. 2011, 7, 4. [Google Scholar] [CrossRef]
- Sivakumar, D.; Korsten, L. Fruit quality and physiological responses of litchi cultivar McLean’s Red to 1-methylcyclopropene pre-treatment and controlled atmosphere storage conditions. LWT-Food Sci. Technol. 2010, 43, 942–948. [Google Scholar] [CrossRef]
- Huang, L.; Tao, S.; Zhu, Y.; Pan, Y.; Zhang, Z.; Yu, Z.; Chen, Y. Regulation of Embden–Meyerhof–Parnas (EMP) Pathway and Tricarboxylic Acid (TCA) Cycle Concerning Aberrant Chilling Injury Behavior in Postharvest Papaya (Carica papaya L.). Int. J. Mol. Sci. 2023, 24, 13898. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Huang, Q.; Shu, W. Phosphoenolpyruvate carboxylase activity in ear organs is related to protein concentration in grains of winter wheat. J. Cereal Sci. 2008, 47, 386–391. [Google Scholar] [CrossRef]
- Patel, M.S.; Nemeria, N.S.; Furey, W.; Jordan, F. The pyruvate dehydrogenase complexes: Structure-based function and regulation. J. Biol. Chem. 2014, 289, 16615–16623. [Google Scholar] [CrossRef]
- Ancai, L.; Yang, X. Organic acid concentrations and the relative enzymatic changes during the development of citrus fruits. Sci. Agric. Sin. 2003, 36, 941–944. [Google Scholar]
- Sadka, A.; Dahan, E.; Cohen, L.; Marsh, K.B. Aconitase activity and expression during the development of lemon fruit. Physiol. Plant. 2000, 108, 255–262. [Google Scholar] [CrossRef]
- Juan, K.; Wang, H.-M.; Jin, C.-H.; Xie, H.-Y. Changes of reactive oxygen species and related enzymes in mitochondria respiratory metabolism during the ripening of peach fruit. Agric. Sci. China 2010, 9, 138–146. [Google Scholar]
- De Vasconcelos, A.C.F.; Chaves, L.H.G. Biostimulants and their role in improving plant growth under abiotic stresses. In Biostimulants in Plant Science; BoD—Books on Demand: Norderstedt, Germany, 2019; pp. 3–16. [Google Scholar]
- Teixeira, W.F.; Fagan, E.B.; Soares, L.H.; Umburanas, R.C.; Reichardt, K.; Neto, D.D. Foliar and seed application of amino acids affects the antioxidant metabolism of the soybean crop. Front. Plant Sci. 2017, 8, 245273. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, E.; Blaszczak, A.; Wiatrak, P.; Canady, M. Biostimulant mode of action: Impact of biostimulant on whole-plant level. In The Chemical Biology of Plant Biostimulants; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 205–227. [Google Scholar]
- Zhang, Y.; Kong, Q.; Niu, B.; Liu, R.; Chen, H.; Xiao, S.; Wu, W.; Zhang, W.; Gao, H. The dual function of calcium ion in fruit edible coating: Regulating polymer internal crosslinking state and improving fruit postharvest quality. Food Chem. 2024, 447, 138952. [Google Scholar] [CrossRef]
- De Freitas, S.T.; Amarante, C.; Mitcham, E.J. Calcium deficiency disorders in plants. In Postharvest Ripening Physiology of Crops; CRC Press: Boca Raton, FL, USA, 2016; pp. 477–502. [Google Scholar]
- Farhat, N.; Elkhouni, A.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Rabhi, M. Effects of magnesium deficiency on photosynthesis and carbohydrate partitioning. Acta Physiol. Plant. 2016, 38, 145. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crop. 2010, 94, 23–25. [Google Scholar]
- Saleem, S.; Anayat, R.; Mushtaq, F.; Mustafa, I.; Khan, S.R.; Farwah, S.; Hussain, S. Effect of foliar application of calcium and magnesium on growth and yield of tomato (Solanum lycopersicum L.) variety Marglobe. IJCS 2019, 7, 1555–1561. [Google Scholar]
- Luo, T.; Shuai, L.; Lai, T.; Liao, L.; Li, J.; Duan, Z.; Xue, X.; Han, D.; Wu, Z. Up-regulated glycolysis, TCA, fermentation and energy metabolism promoted the sugar receding in ‘Shixia’longan (Dimocarpus longan Lour.) pulp. Sci. Hortic. 2021, 281, 109998. [Google Scholar] [CrossRef]
- Liaquat, M.; Ahmad, S.; Khan, A.S.; Ahmed, R. Reduction in fruit rot and enhancement in fruit quality of Kinnow mandarin by calcium chloride application. Pak. J. Agric. Sci. 2019, 56, 367–376. [Google Scholar]
- Wang, Z.; Hassan, M.U.; Nadeem, F.; Wu, L.; Zhang, F.; Li, X. Magnesium fertilization improves crop yield in most production systems: A meta-analysis. Front. Plant Sci. 2020, 10, 495191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, W.; Muneer, M.A.; Ji, Z.; Tong, L.; Zhang, X.; Li, X.; Wang, W.; Zhang, F.; Wu, L. Integrated use of lime with Mg fertilizer significantly improves the pomelo yield, quality, economic returns and soil physicochemical properties under acidic soil of southern China. Sci. Hortic. 2021, 290, 110502. [Google Scholar] [CrossRef]
- Chen, F.-X.; Liu, X.-H.; Chen, L.-S. Developmental changes in pulp organic acid concentration and activities of acid-metabolising enzymes during the fruit development of two loquat (Eriobotrya japonica Lindl.) cultivars differing in fruit acidity. Food Chem. 2009, 114, 657–664. [Google Scholar] [CrossRef]
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, nutrition, detection, and social impact of diseases linked to its deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef] [PubMed]
- Cronje, R.B. Effect of fruit development, maturity and harvesting of litchi (Litchi chinensis Sonn.) on postharvest fruit quality. Stewart Postharvest Rev. 2008, 4, 1–10. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Yuan, M.; Zhou, K. Effects of magnesium foliar application on pericarp coloring of Litchi chinensis Sonn. cv. Feizixiao and contents of potassium, calcium and magnesium in pericarp. J. South. Agric. 2017, 48, 854–860. [Google Scholar]
- Gao, H.; Jia, Y.; Guo, S.; Lv, G.; Wang, T.; Juan, L. Exogenous calcium affects nitrogen metabolism in root-zone hypoxia-stressed muskmelon roots and enhances short-term hypoxia tolerance. J. Plant Physiol. 2011, 168, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Kou, J.; Wei, Y.; He, X.; Xu, J.; Xu, F.; Shao, X. Infection of post-harvest peaches by Monilinia fructicola accelerates sucrose decomposition and stimulates the Embden–Meyerhof–Parnas pathway. Hortic. Res. 2018, 5, 46. [Google Scholar] [CrossRef]
- Chumyam, A.; Shank, L.; Uthaibutra, J.; Saengnil, K. Effects of chlorine dioxide on mitochondrial energy levels and redox status of ‘Daw’longan pericarp during storage. Postharvest Biol. Technol. 2016, 116, 26–35. [Google Scholar] [CrossRef]
- Lu, X.; Meng, G.; Jin, W.; Gao, H. Effects of 1-MCP in combination with Ca application on aroma volatiles production and softening of ‘Fuji’apple fruit. Sci. Hortic. 2018, 229, 91–98. [Google Scholar] [CrossRef]
- Pasquariello, M.S.; Rega, P.; Migliozzi, T.; Capuano, L.R.; Scortichini, M.; Petriccione, M. Effect of cold storage and shelf life on physiological and quality traits of early ripening pear cultivars. Sci. Hortic. 2013, 162, 341–350. [Google Scholar] [CrossRef]
- Juan, K.; Wang, H.-M.; Jin, C.-H. Changes of reactive oxygen species and related enzymes in mitochondrial respiration during storage of harvested peach fruits. Agric. Sci. China 2011, 10, 149–158. [Google Scholar]

| Primer Names | Right Primer Sequences (5′ to 3′) | Left Primer Sequences (5′ to 3′) | Amplification Length |
|---|---|---|---|
| Cluster-6206.72872 | TGGAAAACGGTGGTGTGGAA | AACAGCTTCTCCACCATCGG | 100–200 bp |
| Cluster-6206.110990 | TTGTGGCTGAGTGCACTAGG | TCCACCACTTTCCCAACTGG | 100–200 bp |
| Cluster-6206.117009 | TCCCCAACAATGAACCAGGG | CACTCGAATTCAAGCGTCGC | 100–200 bp |
| β-Actin | AGTTTGGTTGATGTGGGAGAC | TGGCTGAACCCGAGATGAT | 100–200 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajjad, M.; Tahir, H.; Ma, W.; Shaopu, S.; Farooq, M.A.; Zeeshan Ul Haq, M.; Sajad, S.; Zhou, K. Effects of Foliar Ca and Mg Nutrients on the Respiration of ‘Feizixiao’ Litchi Pulp and Identification of Differential Expression Genes Associated with Respiration. Agronomy 2024, 14, 1347. https://doi.org/10.3390/agronomy14071347
Sajjad M, Tahir H, Ma W, Shaopu S, Farooq MA, Zeeshan Ul Haq M, Sajad S, Zhou K. Effects of Foliar Ca and Mg Nutrients on the Respiration of ‘Feizixiao’ Litchi Pulp and Identification of Differential Expression Genes Associated with Respiration. Agronomy. 2024; 14(7):1347. https://doi.org/10.3390/agronomy14071347
Chicago/Turabian StyleSajjad, Muhammad, Hassam Tahir, Wuqiang Ma, Shi Shaopu, Muhammad Aamir Farooq, Muhammad Zeeshan Ul Haq, Shoukat Sajad, and Kaibing Zhou. 2024. "Effects of Foliar Ca and Mg Nutrients on the Respiration of ‘Feizixiao’ Litchi Pulp and Identification of Differential Expression Genes Associated with Respiration" Agronomy 14, no. 7: 1347. https://doi.org/10.3390/agronomy14071347







