Phenotypic Characterization and Yield Screening of Quinoa Germplasms in Diverse Low-Altitude Regions: A Preliminary Study
Abstract
1. Introduction
2. Results
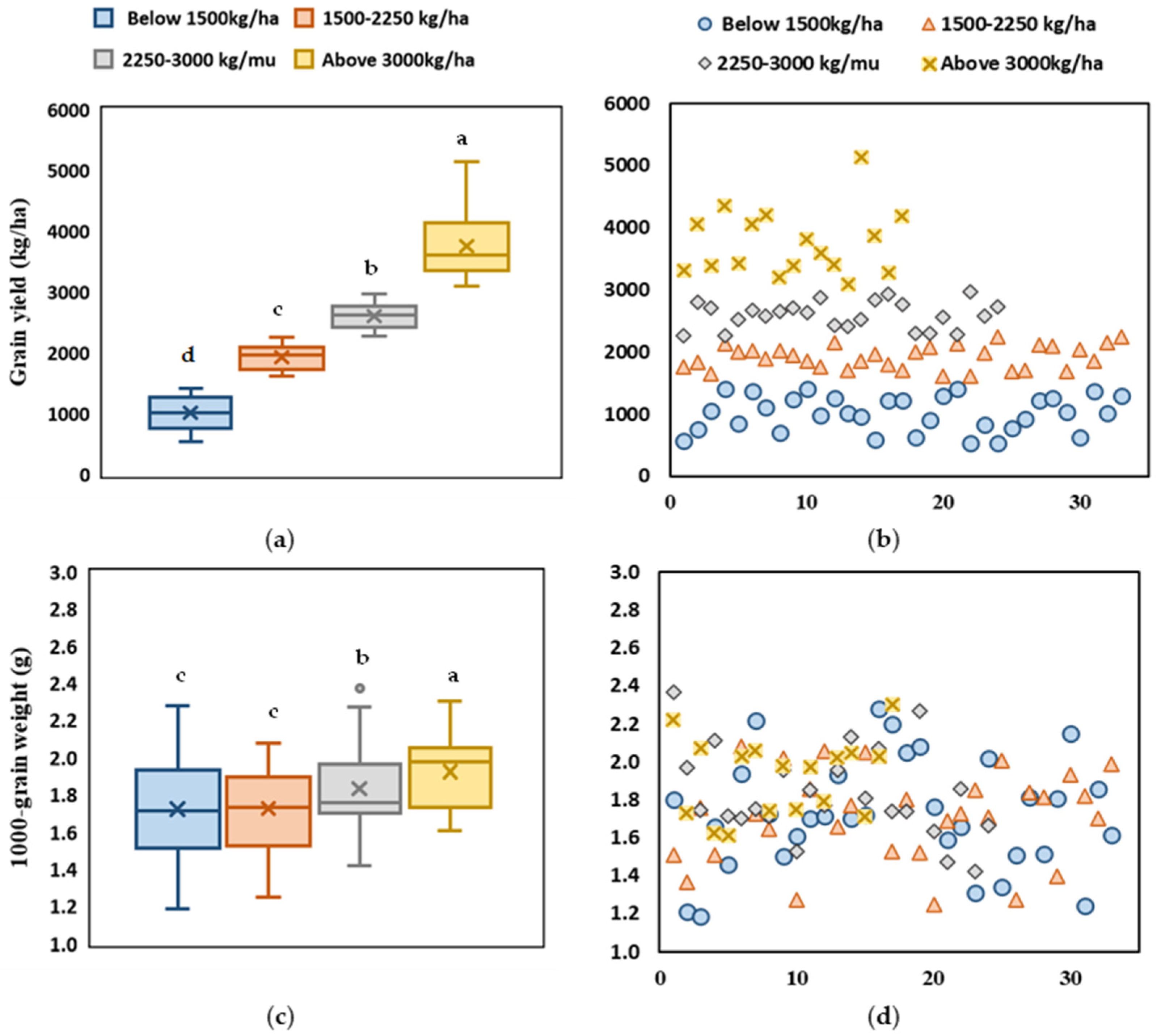
2.1. Grain Weight and Yield
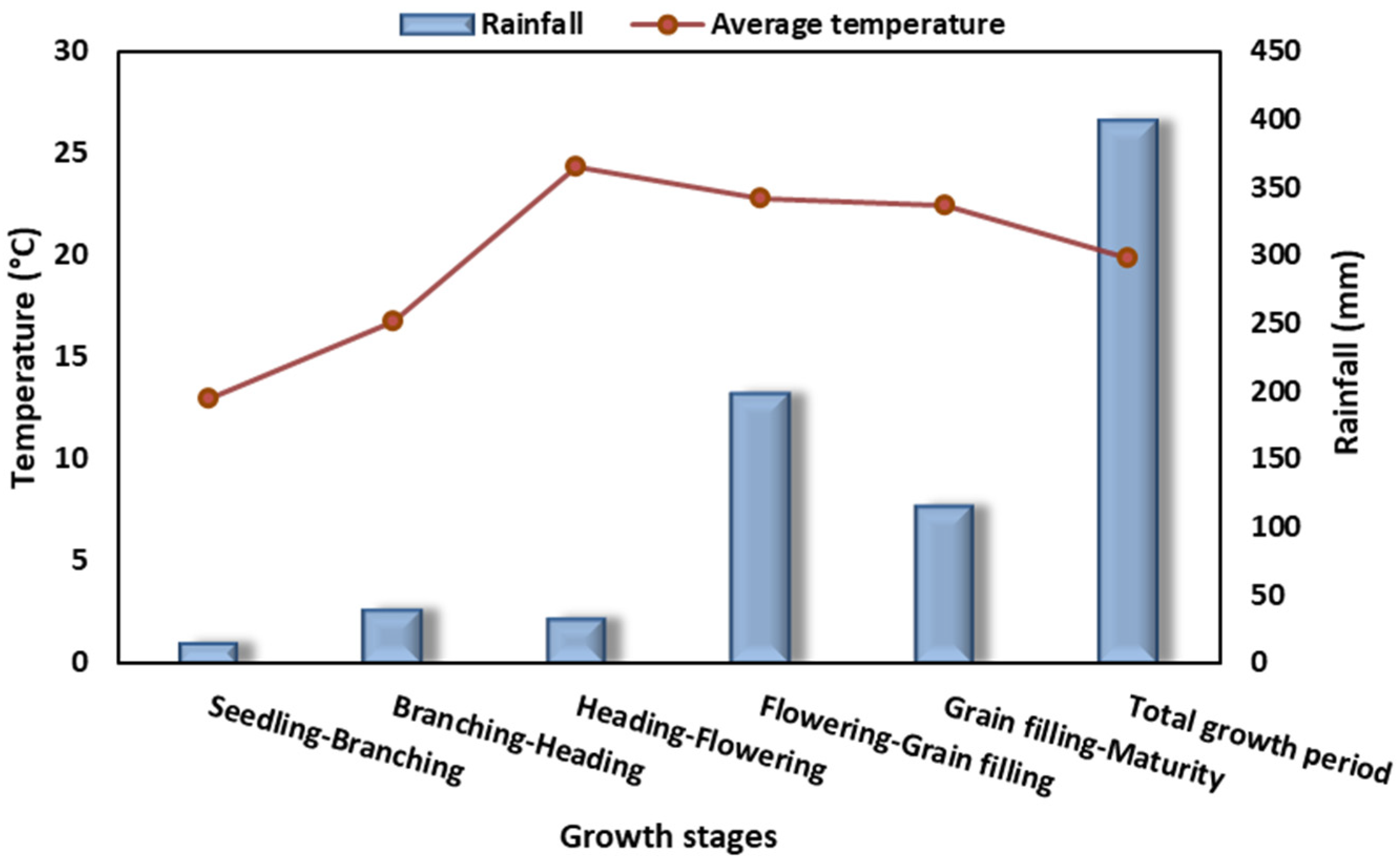
2.2. Growth Period and Accumulated Temperature Characteristics of Different Quinoa Accessions
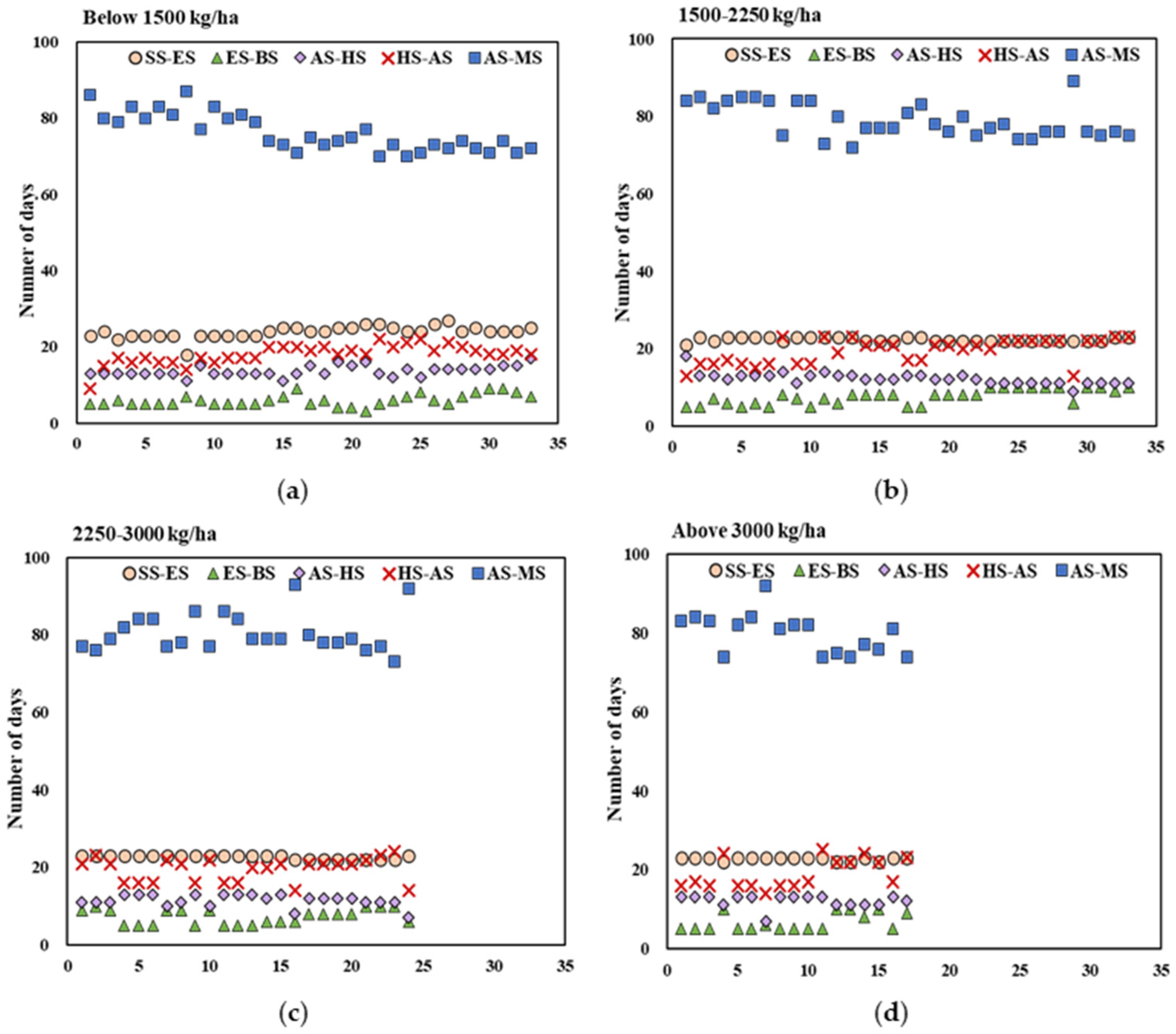
2.2.1. Phenological Developmental Period for Different Quinoa Accessions
2.2.2. Accumulated Temperature at Each Growth Stage
2.3. Differences in Root Characteristics of Different Quinoa Accessions at the Maturity Stage
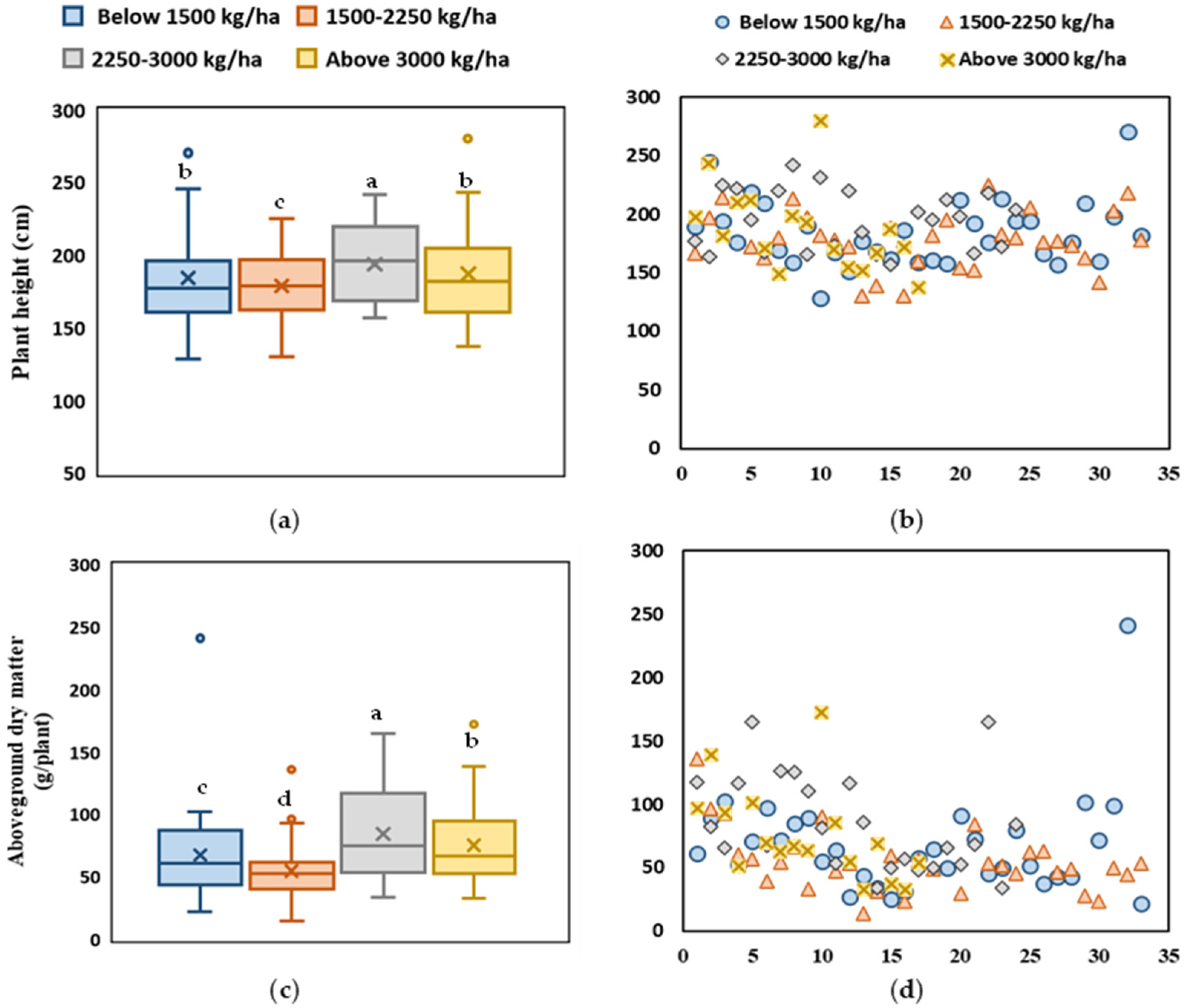
2.4. Stem Characteristics
2.5. Plant Height and Aboveground Dry Weight
2.6. Panicle Characteristics
2.7. Comprehensive Analysis of Agronomic Characters and 1000-Grain Weight of Quinoa Accessions at Different Yield Levels
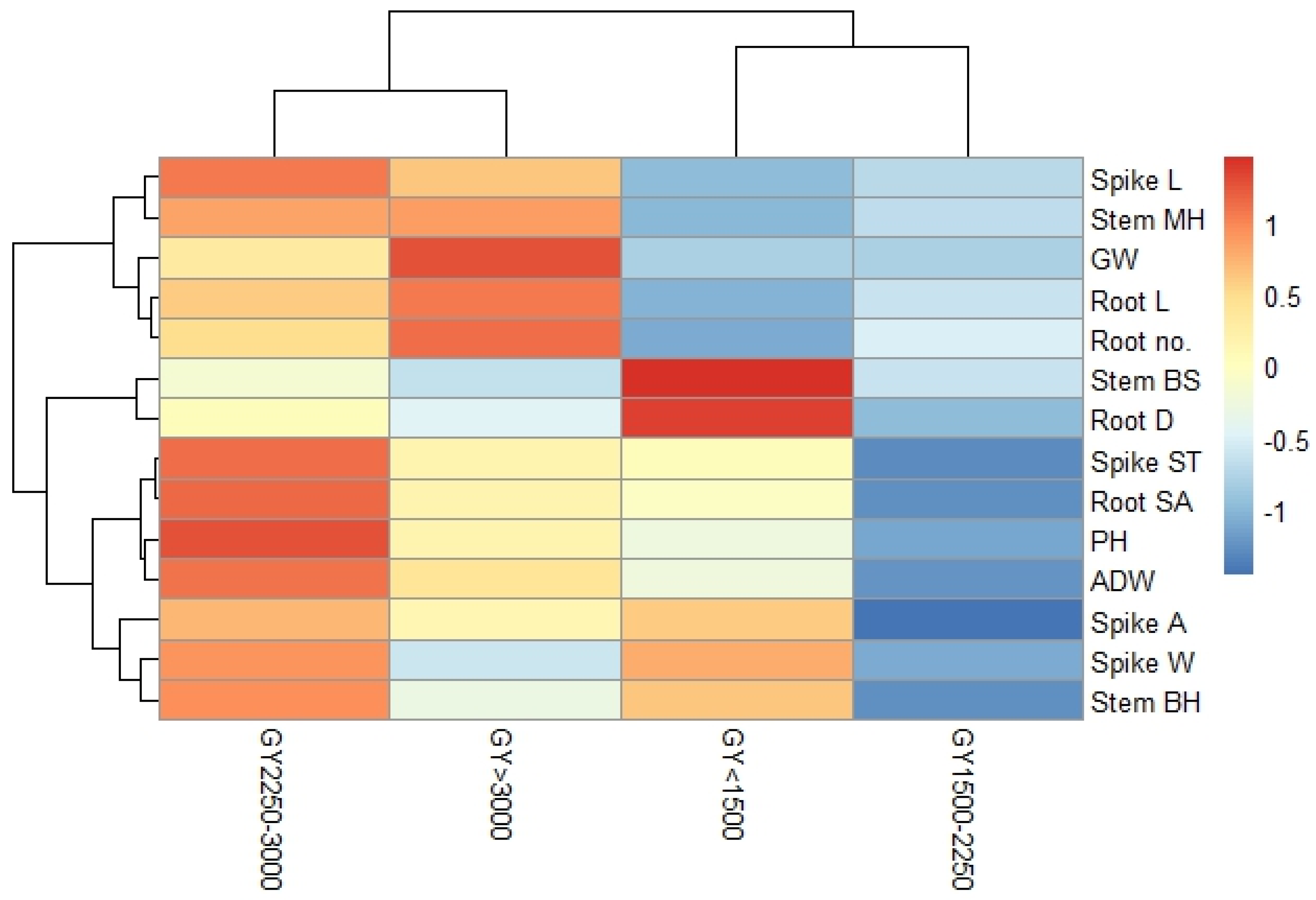
2.8. Heatmap Clustering and Correlation
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Measurements
4.2.1. Agronomic Characteristic
4.2.2. Root Characteristics
4.2.3. Stem Strength Characteristics
4.2.4. Panicle Characteristics
4.2.5. Yield and 1000-Grain Weight
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar] [CrossRef]
- Lin, C.; Liu, Z.J.; Dong, Y.M.; Vales, M.; Mao, Z.C. Domesticated cultivation and genetic breeding of Chenopodium quinoa. Yi Chuan Hered. 2019, 41, 1009–1022. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Yadav, R.; Vishwakarma, H.; Sharma, K.; Chandora, R.; Rana, J.C.; Riar, A. Agro-morphological and nutritional assessment of chenopod and quinoa germplasm-Highly adaptable potential crops. Food Sci. Nutr. 2023, 11, 5446–5459. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). Assessment of the International Year of Quinoa 2013. Hundred and Forty-Ninth Session; CL 149/10;2014. Available online: https://www.fao.org/quinoa/en/ (accessed on 1 April 2024).
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusairi, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Otambekova, M.; et al. Worldwide evaluations of quinoa: Preliminary results from post international year of quinoa FAO projects in nine countries. Front. Plant Sci. 2016, 7, 850. [Google Scholar] [CrossRef]
- Präger, A.; Boote, K.J.; Munz, S.; Graeff-Hönninger, S. Simulating growth and development processes of Quinoa (Chenopodium quinoa Willd.): Adaptation and evaluation of the CSM-CROPGRO model. Agronomy 2019, 9, 832. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Iafelice, G.; Lavini, A.; Pulvento, C.; Caboni, M.F.; Marconi, E. Phenolic compounds and saponins in quinoa samples (Chenopodium quinoa Willd.) grown under different saline and nonsaline irrigation regimens. J. Agric. Food Chem. 2012, 60, 4620–4627. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.I.; Muscolo, A.; Ahmed, M.; Asghar, M.A.; Al-Dakheel, A.J. Agro-morphological, yield and quality traits and interrelationship with yield stability in quinoa (Chenopodium quinoa Willd.) genotypes under saline marginal environment. Plants 2020, 9, 1763. [Google Scholar] [CrossRef]
- Bertero, H.D. Effects of photoperiod, temperature and radiation on the rate of leaf appearance in quinoa (Chenopodium quinoa Willd.) under field conditions. Ann. Bot. 2011, 87, 495–502. [Google Scholar] [CrossRef]
- Hafeez, M.B.; Iqbal, S.; Li, Y.; Saddiq, M.S.; Basra, S.M.; Zhang, H.; Zahra, N.; Akram, M.Z.; Bertero, D.; Curti, R.N. Assessment of phenotypic diversity in the USDA collection of quinoa links genotypic adaptation to germplasm origin. Plants 2022, 11, 738. [Google Scholar] [CrossRef]
- Christensen, S.A.; Pratt, D.B.; Pratt, C.; Nelson, P.T.; Stevens, M.R.; Jellen, E.N.; Coleman, C.E.; Fairbanks, D.J.; Bonifacio, A.; Maughan, P.J. Assessment of genetic diversity in the USDA and CIP-FAO international nursery collections of quinoa (Chenopodium quinoa Willd.) using microsatellite markers. Plant Genet. Resour. 2007, 5, 82–95. [Google Scholar] [CrossRef]
- Dostalíková, L.; Hlásná Čepková, P.; Janovská, D.; Svoboda, P.; Jágr, M.; Dvořáček, V.; Viehmannová, I. Nutritional evaluation of quinoa genetic resources growing in the climatic conditions of Central Europe. Foods 2023, 12, 1440. [Google Scholar] [CrossRef] [PubMed]
- Hour, A.L.; Hsieh, W.H.; Chang, S.H.; Wu, Y.P.; Chin, H.S.; Lin, Y.R. Genetic diversity of landraces and improved varieties of rice (Oryza sativa L.) in Taiwan. Rice 2020, 13, 82. [Google Scholar] [CrossRef]
- Alkhamisi, S.A.; Nadaf, S.K.; Al-Jabri, N.M.; Al-Hashmi, K.S.; Al-Shirawi, A.I.; Khan, R.R.; Al-Sulaimi, H.A.; Al-Azri, M.S. Productivity of quinoa genotypes across different agro-ecological regions of Oman. Open Agric. J. 2021, 15, 98–109. [Google Scholar] [CrossRef]
- Deng, Y.; Sun, X.; Zhang, Q.; Anwar, S.; Lu, J.; Guo, H.; Qin, L.; Zhang, L.; Wang, C. Comprehensive evaluation and physiological response of quinoa genotypes to low nitrogen. Agronomy 2023, 13, 1597. [Google Scholar] [CrossRef]
- Spehar, C.R.; da Silva Rocha, J.E. Exploiting genotypic variability from low-altitude Brazilian Savannah-adapted Chenopodium quinoa. Euphytica 2010, 175, 13–21. [Google Scholar] [CrossRef]
- Burbano-Erazo, E.; Cordero, C.; Pastrana, I.; Espitia, L.; Gomez, E.; Morales, A.; Pérez, J.; López, L.; Rosero, A. Interrelation of ecophysiological and morpho-agronomic parameters in low altitude evaluation of selected ecotypes of sweet potato (Ipomoea batatas [L.] Lam.). Horticulturae 2022, 6, 99. [Google Scholar] [CrossRef]
- Li, X.; Ran, R.; Chen, G.; Zhao, P. Genomic variation underlying the breeding selection of quinoa varieties Longli-4 and CA3-1 in China. Int. J. Mol. Sci. 2022, 23, 14030. [Google Scholar] [CrossRef]
- Fuentes, F.; Bhargava, A. Morphological analysis of quinoa germplasm grown under lowland desert conditions. J. Agron. Crop. Sci. 2011, 197, 124–134. [Google Scholar] [CrossRef]
- Rojas, W.; Pinto, M.; Alanoca, C.; Gómez-Pando, L.; León-Lobos, P.; Alercia, A.; Diulgheroff, S.; Padulosi, S.; Bazile, D. Quinoa genetic resources and ex situ conservation. In State of the Art Report on Quinoa Around the World 2013; Bazile, D., Bertero, D., Nieto, C., Eds.; FAO: Rome, Italy, 2015; pp. 56–82. [Google Scholar]
- Shah, S.S.; Shi, L.; Li, Z.; Ren, G.; Zhou, B.; Qin, P. Yield, agronomic and forage quality traits of different quinoa (Chenopodium quinoa Willd.) genotypes in northeast China. Agronomy 2020, 10, 1908. [Google Scholar] [CrossRef]
- Coronado, A.C.M.; Hernández, E.H.M.; Coronado, Y.M. Phenotypic diversity of agromorphological characteristics of quinoa (Chenopodium quinoa Willd.) germplasm in Colombia. Sci. Agric. 2021, 79, e20210017. [Google Scholar] [CrossRef]
- Xiu-Shi, Y.; Pei-You, Q.; Hui-Min, G.; Gui-Xing, R. Quinoa industry development in China. Int. J. Agric. Natural Res. 2019, 46, 208–219. [Google Scholar] [CrossRef]
- Capelin, M.A.; Madella, L.A.; Panho, M.C.; Meira, D.; Fernandes, R.A.; Colonelli, L.L.; Menegazzi, C.P.; Rosa, A.C.; Rodrigues, A.P.D.A.C.; Benin, G. Impact of altitude on grain yield, oil, and protein content of soybean. Aust. J. Crop. Sci. 2022, 16, 273–279. [Google Scholar] [CrossRef]
- Sivirihauma, C.; Blomme, G.; Ocimati, W.; Vutseme, L.; Sikyolo, I.; Valimuzigha, K.; De Langhe, E.; Turner, D.W. Altitude effect on plantain growth and yield during four production cycles in North Kivu, eastern Democratic Republic of Congo. In Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014), Brisbane, Australia, 2014; IX 1114. pp. 139–148. [Google Scholar]
- Keleş, S.Ö. The effect of altitude on the growth and development of Trojan fir (Abies nordmanniana subsp. equi-trojani [Asch. & Sint. ex Boiss] Coode & Cullen) saplings. Cerne 2020, 26, 381–392. [Google Scholar]
- Mei, L.; Bao, G.; Tong, S.; Yin, S.; Bao, Y.; Jiang, K.; Hong, Y.; Tuya, A.; Huang, X. Elevation-dependent response of spring phenology to climate and its legacy effect on vegetation growth in the mountains of northwest Mongolia. Ecol. Indic. 2021, 126, 107640. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, L.X.; Ai, J.; Zhang, Z.F.; Deng, J.; Zhang, Y.B. Climate variations in the low-latitude Plateau contribute to different sugarcane (Saccharum spp.) yields and sugar contents in China. Plants 2023, 12, 2712. [Google Scholar] [CrossRef] [PubMed]
- Ntawuruhunga, P.; Whyte, J.B.A.; Rubaihayo, P. Effect of altitude and plant age on cyanogenic potential, dry matter, starch and sugar content in cassava genotypes. In Proceedings of the 13th ISTRC Symposium, Arusha, Tanzania, 9–14 November 2007; pp. 177–185. [Google Scholar]
- Razafindrazaka, A.; Stuerz, S.; Cotter, M.; Rajaona, A.; Asch, F. Genotypic yield responses of lowland rice in high-altitude cropping systems. J. Agron. Crop. Sci. 2020, 206, 444–455. [Google Scholar] [CrossRef]
- Spehar, C.R.; Santos, R.L.B. Quinoa (Chenopodium quinoa Willd) BRS Piabiru alternative for crop diversification. Pesqui. Agropecu. Bras. 2002, 37, 889–893. [Google Scholar]
- Oustani, M.; Mehda, S.; Halilat, M.T.; Chenchouni, H. Yield, growth development and grain characteristics of seven Quinoa (Chenopodium quinoa Willd.) genotypes grown in open-field production systems under hot-arid climatic conditions. Sci. Rep. 2023, 13, 1991. [Google Scholar] [CrossRef]
- Colque-Little, C.; Abondano, M.C.; Lund, O.S.; Amby, D.B.; Piepho, H.P.; Andreasen, C.; Schmöckel, S.; Schmid, K. Genetic variation for tolerance to the downy mildew pathogen Peronospora variabilis in genetic resources of quinoa (Chenopodium quinoa). BMC Plant Biol. 2021, 21, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.U.; Reynolds, T.P.; Ramage, M.H. The strength of plants: Theory and experimental methods to measure the mechanical properties of stems. J. Exp. Bot. 2017, 68, 4497–4516. [Google Scholar] [CrossRef]
- Irum, R.; Daowu, H.; Adeel, A.; Hongge, L.; Shoupu, H.; Mian Faisal, N.; Xiaoyang, W.; Yinhua, J.; Zhaoe, P.; Peng, Z.; et al. Correlation analysis of stem hardness traits with fiber and yield-related traits in core collections of Gossypium hirsutum. J. Cotton. Res. 2021, 4, 1–10. [Google Scholar]
- Ishimaru, K.; Togawa, E.; Ookawa, T.; Kashiwagi, T.; Madoka, Y.; Hirotsu, N. New target for rice lodging resistance and its effect in a typhoon. Plant 2008, 227, 601–609. [Google Scholar] [CrossRef]
- Beeck, C.P.; Wroth, J.; Cowling, W.A. Genetic variation in stem strength in field pea (Pisum sativum L.) and its association with compressed stem thickness. Aust. J. Agric. Res. 2006, 57, 193–199. [Google Scholar] [CrossRef]
- Dierig, D.A.; Adam, N.R.; Mackey, B.E.; Dahlquist, G.H.; Coffelt, T.A. Temperature and elevation effects on plant growth, development, and seed production of two Lesquerella species. Indus. Crops Prod. 2006, 24, 17–25. [Google Scholar] [CrossRef]
- Altuhaish, A.A.K.; Yahya, S. Field adaptation of some introduced wheat (Triticum aestivum L.) genotypes in two altitudes of tropical agro-ecosystem environment of Indonesia. Hayati, J. Biosci. 2014, 21, 31–38. [Google Scholar] [CrossRef]
- Tang, P.; Ren, A.; Jiang, Z.; Wang, R.; Cui, K.; Wu, X.; Sun, M.; Gao, Z.; Anwar, S. Evaluation of Quinoa Varieties for Adaptability and Yield Potential in Low Altitudes and Correlation with Agronomic Traits. Agronomy 2024, 14, 852. [Google Scholar] [CrossRef]
- Bazile, D. Global trends in the worldwide expansion of quinoa cultivation. Biol Life Sci Forum 2023, 25, 13. [Google Scholar] [CrossRef]
- Taaime, N.; Rafik, S.; El Mejahed, K.; Oukarroum, A.; Choukr-Allah, R.; Bouabid, R.; El Gharous, M. Worldwide development of agronomic management practices for quinoa cultivation: A systematic review. Front. Agron. 2023, 5, 1215441. [Google Scholar] [CrossRef]
- Christiansen, J.L.; Jacobsen, S.E.; Jørgensen, S.T. Photoperiodic effect on flowering and seed development in quinoa (Chenopodium quinoa Willd.). Acta Agric. Scand. Sec. B-Soil Plant Sci. 2010, 60, 539–544. [Google Scholar]
- Pathan, S.; Ndunguru, G.; Clark, K.; Ayele, A.G. Yield and nutritional responses of quinoa (Chenopodium quinoa Willd.) genotypes to irrigated, rainfed, and drought-stress environments. Front. Sustain. Food Sys. 2023, 7, 1242187. [Google Scholar] [CrossRef]
- El-Sadek, A. Multi-environmental evaluation for grain yield and its components of quinoa genotypes across the north western coast of Egypt. Egypt J. Desert Res. 2017, 67, 65–82. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Q.; Sun, X.; Deng, Y.; Guo, H.; Wang, C.; Zhao, L. Study on the mechanism of slow-release fertilizer and nitrogen fertilizer on the senescence characteristics of quinoa Leaves. Agronomy 2024, 14, 884. [Google Scholar] [CrossRef]
- García-Parra, M.; Roa-Acosta, D.; Bravo-Gómez, J.E. Effect of the altitude gradient on the physiological performance of quinoa in the Central Region of Colombia. Agronomy 2022, 12, 2112. [Google Scholar] [CrossRef]
- Maamri, K.; Zidane, O.D.; Chaabena, A.; Fiene, G.; Bazile, D. Adaptation of some quinoa genotypes (Chenopodium quinoa Willd.), grown in a saharan climate in Algeria. Life 2022, 12, 1854. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Temel, S. Performance of some quinoa (Chenopodium quinoa Willd.) genotypes grown in different climate conditions. Turk. J. Field Crops 2018, 23, 180–186. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Stølen, O. Quinoa—Morphology and phenology and prospects for its production as a new crop in Europe. Eur. J. Agron. 1993, 2, 19–29. [Google Scholar] [CrossRef]
- Jacobsen, S.E. The scope for adaptation of quinoa in Northern Latitudes of Europe. J. Agrn. Crop. Sci. 2017, 203, 603–613. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Ohri, D. Genetic variability and interrelationship among various morphological and quality traits in quinoa (Chenopodium quinoa Willd.). Field Crop Res. 2007, 101, 104–116. [Google Scholar] [CrossRef]
- Hou, P.; Liu, Y.; Xie, R.; Ming, B.; Ma, D.; Li, S.; Mei, X. Temporal and spatial variation in accumulated temperature requirements of maize. Field Crops Res. 2014, 158, 55–64. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, P.; Xie, R.; Hao, W.; Li, S.; Mei, X. Spatial variation and improving measures of the utilization efficiency of accumulated temperature. Crop Sci. 2015, 55, 1806–1817. [Google Scholar] [CrossRef]
- García-Parra, M.A.; Roa-Acosta, D.F.; Stechauner-Rohringer, R.; García-Molano, F.; Bazile, D.; Plazas-Leguizamón, N. Effect of temperature on the growth and development of quinoa plants (Chenopodium quinoa Willd.): A review on a global scale. Sylwan 2020, 164, 411–433. [Google Scholar]
- Proffitt, C.E.; Travis, S.E.; Edwards, K.R. Genotype and elevation influence Spartina alterniflora colonization and growth in a created salt marsh. Ecol. Appl. 2003, 13, 180–192. [Google Scholar] [CrossRef]
- Mahmood, R.S.; AL-Taweel, S.K. Physiological response of genotypes and sowing dates in the growth and yield of Chenopodium quinoa Willd. IOP Conf. Ser. Earth Environ. Sci. 2022, 1060, 01211. [Google Scholar] [CrossRef]
- Manugade, R.P.; Bhalekar, N.B.; Hamane, G.M. Screening of quinoa (Chenopodium quinoa Willd.) genotypes for morphological parameters. Pharma. Innov. J. 2023, 12, 2717–2719. [Google Scholar]
- Yan, D.; Juan-Ling, W.; Anwar, S.; Chuang-Yun, W.; Li, Z.; Li-Guang, Z.; Hong-Xia, G.; Li-Xia, Q.; Hua, L.; Mei-Xia, W. Phenology, lodging and yield traits of Chenopodium quinoa under the effect of planting density and row spacings. Fresenius Env. Bull 2021, 30, 11757–11767. [Google Scholar]
- Khobra, R.; Sareen, S.; Meena, B.K.; Kumar, A.; Tiwari, V.; Singh, G.P. Exploring the traits for lodging tolerance in wheat genotypes: A review. Physiol. Mol. Biol. Plants 2019, 25, 589–600. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shaikh, C.G.; Ponnaiah, G.; Kalleshappa, C.C.; Kholova, J.; Jayalekha, A.K.; Verma, Y.S. Culm Strength: Key Trait to Identify Lodging Tolerant Genotypes in Pearl Millet. In Proceedings of the 16th ROME International Conference on Advances in Agricultural, Biological and Environmental Sciences, RAABE-20, Rome, Italy, 3–5 February 2020; pp. 91–92. [Google Scholar]
- Muhammad, A.; Hao, H.; Xue, Y.; Alam, A.; Bai, S.; Hu, W.; Sajid, M.; Hu, Z.; Samad, R.A.; Li, Z.; et al. Survey of wheat straw stem characteristics for enhanced resistance to lodging. Cellulose 2020, 27, 2469–2484. [Google Scholar] [CrossRef]
- Wang, D.; Ding, W.H.; Feng, S.W.; Hu, T.Z.; Li, G.; Li, X.H.; Yang, Y.Y.; Ru, Z.G. Stem characteristics of different wheat cultivars and their relationship with lodging resistance. Chin. J. Appl. Ecol. 2016, 27, 1496–1502. [Google Scholar]
- Ahmad, S.; Jabbar, M.; Khalique, A.; Shahzad, F.; Ahmad, N.; Fiaz, M.; Younas, U. Effect of different levels of NDF on voluntary feed intake, dry matter digestibility and nutrients utilization in dry Nili Ravi buffaloes. JAPS 2014, 24, 1602–1605. [Google Scholar]
- Deng, Y.; Zhao, L.; Anwar, S.; Zhang, L.G.; Shafiq, F.; Guo, H.X.; Qin, L.X.; Wang, M.X.; Wang, C.Y. Phosphorus fertigation conferred lodging tolerance and improved grain quality in Chenopodium quinoa via enhanced root proliferation and stalk strength. J. Soil Sci. Plant Nutr. 2022, 22, 5099–5110. [Google Scholar] [CrossRef]
- Ali, S.; Chattha, M.U.; Hassan, M.U.; Erils, L. Growth, Biomass Production, and Yield Potential of Quinoa (Chenopodium quinoa Willd.) as Affected by Planting Techniques Under Irrigated Conditions. Int. J. Plant Prod. 2020, 14, 427–441. [Google Scholar] [CrossRef]





| Yield Level (kg/ha) | No. of Accessions | SS-ES | ES-BS | BS-HS | HS-AS | AS-MS | TS |
|---|---|---|---|---|---|---|---|
| Below 1500 | 33 | 23.94 a | 6.00 b | 13.61 a | 18.03 b | 76.18 b | 137.76 b |
| 1500–2250 | 33 | 22.39 b | 7.61 a | 12.21 b | 19.45 a | 79.00 a | 140.67 a |
| 2250–3000 | 24 | 22.67 b | 7.33 a | 11.50 b | 19.50 a | 80.54 a | 141.54 a |
| Above 3000 | 17 | 22.76 b | 6.65 b | 12.00 b | 19.00 ab | 79.88 a | 140.29 a |
| Yield Level (kg/ha) | No. of Accessions | SS-ES | ES-BS | BS-HS | HS-AS | AS-MS | TS |
|---|---|---|---|---|---|---|---|
| Below 1500 | 33 | 408.05 a | 99.71 c | 274.18 c | 395.82 c | 1784.36 b | 2962.12 c |
| 1500–2250 | 33 | 381.98 b | 126.98 a | 244.44 a | 427.11 a | 1801.38 a | 2981.89 b |
| 2250–3000 | 24 | 387.17 b | 123.00 a | 230.17 b | 425.81 a | 1837.17 a | 3003.31 a |
| Above 3000 | 17 | 389.03 b | 106.35 b | 239.38 a | 413.59 b | 1824.32 a | 2972.68 b |
| Yield below 1500 kg/ha | Yield of 1500–2250 kg/ha | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sr. No. | Anthesis–Maturity > 80 Days | RSA at 0–20 cm > Average | SST > Average | AMS > Average | TGW > 2 g | Anthesis–Maturity > 80 Days | RSA at 0–20 cm > Average | SST > Average | AMS > Average | TGW > 2 g |
| 1 | JQ-00085 | JQ-00085 | JQ-00085 | JQ-00085 | -- | JQ-00077 | JQ-00077 | JQ-00077 | JQ-00077 | -- |
| 2 | JQ-00477 | JQ-00477 | -- | -- | -- | JQ-00080 | JQ-00080 | JQ-00080 | JQ-00080 | -- |
| 3 | -- | JQ-00493 | JQ-00493 | JQ-00493 | -- | JQ-00159 | JQ-00159 | JQ-00159 | -- | -- |
| 4 | JQ-00651 | -- | JQ-00651 | -- | -- | JQ-00425 | -- | -- | -- | -- |
| 5 | JQ-00635 | -- | -- | -- | -- | JQ-00519 | JQ-00519 | -- | JQ-00519 | -- |
| 6 | JQ-00573 | JQ-00573 | JQ-00573 | -- | JQ-00664 | -- | -- | JQ-00664 | JQ-00664 | |
| 7 | JQ-00694 | -- | -- | -- | JQ-00694 | JQ-00610 | JQ-00610 | -- | JQ-00610 | -- |
| 8 | JQ-00711 | -- | -- | -- | -- | -- | JQ-00713 | JQ-00713 | -- | -- |
| 9 | JQ-00750 | -- | JQ-00750 | -- | JQ-00715 | JQ-00715 | -- | -- | JQ-00715 | |
| 10 | JQ-00759 | -- | JQ-00759 | -- | JQ-00740 | -- | JQ-00740 | JQ-00740 | -- | |
| 11 | JQ-00895 | JQ-00895 | JQ-00895 | JQ-00895 | -- | JQ-00805 | -- | -- | -- | -- |
| 12 | JQ-00859 | -- | -- | -- | -- | JQ-00782 | -- | -- | JQ-00782 | JQ-00782 |
| 13 | -- | JQ-00908 | -- | -- | -- | -- | -- | -- | -- | -- |
| 14 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 15 | -- | JQ-00955 | -- | JQ-00955 | -- | -- | -- | -- | -- | JQ-00960 |
| 16 | -- | -- | -- | -- | JQ-00957 | -- | -- | -- | -- | JQ-00979 |
| 17 | -- | JQ-00972 | -- | -- | JQ-00972 | JQ-00987 | JQ-00987 | -- | -- | |
| 18 | -- | JQ-00984 | JQ-00984 | JQ-00984 | JQ-00984 | JQ-01238 | JQ-01238 | -- | JQ-01238 | -- |
| 19 | -- | JQ-00988 | JQ-00988 | JQ-00988 | JQ-00988 | -- | -- | -- | -- | -- |
| 20 | -- | JQ-01239 | JQ-01239 | JQ-01239 | -- | -- | -- | -- | -- | -- |
| 21 | -- | JQ-01233 | -- | JQ-01233 | -- | JQ-01298 | JQ-01298 | JQ-01298 | JQ-01298 | -- |
| 22 | -- | -- | JQ-01250 | JQ-01250 | -- | -- | -- | -- | JQ-01347 | -- |
| 23 | -- | JQ-01349 | -- | JQ-01349 | -- | -- | -- | -- | -- | -- |
| 24 | -- | JQ-01359 | JQ-01359 | JQ-01359 | JQ-01359 | -- | -- | -- | -- | -- |
| 25 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 26 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 27 | -- | JQ-01891 | -- | -- | -- | -- | -- | -- | -- | -- |
| 28 | -- | -- | JQ-01921 | -- | -- | -- | -- | -- | -- | -- |
| 29 | -- | JQ-02004 | JQ-02004 | JQ-02004 | -- | JQ-02267 | -- | -- | -- | -- |
| 30 | -- | -- | JQ-02259 | -- | JQ-02259 | -- | -- | -- | -- | -- |
| 31 | -- | JQ-02226 | JQ-02226 | JQ-02226 | -- | -- | -- | JQ-02319 | -- | -- |
| 32 | -- | JQ-02081 | JQ-02081 | JQ-02081 | -- | -- | -- | JQ-02388 | JQ-02388 | -- |
| 33 | -- | -- | JQ-01704 | -- | -- | -- | JQ-01704 | JQ-01704 | -- | |
| Sr. no. | Anthesis–Maturity > 80 Days | RSA at 0–20 cm > Average | SST > Average | Plant Height < Average | AMS > Average | TGW > 2 g |
|---|---|---|---|---|---|---|
| 1 | -- | JQ-00136 | JQ-00136 | JQ-00136 | -- | JQ-00136 |
| 2 | -- | -- | JQ-00518 | JQ-00518 | -- | -- |
| 3 | -- | JQ-00174 | JQ-00174 | -- | JQ-00174 | -- |
| 4 | JQ-00294 | JQ-00294 | JQ-00294 | -- | -- | JQ-00294 |
| 5 | JQ-00312 | JQ-00312 | JQ-00312 | -- | JQ-00312 | -- |
| 6 | JQ-00313 | -- | -- | JQ-00313 | -- | -- |
| 7 | -- | JQ-00316 | JQ-00316 | -- | JQ-00316 | -- |
| 8 | -- | JQ-00317 | JQ-00317 | -- | JQ-00317 | -- |
| 9 | JQ-00337 | JQ-00337 | JQ-00337 | JQ-00337 | -- | -- |
| 10 | -- | JQ-00492 | JQ-00492 | JQ-00492 | -- | |
| 11 | JQ-00646 | -- | -- | JQ-00646 | JQ-00646 | -- |
| 12 | JQ-00593 | JQ-00593 | JQ-00593 | JQ-00593 | -- | |
| 13 | -- | -- | -- | JQ-00783 | -- | -- |
| 14 | -- | JQ-00769 | -- | JQ-00769 | -- | JQ-00769 |
| 15 | -- | JQ-00910 | JQ-00910 | JQ-00910 | JQ-00910 | |
| 16 | JQ-01141 | JQ-01141 | -- | JQ-01141 | -- | JQ-01141 |
| 17 | JQ-01096 | JQ-01096 | -- | -- | -- | -- |
| 18 | -- | JQ-01279 | JQ-01279 | -- | -- | -- |
| 19 | -- | JQ-01394 | JQ-01394 | -- | JQ-01394 | JQ-01394 |
| 20 | -- | -- | -- | -- | -- | |
| 21 | -- | -- | -- | JQ-01705 | JQ-01705 | -- |
| 22 | -- | JQ-02054 | JQ-02054 | -- | JQ-02054 | -- |
| 23 | -- | -- | -- | JQ-02307 | -- | |
| 24 | JQ-02342 | JQ-02342 | JQ-02342 | -- | JQ-02342 | -- |
| Anthesis–Maturity > 80 Days | RSA at 0–20 cm > Average | SST > Average | Plant Height < Average | AMS > Average | TGW > 2 g | |
|---|---|---|---|---|---|---|
| 1 | JQ-00314 | -- | JQ-00314 | -- | -- | JQ-00314 |
| 2 | JQ-00315 | JQ-00315 | JQ-00315 | -- | JQ-00315 | -- |
| 3 | JQ-00084 | JQ-00084 | JQ-00084 | JQ-00084 | JQ-00084 | JQ-00084 |
| 4 | -- | -- | JQ-00106 | -- | -- | -- |
| 5 | JQ-00470 | JQ-00470 | -- | -- | JQ-00470 | -- |
| 6 | JQ-00520 | -- | -- | JQ-00520 | -- | JQ-00520 |
| 7 | JQ-00521 | JQ-00521 | JQ-00521 | -- | JQ-00521 | |
| 8 | JQ-00563 | JQ-00563 | JQ-00563 | -- | -- | -- |
| 9 | JQ-00729 | -- | -- | -- | -- | -- |
| 10 | JQ-01029 | JQ-01029 | JQ-01029 | -- | JQ-01029 | -- |
| 11 | -- | JQ-01403 | JQ-01403 | JQ-01403 | JQ-01403 | -- |
| 12 | -- | -- | -- | JQ-01592 | -- | -- |
| 13 | -- | -- | -- | JQ-01961 | -- | JQ-01961 |
| 14 | -- | -- | -- | JQ-02068 | JQ-02068 | JQ-02068 |
| 15 | -- | JQ-02261 | -- | -- | -- | -- |
| 16 | JQ-02405 | -- | JQ-02405 | JQ-02405 | -- | JQ-02405 |
| 17 | -- | -- | -- | CK1 | CK1 | CK1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, A.; Jiang, Z.; Dai, J.; Sun, M.; Anwar, S.; Tang, P.; Wang, R.; Ding, P.; Li, L.; Wu, X.; et al. Phenotypic Characterization and Yield Screening of Quinoa Germplasms in Diverse Low-Altitude Regions: A Preliminary Study. Agronomy 2024, 14, 1354. https://doi.org/10.3390/agronomy14071354
Ren A, Jiang Z, Dai J, Sun M, Anwar S, Tang P, Wang R, Ding P, Li L, Wu X, et al. Phenotypic Characterization and Yield Screening of Quinoa Germplasms in Diverse Low-Altitude Regions: A Preliminary Study. Agronomy. 2024; 14(7):1354. https://doi.org/10.3390/agronomy14071354
Chicago/Turabian StyleRen, Aixia, Zhijun Jiang, Jing Dai, Min Sun, Sumera Anwar, Peng Tang, Rongzhen Wang, Pengcheng Ding, Linghong Li, Xiangyun Wu, and et al. 2024. "Phenotypic Characterization and Yield Screening of Quinoa Germplasms in Diverse Low-Altitude Regions: A Preliminary Study" Agronomy 14, no. 7: 1354. https://doi.org/10.3390/agronomy14071354
APA StyleRen, A., Jiang, Z., Dai, J., Sun, M., Anwar, S., Tang, P., Wang, R., Ding, P., Li, L., Wu, X., & Gao, Z. (2024). Phenotypic Characterization and Yield Screening of Quinoa Germplasms in Diverse Low-Altitude Regions: A Preliminary Study. Agronomy, 14(7), 1354. https://doi.org/10.3390/agronomy14071354






