Transcriptomic Change in the Effects of Dichloroquinolinic Acid on the Development and Growth of Nicotiana tabacum
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and RNA Isolation
2.2. RNA-Seq Library Construction and Sequencing
2.3. Data Analysis
2.4. qRT-PCR
2.5. Gene Editing and Phenotypic Analysis of Dichloroquinolinic Acid Resistance in Edited Material
3. Results
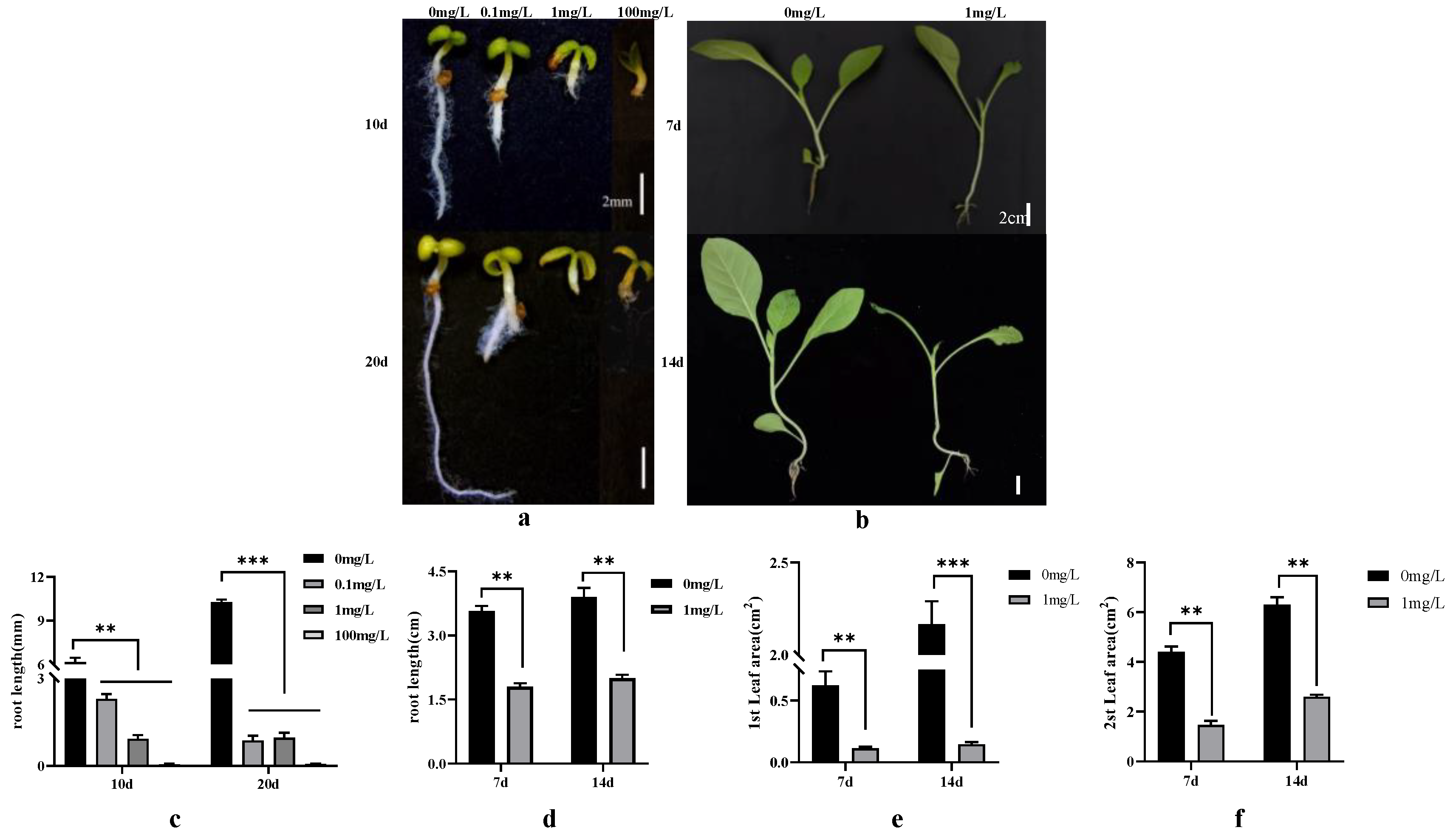
3.1. Effects of Different Dichloroquinolinic Acid and IAA Concentrations on Tobacco Seed Germination and Seedling Growth
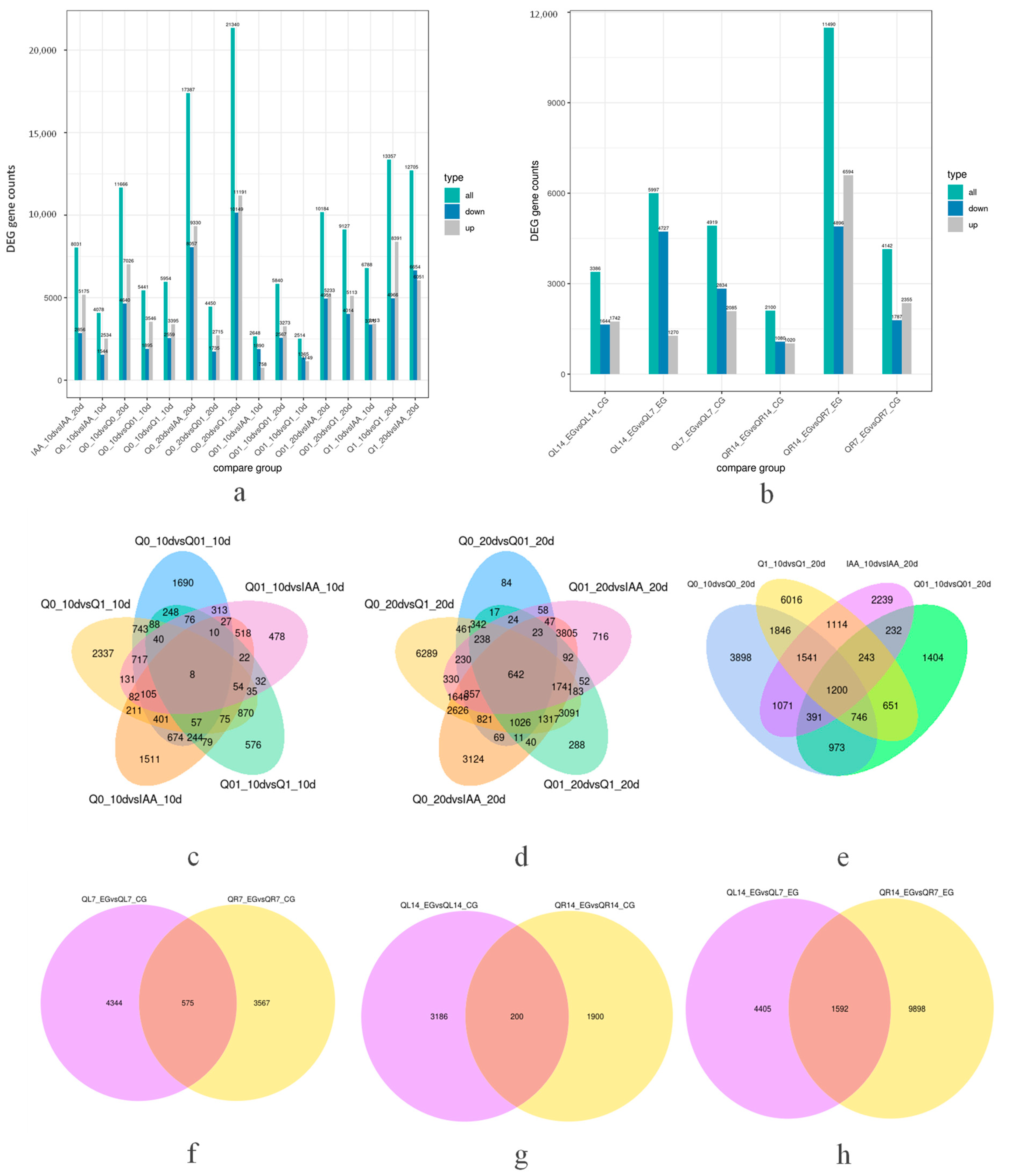
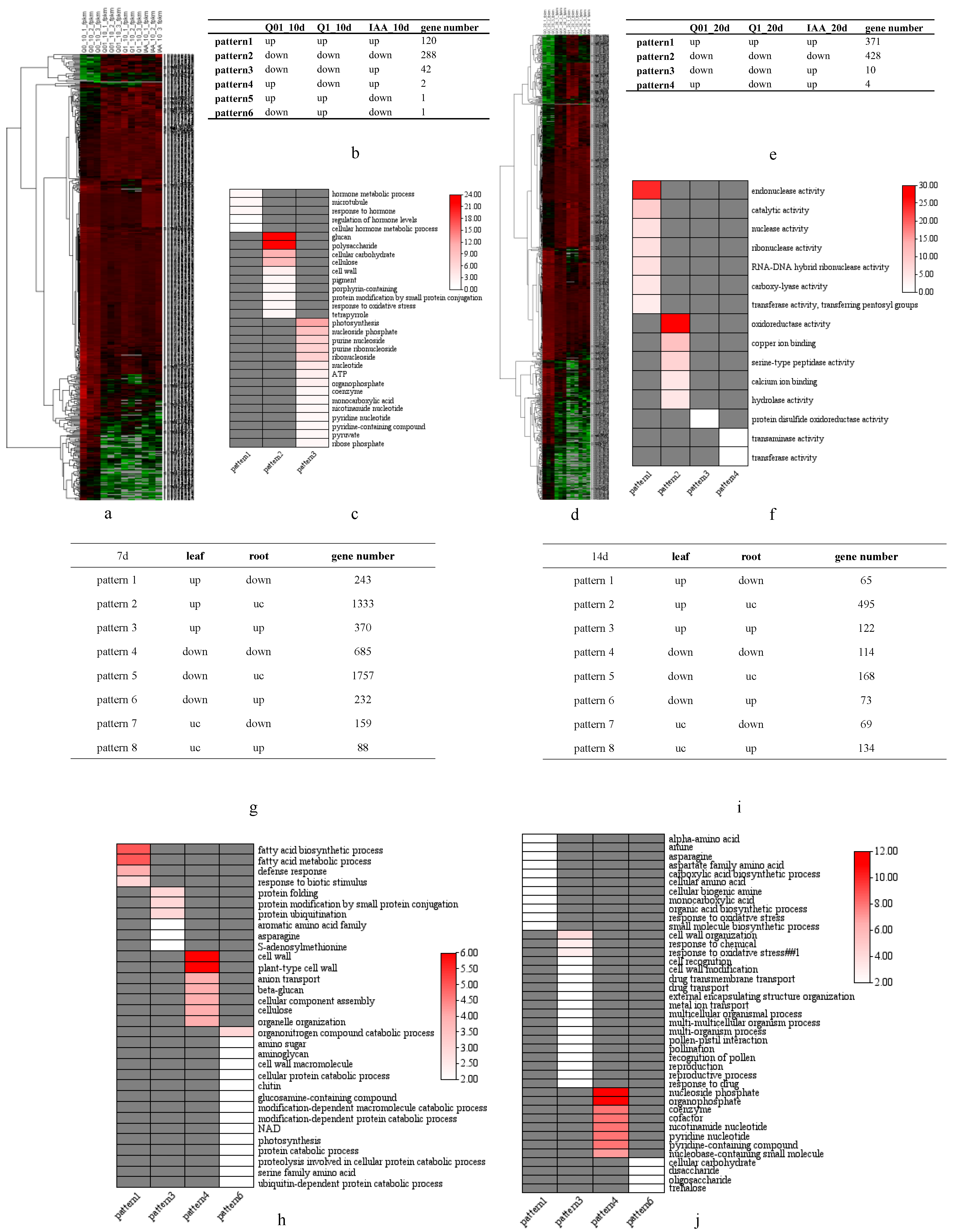
3.2. Illumina HiSeq Transcriptome Sequencing Analysis
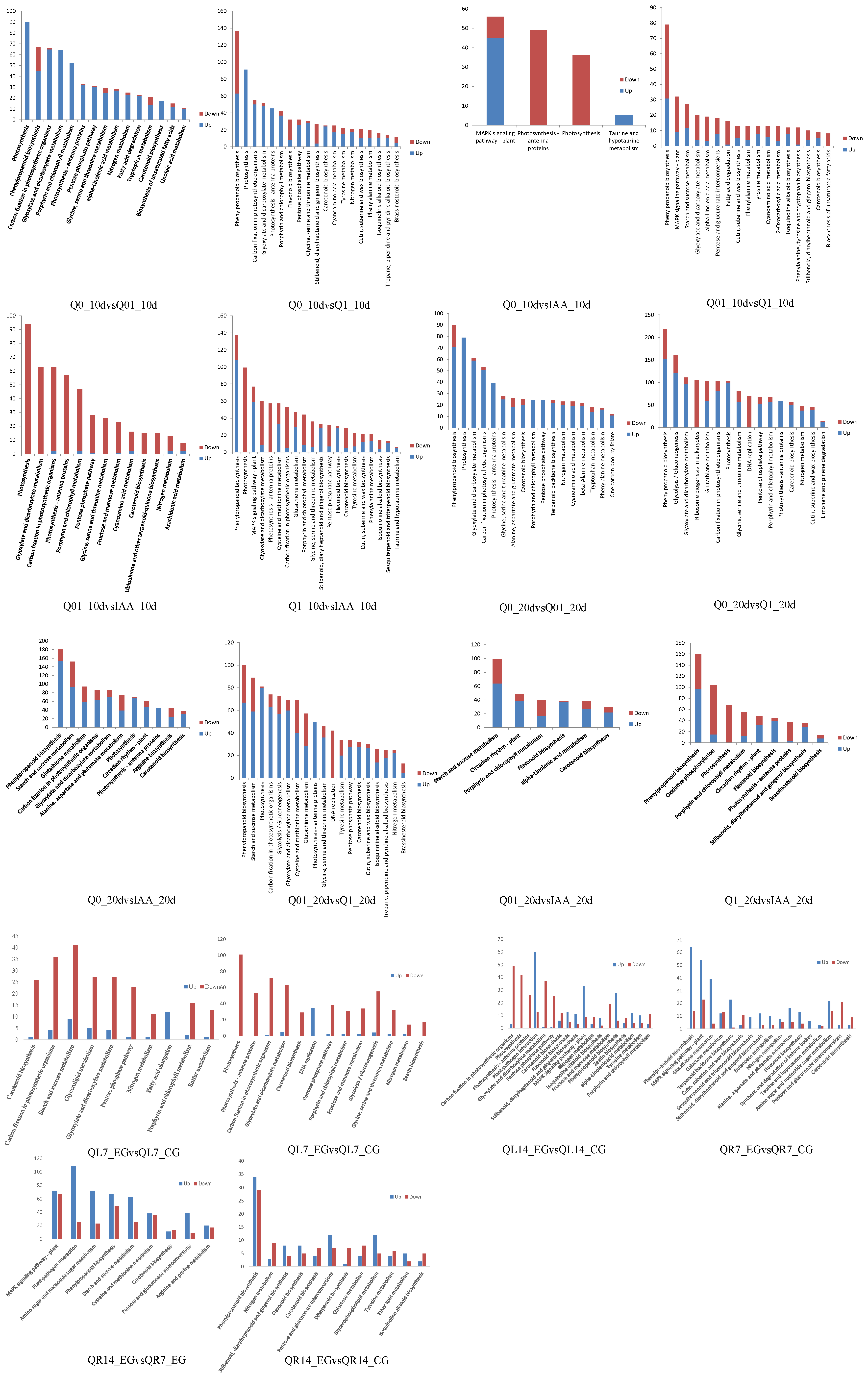
3.3. KEGG Pathway Enrichment Analysis
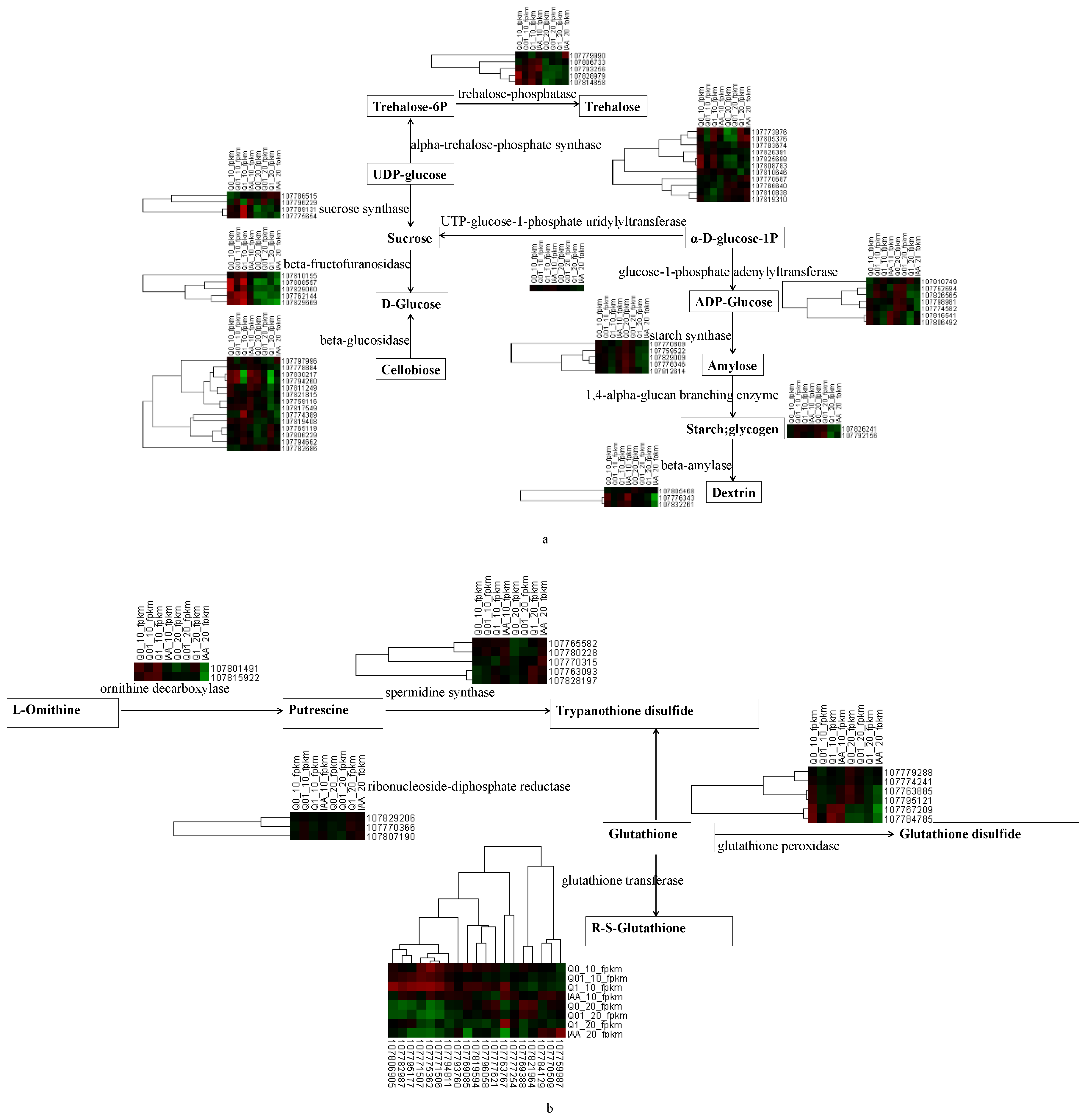
3.4. Rapid Systemic Transcriptional Response of Tobacco to Dichloroquinolinic Acid
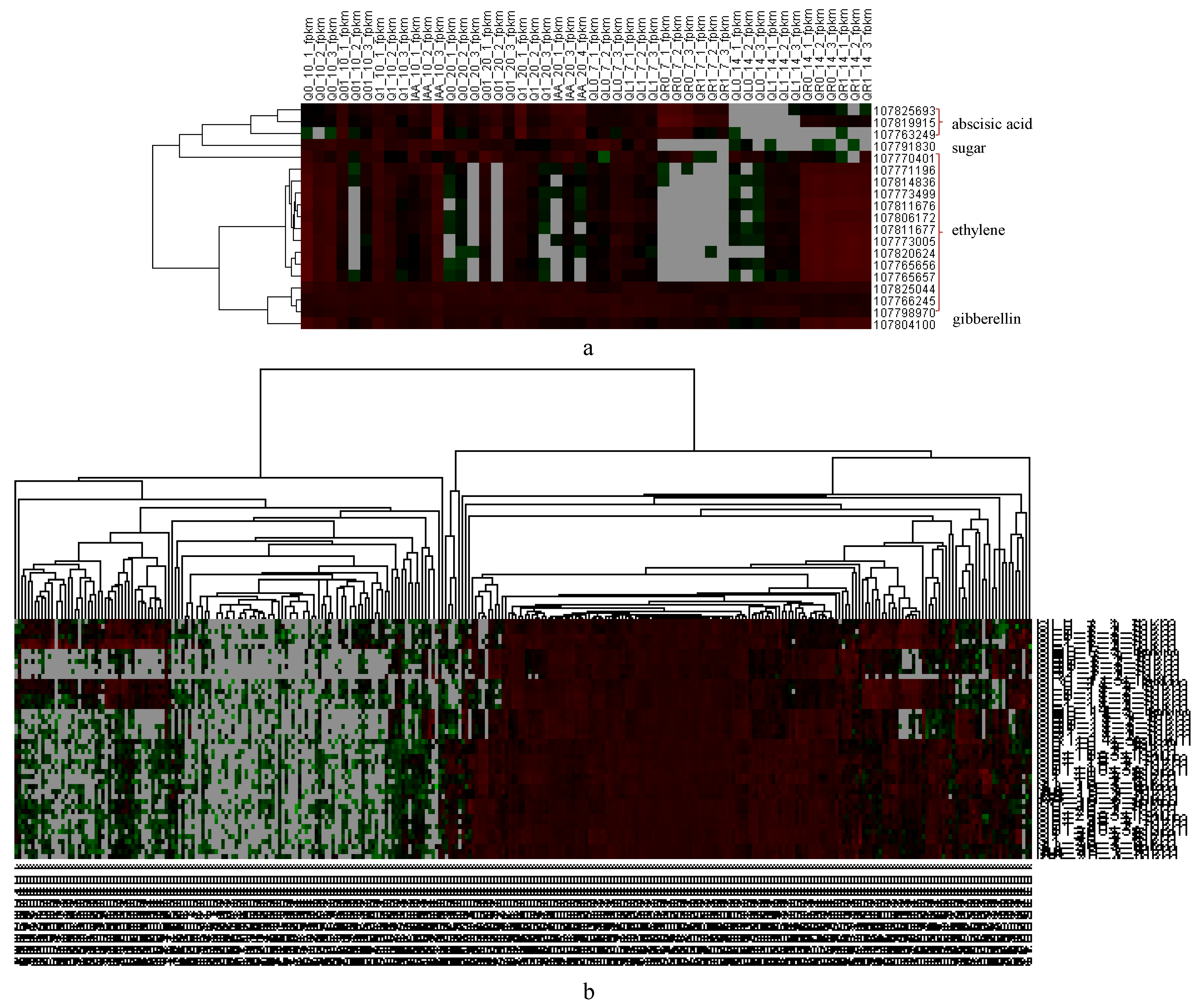
3.5. Significant Changes in Hormone-Related Gene Expression
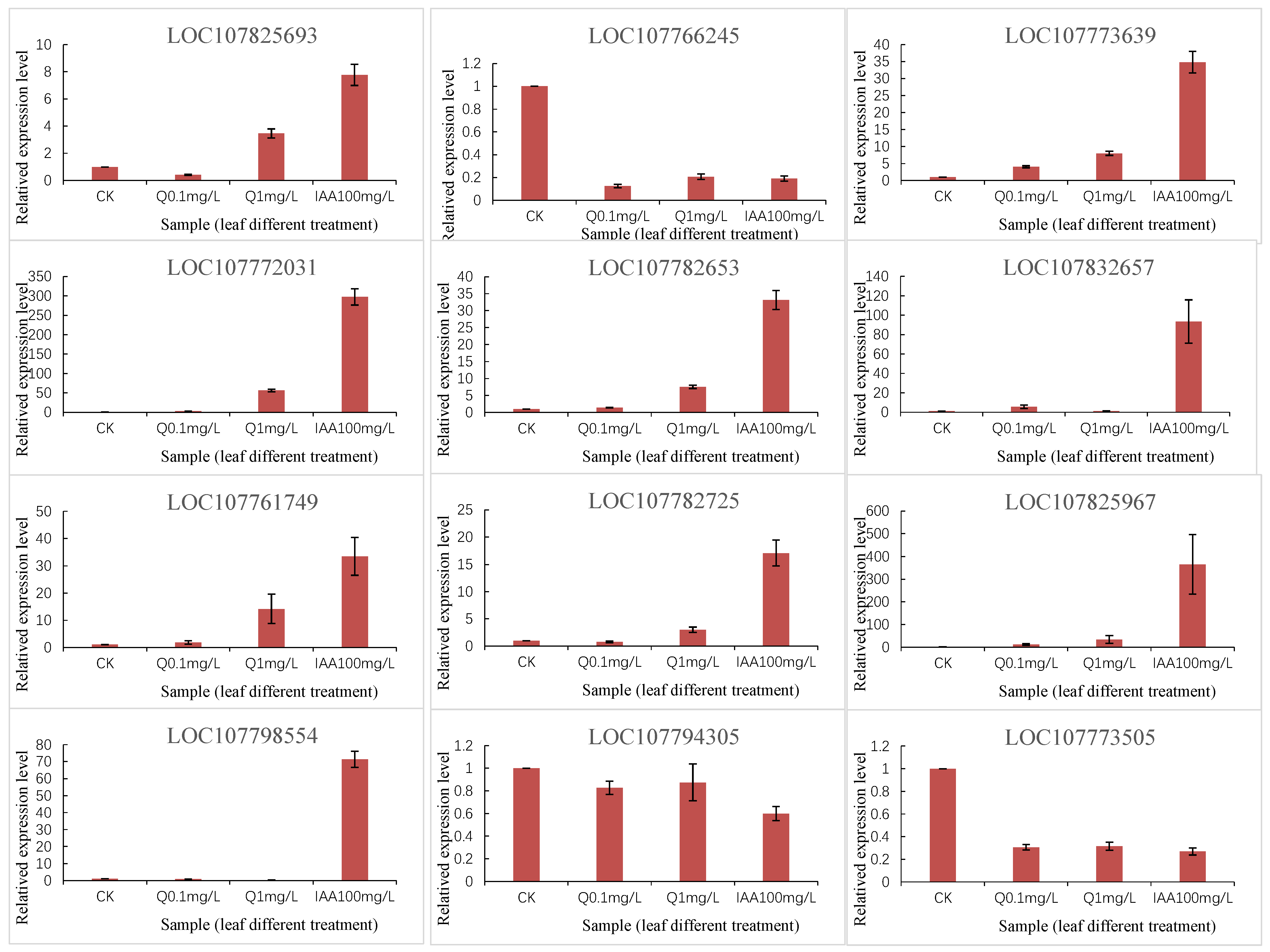
3.6. Verification of Gene Expression via qRT-PCR
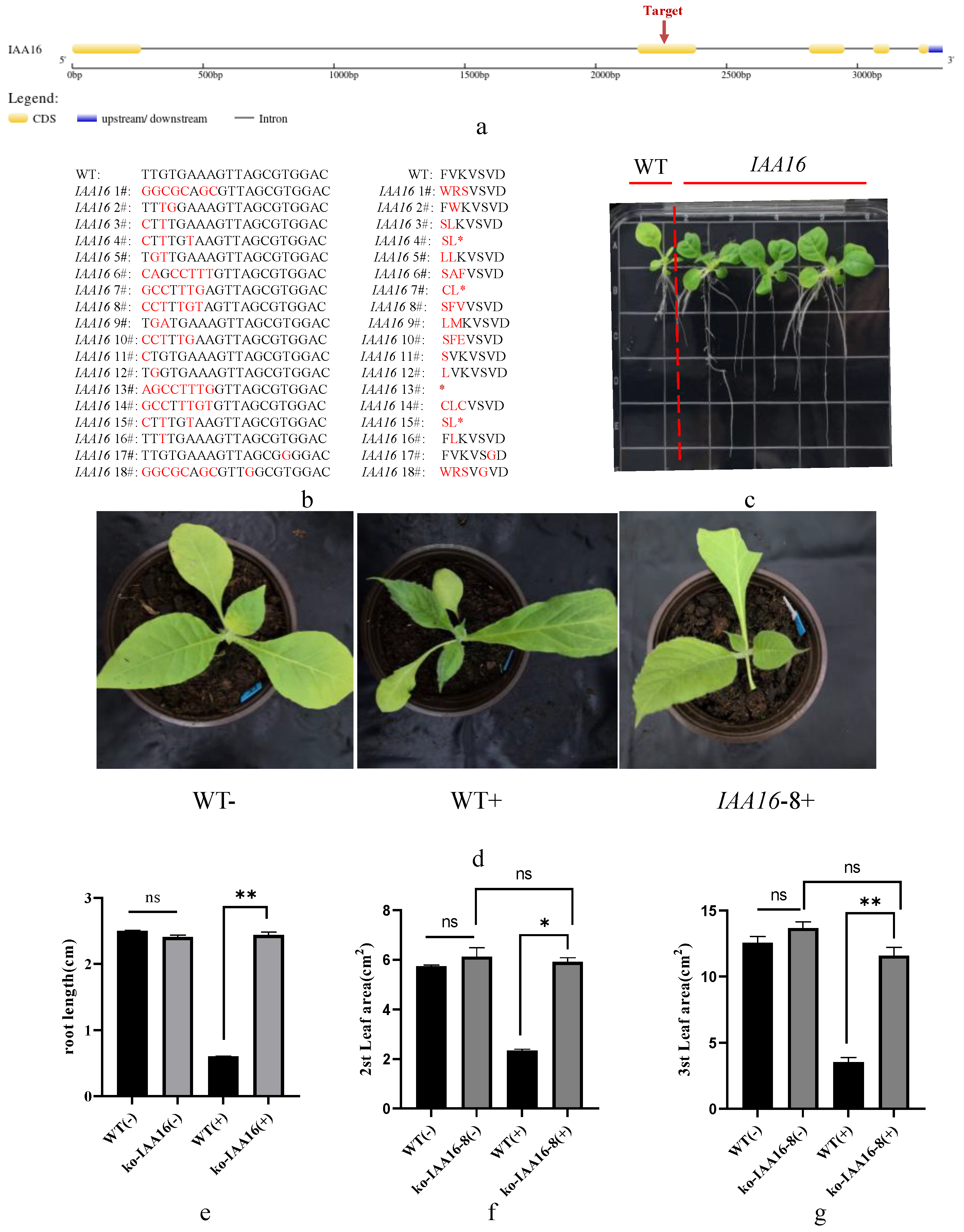
3.7. Verification of Dichloroquinolinic Acid Resistance in IAA-Related Gene Editing Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Lv, M.; Islam, F.; Gill, R.A.; Yang, C.; Ali, B.; Zhou, W. Salicylic acid mediates antioxidant defense system and ABA pathway related gene expression in Oryza sativa against quinclorac toxicity. Ecotoxicol. Environ. Saf. 2016, 133, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Lovelace, M.L.; Hoagland, R.E.; Talbert, R.E.; Scherder, E.F. Influence of Simulated Quinclorac Drift on the Accumulation and Movement of Herbicide in Tomato (Lycopersicon esculentum) Plants. J. Agric. Food Chem. 2009, 57, 6349–6355. [Google Scholar] [CrossRef] [PubMed]
- Sunohara, Y.; Matsumoto, H. Quinclorac-induced cell death is accompanied by generation of reactive oxygen species in maize root tissue. Phytochemistry 2008, 69, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Sunohara, Y.; Matsumoto, H. Comparative Physiological Effects of Quinclorac and Auxins, and Light Involvement in Quinclorac-Induced Chlorosis in Corn Leaves. Pestic. Biochem. Physiol. 1997, 58, 125–132. [Google Scholar] [CrossRef]
- Vignola, M.D.; Sainz, M.; Saldain, N.E.; Marchesi, C.; Bonnecarrère, V.; Gadea, P.D. Limited induction of ethylene and cyanide synthesis are observed in quinclorac-resistant barnyardgrass (Echinochloa crus-galli) in Uruguay. Weed Sci. 2020, 68, 348–357. [Google Scholar] [CrossRef]
- Song, D.; Jiang, X.; Wang, D.; Fang, S.; Zhou, H.; Kong, F. From the effective herbicide to the environmental contaminant: A review of recent studies on quinclorac. Environ. Exp. Bot. 2022, 193, 104706. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.; Song, Q.; Xu, P.; Lv, J.; Cui, M.; Sun, Y.; Dai, C. Screening and Identification of Quinclorac Resistance Lines in Tobacco. Chin. Tob. Sci. 2022, 43, 9–14. [Google Scholar] [CrossRef]
- Zhang, Q.; Guo, W.; Song, C.; Guan, Z.; Wang, J.; Mou, S.; Wang, G.; Li, Y. Degradation Dynamics of Quinclorac and its Influencing Factors in Four Soils. Chin. Tob. Sci. 2013, 34, 83–88. [Google Scholar]
- Kohlhase, D.R.; O’rourke, J.A.; Owen, M.D.K.; Graham, M.A. Using RNA-seq to characterize responses to 4-hydroxyphenylpyruvate dioxygenase (HPPD) inhibitor herbicide resistance in waterhemp (Amaranthus tuberculatus). BMC Plant Biol. 2019, 19, 182. [Google Scholar] [CrossRef]
- Baek, Y.S.; Goodrich, L.V.; Brown, P.J.; James, B.T.; Moose, S.P.; Lambert, K.N.; Riechers, D.E. Transcriptome Profiling and Genome-Wide Association Studies Reveal GSTs and Other Defense Genes In-volved in Multiple Signaling Pathways Induced by Herbicide Safener in Grain Sorghum. Front. Plant Sci. 2019, 10, 192. [Google Scholar] [CrossRef]
- Rangani, G.; Rouse, C.E.; Saski, C.; Noorai, R.E.; Shankar, V.; Lawton-Rauh, A.L.; Werle, I.S.; Roma-Burgos, N. High Resistance to Quinclorac in Multiple-Resistant Echinochloa colona Associated with Elevated Stress Tolerance Gene Expression and Enriched Xenobiotic Detoxification Pathway. Genes 2022, 13, 515. [Google Scholar] [CrossRef]
- Wright, A.A.; Sasidharan, R.; Koski, L.; Rodriguez-Carres, M.; Peterson, D.G.; Nandula, V.K.; Ray, J.D.; Bond, J.A.; Shaw, D.R. Transcriptomic changes in Echinochloa colona in response to treatment with the herbicide imazamox. Planta 2018, 247, 369–379. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.; Bei, F.; Jin, T.; Jia, S.; Bu, R.; Liu, W. Investigating the Metabolic Mesosulfuron-Methyl Resistance in Aegilops tauschii Coss. by Transcriptome Sequencing Combined with the Reference Genome. J. Agric. Food Chem. 2022, 70, 11429–11440. [Google Scholar]
- Casey, A.; Köcher, T.; Caygill, S.; Champion, C.; Bonnot, C.; Dolan, L. Transcriptome changes in chlorsulfuron-treated plants are caused by acetolactate synthase inhibition and not induction of a herbicide detoxification system in Marchantia polymorpha. bioRxiv 2022. bioRxiv:2022.08.31.505973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Park, C.; Bennett, C.; Thornton, M.; Kim, D. Rapid and accurate alignment of nucleotide conversion sequencing reads with HISAT-3N. Genome Res. 2021, 31, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Damgaard, M.V.; Treebak, J.T. Protocol for qPCR analysis that corrects for cDNA amplification efficiency. STAR Protoc. 2022, 3, 101515. [Google Scholar] [CrossRef]
- Li, B.; Shang, Y.; Wang, L.; Lv, J.; Wang, F.; Chao, J.; Mao, J.; Ding, A.; Wu, X.; Cui, M.; et al. Highly efficient multiplex genome editing in dicots using improved CRISPR/Cas systems. bioRxiv 2022. [Google Scholar] [CrossRef]
- Miyazawa, Y.; Kutsuna, N.; Inada, N.; Kuroiwa, H.; Kuroiwa, T.; Yoshida, S. Dedifferentiation of starch-storing cultured tobacco cells: Effects of 2,4-dichlorophenoxy acetic acid on multiplication, starch content, organellar DNA content, and starch synthesis gene expression. Plant Cell Rep. 2002, 21, 289–295. [Google Scholar] [CrossRef]
- Baldwin, A.; Rogers, H.J.; Francis, D.; Harwood, J.L. Fatty acid elongation is important in the activity of thiocarbamate herbicides and in safening by dichlormid. J. Exp. Bot. 2003, 54, 1289–1294. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Chen, Z.; Wen, Y.; Liu, W. Enantioselective Phytotoxic Disturbances of Fatty Acids in Arabidopsis thaliana by Dichlorprop. Environ. Sci. Technol. 2019, 53, 9252–9259. [Google Scholar] [CrossRef] [PubMed]
- Domaradzki, K.; Marczewska-Kolasa, K.; Bortniak, M. Evaluation of the effectiveness of a mixture of herbicides and biostimulators in the cultivation of sugar beet. Przem. Chem. 2015, 94, 787–792. [Google Scholar]
- Cao, J.; Peng, Q.; Yang, X.; Yang, Q.; Bai, L.; Li, Y.; Zhang, Z.; Gu, T. Differences in exogenous methyl jasmonate-induced quinclorac resistance between resistant and sensitive barnyardgrass and the underlying mechanism. Chin. J. Appl. Ecol. 2020, 31, 2293–2298. [Google Scholar]
- Figueiredo, M.R.D.; Küpper, A.; Malone, J.M.; Petrovic, T.; Figueiredo, A.B.T.D.; Campagnola, G.; Gaines, T.A. An in-frame deletion mutation in the degron tail of auxin coreceptor IAA2 confers resistance to the herbicide 2,4-D in Sisymbrium orientale. Proc. Natl. Acad. Sci.USA 2022, 119, e2105819119. [Google Scholar] [CrossRef] [PubMed]
- Van Eerd, L.L.; McLean, M.D.; Stephenson, G.R.; Hall, J.C. Resistance to quinclorac and ALS-inhibitor herbicides in Galium spurium is conferred by two distinct genes. Weed Res. 2010, 44, 355–365. [Google Scholar] [CrossRef]
- Koo, S.J.; Neal, J.C.; DiTomaso, J.M. Mechanism of Action and Selectivity of Quinclorac in Grass Roots. Pestic. Biochem. Physiol. 1997, 57, 44–53. [Google Scholar] [CrossRef]
- Hansen, H.; Grossmann, K. Auxin-Induced Ethylene Triggers Abscisic Acid Biosynthesis and Growth Inhibition. Plant Physiol. 2000, 124, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Prathap, V.; Ali, K.; Singh, A.; Vishwakarma, C.; Krishnan, V.; Chinnusamy, V.; Tyagi, A. Starch accumulation in rice grains subjected to drought during grain filling stage. Plant Physiol. Biochem. 2019, 142, 440–451. [Google Scholar] [CrossRef]
- Saucedo, M.C.C.; Alvarado, A.D.; Téllez, L.C.; Hernández, V.G.; Tapia-Campos, E.; Varela, A.S.; García-De Los Santos, G. Changes in carbohydrate concentration in leaves, PODS and seeds of dry bean plants under drought stress. Interciencia 2012, 37, 168–175. [Google Scholar]
- Katerova, Z.I.; Miteva, P.E. Glutathione and Herbicide Resistance in Plants. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Springer: Dordrecht, The Netherlands, 2010; pp. 191–207. [Google Scholar]
- Nemat Alla, M.M.; Badawi, A.H.M.; Hassan, N.M.; El-Bastawisy, Z.M.; Badran, E.G. Herbicide tolerance in maize is related to increased levels of glutathione and glutathione-associated enzymes. Acta Physiol. Plant. 2008, 30, 371–379. [Google Scholar] [CrossRef]
- Hu, T.; Qv, X.; Xiao, G.; Huang, X. Enhanced tolerance to herbicide of rice plants by over-expression of a glutathione S-transferase. Mol. Breed. 2009, 24, 409–418. [Google Scholar] [CrossRef]
- Miteva, L.P.-E.; Ivanov, S.V.; Alexieva, V.S.; Karanov, E.N. Effect of atrazine on glutathione levels, glutathione s-transferase and glutathione reductase activities in pea and wheat plants. Plant Prot. Sci. 2004, 40, 160. [Google Scholar] [CrossRef]
- Jin, M.; Liu, Y.; Shi, B.; Yuan, H. Exogenous IAA improves the seedling growth of Syringa villosa via regulating the endogenous hormones and enhancing the photosynthesis. Sci. Hortic. 2023, 308, 111585. [Google Scholar] [CrossRef]
| Name | Sequence (5′–3′) | |
|---|---|---|
| F | R | |
| 107825693 | GTATGGGTATGGGTGGTGGA | CCTCCTGGCATCTTCTCCAT |
| 107766245 | CCCTTCCTCTGAGCCTACTG | GGCTGTACAACCCCTCTCTT |
| 107773639 | GGAAAGGCAGCAGATAAGGC | TGCTCTGTCCTTTGTCGTCT |
| 107772031 | GGTCCTTCAGCTACCCAAGT | AGTGATGACAGCCCCGTTAA |
| 107782653 | CCCACGAACAGAGCTACAGA | GGTTTCCTGAGCTGCATCTG |
| 107832657 | GCGATATCCTGGAGACTGCT | AAGCAACAGCAGCATCCAAA |
| 107761749 | GACTTGAGGGTGACATGGGA | CTAACCTCAAATCCGCCACG |
| 107782725 | GAGCATCATCCACTGCAAGG | CCTCCTTTGGGCTAGTTGGA |
| 107825967 | ACAAGGAGAGACGGTGGTTC | GCACGTTCACCACCAGATTT |
| 107798554 | ACATTCCAGTCATCCCAGCA | AGAACCTCCTTGAAGTGCGA |
| 107794305 | GAAATGATGGAGGCAGCAGG | TGGAGGCCTTGGACTTCATT |
| 107773505 | GGTGGGGAAGGAGGAGAAAG | AGTGGGAGAATTGGGGTGAG |
| NtIAA-sg | GGCCACCAGTGAGATCATTC | GTCCACGCTAACTTTCACAA |
| Category | Treatment |
|---|---|
| Q0_10 | Tobacco seedlings 10 days after sowing on petri dishes containing 0 mg/L dichloroquinolinic acid |
| Q01_10 | Tobacco seedlings 10 days after sowing on petri dishes containing 0.1 mg/L dichloroquinolinic acid |
| Q1_10 | Tobacco seedlings 10 days after sowing on petri dishes containing 1 mg/L dichloroquinolinic acid |
| IAA_10 | Tobacco seedlings 10 days after sowing on petri dishes containing 100 mg/L IAA |
| Q0_20 | Tobacco seedlings 20 days after sowing on petri dishes containing 0 mg/L dichloroquinolinic acid |
| Q01_20 | Tobacco seedlings 20 days after sowing on petri dishes containing 0.1 mg/L dichloroquinolinic acid |
| Q1_20 | Tobacco seedlings 20 days after sowing on petri dishes containing 1 mg/L dichloroquinolinic acid |
| IAA_20 | Tobacco seedlings 20 days after sowing on petri dishes containing 100 mg/L IAA |
| QL0_7 | The leaves of tobacco seedlings 7 days after sowing in seedling trays and saturated with 0 mg/L dichloroquinolinic acid |
| QL1_7 | The leaves of tobacco seedlings 7 days after sowing in seedling trays and saturated with 1 mg/L dichloroquinolinic acid |
| QR0_7 | The roots of tobacco seedlings 7 days after sowing in seedling trays and saturated with 0 mg/L dichloroquinolinic acid |
| QR1_7 | The roots of tobacco seedlings 7 days after sowing in seedling trays and saturated with 1 mg/L dichloroquinolinic acid |
| QL0_14 | The leaves of tobacco seedlings 14 days after sowing in seedling trays and saturated with 0 mg/L dichloroquinolinic acid |
| QL1_14 | The leaves of tobacco seedlings 14 days after sowing in seedling trays and saturated with 1 mg/L dichloroquinolinic acid |
| QR0_14 | The roots of tobacco seedlings 14 days after sowing in seedling trays and saturated with 0 mg/L dichloroquinolinic acid |
| QR1_14 | The roots of tobacco seedlings 14 days after sowing in seedling trays and saturated with 1 mg/L dichloroquinolinic acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Ding, A.; Wang, W.; Cui, M.; Sun, Y.; Lv, J.; Dai, C. Transcriptomic Change in the Effects of Dichloroquinolinic Acid on the Development and Growth of Nicotiana tabacum. Agronomy 2024, 14, 1364. https://doi.org/10.3390/agronomy14071364
Li B, Ding A, Wang W, Cui M, Sun Y, Lv J, Dai C. Transcriptomic Change in the Effects of Dichloroquinolinic Acid on the Development and Growth of Nicotiana tabacum. Agronomy. 2024; 14(7):1364. https://doi.org/10.3390/agronomy14071364
Chicago/Turabian StyleLi, Bingjie, Anming Ding, Weifeng Wang, Mengmeng Cui, Yuhe Sun, Jing Lv, and Changbo Dai. 2024. "Transcriptomic Change in the Effects of Dichloroquinolinic Acid on the Development and Growth of Nicotiana tabacum" Agronomy 14, no. 7: 1364. https://doi.org/10.3390/agronomy14071364
APA StyleLi, B., Ding, A., Wang, W., Cui, M., Sun, Y., Lv, J., & Dai, C. (2024). Transcriptomic Change in the Effects of Dichloroquinolinic Acid on the Development and Growth of Nicotiana tabacum. Agronomy, 14(7), 1364. https://doi.org/10.3390/agronomy14071364





