Physiological Mechanisms of BvCPD Regulation in Sugar Beet Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of Photosynthetic Performance
2.3. Determination of Respiration Rate
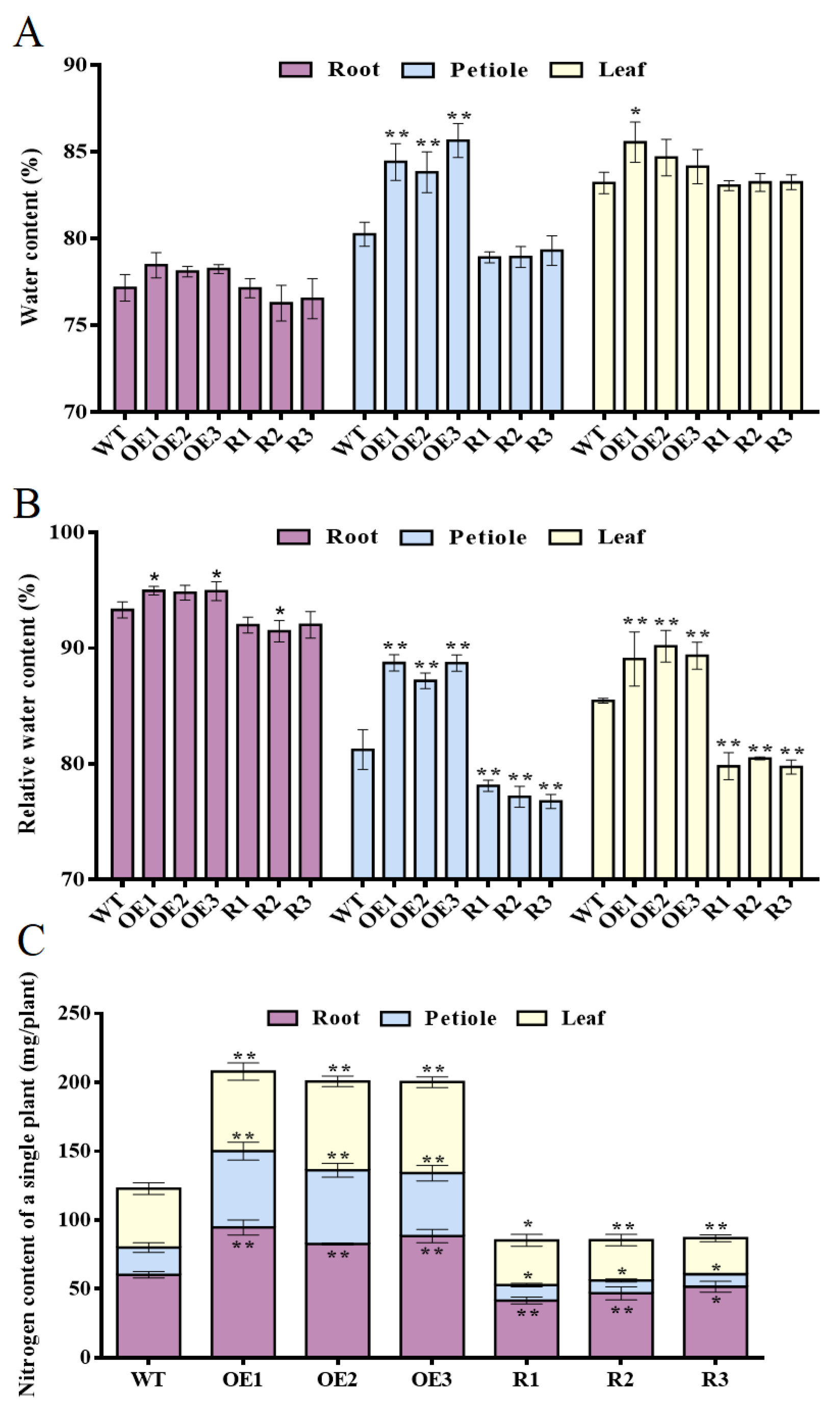
2.4. Determination of Moisture and Nitrogen Content
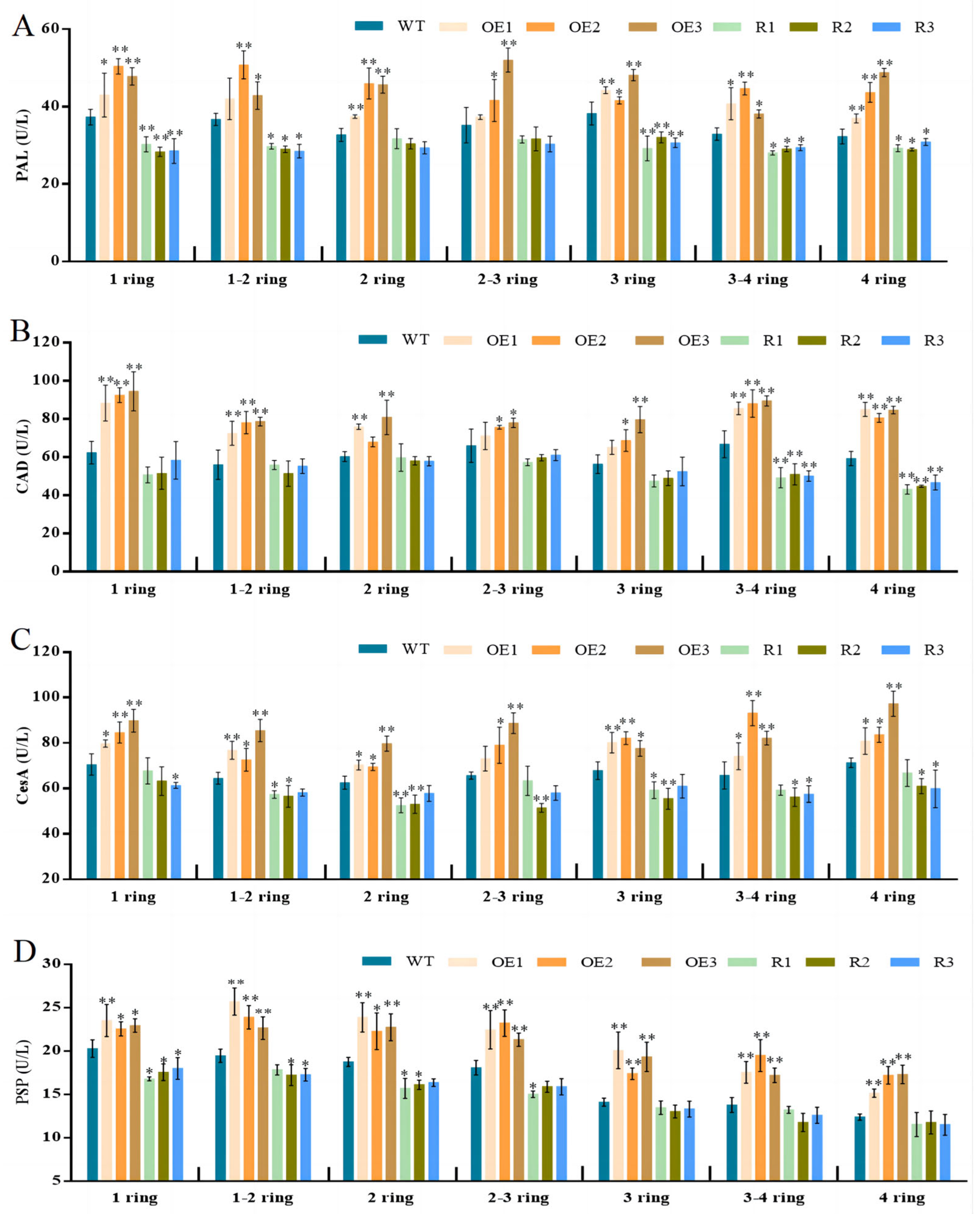
2.5. Determination of ATP Content and Enzyme Activity
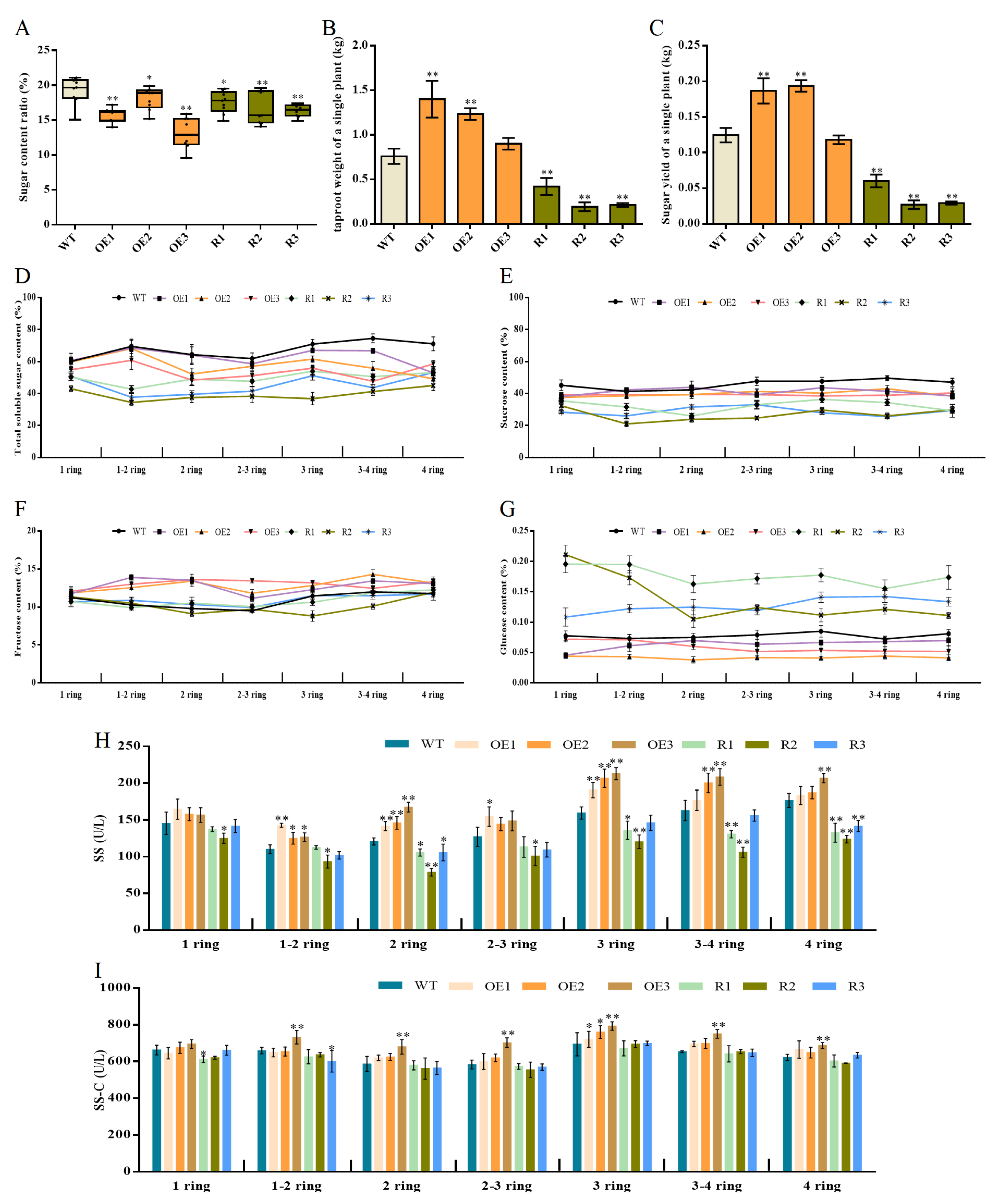
2.6. Determination of Sugar Fractions
- (1)
- Extraction of soluble sugars: Mother roots from each transgenic line were utilized for analysis. BvCPD transgenic sugar beets and WT taproots were shredded and shade-dried, followed by oven drying at 105 °C for 30 min and then 85 °C for 12 h until a constant weight was attained. The dried samples were then ground into a powder. For extraction, 0.05 g of the powdered sample was weighed into a 10 mL centrifuge tube, 4 mL of 80% ethanol solution was added, and the mixture was placed in an 80 °C water bath for 40 min. After centrifugation, the supernatant was collected. The extraction process was repeated using 80% ethanol solution until a final volume of 10 mL was achieved, yielding the solution to be measured.
- (2)
- Determination of total soluble sugar content: Two milliliters of the solution to be measured was taken and mixed with 0.5 mL of anthrone ethyl acetate reagent (prepared by dissolving 1 g of anthrone in 50 mL of ethyl acetate solution) and 5 mL of concentrated sulfuric acid. The mixture was then subjected to a 100 °C water bath for 1 min, followed by cooling. The optical density (OD) value was determined at 625 nm.
- (3)
- Determination of sucrose content: A volume of 150 μL of the solution to be measured was mixed with 150 μL of 2 mol/L NaOH and subjected to a 100 °C water bath for 5 min. After cooling, 2.1 mL of 10 mol/L HCl solution and 0.6 mL of 0.1% resorcinol were added, followed by thorough shaking. The mixture was then subjected to an 80 °C water bath for 10 min, and the OD value was determined at 480 nm.
- (4)
- Determination of fructose content: A volume of 0.4 mL of the solution to be measured was mixed with 2.8 mL of 2 mol/L HCl solution and 0.8 mL of 0.1% resorcinol. The mixture was subjected to an 80 °C water bath for 10 min, and the OD value was determined at 480 nm.
- (5)
- Determination of glucose content: The glucose content was determined according to the instructions of the glucose content detection kit (ADS-W-TDX002) manufactured by Jiangsu Edison Biological Co. in Nanjing, China.
2.7. Determination of Yield
2.8. Statistical Analysis
3. Results
3.1. BvCPD Promotes Photosynthetic Properties of Sugar Beet Leaves
3.2. BvCPD Promotes Energy Metabolism in Taproots
3.3. BvCPD Regulates Sugar Metabolism in Sugar Beets
3.4. BvCPD Can Improve Water and Nitrogen Uptake in Sugar Beets
3.5. BvCPD Enhances the Activity of Enzymes Involved in Cellular Development
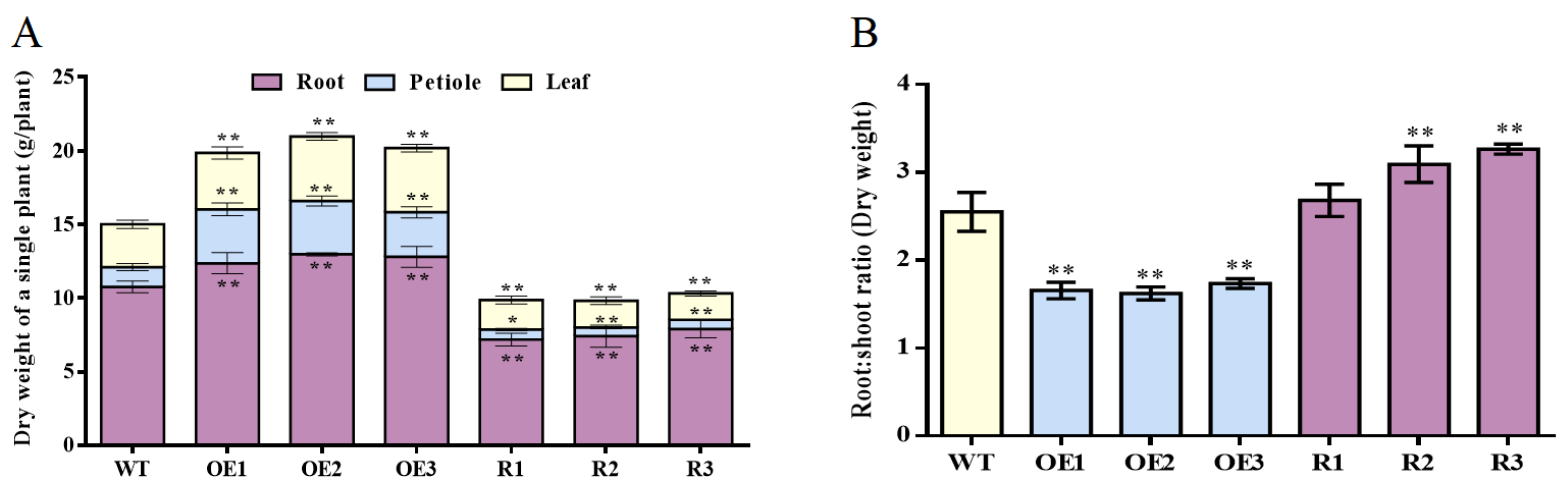
3.6. BvCPD Regulates the Accumulation and Distribution of Dry Matter in Sugar Beet
4. Discussion
4.1. BvCPD Can Improve the “Source-Sink-Flow” Balance in Sugar Beets
4.2. Regulation of Energy and Sugar Metabolism by BvCPD in Sugar Beets
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dohm, J.C.; Minoche, A.E.; Holtgräwe, D.; Capella-Gutiérrez, S.; Zakrzewski, F.; Tafer, H.; Rupp, O.; Sörensen, T.R.; Stracke, R.; Reinhardt, R.; et al. The genome of the recently domesticated crop plant sugar beet (Beta vulgaris). Nature 2014, 505, 546–549. [Google Scholar] [CrossRef]
- Shao, J.W. Physiology of the Sweetbrier; Agricultural Press: Beijing, China, 1991. [Google Scholar]
- Wang, W.; Sun, Y.; Li, G.; Zhang, S. Brassinosteroids promote parenchyma cell and secondary xylem development in sugar beet (Beta vulgaris L.) root. Plant Direct 2021, 5, e340. [Google Scholar] [CrossRef]
- Hosseini, L.; Maleki, A.; Mozafari, A.; Mirzaeiheydari, M.; Sadeghi, S.M. Evaluation of Water and Nitrogen Use Efficiency, Digestibility and Some Quantitative and Qualitative Characteristics of Forage Beet Cultivars under Different Irrigation Methods and Nitrogen Levels. Gesunde Pflanz. 2021, 74, 177–191. [Google Scholar] [CrossRef]
- Zewail, R.M.Y.; El Gmal, I.S.; Khaitov, B.; El Desouky, H.S.A. Micronutrients through foliar application enhance growth, yield and quality of sugar beet (Beta vulgaris L.). J. Plant Nutr. 2020, 43, 2275–2285. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, M.; Zhang, L.; Li, B.; Han, M.; Alva, A.K.; Aahraf, M. Role of exogenous glycinebetaine and humic acid in mitigating drought stress-induced adverse effects in Malus robusta seedlings. Turk. J. Bot. 2013, 37, 920–929. [Google Scholar] [CrossRef]
- Shahram, K.; Mohammad, A.C.; Afshin, S.; Hossein, A.N.; Saeed, S.H. Influence of Foliar-Applied Humic Acid and Some Key Growth Regulators on Sugar Beet (Beta vulgaris L.) under Drought Stress: Antioxidant Defense System, Photosynthetic Characteristics and Sugar Yield. Sugar Tech 2020, 22, 765–772. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, L.; Wang, X.; Wang, Z.; Zhang, H.; Chen, J.; Liu, X.; Wang, Y.; Li, C. Beneficial Effects of Exogenous Melatonin on Overcoming Salt Stress in Sugar Beets (Beta vulgaris L.). Plants 2021, 10, 886. [Google Scholar] [CrossRef]
- Ghaffari, H.; Tadayon, M.R.; Razmjoo, J.; Bahador, M.; Karimzadeh, S.H.; Yuan, T. Impact of Jasmonic Acid on Sugar Yield and Physiological Traits of Sugar Beet in Response to Water Deficit Regimes: Using Stepwise Regression Approach. Russ. J. Plant Physiol. 2020, 67, 482–493. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Li, G.L.; Wang, X.F.; Sun, Y.Q.; Zhang, S.Y. Transcriptomic profiling of taproot growth and sucrose accumulation in sugar beet (Beta vulgaris L.) at different developmental stages. PLoS ONE 2017, 12, e0175454. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.; Li, N.; Li, G.; Sun, Y.; Zhang, S. BvCPD promotes parenchyma cell and vascular bundle development in sugar beet (Beta vulgaris L.) taproot. Front. Plant Sci. 2023, 14, 1271329. [Google Scholar] [CrossRef]
- Wu, H.; Xu, D.; Si, J.; Lian, G.; Qin, Y.; Chen, X.; Jiang, Y.; Wang, X. Cloning, characterization, and functional analysis of Populus euphratica CPD (PeCPD), a key brassinosteroid (BR) biosynthesis gene. Chin. Soc. Cell Biol. 2013, 260, 252. [Google Scholar]
- Zhan, H.; Lu, M.; Luo, Q.; Tan, F.; Zhao, Z.; Liu, M.; He, Y. OsCPD1 and OsCPD2 are functional brassinosteroid biosynthesis genes in rice. Plant Sci. 2022, 325, 111482. [Google Scholar] [CrossRef]
- NY/T 2017-2011; Determination of Nitrogen, Phosphorus and Potassium in Plants. China Agricultural Press: Beijing, China, 2011.
- GB/T 10496-2018; Sugar Beet. China Standard Publishing House: Beijing, China, 2018.
- Wu, X.; Tong, L.; Kang, S.; Du, T.; Ding, R.; Li, S.; Chen, Y. Combination of suitable planting density and nitrogen rate for high yield maize and their source-sink relationship in Northwest China. J. Sci. Food Agric. 2023, 103, 5300–5311. [Google Scholar] [CrossRef]
- Wang, L.; Xia, H.; Li, X.; Qiao, Y.; Xue, Y.; Jiang, X.; Yan, W.; Liu, Y.; Xue, Y.; Kong, L. Source-Sink Manipulation Affects Accumulation of Zinc and Other Nutrient Elements in Wheat Grains. Plants 2021, 10, 1032. [Google Scholar] [CrossRef]
- Zhai, L.; Yan, A.; Shao, K.; Wang, S.; Wang, Y.; Chen, Z.H.; Xu, J. Large Vascular Bundle Phloem Area 4 enhances grain yield and quality in rice via source-sink-flow. Plant Physiol. 2023, 191, 317–334. [Google Scholar] [CrossRef]
- Li, W.; Xiong, B.; Wang, S.; Deng, X.; Yin, L.; Li, H. Regulation Effects of Water and Nitrogen on the Source-Sink Relationship in Potato during the Tuber Bulking Stage. PLoS ONE 2016, 11, e0146877. [Google Scholar] [CrossRef]
- Qin, A.; Aluko, O.O.; Liu, Z.; Yang, J.; Hu, M.; Guan, L.; Sun, X. Improved cotton yield: Can we achieve this goal by regulating the coordination of source and sink? Front. Plant Sci. 2023, 14, 1136636. [Google Scholar] [CrossRef]
- Hu, X.; Gu, T.; Khan, I.; Zada, A.; Jia, T. Research Progress in the Interconversion, Turnover and Degradation of Chlorophyll. Cells 2021, 10, 3134. [Google Scholar] [CrossRef]
- Melkozernov, A.N.; Blankenship, R.E. Photosynthetic functions of chlorophylls. Adv. Photosynth. Respir. 2006, 25, 397–412. [Google Scholar]
- Jian, C. Regulation of Sugar Beet Growth and Yield Quality by Four Plant Growth Regulators. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2017. [Google Scholar]
- Ibañes, M.; Fàbregas, N.; Chory, J.; Caño-Delgado, A.I. Brassinosteroid signaling and auxin transport are required to establish the periodic pattern of Arabidopsis shoot vascular bundles. Proc. Natl. Acad. Sci. USA 2009, 106, 13630–13635. [Google Scholar] [CrossRef]
- O’Leary, B.; Plaxton, W.C. Multifaceted functions of post-translational enzyme modifications in the control of plant glycolysis. Curr. Opin. Plant Biol. 2020, 55, 28–37. [Google Scholar] [CrossRef]
- Bar-Even, A.; Flamholz, A.; Noor, E.; Milo, R. Rethinking glycolysis: On the biochemical logic of metabolic pathways. Nat. Chem. Biol. 2012, 8, 509–517. [Google Scholar] [CrossRef]
- Banks, R.D.; Blake, C.C.; Evans, P.R.; Haser, R.; Rice, D.W.; Hardy, G.W.; Merrett, M.; Phillips, A.W. Sequence, structure and activity of phosphoglycerate kinase: A possible hinge-bending enzyme. Nature 1979, 279, 773–777. [Google Scholar] [CrossRef]
- Massange-Sánchez, J.A.; Casados-Vázquez, L.E.; Juarez-Colunga, S.; Sawers, R.J.H.; Tiessen, A. The Phosphoglycerate Kinase (PGK) Gene Family of Maize (Zea mays var. B73). Plants 2020, 9, 1639. [Google Scholar] [CrossRef]
- Wei, H.; Movahedi, A.; Yang, J.; Zhang, Y.; Liu, G.; Zhu, S.; Yu, C.; Chen, Y.; Zhong, F.; Zhang, J. Characteristics and molecular identification of glyceraldehyde-3-phosphate dehydrogenases in poplar. Int. J. Biol. Macromol. 2022, 219, 185–198. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Huang, S.N.; Wang, G.; Xuan, J.P.; Guo, Z.R. Overexpression of Actinidia deliciosa pyruvate decarboxylase 1 gene enhances waterlogging stress in transgenic Arabidopsis thaliana. Plant Physiol. Biochem. PPB 2016, 106, 244–252. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Huang, S.N.; Chen, Y.H.; Wang, G.; Guo, Z.R. Identification and characterization of two water logging responsive alcohol dehydrogenase genes (AdADH1 and AdADH2) in Actinidia deliciosa. Mol. Breed. 2017, 37, 52. [Google Scholar] [CrossRef]
- Xing, M.; Huang, K.; Zhang, C.; Xi, D.; Luo, H.; Pei, J.; Ruan, R.; Liu, H. Transcriptome Analysis Reveals the Molecular Mechanism and Responsive Genes of Waterlogging Stress in Actinidia deliciosa Planch Kiwifruit Plants. Int. J. Mol. Sci. 2023, 24, 15887. [Google Scholar] [CrossRef]
- Zheng, L.W. Research on the Regulatory Function of Oleuropein Lactone on the Growth and Development of Young Apple Trees. Ph.D. Thesis, Northwest Agriculture and Forestry University, Xianyang, China, 2020. [Google Scholar] [CrossRef]

| Line Names | Chlorophyll A (mg·g−1 FW) | Chlorophyll B (mg·g−1 FW) | Chlorophyll (mg·g−1 FW) | Pn (μmol·m−2·s−1) | Fv/Fm |
|---|---|---|---|---|---|
| WT | 1.67 ± 0.07 | 0.53 ± 0.03 | 2.20 ± 0.10 | 16.20 ± 0.44 | 0.82 ± 0.01 |
| OE1 | 2.05 ± 0.10 ** | 0.70 ± 0.06 ** | 2.75 ± 0.16 ** | 15.97 ± 0.06 | 0.84 ± 0.00 ** |
| OE2 | 2.16 ± 0.08 ** | 0.74 ± 0.07 ** | 2.91 ± 0.14 ** | 15.70 ± 0.44 | 0.84 ± 0.00 ** |
| OE3 | 1.90 ± 0.16 ** | 0.62 ± 0.05 ** | 2.52 ± 0.21 ** | 16.47 ± 0.32 | 0.84 ± 0.01 ** |
| R1 | 1.23 ± 0.06 ** | 0.43 ± 0.03 ** | 1.65 ± 0.09 ** | 15.90 ± 0.69 | 0.81 ± 0.01 |
| R2 | 1.17 ± 0.07 ** | 0.43 ± 0.02 ** | 1.61 ± 0.09 ** | 15.97 ± 0.15 | 0.81 ± 0.01 * |
| R3 | 1.41 ± 0.10 ** | 0.52 ± 0.04 | 1.93 ± 0.13 * | 16.43 ± 0.25 | 0.79 ± 0.00 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Li, G.; Sun, Y.; Li, N.; Zhang, S. Physiological Mechanisms of BvCPD Regulation in Sugar Beet Growth. Agronomy 2024, 14, 1367. https://doi.org/10.3390/agronomy14071367
Guo X, Li G, Sun Y, Li N, Zhang S. Physiological Mechanisms of BvCPD Regulation in Sugar Beet Growth. Agronomy. 2024; 14(7):1367. https://doi.org/10.3390/agronomy14071367
Chicago/Turabian StyleGuo, Xiaotong, Guolong Li, Yaqing Sun, Ningning Li, and Shaoying Zhang. 2024. "Physiological Mechanisms of BvCPD Regulation in Sugar Beet Growth" Agronomy 14, no. 7: 1367. https://doi.org/10.3390/agronomy14071367
APA StyleGuo, X., Li, G., Sun, Y., Li, N., & Zhang, S. (2024). Physiological Mechanisms of BvCPD Regulation in Sugar Beet Growth. Agronomy, 14(7), 1367. https://doi.org/10.3390/agronomy14071367





