Quantitative Trait Loci Mapping and Association Analysis of Solanesol Content in Tobacco (Nicotiana tabacum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Field Trial Design
2.3. Sampling and Treatment
2.4. UPLC-Based Quantification of Solanesol Content
2.5. DNA Extraction and Selection of Polymorphic Primers
2.6. Molecular Data Obtained
2.7. Analysis of Phenotypic Data
2.8. Construction of the Genetic Map
2.9. QTL Mapping
2.10. Association Analysis
3. Results
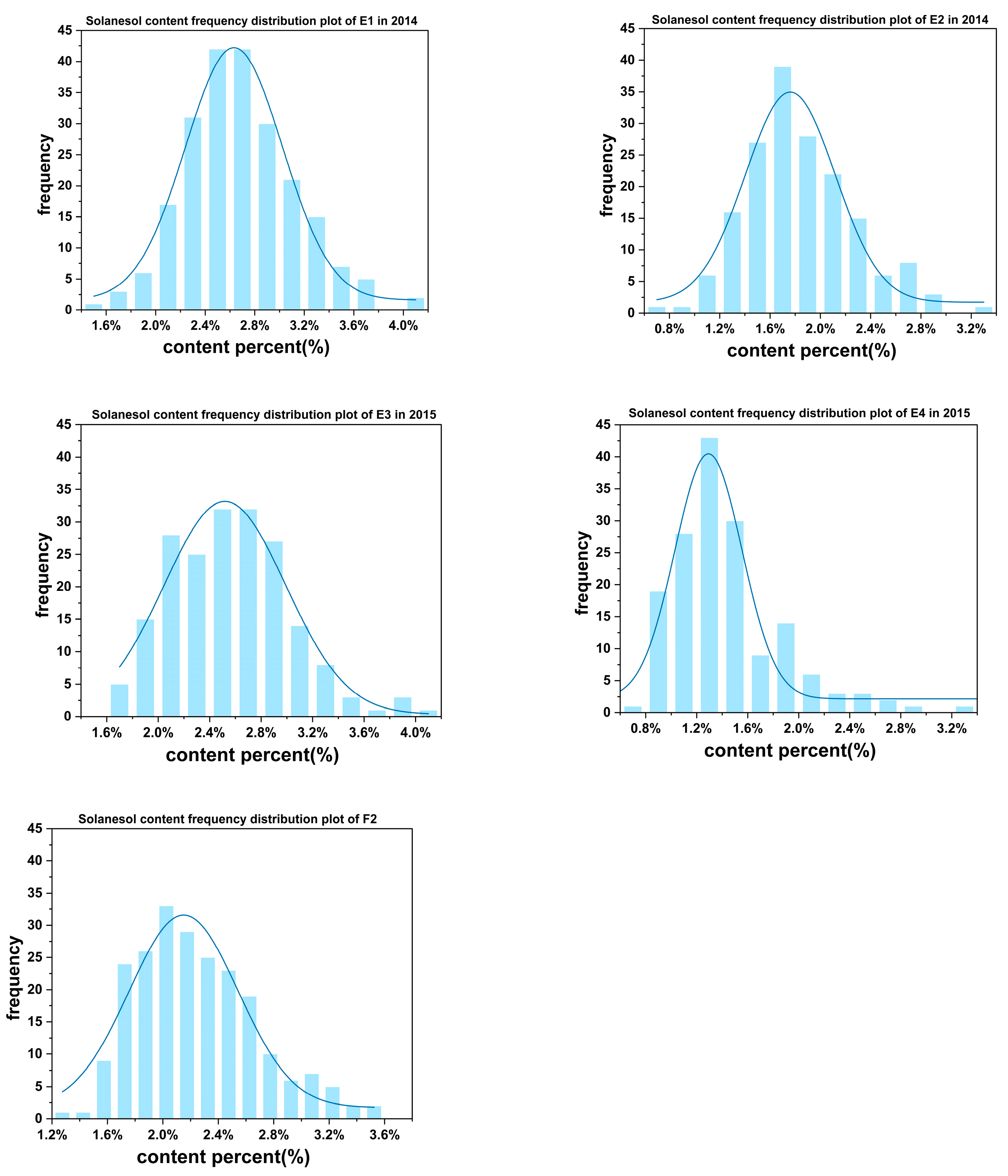
3.1. Analysis of Phenotypic Data
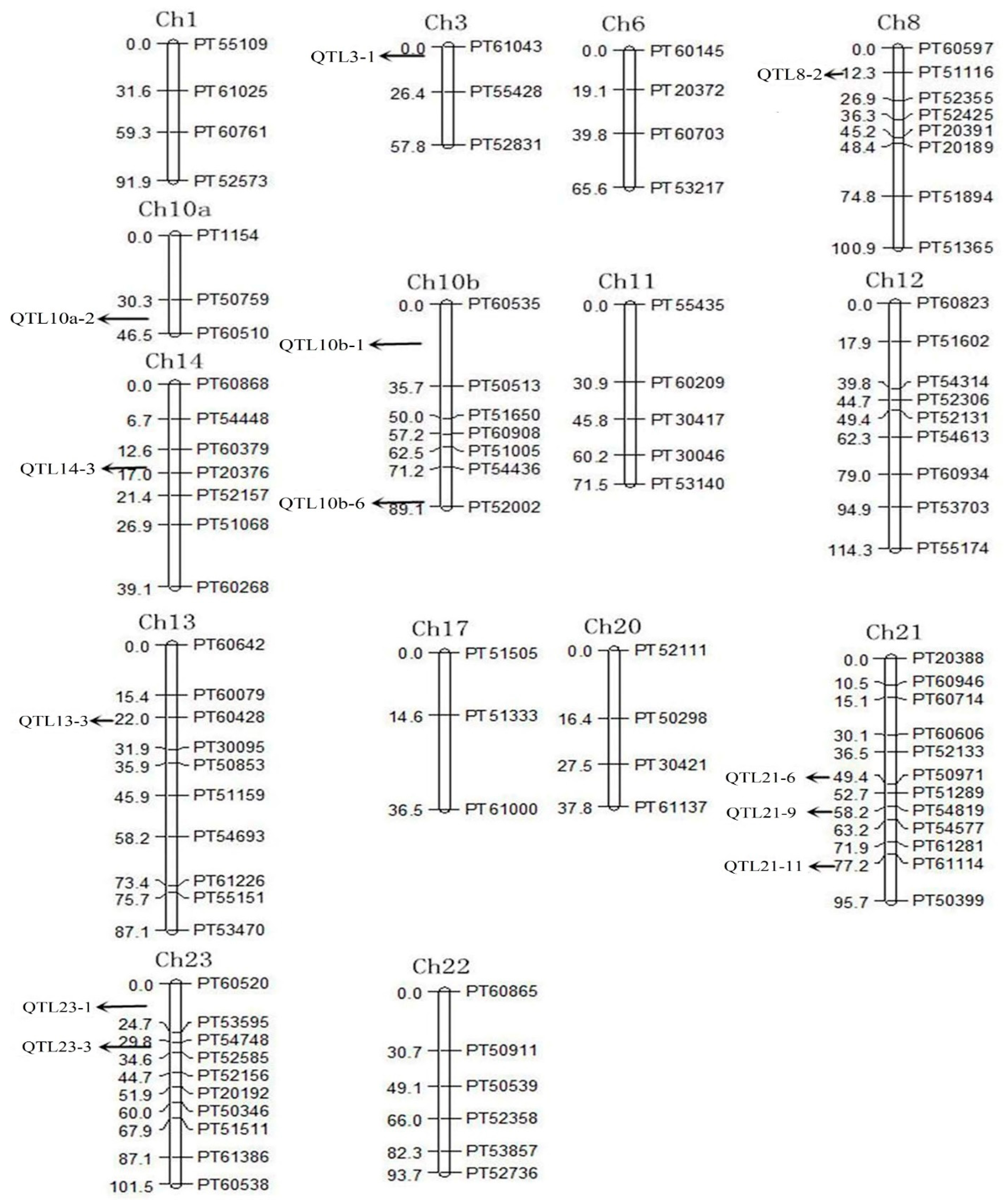
3.2. Quantitative Trait Locus Mapping for Solanesol Content
3.3. Association Analysis of SSRs and Solanesol Content
3.4. The Confirmed Markers Related to Solanesol Content by Two Methods
4. Discussion
4.1. Solanesol of Tobacco Leaves
4.2. QTL Mapping and Association Analysis for Tobacco Traits
4.3. Analysis of Plant Metabolites
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Name | No. | Name | No. | Name | No. | Name |
|---|---|---|---|---|---|---|---|
| 1 | NC82 | 57 | 86-3002 | 113 | Speight G-41 | 169 | Jintai66-4 |
| 2 | V2 | 58 | 82-3041 | 114 | Virginia 182 | 170 | Wenganpipaye |
| 3 | Xiaohuangjin1025 | 59 | 7514 | 115 | P3 | 171 | G80B |
| 4 | Speight G-28 | 60 | C151 | 116 | Qiannan7 | 172 | Wenganzhongpingkaoyan |
| 5 | Dabaijin599 | 61 | 7411 | 117 | T.I.706 | 173 | Fuquangaojiaohuang |
| 6 | Zhongyan90 | 62 | B22 | 118 | Yunduo1 | 174 | Jintai88 |
| 7 | Zhongyan15 | 63 | CV58 | 119 | Xilouyan | 175 | Chunlei1 |
| 8 | 9201 | 64 | K149 | 120 | Dabaijin2522 | 176 | Bijin1 |
| 9 | NC567 | 65 | NC 2326 | 121 | Chunlei4 | 177 | Liaoyan11 |
| 10 | K346 | 66 | Yongding1 | 122 | Changbaziyan | 178 | Wanye |
| 11 | Baihua205 | 67 | Danyu2 | 123 | Baijinhuangmiao | 179 | JB-200 |
| 12 | Coker 176 | 68 | Guanghuang54 | 124 | Wuming2 | 180 | Dixie Bright 101 |
| 13 | Yunyan85 | 69 | Jinxing6007 | 125 | Red Hort | 181 | Mudan79-2 |
| 14 | Cuibi1 | 70 | Panyuanhuang | 126 | H68E-1 | 182 | H80A432 |
| 15 | CF80 | 71 | Puyou1 | 127 | Vesta 30 | 183 | TI1112 |
| 16 | NC89 | 72 | Yunyan2 | 128 | Zhubo1 | 184 | CNH-NO.7 |
| 17 | K326 | 73 | CF90NF | 129 | Kuiyan2487 | 185 | CU263 |
| 18 | CF965 | 74 | Changbohuang | 130 | Liaoyan9 | 186 | K358 |
| 19 | Va116 | 75 | Tailifu1060 | 131 | Anqiumanwuxiang | 187 | MRS-2 |
| 20 | Yunyan87 | 76 | Lushanxiaoliuye | 132 | Changmaohuang | 188 | NC1108 |
| 21 | NC55 | 77 | Gexin5 | 133 | Majiangliyan | 189 | NCTG60 |
| 22 | RG13 | 78 | Special 401 | 134 | Oxford 4 | 190 | NCTG61 |
| 23 | CV088 | 79 | Qinyan95 | 135 | Damiaohuang | 191 | OX2028 |
| 24 | CV87 | 80 | Qinyan96 | 136 | Gedajinyan | 192 | OX2101 |
| 25 | FC8 | 81 | Longjiang851 | 137 | Wengantieganyan | 193 | RG3414 |
| 26 | Zhongyan14 | 82 | Longjiang925 | 138 | Hicks 55 | 194 | SPG-169 |
| 27 | RG8 | 83 | Longjiang935 | 139 | 8813 | 195 | SPG-172 |
| 28 | RG89 | 84 | Yuyan3 | 140 | Daliutiao | 196 | TI1597 |
| 29 | Zhongyan102 | 85 | Special 400 | 141 | Anxuan4 | 197 | Va80 |
| 30 | 9111-21 | 86 | Cash | 142 | Taoliuzi | 198 | Va411 |
| 31 | T.T.9 | 87 | Qiaozhuangduoye | 143 | Wangengzi | 199 | Chunlei3 |
| 32 | T.T.11 | 88 | Black Shank Resistant | 144 | Pingbanliuye | 200 | Damo |
| 33 | Honghuadajinyuan | 89 | Harrison Special | 145 | Luodihuang | 201 | TI 448A |
| 34 | Speight G-80 | 90 | Longyan1 | 146 | ETWM 10 | 202 | Fandi3-bing |
| 35 | RG11 | 91 | Kutsaga E1 | 147 | NC71 | 203 | 84-3117 |
| 36 | RG17 | 92 | Virginia Gold | 148 | Criollo c-1-1 | 204 | Enshu |
| 37 | Yanyan97 | 93 | SH.86-1 | 149 | Xiaohuangjin0138 | 205 | K730 |
| 38 | 09-53 | 94 | NC-22-NF | 150 | Y-2 | 206 | OX2007 |
| 39 | Gexin3 | 95 | TL 106 | 151 | TI1500 | 207 | SPG-168 |
| 40 | Jingyehuang | 96 | 78-3012 | 152 | Tailifu1011 | 208 | Changgeliuye |
| 41 | T.T.8 | 97 | Baisezhong | 153 | 8022 | 209 | Dashuba(Straight) |
| 42 | Zhongyan86 | 98 | I-35 | 154 | Changboyan | 210 | Dashuba2106 |
| 43 | Zhongyan103 | 99 | Tailifu1061 | 155 | Heimiaoshuba2104 | 211 | Yuyeshuba2109 |
| 44 | Kang88 | 100 | Longshe | 156 | 77089-12 | 212 | Kaiyangtuanyuye |
| 45 | CV91 | 101 | Heiyeyan | 157 | Va458 | 213 | Huangpingmaoganyan |
| 46 | Speight G-140 | 102 | Yuanyeyan | 158 | Coker187-Hicks | 214 | Fuquanzheyan |
| 47 | Beinhart 1000-1 | 103 | Daqingjin | 159 | Jiyan5 | 215 | Fuquandapipa |
| 48 | T.I.245 | 104 | Xiaojianshao | 160 | Coker 206 | 216 | Huangpingdaliuye |
| 49 | Kang66 | 105 | Liuyejian2017 | 161 | Manguangliuyejian | 217 | Fuquandajiwei |
| 50 | Tiebaziyan | 106 | Dashuba2101 | 162 | CT709 | 218 | Fuquanchaotianli |
| 51 | Manwuxiang | 107 | Huangmiaoyu2235 | 163 | Xiaohuangyan | 219 | Lushandawojuye |
| 52 | Hicks(Broad Leaf) | 108 | Fuquanhoujieba | 164 | Renshenyan | 220 | K394 |
| 53 | NC 95 | 109 | Jintai49 | 165 | Coker9 | 221 | Xiaoyehuang |
| 54 | Coker 319 | 110 | Pelo De Oro P-1-6 | 166 | Boheyan | 222 | Hicks |
| 55 | Coker 139 | 111 | Jiulouyan | 167 | Hyco Ruce | ||
| 56 | By 4 | 112 | Ky 151 | 168 | Wajiaoyan |
| Marker | Na [a] | PIC [b] | Marker | Na | PIC | Marker | Na | PIC |
|---|---|---|---|---|---|---|---|---|
| PT54245 | 2 | 0.323 | PT53936 | 3 | 0.473 | PT53595 | 3 | 0.558 |
| PT51682 | 4 | 0.660 | PT53089 | 2 | 0.308 | PT50136 | 2 | 0.355 |
| PT61187 | 2 | 0.232 | PT51206 | 3 | 0.304 | PT50727 | 5 | 0.546 |
| PT61210 | 2 | 0.375 | PT60435 | 3 | 0.466 | PT50472 | 6 | 0.725 |
| PT60345 | 3 | 0.485 | PT50670 | 2 | 0.370 | PT55266 | 2 | 0.310 |
| PT50392 | 3 | 0.439 | PT51144 | 6 | 0.688 | PT61428 | 4 | 0.547 |
| PT61010 | 3 | 0.474 | PT53796 | 2 | 0.354 | PT54887-1 | 2 | 0.254 |
| PT52958 | 2 | 0.260 | PT53362 | 3 | 0.359 | PT54887-2 | 2 | 0.344 |
| PT60369 | 3 | 0.345 | PT61564 | 6 | 0.757 | PT54887-3 | 3 | 0.484 |
| PT55030 | 4 | 0.598 | PT61061 | 4 | 0.521 | PT55296 | 3 | 0.272 |
| PT61396 | 2 | 0.277 | PT51145 | 3 | 0.402 | PT53026 | 3 | 0.436 |
| PT60172 | 2 | 0.369 | PT60080 | 6 | 0.520 | PT53915 | 2 | 0.245 |
| PT61499 | 3 | 0.412 | PT60606 | 8 | 0.772 | PT61339-1 | 2 | 0.358 |
| PT60863 | 2 | 0.370 | PT30355 | 8 | 0.788 | PT61339-2 | 3 | 0.475 |
| PT60868 | 4 | 0.505 | PT20388 | 4 | 0.299 | PT60114-1 | 2 | 0.284 |
| PT53829 | 2 | 0.230 | PT54644 | 4 | 0.412 | PT60114-2 | 5 | 0.587 |
| PT52906 | 2 | 0.366 | PT50077 | 4 | 0.491 | PT30311 | 3 | 0.471 |
| PT54448 | 2 | 0.348 | PT51415 | 3 | 0.408 | PT60177-1 | 3 | 0.514 |
| PT51411 | 3 | 0.382 | PT55319 | 5 | 0.676 | PT60177-2 | 2 | 0.269 |
| PT54811 | 2 | 0.347 | PT51085 | 3 | 0.554 | PT30380 | 4 | 0.479 |
| PT53384 | 3 | 0.382 | PT54778 | 5 | 0.711 | PT50245 | 4 | 0.561 |
| PT61286 | 3 | 0.511 | PT60427 | 4 | 0.648 | PT61386 | 3 | 0.279 |
| PT60494 | 2 | 0.317 | PT52838 | 4 | 0.623 | PT60520 | 3 | 0.557 |
| PT50500 | 2 | 0.195 | PT54819 | 3 | 0.589 | PT55117 | 3 | 0.379 |
| PT60271 | 3 | 0.403 | PT51951 | 5 | 0.693 | PT20445 | 5 | 0.561 |
| PT51976 | 2 | 0.254 | PT61192 | 2 | 0.151 | PT52002 | 2 | 0.364 |
| PT61584 | 2 | 0.375 | PT51054 | 2 | 0.370 | PT54336 | 3 | 0.374 |
| PT54342 | 2 | 0.141 | PT51289 | 3 | 0.412 | PT61043 | 4 | 0.501 |
| PT52736 | 3 | 0.546 | PT55472 | 3 | 0.554 | PT50513 | 3 | 0.446 |
| PT52353 | 3 | 0.448 | PT61114 | 3 | 0.460 | PT60908 | 3 | 0.352 |
| PT52585 | 2 | 0.374 | PT61319 | 4 | 0.512 | PT51005 | 3 | 0.346 |
| PT54629 | 2 | 0.159 | PT50352 | 2 | 0.333 | PT1154 | 2 | 0.368 |
| PT54759 | 2 | 0.270 | PT60946 | 4 | 0.588 | PT54711 | 2 | 0.133 |
| PT54707 | 3 | 0.348 | PT55428 | 4 | 0.681 | PT61612 | 2 | 0.132 |
| PT51152 | 4 | 0.488 | PT53466 | 2 | 0.364 | PT50923 | 2 | 0.363 |
| PT50457 | 4 | 0.430 | PT60524 | 2 | 0.328 | PT52760 | 3 | 0.301 |
| PT60404 | 3 | 0.205 | PT60510 | 3 | 0.471 | PT52418 | 2 | 0.323 |
| PT61367 | 2 | 0.330 | PT50759 | 4 | 0.555 | PT60581 | 3 | 0.361 |
| PT60257 | 2 | 0.201 | PT54061 | 2 | 0.305 | PT53223 | 3 | 0.314 |
| PT60146 | 2 | 0.151 | PT50062 | 4 | 0.513 | PT61160 | 3 | 0.431 |
| PT60886 | 5 | 0.561 | PT55453 | 2 | 0.271 | PT50736 | 2 | 0.326 |
| PT60123 | 5 | 0.648 | PT52718 | 3 | 0.480 | PT51170 | 2 | 0.198 |
| PT52804 | 6 | 0.521 | PT60861 | 3 | 0.547 | PT52318 | 2 | 0.364 |
| PT52536 | 2 | 0.373 | PT52509 | 3 | 0.463 | PT53847 | 3 | 0.447 |
| PT51130 | 10 | 0.586 | PT50346-1 | 5 | 0.736 | PT50501 | 3 | 0.366 |
| PT50434 | 6 | 0.616 | PT50346-2 | 2 | 0.368 | PT61633 | 2 | 0.365 |
| PT55162 | 2 | 0.266 | PT51065 | 3 | 0.479 | PT50488 | 2 | 0.191 |
| PT52133 | 4 | 0.691 | PT52156 | 2 | 0.369 | Mean | 3.133 | 0.422 |
References
- Feng, W.W. Decryption tobacco. Cencer Front. 2014, 11, 16–19. [Google Scholar]
- Jiang, Y.E. Chinese Tobacco Germplasm Resources; China Agriculture Press: Beijing, China, 1997. [Google Scholar]
- Bai, Q.; Yu, J.; Su, M.; Bai, R.; Katumata, G.; Katumata, M.; Chen, X. Antioxidant function of solanesol and its inhibitory effect on tyrosinase. J. Biomed. Eng. 2014, 31, 833–836. [Google Scholar] [CrossRef]
- Taylor, M.A.; Fraser, P.D. Solanesol: Added value from Solanaceous waste. Phytochemistry 2011, 72, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Banožić, M.; Babić, J.; Jokić, S. Recent advances in extraction of bioactive compounds from tobacco industrial waste—A review. Ind. Crop. Prod. 2020, 14, 112009. [Google Scholar] [CrossRef]
- Nidhi, S.; Shubham, U.; Ambika, S.; Rakesh, S.; Nshuman, S.; Bidisha, R.; Sidharth, M. Neuroprotection by solanesol against ethidium bromide-induced multiple sclerosis-like neurobehavioral, molecular, and neurochemical alterations in experimental rats. Phytomed. Plus 2021, 1, 4. [Google Scholar] [CrossRef]
- Withers, S.T.; Keasling, J.D. Biosynthesis and engineering of isoprenoid small molecules. Appl. Microbiol. Bio. 2007, 73, 980–990. [Google Scholar] [CrossRef]
- Lin, F.R.; Zhou, Y.S.; Chen, X.Z. Characterization of solanesol. Jiangsu Chem. Ind. 2008, 36, 27–29. [Google Scholar] [CrossRef]
- Saygili, I.; Kandemir, N.; Kinay, A.; Aytac, S.; Ayan, A.K. SSR marker-based genetic characterization of Turkish oriental tobaccos. Mol. Biol. Rep. 2022, 49, 11351–11358. [Google Scholar] [CrossRef] [PubMed]
- Bindler, G.; Plieske, J.; Bakaher, N.; Gunduz, I.; Ivanov, N.; Van der Hoeven, R.; Ganal, M.; Donini, P. A high density genetic Map of tobacco (Nicotiana tabacum L.) obtained from large scale microsatellite marker development. Theor. Appl. Genet. 2011, 123, 219–230. [Google Scholar] [CrossRef]
- Bindler, G.; van der Hoeven, R.; Gunduz, I.; Plieske, J.; Ganal, M.; Rossi, L.; Gadani, F.; Donini, P. A microsatellite marker based linkage map of tobacco. Theor. Appl. Genet. 2007, 114, 341–349. [Google Scholar] [CrossRef]
- Tong, Z.J.; Jiao, F.C.; Wu, X.F.; Wang, F.Q.; Chen, X.J.; Li, X.Y. Mapping of quantitative trait loci underlying six agronomic traits in flue-cured tobacco (Nicotiana tabacum L.). Acta Agron. Sin. 2012, 38, 1407–1415. [Google Scholar] [CrossRef]
- Vontimitta, V.; Danehower, D.A.; Steede, T.; Moon, H.S.; Lewis, R.S. Analysis of a Nicotiana tabacum L. Genomic region controlling two leaf surface chemistry traits. J. Agric. Food Chem. 2010, 58, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.R.; Yang, A.G.; Jiang, C.H.; Ren, M.; Zhang, Y.; Feng, Q.F. Quantitative Trait Loci Mapping for Plant Height in Tobacco using Linkage and Association Mapping Methods. Crop Sci. 2015, 55, 641–647. [Google Scholar] [CrossRef]
- Julio, E.; Denoyes-Rothan, B.; Verrier, J.L.; Dorlhac de Borne, F. Detection of QTLs linked to leaf and smoke properties in Nicotiana tabacum based on a study of 114 recombinant inbred lines. Mol. Breed. 2006, 18, 69–91. [Google Scholar] [CrossRef]
- Yang, H.J.; Geng, X.Q.; Zhao, S.M.; Shi, H.Z. Genomic diversity analysis and identification of novel SSR markers in four tobacco varieties by high-throughput resequencing. Plant Physiol. Biochem. 2020, 150, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Chen, M.X.; Zhou, D.X.; Chen, S.H.; Tao, A.F.; Li, Y.K. QTL analysis of six important traits in tobacco (Nicotiana tabacum L.). Acta Agron. Sin. 2011, 37, 1577–1584. [Google Scholar] [CrossRef]
- Gao, T.T.; Jiang, C.H.; Luo, C.G.; Yang, A.G.; Cheng, L.R.; Dai, S.S. Mapping of quantitative trait loci affecting resistance to brown spot in tobacco line Beinhart1000-1. Acta Tabacaria Sin. 2014, 20, 104–107. [Google Scholar] [CrossRef]
- Bai, D.; Reeleder, R.; Brandle, J.E. Identification of two RAPD markers tightly linked with the Nicotiana debneyi gene for resistance to black root rot of tobacco. Theor. Appl. Genet. 1995, 91, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Vontimitta, V.; Lewis, R.S. Mapping of quantitative trait loci affecting resistance to Phytophthora nicotianae in tobacco (Nicotiana tabacum L.) line Beinhart-1000. Mol. Breed. 2012, 29, 89–98. [Google Scholar] [CrossRef]
- Xiao, B.G.; Lu, X.P.; Jiao, F.C.; Li, Y.P.; Sun, Y.H.; Guo, Z.K. Preliminary QTL analysis of several chemical components in flue cured tobacco (Nicotiana tabacum L.). Acta Agron. Sin. 2008, 34, 1762–1769. [Google Scholar] [CrossRef]
- Zhang, J.S.; Wang, R.G.; Yang, C.Y.; Wu, C.; Shi, Y.W.; Wang, Z.H.; Wang, Y.; Ren, X.L. Genetic diversity of agronomic traits and association analysis with SRAP markers in flue-cured tobacco (Nicotiana tabacum) varieties from China and abroad. Acta Agron. Sin. 2012, 38, 1029–1041. [Google Scholar] [CrossRef]
- Dadras, A.R.; Sabouri, H.; Nejad, G.M.; Sabouri, A.; Shoai-Deylami, M. Association analysis, genetic diversity and structure analysis of tobacco based on AFLP markers. Mol. Biol. Rep. 2014, 41, 3317–3329. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.D.; Cao, P.J.; Wang, Z.; Gao, J.P.; Wu, M.Z.; Li, X.X.; Zhang, J.F.; Wang, Y.F.; Gong, D.P.; Yang, J. Genome-wide characterization and expression profiling of the PDR gene family in tobacco (Nicotiana tabacum). Gene 2021, 788, 145637. [Google Scholar] [CrossRef]
- Wu, C.; Xia, Y.S.; Li, R.H.; Lv, Y.H.; Yu, Y.W.; Zhao, W.C.; Qiu, M.W.; Guo, P.G. Association analysis of tobacco bacterial wilt resistance with molecular markers. Tob. Sci. Technol. 2015, 48, 1–12. [Google Scholar] [CrossRef]
- Ge, X.L.; Liu, Y.H.; Yao, Z.M.; Du, Y.M.; Yan, N.; Zhang, H.B.; Dai, P.G. Study on the correlation between solanesol accumulation and expression of gene encoding terpenoid synthetic enzymes in tobacco. Chin. Tob. Sci. 2017, 38, 8–14. [Google Scholar] [CrossRef]
- Xiang, D.H.; Zhao, T.Z.; Du, Y.M.; Zhang, Z.F.; Yang, N.; Huang, W.C.; Wang, A.H.; Fu, Q.J.; Gong, Y.N.; Liu, Y.H. Genetic analysis on solanesol content of tobacco. Chin. Tob. Sci. 2015, 36, 1–7. [Google Scholar]
- Xiang, D.H.; Yao, Z.M.; Liu, Y.H.; Gai, X.L.; Du, Y.M.; Yan, N.; Wang, A.H.; Fu, Q.J. Analysis on Solanesol Content and Genetic Diversity of Chinese Flue-Cured Tobacco (Nicotiana tabacum L.). Crop Sci. 2017, 57, 847–855. [Google Scholar] [CrossRef]
- Pan, W.; Liu, W.J.; Weng, B.Q.; Zhan, W.X. Determining solanesolin in tobacco leaves and its extracts using HPLC standardized method. Chin. Tob. Sci. 2013, 34, 60–61. [Google Scholar] [CrossRef]
- Tamari, F.; Hinkley, C.S.; Ramprasbad, N.A. Comparison of DNA extraction methods using petunia hybrida tissues. J. Biomol. Tech. 2013, 24, 113–118. [Google Scholar] [CrossRef]
- Van Ooijen, J.W. JoinMap 4: Software for the Calculation of Genetic Linkage Maps in Experimental Populations; Kyazma B.V.: Wageningen, The Netherlands, 2006. [Google Scholar]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Linked loci and correlated allele frequencies. Genetics 2003, 164, 1567–1587. [Google Scholar] [CrossRef]
- Zhang, F.T.; Zhu, Z.H.; Tong, X.R.; Zhu, Z.X.; Qi, T.; Zhu, J. Mixed Linear Model Approaches of Association Mapping for Complex Traits Based on Omics Variants. Sci. Rep. 2015, 5, 10298. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Zhang, C.; Feng, S. Identification and genetic diversity analysis of hybrid offspring of azalea based on EST-SSR markers. Sci. Rep. 2022, 12, 15239. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.G. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Muse, S.V. PowerMarker: An integrated analysis environment for genetic Marker analysis. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, P.J.; Zhang, Z.W.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2638. [Google Scholar] [CrossRef]
- Rajkhowa, B.; Mehan, S.; Sethi, P.; Prajapati, A.; Suri, M.; Kumar, S.; Bhalla, S.; Narula, A.S.; Alshammari, A.; Alharbi, M.; et al. Activating SIRT-1 Signalling with the Mitochondrial-CoQ10 Activator Solanesol Improves Neurobehavioral and Neurochemical Defects in Ouabain-Induced Experimental Model of Bipolar Disorder. Pharmaceuticals 2022, 15, 959. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.H.; Dong, Y.B.; Li, Y.L.; Wang, Q.L.; Shi, Q.L.; Zhou, Q. Verification of QTL for grain starch content and its genetic correlation with oil content using two connected RIL populations in high-oil maize. PLoS ONE 2013, 8, e53770. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Obara, M.; Tamura, W.; Ebitani, T.; Yano, M.; Sato, T.; Yamaya, T. Fine-mapping of qRL6.1, a major QTL for root length of rice seedlings grown under a wide range of NH4+ Concentrations in hydroponic conditions. Theor. Appl. Genet. 2010, 121, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Q.; Xie, Q.; Li, G.Q.; Jia, H.Y.; Zhou, J.Y.; Kong, Z.X.; Li, N.; Yuan, Y. Germplasms, geneticsand genomics for better control of disastrous wheat Fusarium head blight. Theor. Appl. Genet. 2020, 133, 1541–1568. [Google Scholar] [CrossRef]
- Chai, Q.; Wang, X.; Gao, M.; Zhao, X.; Chen, Y.; Zhang, C.; Jiang, H.; Wang, J.; Wang, Y.; Zheng, M.; et al. A glutathione S-transferase GhTT19 determines flower petal pigmentation via regulating anthocyanin accumulation in cotton. Plant Biotechnol. J. 2023, 21, 433–448. [Google Scholar] [CrossRef]
- Lin, T.Y.; Kao, Y.Y.; Lin, S.; Lin, R.F.; Chen, C.M.; Huang, C.H.; Wang, C.K.; Lin, Y.Z.; Chen, C.M. A genetic linkage map of Nicotiana plumbaginifolia/Nicotiana longiflora based on RFLP and RAPD markers. Theor. Appl. Genet. 2001, 103, 905–911. [Google Scholar] [CrossRef]
- Li, Y.L. Difference Analysis of Aroma Components and Association Analysis with SSR Markers in Flue-Cured Tobacco (Nicotiana tabacum) Varieties. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2015. [Google Scholar]
- Lai, X.; Yan, L.; Lu, Y.; Schnable, J.C. Largely unlinked gene sets targeted by selection for domestication syndrome phenotypes in maize and sorghum. Plant J. 2018, 93, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, S.; Shah, T.; Xie, C.; Hao, Z.; Li, X.; Farkhari, M.; Ribaut, J.M.; Cao, M.; Rong, T.Z.; et al. Joint linkage-linkage disequilibrium mapping is a powerful approach to detecting quantitative trait loci underlying drought tolerance in maize. Proc. Natl. Acad. Sci. USA 2010, 107, 19585–19590. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Buckler, E.S. Genetic association mapping and genome organization of maize. Curr. Opin. Biotechnol. 2006, 17, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Mackay, I.; Powell, W. Methods for linkage disequilibrium mapping in crops. Trends Plant Sci. 2007, 12, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Giraud, H.; Bauland, C.; Falque, M.; Madur, D.; Combes, V.; Jamin, P.; Monteil, C.; Laborde, J.; Palaffre, C.; Gaillard, A.; et al. Linkage Analysis and Association Mapping QTL Detection Models for Hybrids Between Multiparental Populations from Two Heterotic Groups: Application to Biomass Production in Maize (Zea mays L.). G3 Genes Genomes Genet. 2017, 7, 3649–3657. [Google Scholar] [CrossRef] [PubMed]
- Keurentjes, J.J. Genetical metabolomics: Closing in on phenotypes. Curr. Opin. Plant Biol. 2009, 12, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, F.; Okazaki, Y.; Oikawa, A.; Kusano, M.; Saito, K. Dissection of genotype–phenotype associations in rice grains using metabolome quantitative trait loci analysis. Plant J. 2012, 70, 624–636. [Google Scholar] [CrossRef]
- Gao, Z.; Ovchinnikova, O.G.; Huang, B.S.; Liu, F.; Williams, D.E.; Andersen, R.J.; Lowary, T.L.; Whitfield, C.; Withers, S.G. High-Throughput “FP-Tag” Assay for the Identification of Glycosyltransferase Inhibitors. J. Am. Chem. Soc. 2019, 141, 2201–2204. [Google Scholar] [CrossRef]
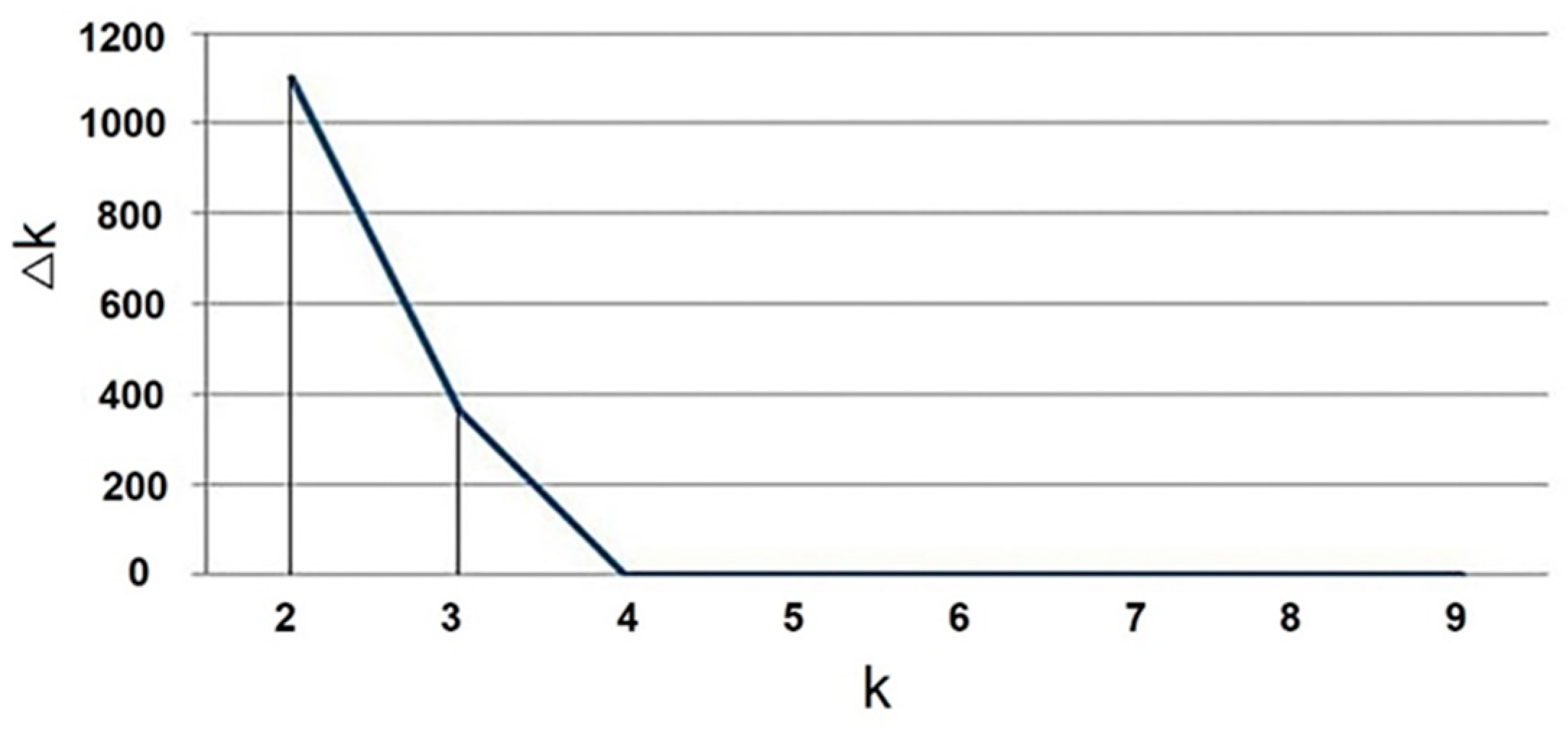
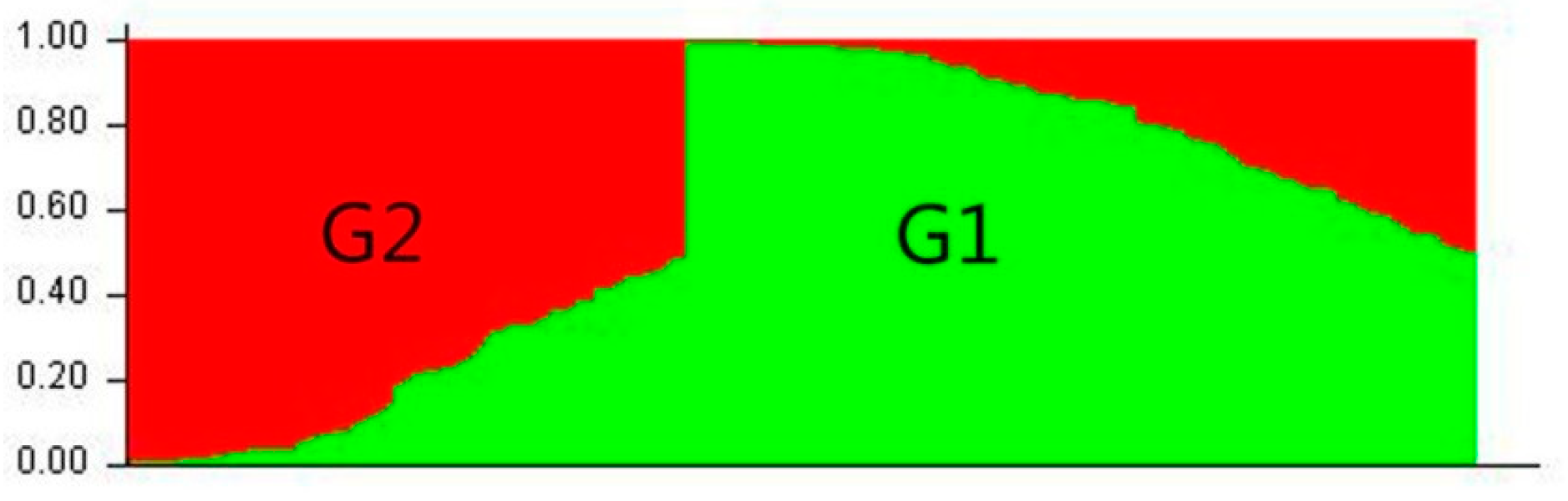
| Populations | Environment | Number | Mean ± SD (%) | Range (%) | CV (%) | Kurtosis | Skewness |
|---|---|---|---|---|---|---|---|
| K326 | Hubei | 50 | 2.909 ± 0.201 A | 2.868–3.05 | 10.215 | ||
| Maryland609 | 50 | 1.126 ± 0.143 C | 0.768–1.788 | 10.484 | |||
| F1 | 50 | 2.63 ± 0.203 A | 2.516–2.823 | 12.548 | |||
| F2 population | 206 | 2.29 ± 0.459 B | 0.531–3.558 | 26.285 | 0.956 | 0.386 | |
| Natural population | E1 | 173 | 1.830 ± 0.438 c | 0.695–3.248 | 33.725 | 0.293 | 0.458 |
| E2 | 222 | 2.683 ± 0.447 a | 1.535–4.131 | 26.782 | 0.404 | 0.389 | |
| E3 | 187 | 1.428 ± 0.402 d | 0.589–3.386 | 34.129 | 0.303 | 0.462 | |
| E4 | 222 | 2.368 ± 0.426 b | 1.690–3.969 | 28.264 | 0.412 | 0.391 | |
| Average | 222 | 2. 077 ± 0.428 b | 1.127–3.1684 | 30.725 | 0.353 | 0.425 |
| QTL | Linkage Group | Left Marker | Right Marke | Position (cm) | LOD Value | A [a] | D [b] | PVE [c] (%) |
|---|---|---|---|---|---|---|---|---|
| QTL3-1 | 3 | PT61043 | PT55428 | 2.0 | 4.34 | 0.0685 | −0.1886 | 5.19 |
| QTL21-6 | 21 | PT50971 | PT51289 | 49.4 | 5.18 | −0.0866 | −0.2010 | 7.59 |
| QTL23-3 | 23 | PT54748 | PT52585 | 30.8 | 8.6 | 0.1820 | 0.0463 | 10.05 |
| Marker | LG | Position | E1 | E2 | E3 | E4 | Average | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | R2 (%) | p | R2 (%) | p | R2 (%) | p | R2 (%) | p | R2 (%) | |||
| PT50457 | 1 | 100.72 | 8.50 × 10−4 | 8.31 | ns | ns | ns | ns | 4.77 × 10−6 | 12.47 | 5.67 × 10−7 | 12.69 |
| PT55428 | 3 | 114.06 | ns | ns | 9.43 × 10−5 | 7.29 | ns | ns | ns | ns | 4.67 × 10−5 | 10.38 |
| PT55117 | 3 | 114.06 | ns | ns | 1.67 × 10−4 | 9.33 | ns | ns | ns | ns | ns | ns |
| PT60524 | 3 | 122.13 | ns | ns | 3.02 × 10−5 | 10.30 | 5.48 × 10−5 | 10.38 | ns | ns | 2.12 × 10−6 | 10.56 |
| PT54245 | 4 | 38.74 | 1.48 × 10−4 | 8.43 | ns | ns | 4.55 × 10−4 | 7.74 | ns | ns | ns | ns |
| PT61187 | 5 | 130.24 | 8.67 × 10−6 | 11.46 | ns | ns | 1.02 × 10−6 | 14.58 | ns | ns | 1.14 × 10−7 | 12.45 |
| PT50923 | 6 | 136.38 | ns | ns | 9.89 × 10−7 | 11.47 | 7.62 × 10−5 | 10.20 | ns | ns | 6.97 × 10−5 | 7.73 |
| PT50392 | 9 | 48.65 | ns | ns | 6.52 × 10−5 | 8.73 | 2.04 × 10−4 | 10.51 | ns | ns | 5.45 × 10−6 | 10.85 |
| PT60510 | 10 | 0.00 | 3.31 × 10−4 | 11.92 | ns | ns | 8.85 × 10−5 | 14.49 | 1.58 × 10−5 | 13.99 | 3.14 × 10−8 | 18.34 |
| PT50759 | 10 | 1.65 | ns | ns | ns | ns | ns | ns | 4.02 × 10−4 | 8.63 | 4.06 × 10−4 | 8.62 |
| PT61154 | 10 | 4.16 | ns | ns | 7.88 × 10−5 | 7.30 | 3.98 × 10−4 | 7.21 | 1.01 × 10−4 | 7.23 | 7.68 × 10−8 | 13.35 |
| PT61061 | 10 | 9.84 | 3.98 × 10−4 | 12.21 | ns | ns | ns | ns | 7.68 × 10−8 | 13.35 | 3.97 × 10−8 | 20.07 |
| PT61339-1 | 10 | 51.35 | ns | ns | 1.81 × 10−4 | 6.30 | ns | ns | 1.28 × 10−6 | 11.76 | 1.54 × 10−8 | 13.80 |
| PT51005 | 10 | 55.23 | ns | ns | ns | ns | ns | ns | 2.25 × 10−4 | 12.73 | 1.34 × 10−5 | 14.26 |
| PT60114-1 | 10 | 57.16 | 2.63 × 10−4 | 13.06 | ns | ns | ns | ns | ns | ns | 1.46 × 10−6 | 12.07 |
| PT60114-2 | 10 | 57.16 | 1.63 × 10−4 | 10.10 | 2.61 × 10−4 | 7.59 | 3.89 × 10−5 | 12.58 | ns | ns | 6.07 × 10−8 | 18.42 |
| PT60172 | 12 | 89.26 | 2.26 × 10−4 | 9.62 | 1.33 × 10−4 | 8.04 | ns | ns | ns | ns | 5.15 × 10−6 | 10.80 |
| PT60863 | 14 | 35.13 | ns | ns | 3.09 × 10−6 | 9.69 | 4.29 × 10−4 | 7.76 | ns | ns | 2.03 × 10−5 | 8.15 |
| PT60868 | 14 | 37.34 | ns | ns | 2.13 × 10−4 | 7.57 | ns | ns | ns | ns | 3.72 × 10−5 | 9.05 |
| PT54448 | 14 | 42.08 | 3.03 × 10−4 | 7.50 | 1.96 × 10−4 | 6.20 | 2.39 × 10−6 | 13.33 | 1.51 × 10−4 | 7.33 | 7.59 × 10−8 | 12.50 |
| PT52906 | 15 | 102.76 | ns | ns | 2.27 × 10−5 | 7.99 | ns | ns | 1.86 × 10−5 | 9.31 | 3.00 × 10−6 | 9.63 |
| PT54811 | 16 | 130.98 | ns | ns | 2.33 × 10−5 | 7.87 | ns | ns | ns | ns | 4.27 × 10−5 | 7.38 |
| PT61633 | 17 | 75.26 | ns | ns | ns | ns | ns | ns | 6.83 × 10−4 | 6.54 | ns | ns |
| PT52838 | 21 | 24.05 | ns | ns | 0.0231 | 4.55 | ns | ns | ns | ns | 0.04700421 | 4.86 |
| PT61192 | 21 | 45.54 | 0.0020 | 5.92 | ns | ns | ns | ns | ns | ns | 0.007355 | 4.22 |
| PT51054 | 21 | 49.70 | ns | ns | 3.53 × 10−8 | 13.33 | ns | ns | 5.41 × 10−4 | 6.25 | 3.88 × 10−9 | 15.06 |
| PT52536 | 21 | 49.70 | ns | ns | 1.48 × 10−4 | 6.83 | ns | ns | ns | ns | 1.96 × 10−5 | 8.57 |
| PT61114 | 21 | 49.70 | ns | ns | 7.52 × 10−4 | 7.81 | ns | ns | ns | ns | 4.98 × 10−5 | 10.30 |
| PT55472 | 21 | 49.70 | ns | ns | ns | ns | ns | ns | ns | ns | 5.09 × 10−4 | 10.67 |
| PT30355 | 21 | 52.43 | ns | ns | 5.50 × 10−4 | 12.50 | ns | ns | ns | ns | ns | ns |
| PT20388 | 21 | 72.14 | ns | ns | 1.45 × 10−5 | 11.56 | ns | ns | 5.84 × 10−7 | 16.39 | 2.61 × 10−8 | 17.03 |
| PT52760 | 22 | 30.24 | ns | ns | ns | ns | 7.13 × 10−5 | 14.60 | ns | ns | 2.97 × 10−4 | 9.32 |
| PT60494 | 22 | 47.55 | ns | ns | 2.43 × 10−4 | 7.23 | ns | ns | 3.57 × 10−4 | 5.67 | 0.03087387 | 7.52 |
| PT52736 | 22 | 68.26 | ns | ns | 1.31 × 10−4 | 8.05 | ns | ns | ns | ns | 1.07 × 10−4 | 8.22 |
| PT54707 | 23 | 23.21 | ns | ns | ns | ns | 1.82 × 10−4 | 10.98 | ns | ns | ns | ns |
| PT60520 | 23 | 63.69 | ns | ns | 3.77 × 10−5 | 8.40 | ns | ns | ns | ns | 6.16 × 10−5 | 7.96 |
| PT61584 | 23 | 61.71 | ns | ns | 4.27 × 10−6 | 9.38 | ns | ns | ns | ns | 3.11 × 10−5 | 7.77 |
| PT51170 | 24 | 49.25 | ns | ns | ns | ns | 5.86 × 10−4 | 7.60 | 3.03 × 10−5 | 9.05 | 2.99 × 10−6 | 9.94 |
| QTL | Linkage Group | Interval | Marker | Location [a] | D [b] (cm) | Molecular Marker Localization |
|---|---|---|---|---|---|---|
| QTL3-1 | 3 | PT61043-PT55428 | PT55428 | 114.06 | 0.00 | 138,644,270–138,644,484 |
| QTL10a-2 | 10a | PT50759-PT60510 | PT60510 | 0.00 | 0.00 | 3,640,102–3,640,139 |
| QTL10b-6 | 10b | PT54436-PT52002 | PT60114-1 | 57.16 | 2.479 | 490,772–490,852 |
| PT60114-2 | 57.16 | 2.479 | 73,974,226–73,974,332 | |||
| QTL14 | 14 | PT60379-PT20376 | PT60868 | 35.13 | −2.48 | 59,135,301–59,135,403 |
| PT60863 | 37.34 | 3.651 | 65,675,209–65,675,347 | |||
| QTL21-6 | 21 | PT50971-PT51289 | PT51054 | 49.70 | 0.00 | 51,260,251–51,260,374 |
| PT52536 | 49.70 | 0.00 | 52,765,747-52,765,930 | |||
| PT55472 | 49.70 | 0.00 | 54,904,073–54,904,263 | |||
| QTL21-9 | 21 | PT54577-PT61281 | PT54707 | 23.21 | 1.49 | 628,952–629,173 |
| QTL21-11 | 21 | PT61114-PT50399 | PT61114 | 49.70 | 0.00 | 108,401,173–108,401,338 |
| PT20388 | 49.70 | 0.00 | 927,449,98–92,745,182 | |||
| QTL23-1 | 23 | PT60520-PT53595 | PT60520 | 63.69 | 0.00 | 40,922,488–40,922,687 |
| QTL23-3 | 23 | PT54748-PT52585 | PT61584 | 61.71 | −0.55 | 330,872 – 331,056 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xiang, D.; Du, Y.; Zhang, Z.; Zhang, H.; Cheng, L.; Fu, Q.; Yan, N.; Ju, F.; Qi, C.; et al. Quantitative Trait Loci Mapping and Association Analysis of Solanesol Content in Tobacco (Nicotiana tabacum L.). Agronomy 2024, 14, 1370. https://doi.org/10.3390/agronomy14071370
Liu J, Xiang D, Du Y, Zhang Z, Zhang H, Cheng L, Fu Q, Yan N, Ju F, Qi C, et al. Quantitative Trait Loci Mapping and Association Analysis of Solanesol Content in Tobacco (Nicotiana tabacum L.). Agronomy. 2024; 14(7):1370. https://doi.org/10.3390/agronomy14071370
Chicago/Turabian StyleLiu, Jing, Dehu Xiang, Yongmei Du, Zhongfeng Zhang, Hongbo Zhang, Lirui Cheng, Qiujuan Fu, Ning Yan, Fuzhu Ju, Chaofan Qi, and et al. 2024. "Quantitative Trait Loci Mapping and Association Analysis of Solanesol Content in Tobacco (Nicotiana tabacum L.)" Agronomy 14, no. 7: 1370. https://doi.org/10.3390/agronomy14071370
APA StyleLiu, J., Xiang, D., Du, Y., Zhang, Z., Zhang, H., Cheng, L., Fu, Q., Yan, N., Ju, F., Qi, C., Lei, Y., Wang, J., & Liu, Y. (2024). Quantitative Trait Loci Mapping and Association Analysis of Solanesol Content in Tobacco (Nicotiana tabacum L.). Agronomy, 14(7), 1370. https://doi.org/10.3390/agronomy14071370






