Histological Variations in Cucumber Grafted Plants and Their Effect on Yield
Abstract
:1. Introduction
2. Materials and Methods
- Assessed variables:
- Micro-anatomic variables:
- (a)
- The average xylem vessel area (AVXR), the number of xylem vessels per vascular bundle (NVXR), the vascular bundle area in the root (AHVR) and the number of vascular bundles in the root (NHVR) were calculated using roots of 3 mm in diameter and 10 cm in depth.
- (b)
- The same variables than in the previous case were estimated, including the xylem vessel area (AVXTB), number of xylem vessels per vascular bundle (NVXTB), vascular bundle area (AHVTB), number of vascular bundles (NHVTB) at the basal stalk, 8 cm below the splitting of the two productive stems, as well as below the grafting point, in all treatments.
- (c)
- At the section between the 5th and 6th leaves, below the plant apex the following variables were estimated: xylem vessel area (AVXTP), number of xylem vessels per vascular bundle (NVXTP), finally the vascular bundle area (AHVTP) and the number of vascular bundles (NHVTP).
3. Results and Discussion
- Micro-anatomic variables
- Correlation among variables:
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Data México. Secretaria de Economía. Pepinos y Pepinillos, Frescos y Refrigerados. 2023. Available online: https://www.economia.gob.mx/datamexico/es/profile/product/cucumbers-and-gherkins-fresh-or-chilled (accessed on 1 February 2024).
- SIAP. Servicio de Información Agroalimentaria y Pesquera. Acciones y Programas. Cierre de la Producción Agrícola. 2022. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 1 February 2024).
- Hernández-González, Z.; Sahagun-Castellanos, J.; Espinosa-Robles, P.; Colinas-León, M.T.; Rodríguez-Pérez, J.E. Efecto del patrón en el rendimiento y tamaño de fruto en pepino injertado. Rev. Fitotec. Mex. 2014, 37, 41–47. [Google Scholar] [CrossRef]
- Secretaría de Agricultura y Desarrollo Rural. Agricultura Protegida ubica a México Entre los Principales Productores de Frutas y Hortalizas. 2022. Available online: https://www.gob.mx/agricultura/prensa/agricultura-protegida-ubica-a-mexico-entre-los-principales-productores-de-frutas-y-hortalizas?idiom=es%C2%A0#:~:text=Detall%C3%B3 (accessed on 22 February 2024).
- Hernández, S.J.L. Auge, consolidación y expansión territorial de la agricultura protegida en México y Zacatecas, 2005–2022. In Nuevas Territorialidades-Economía Sectorial y Reconfiguración Territorial; Egurrola, I., Eduardo, J., Eds.; Universidad Nacional Autonoma de Mexico, Asociación Mexicana de Ciencias para el Desarrollo Regional: Mexico City, Mexico, 2023; pp. 237–254. ISBN UNAM 978-607-30-8315-7. Available online: https://ru.iiec.unam.mx/6133/1/5.%20144-Hern%C3%A1ndez.pdf (accessed on 12 February 2024).
- López, E.J.; Jiménez, J.; Huez, M.A.; Garza, B.O.; Cruz, B.F.; Bautista, O.A. Medidas de control biológico en la producción de pepino, bajo condiciones de invernadero. Idesia 2017, 35, 7–12. [Google Scholar]
- Pratt, L.; Ortega, J.M. Agricultura Protegida en México: Elaboración de la Metodología para el Primer Bono Verde Agrícola Certificado; Nieto, E., Braly-Cartillier, I., Eds.; Banco Interamericano de Desarrollo: Washington, DC, USA, 2019. [Google Scholar]
- Velasco, A.M.D.; Castro, B.R.; Castillo, G.A.M.; Avitia, G.E.; Sahagún, C.J.; Lobato, O.R. Composición mineral, biomasa y rendimiento en tomate (Solanum lycopersicum L.) injertado. Interciencia 2016, 41, 703–708. [Google Scholar]
- Flores, F.B.; Sanchez-Bel, P.; Estañ, M.T.; Martinez-Rodriguez, M.M.; Moyano, E.; Morales, B.; Campos, J.F.; Garcia-Abellán, J.O.; Egea, M.I.; Fernández-Garcia, N.; et al. The effectiveness of grafting to improve tomato fruit quality. Sci. Hortic. 2010, 125, 211–217. [Google Scholar] [CrossRef]
- Al-Harbi, A.R.; Al-Omran, A.M.; Alharbi, K. Grafting improves cucumber water stress tolerance in Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Santarosa, E.; De Souza, P.V.D.; De Araujo Mariath, J.E.; Lourosa, G.V. Physiological interaction between rootstock-scion: Effects on xylem vessels in Cabernet Sauvignon and Merlot grapevines. Am. J. Enol. Vitic. 2016, 67, 65–76. [Google Scholar] [CrossRef]
- Sory, A.; Nieto-Angel, R.; Rodríguez-Pérez, J.E.; Barrientos-Priego, A.F.; Ibañez-Castillo, L.A.; Romabchik, E.; Núñez-Colín, C.A. Variación anatómica del xilema en tallo de cultivares de tomate injertados en un tipo criollo. Rev. Chapingo Ser. Hortic. 2010, 16, 67–76. [Google Scholar]
- Goldschmidt, E.E. Plant grafting: New mechanisms, evolutionary implications. Front. Plant Sci. 2014, 5, 727. [Google Scholar] [CrossRef] [PubMed]
- Miguel, A. Injertos y portainjertos en sandía. In Técnicas de Cultivo y Comercialización de la Sandía; Cajamar Caja Rural, Serie Agricultura; Gázquez Garrido, J.C., Ed.; ReDivia; Instituto Valenciano de Investigaciones Agrarias (IVIA): Valencia, España, 2015; pp. 75–98. [Google Scholar]
- Steiner, A.A. The universal nutrient solution. In Proceedings of the 6th International Congress on Soilless Culture International Soc. for Soilless Culture, ISOSC, Wageningen, The Netherlands, 29 April–5 May 1984; pp. 633–649. [Google Scholar]
- Hernández, S.M. Manual de Laboratorio Citología y Citogenética; Editorial Trillas; Universidad Autónoma Agraria Antonio Narro: Saltillo, Mexico, 1990; 105p. [Google Scholar]
- Jáuregui, D. Manual Práctico de Microtecnia Vegetal; Departamento de Botánica Agrícola. Laboratorio de Botánica, Facultad de Agronomía, Postgrado de Botánica Agrícola, Universidad Central de Venezuela. Maracay: Maraca, Venezuela, 2003; 71p. [Google Scholar]
- Cañizares, A.; Sanabria, M.; y Rojas, E. Anatomía de la hoja de Lima Tahití (Citrus latifolia Tanaka). Rev. Cient. UDO Agric. 2005, 5, 68–73. [Google Scholar]
- Pérez, A.N.; Tomasi, V.H. Tinción con azul brillante de cresilo en secciones vegetales con parafina. Bol. Soc. Argent. Bot. 2002, 37, 211–215.2. [Google Scholar]
- Wilkinson, H. The plant surface (maily leaf). In Anatomy of Dicotiledons; Metcalfe y Chalk, C.R., Ed.; Oxford Cloredons Press: Cary, NC, USA, 1979; Volume 1, pp. 97–165. [Google Scholar]
- Statistical Analysis System Institute. User’s Guide of SAS; SAS Institute Inc.: Cary, NC, USA, 2002; 550p. [Google Scholar]
- Sánchez, C.E.; Torres, A.G.; Flores, M.A.C.; Preciado, P.R.; Márquez, C.Q. Uso de portainjerto sobre el rendimiento, calidad del fruto y resistencia a Phytophthora capsici Leonian en pimiento morrón. Nova Sci. 2015, 7, 227–244. [Google Scholar] [CrossRef]
- Acevedo, C.J.; Sánchez, C.E. Eficiencia del uso de portainjerto sobre el rendimiento y dinámica nutricional foliar de macronutrientes en pimiento morrón. Rev. Mex. Cienc. Agríc. 2017, 8, 685–693. [Google Scholar]
- Suárez, H.Á.M.; Grimald, J.O.; García, L.A.M.; González, M.D.; Huitrón, R.M.V. Evaluación de portainjertos criollos de Lagenaria siceraria en la producción de sandía injertada. Idesia 2017, 35, 39–44. [Google Scholar]
- Farhadi, A.A.; Nemati, H.; Salehi, R.H.; Giuffrida, F. The effectiveness of different rootstocks for improving yield and growth of cucumber cultivated hydroponically in a greenhouse. Horticulturae 2016, 2, 1. [Google Scholar] [CrossRef]
- Albornoz, F.; Gebauer, M.; Ponce, C.; Cabeza, R. LeNRT1.1 improves nitrate uptake in grafted tomato plants under high nitrogen demand. Int. J. Mol. Sci. 2018, 19, 3921. [Google Scholar] [CrossRef] [PubMed]
- Cushman, K.E.; Huan, J. Performance of four triploid watermelon cultivars grafted onto five rootstock genotypes: Yield and fruit quality under commercial growing conditions. Acta Hortic. 2008, 782, 335–341. [Google Scholar] [CrossRef]
- Godoy, H.H.; Castellanos Ramos, J.Z.H.; Alcántar, J.Z.; Sandoval, G.V.M.; Ramos, J.D.M. Efecto del injerto y nutrición de tomate sobre rendimiento, materia seca y extracción de nutrimentos. Terra Latinoamericana. 2009, 27, 1–9. [Google Scholar]
- Calatayud, A.; Marsal, J.I.; Galarza, L.; San Bautista, A.S.; Nebauer, S.G. Efecto del injerto sobre la respuesta a la salinidad de pimiento. Acta Hortic. 2010, 58, 139–143. [Google Scholar]
- Milenković, L.; Mastilović, J.; Kevrešan, Ž.; Bajić, A.; Gledić, A.; Stanojević, L.; Cvetković, D.; Šunić, L.; Ilić, Z.S. Effect of shading and grafting on yield and quality of tomato. J. Sci. Food. Agric. 2020, 100, 623–633. [Google Scholar] [CrossRef]
- Paul, S.; Das, M.K.; Baishya, P.; Ramteke, A.; Farooq, M.; Baroowa, B.; Sunkar, R.; Gogoi, N. Effect of high temperature on yield associated parameters and vascular bundle development in five potato cultivars. Sci. Hortic. 2017, 225, 134–140. [Google Scholar] [CrossRef]
- Albornoz, F.; Pérez-Donoso, A.G.; Leigh Urbina, J.; Monasterio, M.; Gómez, M.; Steinfort, Ú. Nitrate Transport Rate in the Xylem of Tomato Plants Grafted onto a Vigorous Rootstock. Agronomy 2020, 10, 182. [Google Scholar] [CrossRef]
- Lu, X.; Liu, W.; Wang, T.; Zhang, J.; Li, X.; Zhang, W. Systemic Long-Distance Signaling and Communication Between Rootstock and Scion in Grafted Vegetables. Front. Plant Sci. 2020, 11, 460. [Google Scholar] [CrossRef]
- Osugi, A.; Kojima, M.; Takebayashi, Y.; Ueda, N.; Kiba, T.; Sakakibara, H. Systemic transport of trans-zeatin and its precursor have differing roles in Arabidopsis shoots. Nat Plants 2017, 24, 17112. [Google Scholar] [CrossRef] [PubMed]
- Tamilselvi, N.A.; Pugalendhi, L. Graft compatibility and anatomical studies of bitter gourd (Momordica charantia L.) scions with cucurbitaceous rootstocks. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1801–1810. [Google Scholar] [CrossRef]
- Melnyk, C.W. Connecting the plant vasculature to friend or foe. New Phytologist. 2017, 213, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; Fevereiro, P.; Kragler, F.; Pina, A. Plant grafting and graft incompatibility: A review from the grapevine perspective. Sci. Hortic. 2022, 299, 111019. [Google Scholar] [CrossRef]
- Tsaballa, A.; Xanthopoulou, A.; Madesis, P.; Tsaftaris, A.; Nianiou-Obeidat, I. Vegetable Grafting From a Molecular Point of View: The Involvement of Epigenetics in Rootstock-Scion Interactions. Front. Plant Sci. 2021, 11, 621999. [Google Scholar] [CrossRef] [PubMed]
- Ilakiya, T.; Parameswari, E.; Davamani, V.; Yazhini, G.; Singh, S. Grafting Mechanism in Vegetable Crops. Res J. Chem. Environ. Sci. 2021, 9, 18–26. [Google Scholar]
- Martínez-Andújar, C.; Albacete, A.; Pérez-Alfocea, F. Rootstocks for increasing yield stability and sustainability in vegetable crops. Acta Hortic. 2020, 1273, 449–470. [Google Scholar] [CrossRef]
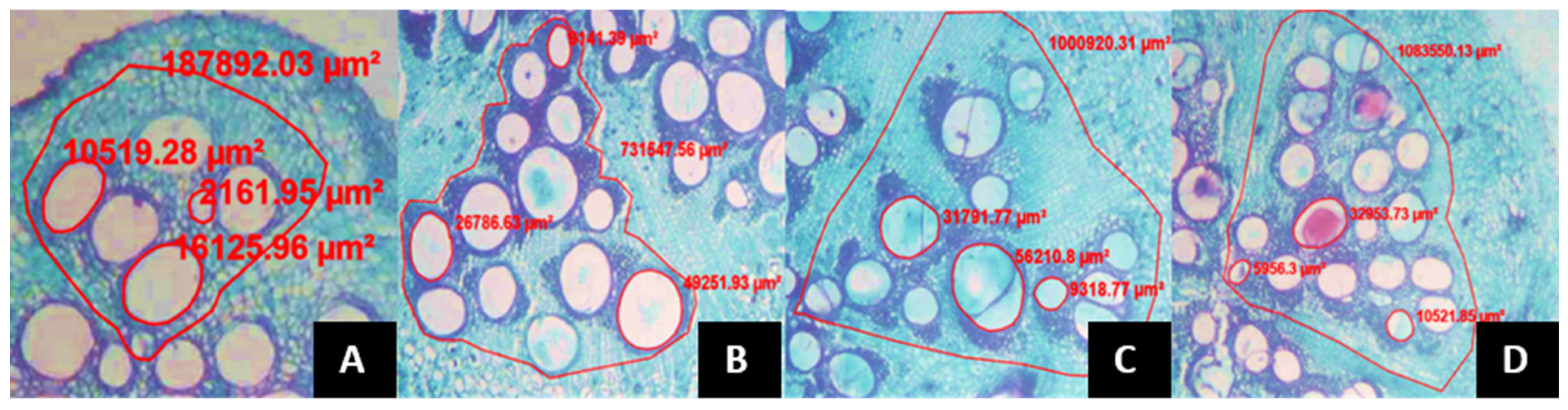
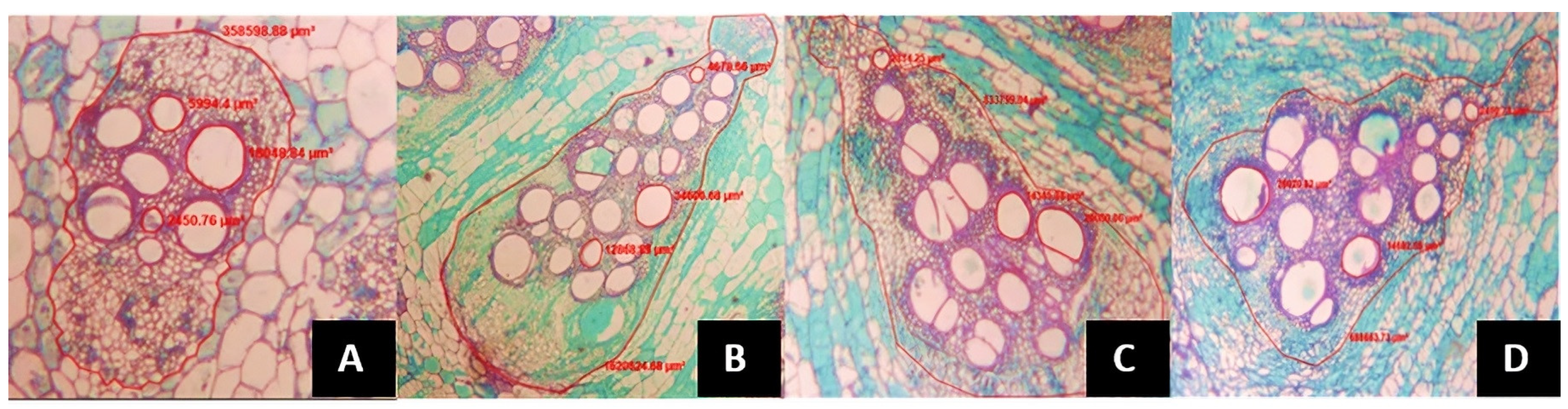
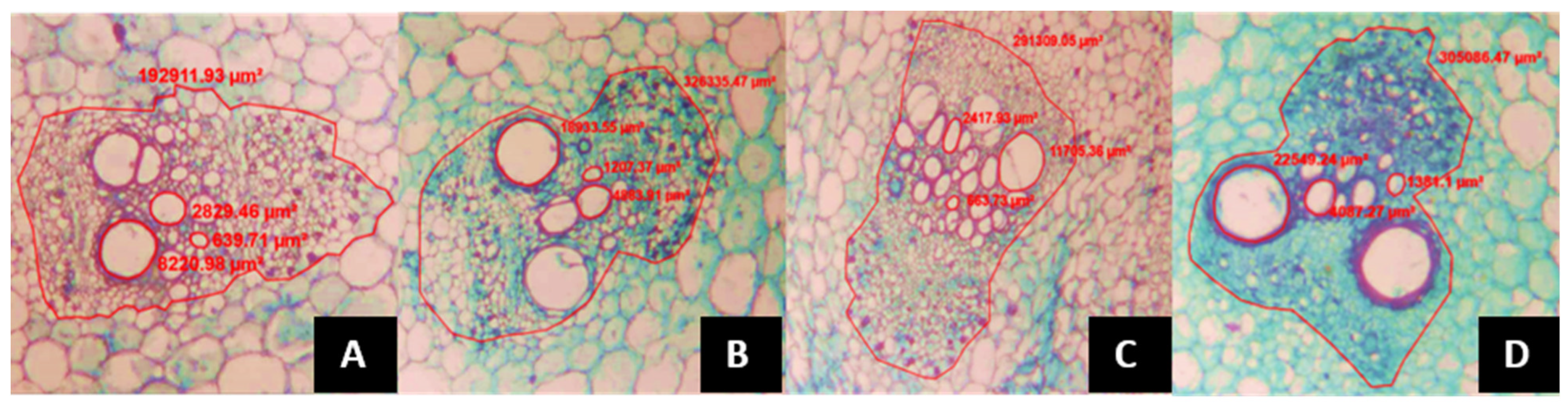
| Treatment | NFPP | PFPP (g) | PPF (g) | PSP (g) |
|---|---|---|---|---|
| T1:PSP = cucumber without rootstock | 13.00 B | 2730.0 B | 208.42 A | 82.80 C |
| T2:P/MA = cucumber/C. maxima | 19.00 A | 5445.0 A | 286.55 A | 196.65 A |
| T3:P/MO = cucumber/C. moschata | 11.33 B | 3503.9 AB | 305.44 A | 153.18 B |
| T4:P/CL = cucumber/C. lanatus | 14.00 AB | 4270.0 AB | 304.92 A | 159.39 B |
| CV (%) | 14.6 | 22.0 | 14.6 | 3.80 |
| DMS | 5.94 | 2484.1 | 114.54 | 15.93 |
| Treatments | Mean Values from the Root | |||
|---|---|---|---|---|
| AVXR (µm2) | NVXR | AHVR (µm2) | NHVR | |
| T1:PSP = cucumber without rootstock | 10,927 B | 12.557 A | 270,894 C | 3.11 B |
| T2:P/MA = cucumber/C. maxima | 27,988 A | 13.223 A | 1,621,251 A | 3.89 A |
| T3:P/MO = cucumber/C. moschata | 25,006 A | 10.000 A | 1,004,008 C | 4.22 A |
| T4:P/CL = cucumber/C. lanatus | 30,063 A | 14.113 A | 567,684 B | 4.33 A |
| CV (%) | 14.81 | 22.34 | 113.25 | 5.87 |
| DMS | 9838.1 | 7.87 | 324,311 | 0.64 |
| Treatments | Mean Values at Basal Stalk | |||
|---|---|---|---|---|
| AVXTB (µm2) | NVXTB | AHVTB (µm2) | NHVTB | |
| T1:PSP = cucumber without rootstock | 8360 C | 13.25 C | 471,927 C | 9.00 A |
| T2:P/MA = cucumber/C. maxima | 22,838 A | 29.00 AB | 1,863,489 A | 7.00 AB |
| T3:P/MO = cucumber/C. moschata | 14,147 BC | 36.50 A | 1,179,155 B | 6.67 B |
| T4:P/CL = cucumber/C. lanatus | 19,338 AB | 20.25 BC | 1,070,061 B | 8.50 AB |
| CV (%) | 12.80 | 22.32 | 10.45 | 9.26 |
| DMS | 5852.6 | 15.61 | 338,541 | 2.03 |
| Treatments | Mean Values in Cucumber Grafting | |||
|---|---|---|---|---|
| AVXTP (µm2) | NVXTP | AHVTP (µm2) | NHVTP | |
| T1:PSP = cucumber without rootstock | 3747 A | 14.917 A | 263,516 A | 9.833 A |
| T2:P/MA = cucumber/C. maxima | 4749 A | 13.250 A | 316,058 A | 10.333 A |
| T3:P/MO = cucumber/C. moschata | 3644 A | 16.667 A | 266,838 A | 9.667 A |
| T4:P/CL = cucumber/C. lanatus | 5008 A | 14.250 A | 261,098 A | 9.000 A |
| CV (%) | 32.30 | 16.61 | 19.62 | 12.76 |
| DMH | 3914.8 | 6.93 | 153,607 | 3.50 |
| Studied Variables | AVXR | NVXR | AHVR | NHVR | AVXTB | NVXTB | AHVTB | NHVTB | AVXTA | NVXTA | AHVTA | NHVTA | NFPP | PFPP | PPF | PSP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AVXR | 1.000 | |||||||||||||||
| NVXR | 0.188 | 1.000 | ||||||||||||||
| AHVR | 0.745 | 0.463 | 1.000 | |||||||||||||
| NHVR | 0.932 | −0.066 | 0.461 | 1.000 | ||||||||||||
| AVXTB | 0.892 | 0.420 | 0.965 | 0.670 | 1.000 | |||||||||||
| NVXTB | 0.578 | −0.648 | 0.331 | 0.660 | 0.416 | 1.000 | ||||||||||
| AHVTB | 0.761 | 0.076 | 0.919 | 0.557 | 0.901 | 0.664 | 1.000 | |||||||||
| NHVTB | −0.541 | 0.578 | −0.452 | −0.551 | −0.482 | −0.974 | −0.766 | 1.000 | ||||||||
| AVXTA | 0.704 | 0.828 | 0.789 | 0.466 | 0.829 | −0.124 | 0.522 | 0.083 | 1.000 | |||||||
| NVXTA | −0.227 | −0.873 | −0.727 | 0.124 | −0.599 | 0.392 | −0.426 | −0.232 | −0.785 | 1.000 | ||||||
| AHVTA | 0.331 | 0.187 | 0.831 | 0.031 | 0.676 | 0.341 | 0.846 | −0.536 | 0.374 | −0.634 | 1.000 | |||||
| NHVTA | −0.227 | −0.243 | 0.315 | −0.412 | 0.098 | 0.278 | 0.454 | −0.471 | −0.253 | −0.235 | 0.788 | 1.000 | ||||
| NFPP | 0.361 | 0.582 | 0.888 | 0.004 | 0.743 | 0.009 | 0.736 | −0.200 | 0.667 | −0.897 | 0.907 | 0.545 | 1.000 | |||
| PFPP | 0.783 | 0.417 | 0.998 | 0.515 | 0.977 | 0.388 | 0.937 | −0.499 | 0.776 | −0.679 | 0.815 | 0.296 | 0.857 | 1.000 | ||
| PPF | 0.950 | −0.109 | 0.534 | 0.999 | 0.721 | 0.739 | 0.657 | −0.654 | 0.451 | 0.095 | 0.155 | −0.279 | 0.088 | 0.588 | 1.000 | |
| PSP | 0.910 | 0.130 | 0.903 | 0.751 | 0.955 | 0.668 | 0.961 | −0.717 | 0.633 | −0.370 | 0.681 | 0.192 | 0.624 | 0.930 | 0.821 | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robledo-Torres, V.; González-Cortés, A.; Luna-García, L.R.; Mendoza-Villarreal, R.; Pérez-Rodríguez, M.Á.; Camposeco-Montejo, N. Histological Variations in Cucumber Grafted Plants and Their Effect on Yield. Agronomy 2024, 14, 1377. https://doi.org/10.3390/agronomy14071377
Robledo-Torres V, González-Cortés A, Luna-García LR, Mendoza-Villarreal R, Pérez-Rodríguez MÁ, Camposeco-Montejo N. Histological Variations in Cucumber Grafted Plants and Their Effect on Yield. Agronomy. 2024; 14(7):1377. https://doi.org/10.3390/agronomy14071377
Chicago/Turabian StyleRobledo-Torres, Valentín, Areli González-Cortés, Laura Raquel Luna-García, Rosalinda Mendoza-Villarreal, Miguel Ángel Pérez-Rodríguez, and Neymar Camposeco-Montejo. 2024. "Histological Variations in Cucumber Grafted Plants and Their Effect on Yield" Agronomy 14, no. 7: 1377. https://doi.org/10.3390/agronomy14071377






