Application of 2-Iminoselenazolidin-4-Ones (ISeA) for Beta vulgaris L. and Brassica rapa L. Plants Se-Biofortification
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
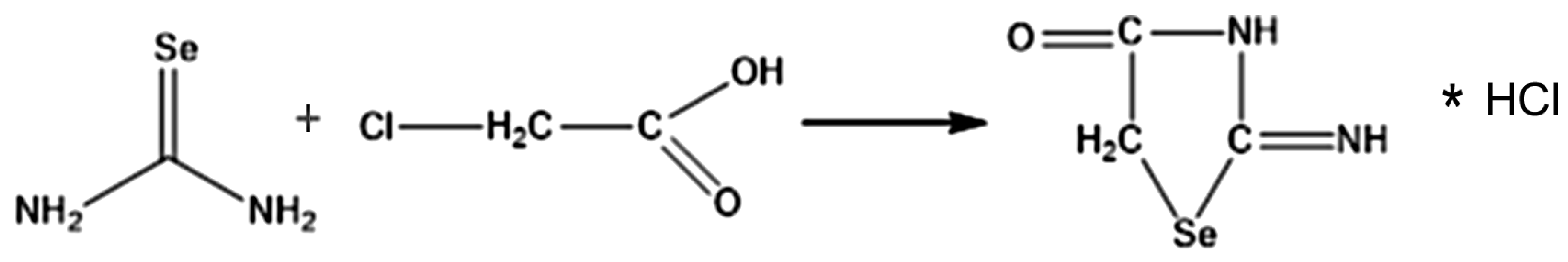
2.2. ISeA Obtaining
2.3. ISeA Particles Characterization
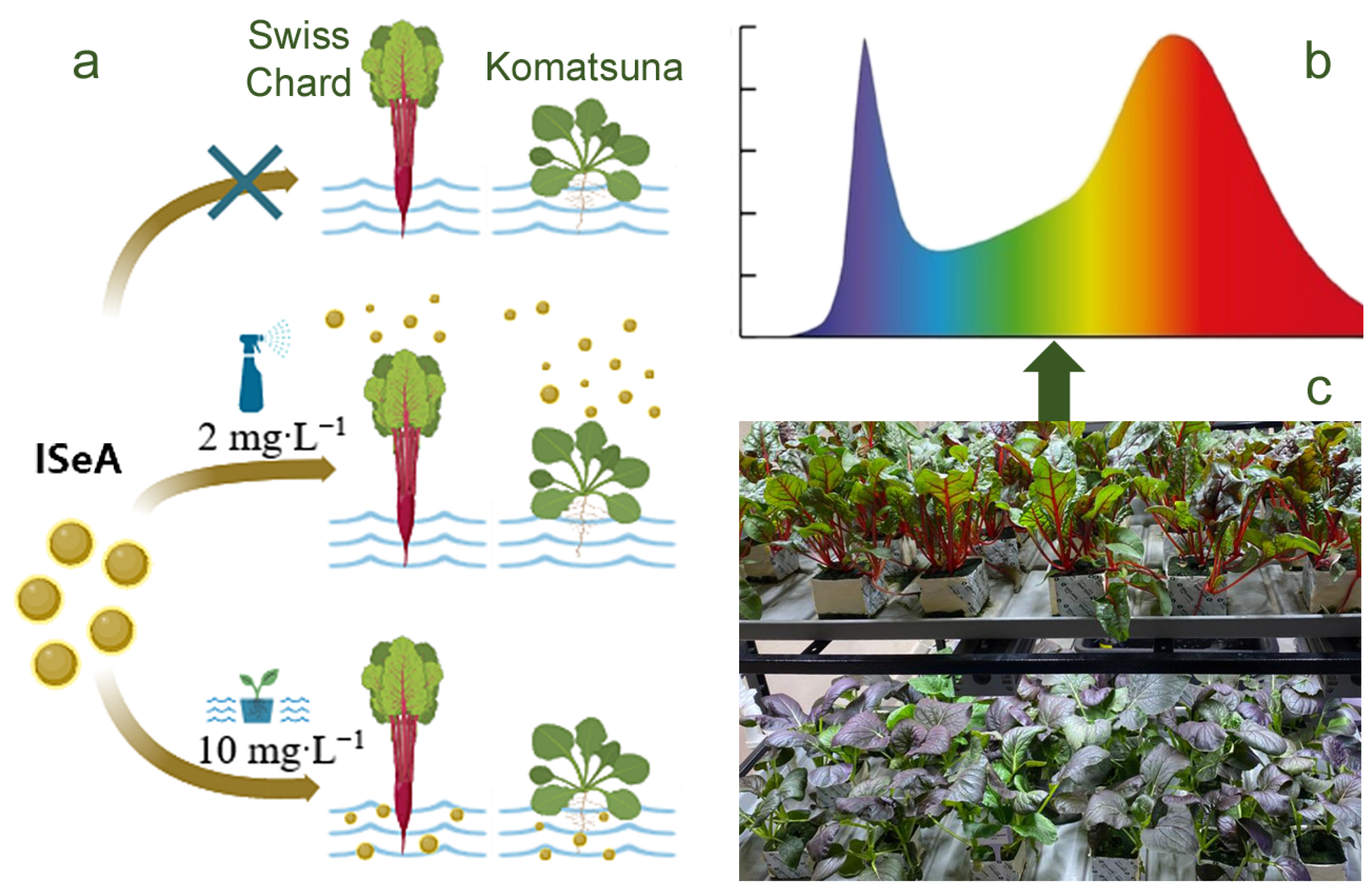
2.4. Plant Cultivation Conditions
2.5. Biometric Indicators
2.6. Pigment Content
2.7. Sugar Content
2.8. Element Analysis
2.9. Statistical Analysis
3. Results
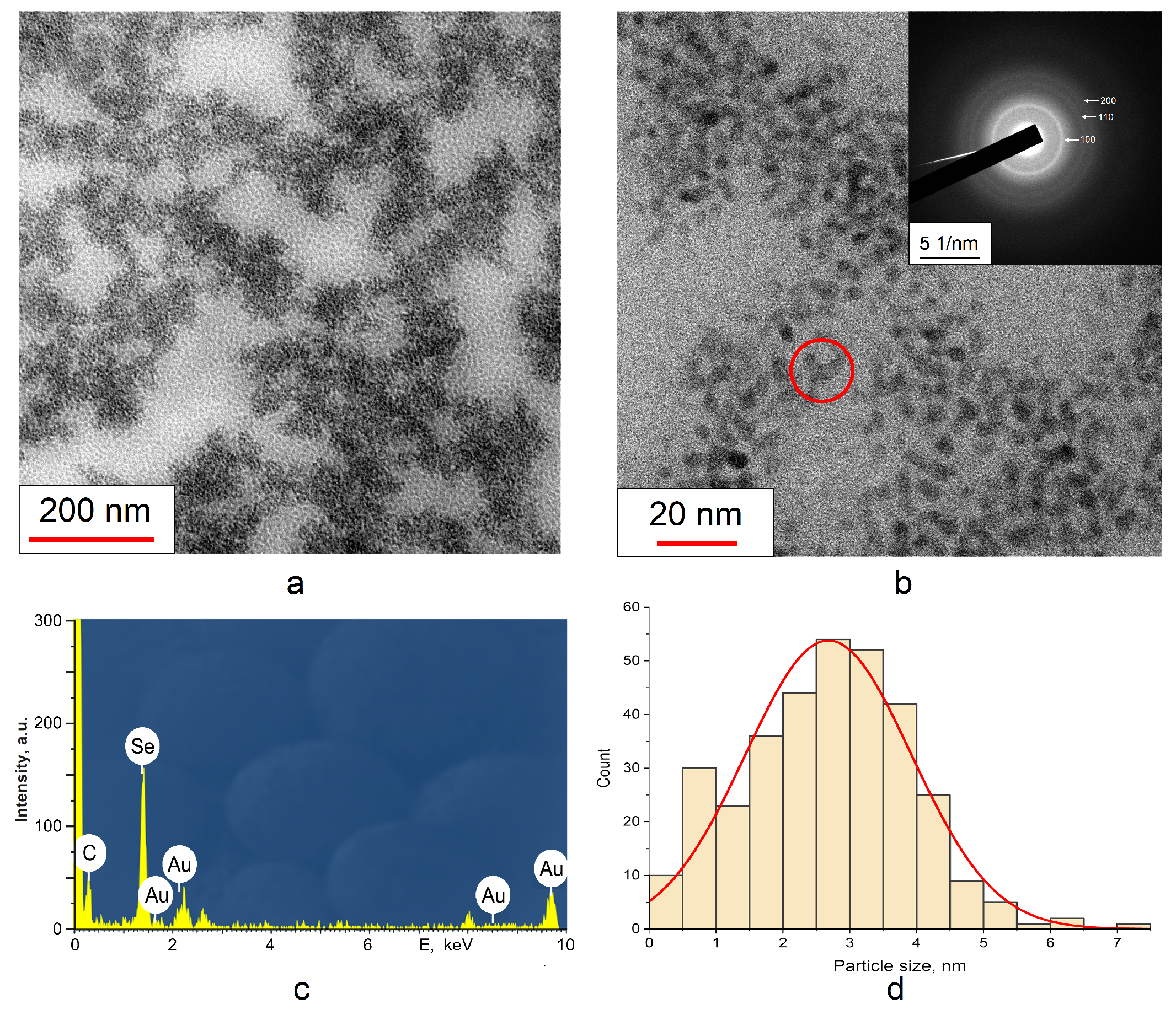
3.1. Characterization of SeNPs
3.2. Effect of ISeA and Processing Method on Plants Biometric Characteristics
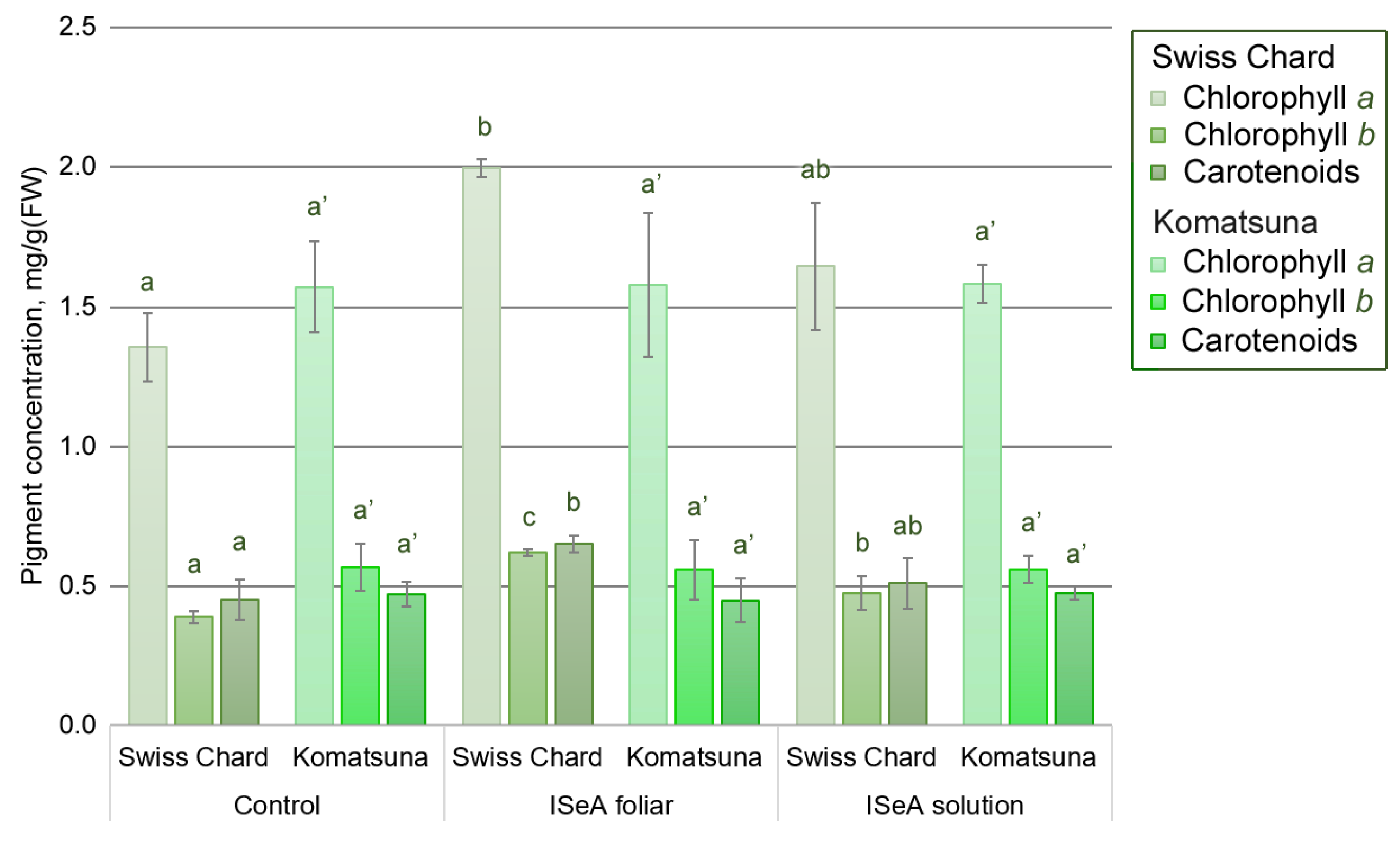
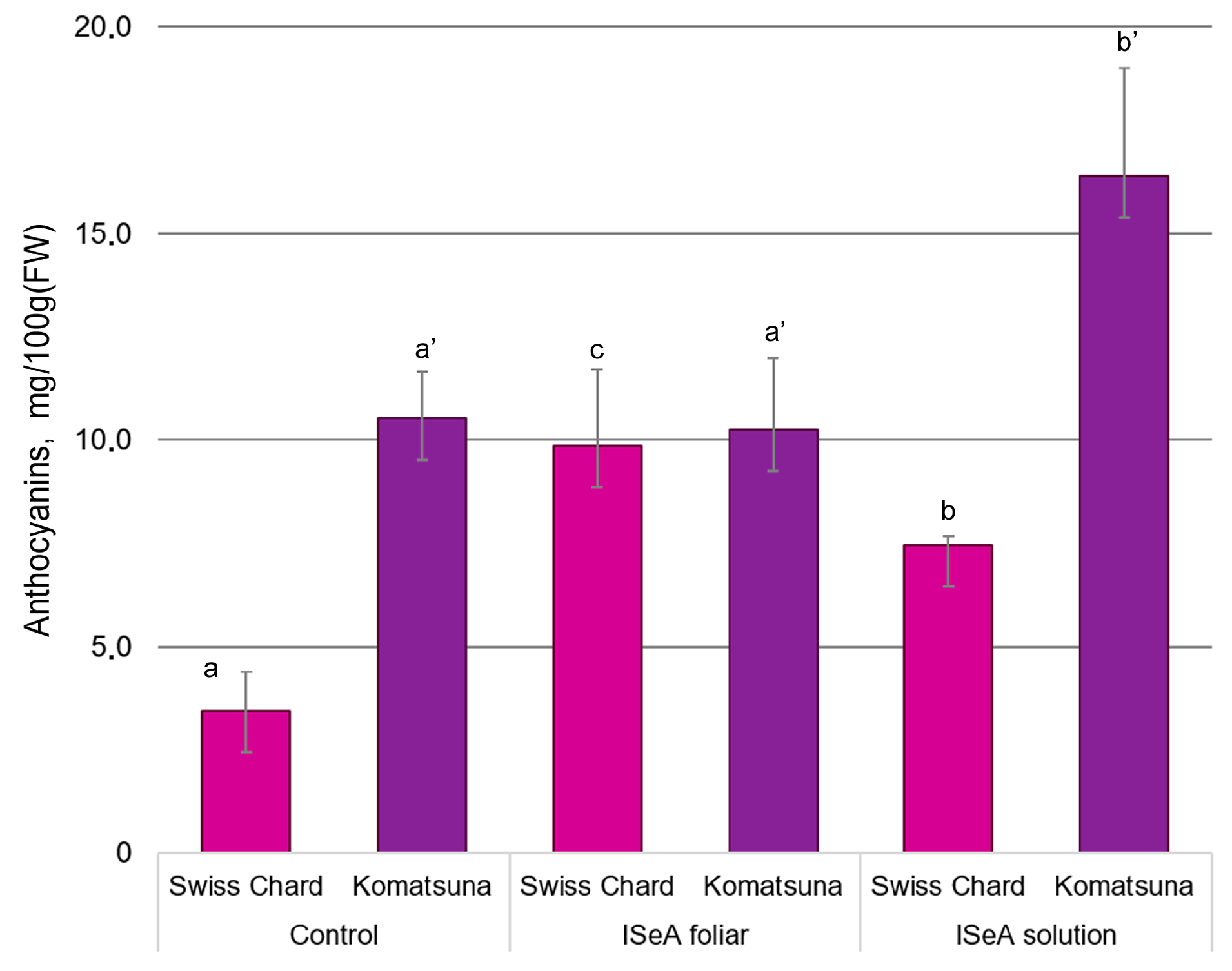
3.3. Effect of Enrichment Method and ISeA on the Biosynthesis of Photosynthetic Pigments and Anthocyanins
3.4. Effect of Enrichment Method and ISeA on Sugar Content
3.5. Impact of ISeA and Enrichment Method on the Absorption and Accumulation of Se and Other Elements
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ISeA | Compound 2-iminoselenazolidin-4-ones |
| FW | Fresh weight of plants |
| DW | Dry weight of plants |
| SeNPs | Selenium nanoparticles |
| P | Petioles of considered plants |
| L | Leaves of considered plants |
References
- Baraboj, V.A. Biological functions, metabolism and mechanisms of selenium effects. Biol. Bull. Rev. 2004, 124, 157–168. [Google Scholar]
- Duntas, L.; Benvenga, S. Selenium: An element for life. Endocrine 2015, 48, 756–775. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. 2014, 39, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Tutelyan, V.A.; Kniazhev, V.A.; Hotimchenko, S.A.; Golubkina, N.A.; Kushlinskiy, N.I.; Sokolov, Y.A. Selenium in the Human Body Metabolism, Antioxidant Properties, Role in Carcinogenesis; RAMS: Moscow, Russia, 2002; 224p. [Google Scholar]
- Newman, R.; Waterland, N.; Moon, Y.; Tou, J.C. Selenium biofortification of agricultural crops and effects on plant nutrients and bioactive compounds important for human health and disease prevention—A review. Plant Foods Hum. Nutr. 2019, 74, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Kekina, H.; Nadegkin, S.M. Prospects of agricultural plants biofortification with iodine and selenium (review). Trace Elem. Med. 2015, 16, 12–19. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Lovkova, M.; Sokolova, S.; Buzuk, G. Medicinal plants concentrating Selenium: Prospects of wider use. Dokl. Biol. Sci. 2008, 418, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Wang, Z.; Yin, X.; Liu, X.; Qin, L.; Farooq, M.R.; Danso, O.P.; Zhang, Z.; Luo, Q.; Sun, C.; et al. A preliminary predictive model for selenium nutritional status in residents based on three selenium biomarkers. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2024, 81, 127347. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium Biofortification and Interaction with Other Elements in Plants: A Review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, X.; Du, H.; Yang, J.; Guo, F.; Wu, F. Impacts, causes and biofortification strategy of rice selenium deficiency based on publication collection. Sci. Total Environ. 2024, 912, 169619. [Google Scholar] [CrossRef]
- Kieliszek, M.; Serrano Sandoval, S.N. The importance of selenium in food enrichment processes. A comprehensive review. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2023, 79, 127260. [Google Scholar] [CrossRef]
- Golubkina, N.; Papazyan, T. Selenium in Nutrition. Plants, Animals, Humans; Pechatny Gorod; Moscow, Russia, 2006; p. 250. (In Russian) [Google Scholar]
- Lanza, M.G.D.B.; Reis, A.R.D. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef]
- Chilimba, A.; Young, S.; Black, C.; Meacham, M.; Lammel, J.; Broadley, M. Agronomic biofortification of maize with selenium (Se) in Malawi. Field Crops Res. 2012, 125, 118–128. [Google Scholar] [CrossRef]
- Ros, G.; van Rotterdam, A.; Bussink, D.; Bindraban, P. Selenium fertilization strategies for bio-fortification of food: An agro-ecosystem approach. Plant Soil 2016, 404, 99–112. [Google Scholar] [CrossRef]
- Revenskiy, V.; Zonkhoeva, E.; Chimitdorzhieva, G. Effect of a complex selenium-zeolite mineral fertilizer of prolonged action on the yield and grain quality of spring wheat. Agrochemistry 2007, 37–40. (In Russian) [Google Scholar]
- Chen, L.; Yang, F.; Xu, J.; Hu, Y.; Hu, Q.; Zhang, Y.; Pan, G. Determination of selenium concentration of rice in china and effect of fertilization of selenite and selenate on selenium content of rice. J. Agric. Food Chem. 2002, 50, 5128–5130. [Google Scholar] [CrossRef] [PubMed]
- Sors, T.; Ellis, D.; Salt, D. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef]
- Silva, V.; Boleta, E.; Martins, J.; dos Santos, F.; da Rocha Silva, A.; Alcock, T.; Wilson, L.; de Sá, M.E.; Young, S.; Broadley, M.; et al. Agronomic biofortification of cowpea with selenium: Effects of selenate and selenite applications on selenium and phytate concentrations in seeds. J. Sci. Food Agric. 2019, 99, 5969–5983. [Google Scholar] [CrossRef]
- Vodyuanitsky, Y. Properties of heavy metals and metalloids in soils. Agrochemistry 2009, 105–107. (In Russian) [Google Scholar]
- Potatueva, Y. Selenium in soils and plants. Agrochemistry 1976, 149–151. (In Russian) [Google Scholar]
- Rodrigues dos Reis, A.; Boleta, E.H.M.; Alves, C.; Cotrim, M.F.; Barbosa, J.Z.; Silva, V.M.; Porto, R.L.; Lanza, M.G.D.B.; Lavres, J.; Gomes, M.H.F.; et al. Selenium toxicity in upland field-grown rice: Seed physiology responses and nutrient distribution using the μ-XRF technique. Ecotoxicol. Environ. Saf. 2020, 190, 110147. [Google Scholar] [CrossRef] [PubMed]
- Eich-Greatorex, S.; Sogn, T.A.; Øgaard, A.F.; Aasen, I. Plant availability of inorganic and organic selenium fertiliser as influenced by soil organic matter content and pH. Nutr. Cycl. Agroecosyst. 2007, 79, 221–231. [Google Scholar] [CrossRef]
- Schiavon, M.; Lima, L.; Jiang, Y.; Hawkesford, M. Selenium in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects. In Effects of Selenium on Plant Metabolism and Implications for Crops and Consumers; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.-Q., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 257–275. [Google Scholar]
- El-Ramady, H.R.; Domokos-Szabolcsy, É.; Shalaby, T.A.; Prokisch, J.; Fári, M. Selenium in agriculture: Water, air, soil, plants, food, animals and nanoselenium. In CO2 Sequestration, Biofuels and Depollution; Springer: Berlin/Heidelberg, Germany, 2015; pp. 153–232. [Google Scholar]
- Broadley, M.R.; Alcock, J.; Alford, J.; Cartwright, P.; Foot, I.; Fairweather-Tait, S.J.; Hart, D.J.; Hurst, R.; Knott, P.; McGrath, S.P.; et al. Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilisation. Plant Soil 2010, 332, 5–18. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium biofortification: Roles, mechanisms, responses and prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef] [PubMed]
- Kikkert, J.; Berkelaar, E. Plant uptake and translocation of inorganic and organic forms of selenium. Arch. Environ. Contam. Toxicol. 2013, 65, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Pilon, M.; Malagoli, M.; Pilon-Smits, E.A. Exploring the importance of sulfate transporters and ATP sulphurylases for selenium hyperaccumulation—A comparison of Stanleya pinnata and Brassica juncea (Brassicaceae). Front. Plant Sci. 2015, 6, 118108. [Google Scholar] [CrossRef] [PubMed]
- Bikkulova, A.; Ishmuratova, G. Bioelementology of s-, p-, d-Elements; Nauka: Saint-Petersburg, Russia, 1999; p. 256. (In Russian) [Google Scholar]
- Bandman, A.L. Selenium and Its Compounds; Harmfull Chemicals. Inorganic Compounds of Elements in Groups V–VIII; Khimia: Leningrad, Russia, 1989. (In Russian) [Google Scholar]
- Comrie, A.; Dingwall, D.; Stenlake, J. 1089. Tautomerism of 2-iminoselenazolidin-4-ones. J. Chem. Soc. 1963, 5713–5717. [Google Scholar] [CrossRef]
- Niedzielski, P.; Rudnicka, M.; Wachelka, M.; Kozak, L.; Rzany, M.; Wozniak, M.; Kaskow, Z. Selenium species in selenium fortified dietary supplements. Food Chem. 2016, 190, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Poluboyarinov, P.; Sindireva, A. Selenium in food crops. Probl. Nutr. 2017, 86, 63–69. (In Russian) [Google Scholar]
- Luo, L.; Zhang, J.; Zhang, K.; Wen, Q.; Ming, K.; Xiong, H.; Ning, F. Peanut selenium distribution, concentration, speciation, and effects on proteins after exogenous selenium biofortification. Food Chem. 2021, 354, 129515. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory: An Indoor Vertical Farming System for Efficient Quality FOOD Production; Academic Press: Cambridge, MA, USA, 2019; pp. 3–5. [Google Scholar] [CrossRef]
- Puccinelli, M.; Rosellini, I.; Malorgio, F.; Pardossi, A.; Pezzarossa, B. Hydroponic Production of Selenium-Enriched Baby Leaves of Swiss Chard (Beta vulgaris var. cicla) and Its Wild Ancestor Sea Beet (Beta vulgaris ssp. maritima). Horticulturae 2023, 9, 909. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Taha, H.S.; Alshaal, T.; El-Henawy, A.; Faizy, S.E.D.A.; Shams, M.S.; Youssef, S.M.; Shalaby, T.; Bayoumi, Y.; et al. Selenium and nano-selenium in plant nutrition. Environ. Chem. Lett. 2016, 14, 123–147. [Google Scholar] [CrossRef]
- Li, D.; An, Q.; Wu, Y.; Li, J.Q.; Pan, C. Foliar application of selenium nanoparticles on celery stimulates several nutrient component levels by regulating the α-linolenic acid pathway. ACS Sustain. Chem. Eng. 2020, 8, 10502–10510. [Google Scholar] [CrossRef]
- Serov, D.A.; Khabatova, V.V.; Vodeneev, V.; Li, R.; Gudkov, S.V. A review of the antibacterial, fungicidal and antiviral properties of selenium nanoparticles. Materials 2023, 16, 5363. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Chen, F.; Yue, L.; Cao, X.; Li, J.; Cheng, B.; Wang, Z.; Xing, B. Selenium content and nutritional quality of Brassica chinensis L enhanced by selenium engineered nanomaterials: The role of surface charge. Environ. Pollut. 2022, 308, 119582. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.Y.; Li, Q.; Chen, H.; Liu, J.; Xing, S.F.; Xu, M.; Yan, Z.; Song, C.; Wang, S.G. Selenium nanoparticles ameliorate Brassica napus L. cadmium toxicity by inhibiting the respiratory burst and scavenging reactive oxygen species. J. Hazard. Mater. 2021, 417, 125900. [Google Scholar] [CrossRef]
- Huang, R.; Bañuelos, G.S.; Zhao, J.; Wang, Z.; Farooq, M.R.; Yang, Y.; Song, J.; Zhang, Z.; Chen, Y.; Yin, X.; et al. Comprehensive evaluation of factors influencing selenium fertilization biofortification. J. Sci. Food Agric. 2024, 104, 6100–6107. [Google Scholar] [CrossRef]
- Zhou, B.; Cao, H.; Wu, Q.; Mao, K.; Yang, X.; Su, J.; Zhang, H. Agronomic and Genetic Strategies to Enhance Selenium Accumulation in Crops and Their Influence on Quality. Foods 2023, 12, 4442. [Google Scholar] [CrossRef]
- Silaev, G.; Krasheninnikov, V.; Shaidulin, A.; Uvarov, O.; Orlovskaya, E.; Orlovskii, Y.; Vainer, Y. Optical and Electron Microscopy of Clusters of Nd3+:LaF3 Nanoparticles Synthesized by the HTMW Method. Phys. Wave Phenom. 2023, 31, 160–170. [Google Scholar] [CrossRef]
- Xia, Q.; Yang, Z.; Shui, Y.; Liu, X.; Chen, J.; Khan, S.; Wang, J.; Gao, Z. Methods of selenium application differentially modulate plant growth, selenium accumulation and speciation, protein, anthocyanins and concentrations of mineral elements in purple-grained wheat. Front. Plant Sci. 2020, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.; Sarimov, R.M.; Astashev, M.E.; Pishchalnikov, R.Y.; Yanykin, D.V.; Simakin, A.V.; Shkirin, A.V.; Serov, D.A.; Konchekov, E.M.; Gusein-zade, N.G.O.; et al. Modern physical methods and technologies in agriculture. Phys. Usp. 2024, 67, 194–210. [Google Scholar] [CrossRef]
- Wettstein, D.v. Chlorophyll-letale und der submikroskopische Formwechsel der Plastiden. Exp. Cell Res. 1957, 12, 427–506. [Google Scholar] [CrossRef] [PubMed]
- Maslennikov, P.; Chupakhina, G.; Skrypnick, L.; Fedurayev, P.; Poltavskaya, R. Content of anthocyanin and carotenoid pigments in medicinal plants. Electron. J. Bull. Mosc. State Reg. Univ. 2013, 1, 6. [Google Scholar]
- Andrade, F.R.; da Silva, G.N.; Guimarães, K.C.; Barreto, H.B.F.; de Souza, K.R.D.; Guilherme, L.R.G.; Faquin, V.; Dos Reis, A.R. Selenium protects rice plants from water deficit stress. Ecotoxicol. Environ. Saf. 2018, 164, 562–570. [Google Scholar] [CrossRef]
- Silva, V.M.; Tavanti, R.F.R.; Gratão, P.L.; Alcock, T.D.; Dos Reis, A.R. Selenate and selenite affect photosynthetic pigments and ROS scavenging through distinct mechanisms in cowpea (Vigna unguiculata (L.) walp) plants. Ecotoxicol. Environ. Saf. 2020, 201, 110777. [Google Scholar] [CrossRef] [PubMed]
- Hemmati, M.; Delkhosh, B.; Rad, A.H.S.; Mohammadi, G.N. Effect of the application of foliar selenium on canola cultivars as influenced by different irrigation regimes. J. Agric. Sci. 2019, 25, 309–318. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Akbar, A.; Parveen, A.; Rasheed, R.; Hussain, I.; Iqbal, M. Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol. Biochem. 2018, 123, 268–280. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Dresler, S.; Rubinowska, K.; Matraszek-Gawron, R.; Woch, W.; Hasanuzzaman, M. Selenium biofortification enhances the growth and alters the physiological response of lamb’s lettuce grown under high temperature stress. Plant Physiol. Biochem. 2018, 127, 446–456. [Google Scholar] [CrossRef]
- Sabatino, L.; Ntatsi, G.; Iapichino, G.; D’Anna, F.; De Pasquale, C. Effect of selenium enrichment and type of application on yield, functional quality and mineral composition of curly endive grown in a hydroponic system. Agronomy 2019, 9, 207. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef]
- Schiavon, M.; Berto, C.; Malagoli, M.; Trentin, A.; Sambo, P.; Dall’Acqua, S.; Pilon-Smits, E.A. Selenium biofortification in radish enhances nutritional quality via accumulation of methyl-selenocysteine and promotion of transcripts and metabolites related to glucosinolates, phenolics, and amino acids. Front. Plant Sci. 2016, 7, 1371. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.d.N.d.; Cidade, M.; Heerdt, G.; Ribessi, R.L.; Morgon, N.H.; Cadore, S. Effect of selenite and selenate application on mineral composition of lettuce plants cultivated under hydroponic conditions: Nutritional balance overview using a multifaceted study. J. Braz. Chem. Soc. 2018, 29, 371–379. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Maggini, R.; Rosellini, I.; Pezzarossa, B. Biofortification of Ocimum basilicum L. plants with selenium. In Proceedings of the III International Symposium on Horticulture in Europe-SHE2016 1242, Chania, Greece, 17–21 October 2016; pp. 663–670. [Google Scholar]
- Ferrarese, M.; Sourestani, M.; Quattrini, E.; Schiavi, M.; Ferrante, A. Biofortification of spinach plants applying selenium in the nutrient solution of floating system. J. Fruit Ornam. Plant Res. 2012, 76, 127–136. [Google Scholar] [CrossRef]
- Saffaryazdi, A.; Lahouti, M.; Ganjeali, A.; Bayat, H. Impact of selenium supplementation on growth and selenium accumulation on spinach (Spinacia oleracea L.) plants. Not. Sci. Biol. 2012, 4, 95–100. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, L.; Xu, J.; Zhang, Y.; Pan, G. Determination of selenium concentration in rice and the effect of foliar application of Se-enriched fertiliser or sodium selenite on the selenium content of rice. J. Sci. Food Agric. 2002, 82, 869–872. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhu, L.N.; Li, K.; Wang, Q.; Wang, K.; Guo, Y.B.; Li, H.F. Absorption and transportation of selenium nanoparticles in wheat and rice. Huan Jing Ke Xue = Huanjing Kexue 2019, 40, 4654–4660. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Y.; Li, K.; Wan, Y.; Wang, Q.; Zhuang, Z.; Guo, Y.; Li, H. Uptake, translocation and biotransformation of selenium nanoparticles in rice seedlings (Oryza sativa L.). J. Nanobiotechnol. 2020, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Martín, G.; Sanz-Landaluze, J.; León-González, M.E.; Madrid, Y. Insights into the accumulation and transformation of Ch-SeNPs by Raphanus sativus and Brassica juncea: Effect on essential elements uptake. Sci. Total Environ. 2020, 725, 138453. [Google Scholar] [CrossRef]
- Nikulina, E.; Tsirulnikova, N.; Semenova, N.; Godyaeva, M.; Akimova, S. Potential Application of Phosphorus-Containing Micronutrient Complexates in Hydroponic System Nutrient Solutions. In International Conference on Intelligent Computing & Optimization; Springer: Cham, Switzerland, 2023; pp. 317–324. [Google Scholar] [CrossRef]
- Makarova, A.; Nikulina, E.; Tsirulnikova, N.; Pishchaeva, K.; Fedoseev, A. Effect of monoethanolamine salt-containing dicarboxylic acid and plant growth regulators on the absorption and accumulation of mercury. Saudi J. Biol. Sci. 2022, 29, 3448–3455. [Google Scholar] [CrossRef]
- Makarova, A.; Nikulina, E.; Fedotov, P. Induced phytoextraction of mercury. Sep. Purif. Rev. 2022, 51, 174–194. [Google Scholar] [CrossRef]



| ISeA Treatment | FW (g) | DW/FW (%) | Number of Leaves | Leaf Area (cm2) | Plant Height (cm) |
|---|---|---|---|---|---|
| Chard | |||||
| Control | a | a | 1 a | a | a |
| Foliar | b | b | a | a | ab |
| Solution | b | b | a | b | b |
| Komatsuna | |||||
| Control | a′ | a′ | a′ | a′ | a′ |
| Foliar | ab′ | a′ | b′ | b′ | a′ |
| Solution | b′ | b′ | b′ | b′ | a′ |
| Treatment | Part of Plant | Ca | K | Mg |
|---|---|---|---|---|
| Chard | ||||
| Control | P | a | b | b |
| L | b | a | d | |
| Foliar | P | a | a | a |
| L | b | ab | c | |
| Solution | P | a | b | a |
| L | b | b | c | |
| Komatsuna | ||||
| Control | P | a | d | a |
| L | b | ab | b | |
| Foliar | P | b | b | a |
| L | c | a | b | |
| Solution | P | bc | c | b |
| L | bc | a | b |
| Treatment | Part of Plant | Se | Fe | Cu | Mo |
|---|---|---|---|---|---|
| Chard | |||||
| Control | P | - | b | b | a |
| L | - | b | c | c | |
| Foliar | P | a | a | a | a |
| L | c | ab | b | c | |
| Solution | P | b | a | b | a |
| L | c | ab | b | b | |
| Komatsuna | |||||
| Control | P | - | c | a | a |
| L | - | c | a | b | |
| Foliar | P | a | a | a | a |
| L | c | b | a | bc | |
| Solution | P | d | c | a | c |
| L | b | b | a | a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semenova, N.A.; Nikulina, E.A.; Tsirulnikova, N.V.; Godyaeva, M.M.; Uyutova, N.I.; Baimler, I.V.; Simakin, A.V.; Stepanova, E.V.; Gudkov, S.V. Application of 2-Iminoselenazolidin-4-Ones (ISeA) for Beta vulgaris L. and Brassica rapa L. Plants Se-Biofortification. Agronomy 2024, 14, 1407. https://doi.org/10.3390/agronomy14071407
Semenova NA, Nikulina EA, Tsirulnikova NV, Godyaeva MM, Uyutova NI, Baimler IV, Simakin AV, Stepanova EV, Gudkov SV. Application of 2-Iminoselenazolidin-4-Ones (ISeA) for Beta vulgaris L. and Brassica rapa L. Plants Se-Biofortification. Agronomy. 2024; 14(7):1407. https://doi.org/10.3390/agronomy14071407
Chicago/Turabian StyleSemenova, Natalia A., Elena A. Nikulina, Nina V. Tsirulnikova, Maria M. Godyaeva, Nadezhda I. Uyutova, Ilya V. Baimler, Aleksander V. Simakin, Eugenia V. Stepanova, and Sergey V. Gudkov. 2024. "Application of 2-Iminoselenazolidin-4-Ones (ISeA) for Beta vulgaris L. and Brassica rapa L. Plants Se-Biofortification" Agronomy 14, no. 7: 1407. https://doi.org/10.3390/agronomy14071407
APA StyleSemenova, N. A., Nikulina, E. A., Tsirulnikova, N. V., Godyaeva, M. M., Uyutova, N. I., Baimler, I. V., Simakin, A. V., Stepanova, E. V., & Gudkov, S. V. (2024). Application of 2-Iminoselenazolidin-4-Ones (ISeA) for Beta vulgaris L. and Brassica rapa L. Plants Se-Biofortification. Agronomy, 14(7), 1407. https://doi.org/10.3390/agronomy14071407









