Abstract
The characteristics and responses of soil bacterial communities and potato productivity to different fertilization treatments in farmlands in the agropastoral zone of Inner Mongolia were investigated. Moreover, the diversity and structure of soil bacterial communities and potato productivity under different fertilization treatments (no fertilization, CK; phosphorus-deficient treatment, NK; conventional fertilization, NPK; and organic–inorganic combination, NPKM) were assessed using Illumina high-throughput sequencing. The results revealed that soil pH, organic matter (SOM), total nitrogen (TN), and total phosphorus (TP) content, and potato productivity were significantly increased under fertilizer treatments (NK, NPK, and NPKM) compared with those under CK, with NPKM treatment having the best enhancement effect. The application of organic fertilizers significantly increased the Shannon, evenness, Chao1, and Ace indices of soil bacterial communities and reshaped the bacterial community structure. Random forest model analysis revealed that soil pH and TP significantly affected soil bacterial diversity, whereas soil pH, SOM, TP, and TN significantly affected soil bacterial community structure. Correlation and structural equation modeling analyses revealed that soil TP and SOM indirectly affected potato productivity by changing soil bacterial diversity and community composition. The results of this study provide a scientific basis for improving the quality and productivity of farmland soil to guide the rational fertilization of farmlands in the agropastoral zone of northern China.
1. Introduction
An ecologically fragile area and a major agricultural production area in China, the agricultural and livestock intertwined zone in Inner Mongolia has rich ecological and production values [1]. However, severe wind erosion, declining land quality, and overloaded production in this region have rapidly reduced the levels of soil organic matter and nutrients, such as nitrogen, phosphorus, and potassium, thereby limiting the potential for agricultural production in the northern agropastoral belt [2]. In most parts of the country, chemical fertilizers have been used to increase crop yield; however, long-term, excessive, and irrational application of chemical fertilizers has intensified soil organic matter loss and reduced soil biodiversity, arable land production capacity, and food quality [3]. Tang et al. [4], using random-effect model regression analysis, found that the increased application of chemical fertilizers increased difficulties in food production. Organic fertilizers can effectively improve soil aggregate structure, increase the content of organic matter, stimulate the biological function of soil, and improve the productivity of arable land [5].
Soil microbial communities can participate in soil nutrient cycling and material transformation by releasing various active metabolites [6]. The structure, composition, and diversity of soil microbial communities are considered to be the most sensitive indicators of soil fertility changes and health [7]. Wu et al. [8] and Liu et al. [9] found that the application of organic fertilizers can significantly increase the content of nitrogen, phosphorus, and potassium in the soil, directly transforming the structure of the soil bacterial community. The bacterial diversity index also showed a positive feedback effect. However, a previous study reported that different soil types have different responses to the microbial community structure and diversity index after the application of organic fertilizer [10]. Therefore, the effects of different organic fertilizer treatments on soil bacterial diversity and community structure remain controversial. Consequently, it is important to study the changes in soil bacterial diversity and community structure under different fertilizer treatments to maintain the multifunctionality of farmland ecosystems and stabilize grain yield in the agropastoral zone.
Improvement in soil microbial diversity helps increase the multifunctionality of agricultural soil ecosystems and their stability, thereby playing a key role in promoting soil biological functions and crop growth [11]. Organic fertilizer treatment helps in the proliferation of beneficial microorganisms in the soil, thereby optimizing the structure of the soil bacterial community [12]. Han et al. [13] found that organic fertilizer significantly increased the abundance of functional microorganisms related to nitrogen metabolism, thereby enhancing crop yield through accelerating soil nitrogen transformation. Although organic fertilizer has an important effect on improving soil microbial function and crop productivity, the mechanism of microbial community effect on potato productivity under organic fertilizer treatment has not been systematically revealed. In this study, we used Illumina high-throughput sequencing to investigate soil fertility, soil bacterial diversity, and soil community structure under different organic fertilizer treatments and their potential effects on potato productivity. We aimed to provide a theoretical basis for the establishment of reasonable fertilizer cultivation measures for farmlands in the agricultural and pastoral areas of the northern foothills of the Yinshan Mountains, rapid improvement in arable land quality in the region, and safeguarding of food security.
2. Materials and Methods
2.1. Overview of the Study Area
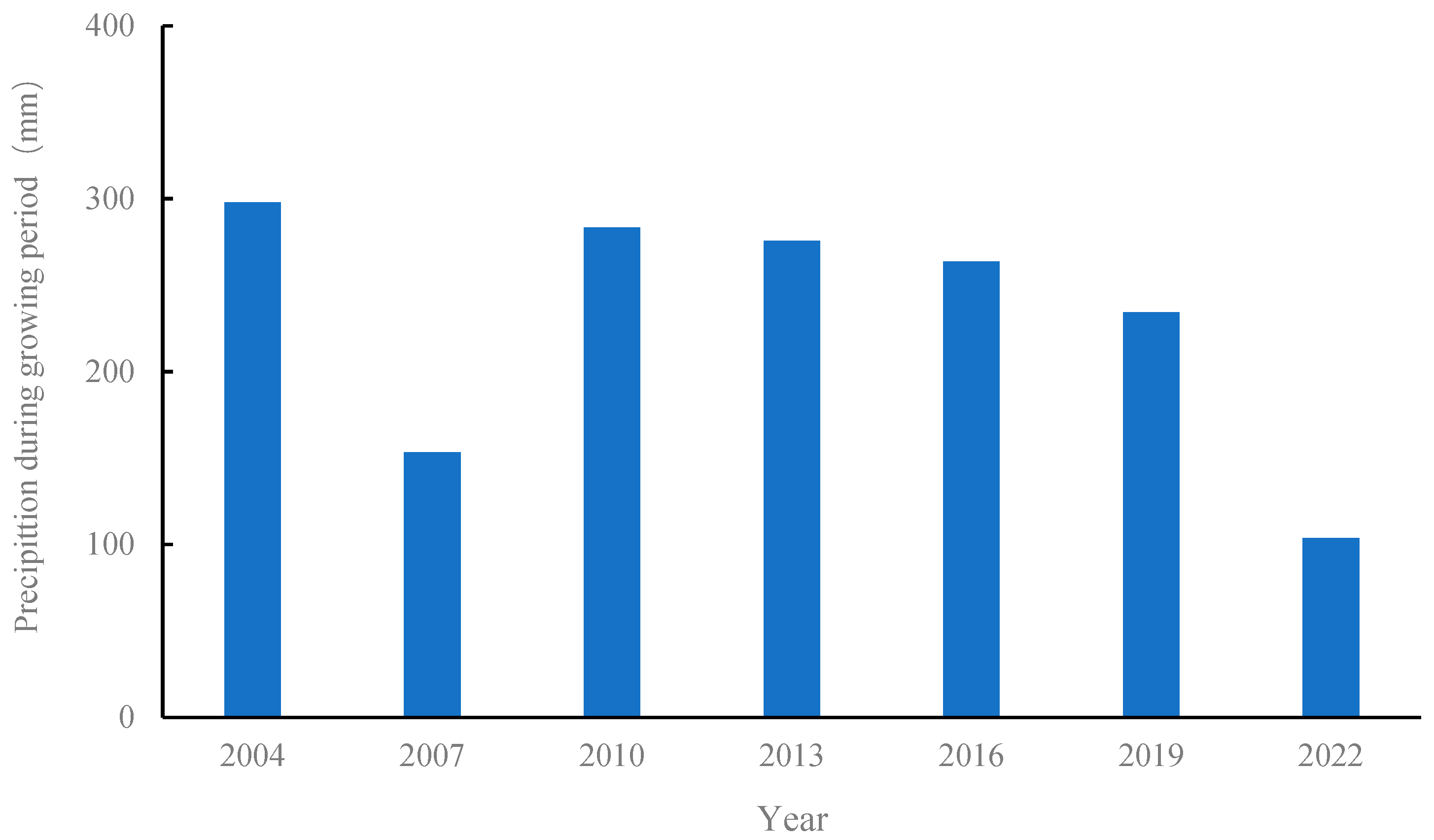
The experiment was conducted at the Wuchuan dry farming Experimental Station of the Inner Mongolia Academy of Agricultural and Animal Husbandry Sciences (41°08′23″ Nphia 111°17′44″ E). This region has a mid-temperate continental monsoon climate. The annual average temperature is 2.4 °C, the coldest month is January, with an average temperature of −14.8 °C, and the hottest month is July, with an average temperature of 18.8 °C. The frost-free period is approximately 110 days, and the annual accumulated temperature with an average monthly temperature of ≥5 °C is 1800 °C over the years. The average precipitation over the years is approximately 350 mm, concentrated mainly between July and September. It sits at an elevation of 1570 m and has a cold and arid climate, a typical semiarid ecotone between agriculture and animal husbandry. Figure 1 shows the changes in precipitation over the years. During the growth periods of 2007 and 2022, there was less precipitation and a dry climate, whereas the other years had normal precipitation. The experiment was conducted in 2004 using a potato–rape–naked oats three-year rotation system, one crop per year, and potato crops in 2022. Potato crop productivity is expressed using potato yield data.
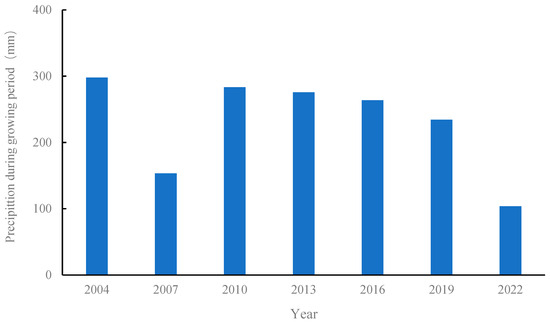
Figure 1.
Precipitation during the potato growing season. Note: The fertile period is defined as May through September each year.
The soil type is calcium chestnut. At the beginning of the experiment, the soil in the plow layer contained 12.5 g/kg organic matter, 75 mg/kg hydrolyzable nitrogen, 7.5 mg/kg available phosphorus (AP), and 70.4 mg/kg available potassium.
2.2. Experimental Design
The experiment was divided into four treatments: no fertilization (CK); nitrogen and potassium fertilizer (NK); nitrogen, phosphorus, and potassium fertilizer (NPK); and nitrogen, phosphorus, and potassium fertilizer combined with sheep manure (NPKM). Table 1 shows the fertilization results of the different treatments. Each treatment was repeated thrice and arranged at random, with a plot area of 50 NPK. The organic fertilizer used was sheep manure, and the average nutrient content of N, P2O5, and K2O was 0.57%, 0.24%, and 1.05%, respectively. The fertilizer was evenly spread on the surface after the annual crop harvest and manually turned into the soil. Urea (N, 46.0%) was used for nitrogen fertilizer, heavy superphosphate (P2O5, 18–46%) for phosphate fertilizer, potassium chloride (K2O, 60%) for potash fertilizer, with deep application of chemical fertilizer on the side of the ditch during sowing, and all fertilizers were applied as base fertilizer at once. Field trials are usually sown in early May and harvested in mid-September.

Table 1.
Annual application rates of chemical nutrients and organic manure in different treatments (kg/hm2).
2.3. Sample Collection
Fresh soil samples were collected after the potato harvest in 2022, impurities were removed from the soil samples, and soil samples at a depth of 0–20 cm in different treatments were collected using the multipoint sampling method. To reduce the error between treatments, six independent samples were collected from each experimental plot, totaling 24 soil samples. They were placed in an icebox and brought back to the laboratory. Some soil samples were sifted using a 1 mm sieve and stored in a refrigerator at −80 °C for the analysis of soil bacterial community structure and diversity. Some samples were stored at 2 °C–8 °C for the determination of soil ammonium nitrogen and nitrate nitrogen. Other samples were naturally air-dried to determine the physical and chemical properties of the soil.
2.4. Determination Items and Methods
2.4.1. Determination of the Physical and Chemical Properties of Soil
Soil total nitrogen, organic matter, total phosphorus (TP), AP, alkali hydrolyzable nitrogen, and available potassium were determined using the Kjeldahl method, potassium dichromate capacity–external heating method, H2SO4-HCl O4 digestion–molybdenum–antimony resistance colorimetric method, Olsen method, alkali hydrolysis diffusion method, and NH4OAc extraction–flame photometric method [14]. Soil pH was determined using a PHSJ-4F pH meter (Shanghai instrument Electric Scientific instrument Co., Ltd., Shanghai, China) (soil–water ratio of 1:5). Soil ammonium nitrogen and nitrate nitrogen were extracted with 1 mol·L−1 KCl, and determined using a continuous flow injection analyzer (AMS Alliance Futura).
2.4.2. DNA Extraction and PCR Amplification of the Soil Samples
- (1)
- DNA extraction
DNA was extracted using a TGuide S96 Magnetic Soil/Stool DNA Kit (Tiangen Biotech (Beijing, China) Co., Ltd.) according to manufacturer instructions. The DNA concentration of the samples was measured using the Qubit dsDNA HS Assay Kit and Qubit 4.0 Fluorometer (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA).
- (2)
- Amplicon sequencing
The 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) universal primer set was used to amplify the V3–V4 region of the 16S rRNA gene from the genomic DNA extracted from each sample. Both the forward and reverse 16S primers were tailed with sample-specific Illumina index sequences to allow for deep sequencing. PCR was performed in a total reaction volume of 10 μL consisting of DNA template (5–50 ng), 0.3 μL *Vn F (10 μM), 0.3 μL *Vn R (10 μM), 5 μL KOD FX Neo Buffer, 2 μL dNTP (2 mM each), 0.2 μL KOD FX Neo, and ddH2O. Vn F and Vn R were selected according to the amplification area. Thermal cycling started with initial denaturation at 95 °C for 5 min, followed by 25 cycles of denaturation at 95 °C for 30 s, annealing at 50 °C for 30 s, and extension at 72 °C for 40 s, and a final extension step at 72 °C for 7 min. PCR amplicons were purified with Agencourt AMPure XP Beads (Beckman Coulter, Indianapolis, IN, USA) and quantified using the Qubit dsDNA HS Assay Kit and Qubit 4.0 Fluorometer (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). After quantifying each sample, the amplicons were pooled together in equal amounts. The library was sequenced using Illumina novaseq 6000 (Illumina, Santiago, CA, USA).
- (3)
- Bioinformatic Analysis
Bioinformatics was performed using BMK Cloud (Biomarker Technologies Co., Ltd., Beijing, China). Raw data were primarily filtered using Trimmomatic [1] (version 0.33) based on the quality of each nucleotide. Primer sequences were identified and removed using Cutadapt [2] (version 1.9.1). PE reads obtained from previous steps were assembled using USEARCH [5] (version 10). Chimeras were removed using UCHIME [4] (version 8.1). The high-quality reads generated from the above steps were used in subsequent analysis. Sequences with similarity values ≥97% were clustered into the same operational taxonomic unit (OTU) using USEARCH [1] (v10.0), and the OTUs with relative abundance <0.005% were filtered out. OTUs were annotated taxonomically using the naïve Bayes classifier in QIIME2 [3] and the SILVA database [4] (release 132) with a confidence threshold of 70%. Alpha diversity was calculated and displayed using QIIME2 and R software, respectively. Beta diversity was determined to evaluate the degree of similarity among microbial communities from different samples using QIIME. Principal coordinate analysis (PCoA), heat maps, UPGMA, and nonmetric multidimensional scaling were used to analyze the beta diversity. Furthermore, we employed linear discriminant analysis (LDA) effect size (LEfSe [5]) to assess the taxonomic differences among groups. A logarithmic LDA score of 4.0 was set as the threshold for discriminating between groups. The levels of dissimilarity among the microbiomes of different factors were assessed by performing redundancy analysis (RDA) in R using the package “vegan”.
2.5. Statistical Analysis
The data were processed using Microsoft Excel 2021, and the differences in soil physical and chemical properties, bacterial community composition, and diversity index were tested using one-way ANOVA and Dunn–Sidak test. Pearson correlation analysis was performed using SPSS 26.0. After 999 random Monte Carlo replacements, beta diversity of the soil bacterial community was analyzed using the ANOSIM test, and RDA was conducted using Canoco 5.0. At a 95% confidence level, AMOS V22.0 was used to construct a structural equation model (SEM) to analyze the hypothetical causal relationship, significance, and effect between soil physical and chemical properties and bacterial community structure and diversity under the influence of different long-term fertilization methods. The libraries of the R environment in the random forest package were used for random forest analysis. Origin 2021 was used to draw.
3. Results and Analyses
3.1. Soil Properties and Potato Productivity under Different Fertilizer Treatments
As shown in Table 2, different organic fertilizer treatments significantly affected the physicochemical properties of the soil and potato productivity. Soil pH increased to 8.34 and 8.41 in the conventional (NPK) and organic–inorganic treatments (NPKM), respectively, compared with that in the CK treatment. The content of soil nutrients, including SOM, TN, and TP, increased significantly (p < 0.05) in different fertilizer treatments (NK, NPK, and NPKM), and the trend followed the order of NPKM > NPK > NK > CK. The SOM content in the NK, NPK, and NPKM treatments increased by 2.96, 3.17, and 4.39 times, the AN content increased by 0.47, 0.67, and 1.18 times, and the AP content increased by 0.04, 1.50, and 2.64 times, respectively, compared with those in the CK treatment. In addition, with the increase in organic fertilizer application, potato productivity significantly increased, and potato productivity under NPK and NPKM treatments increased by 0.89 and 1.26 times, respectively, compared with that in the CK treatment.

Table 2.
Soil chemical properties and potato productivity under different organic fertilization treatments.
3.2. Characteristics of Soil Bacterial Community under Different Fertilizer Treatments
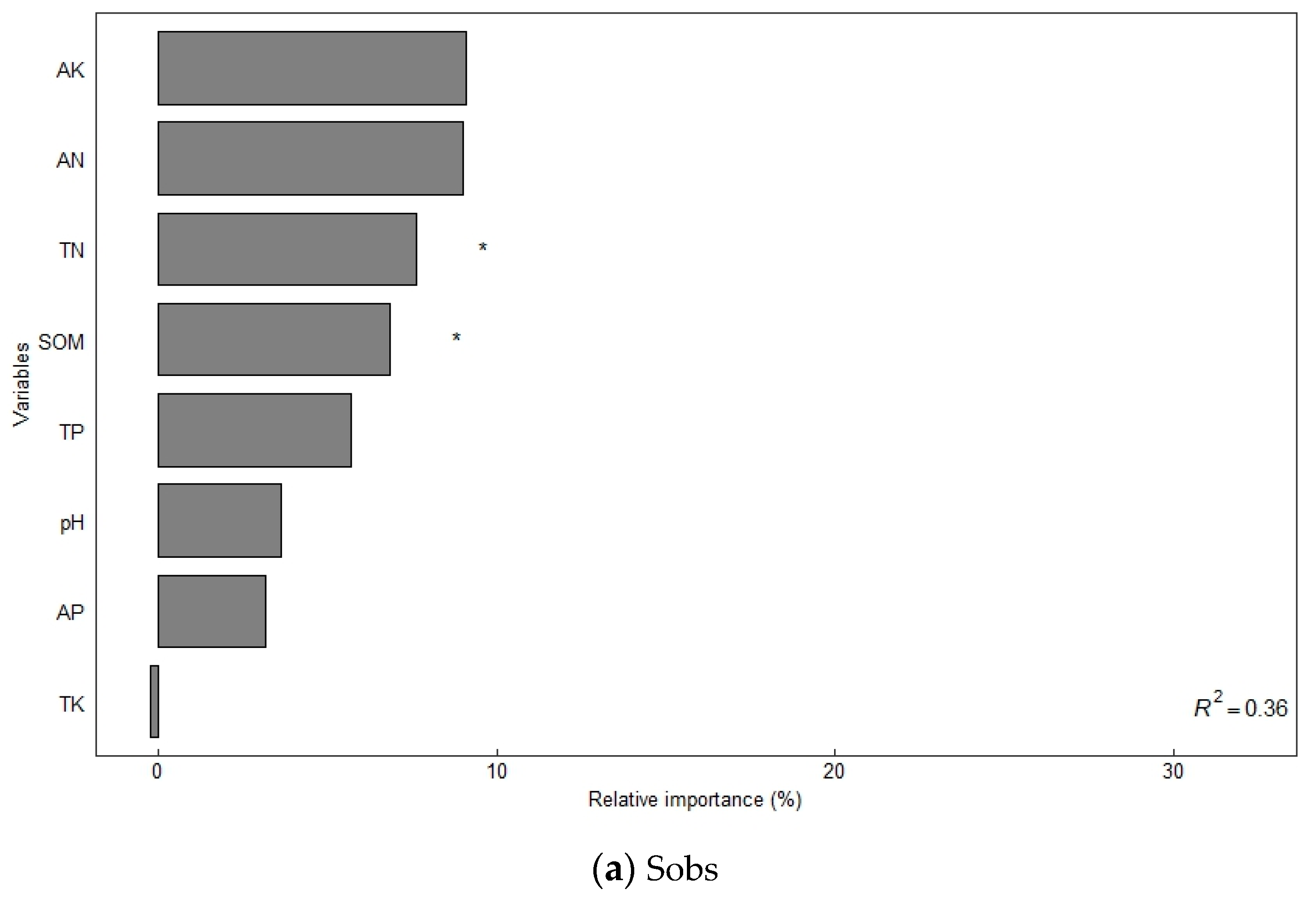
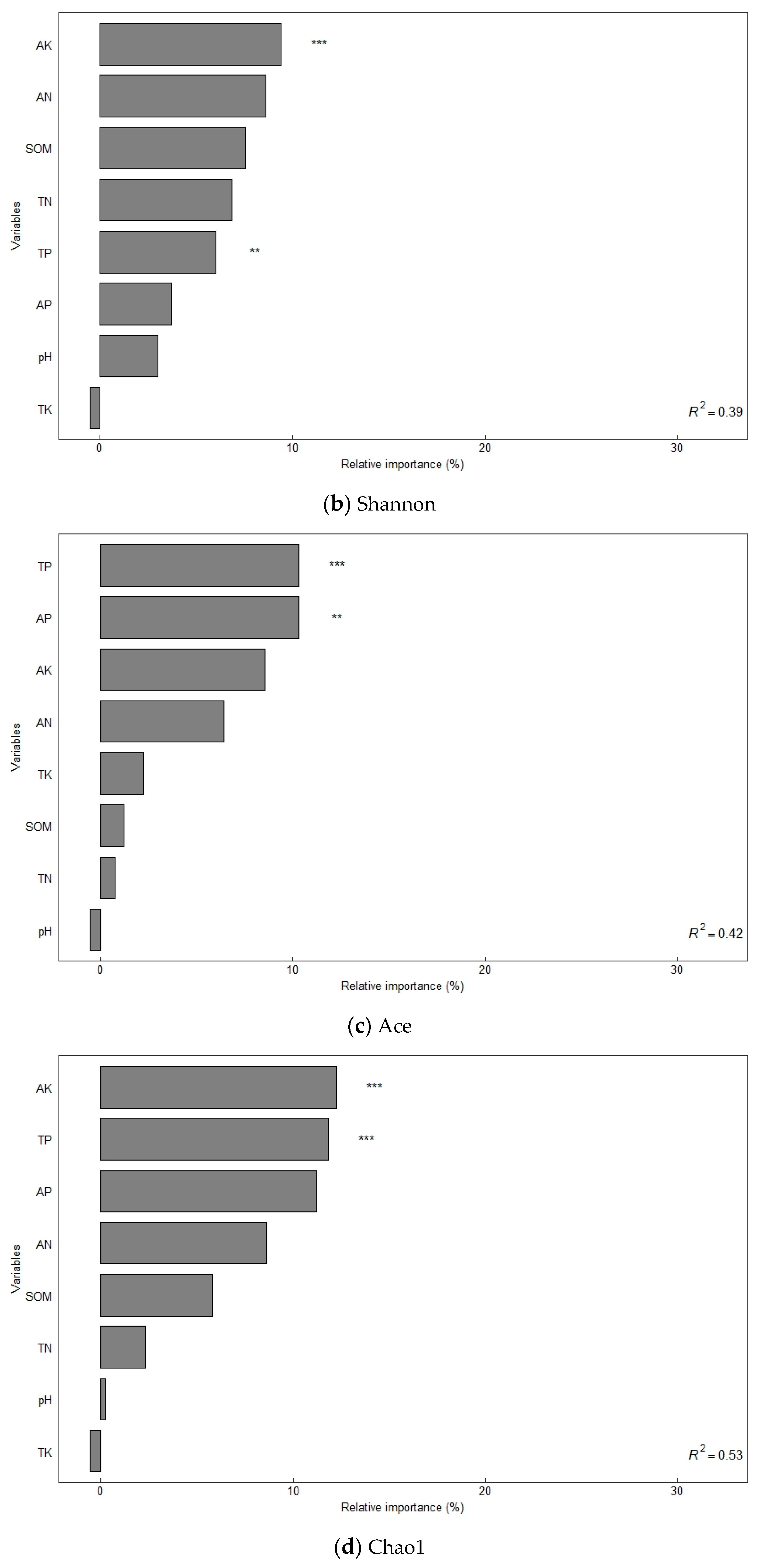
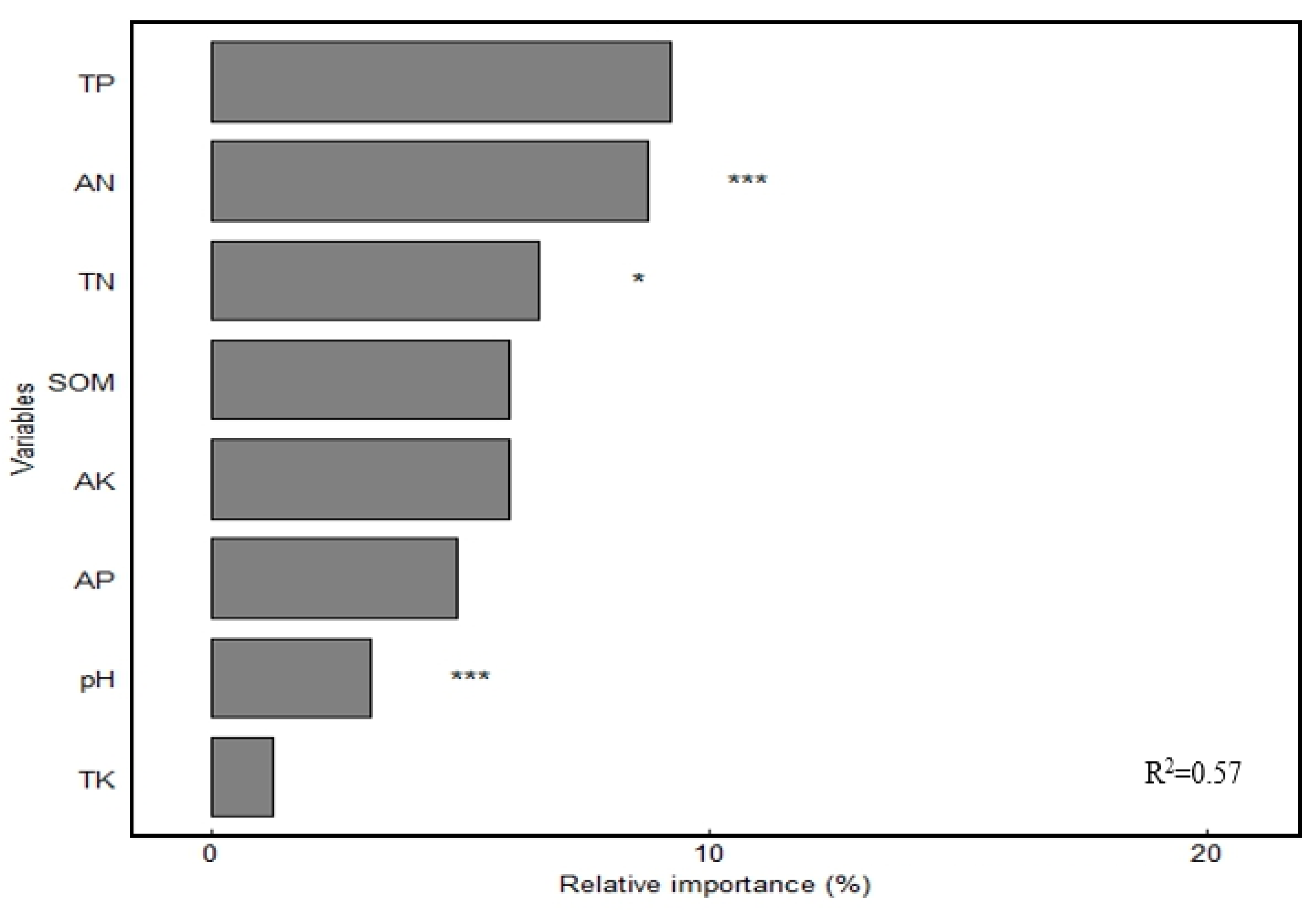
The alpha diversity of soil bacterial communities increased significantly under different organic fertilizer treatments (Table 3). The Sobs, Shannon, Ace, and Chao1 indices of soil bacteria were significantly higher in the NK, NPK, and NPKM treatments from those in the CK treatment (p < 0.05), and the trend of the changes followed the order of NPKM > NPK > NK > CK. A random forest model was used to assess the effect of environmental factors on soil bacterial diversity (increased mean square value, Figure 2), and the results showed that SOM (7.31–8.17%) and soil TN (8.59–8.67%) were the main variables determining the soil bacterial Sobs index (p < 0.05). Soil TP (7.32–12.49%) and AK (9.95–13.21%) were the main variables that determined the Shannon and Chao1 indices of soil bacteria (p < 0.05). Soil TP (11.05%) and AP (11.21%) were the main variables that determined the Ace index of soil bacteria (p < 0.05).

Table 3.
Soil bacterial diversity under different organic fertilizer treatments.
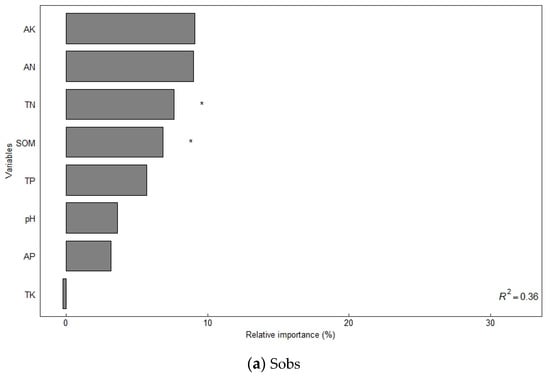
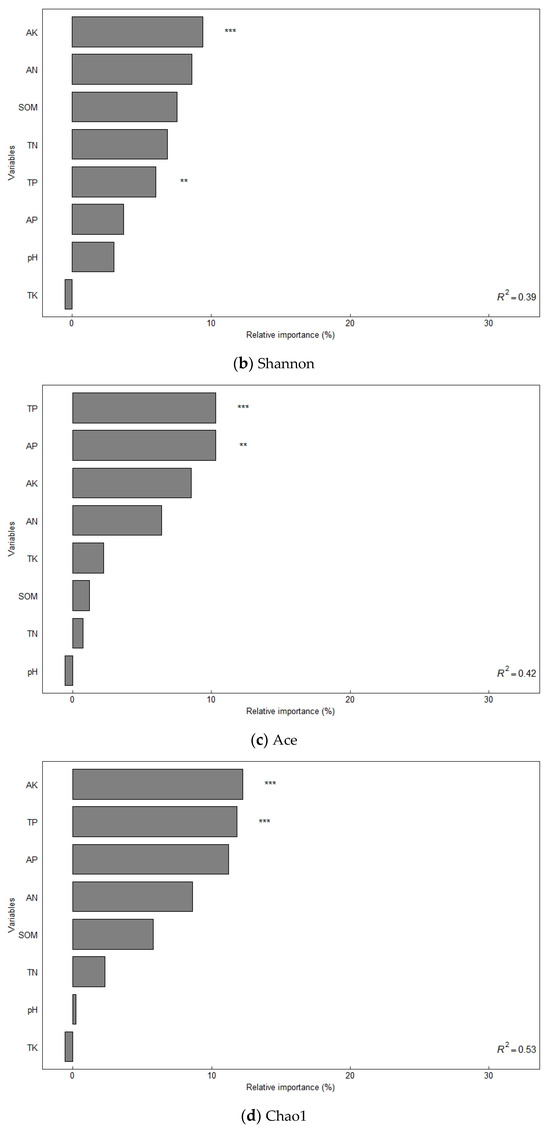
Figure 2.
Factors influencing soil bacterial diversity determined using random forest modeling analysis. Notes: * Significant correlation at the p < 0.05 level; ** significant correlation at the p < 0.01 level; *** significant correlation at the p < 0.001 level.
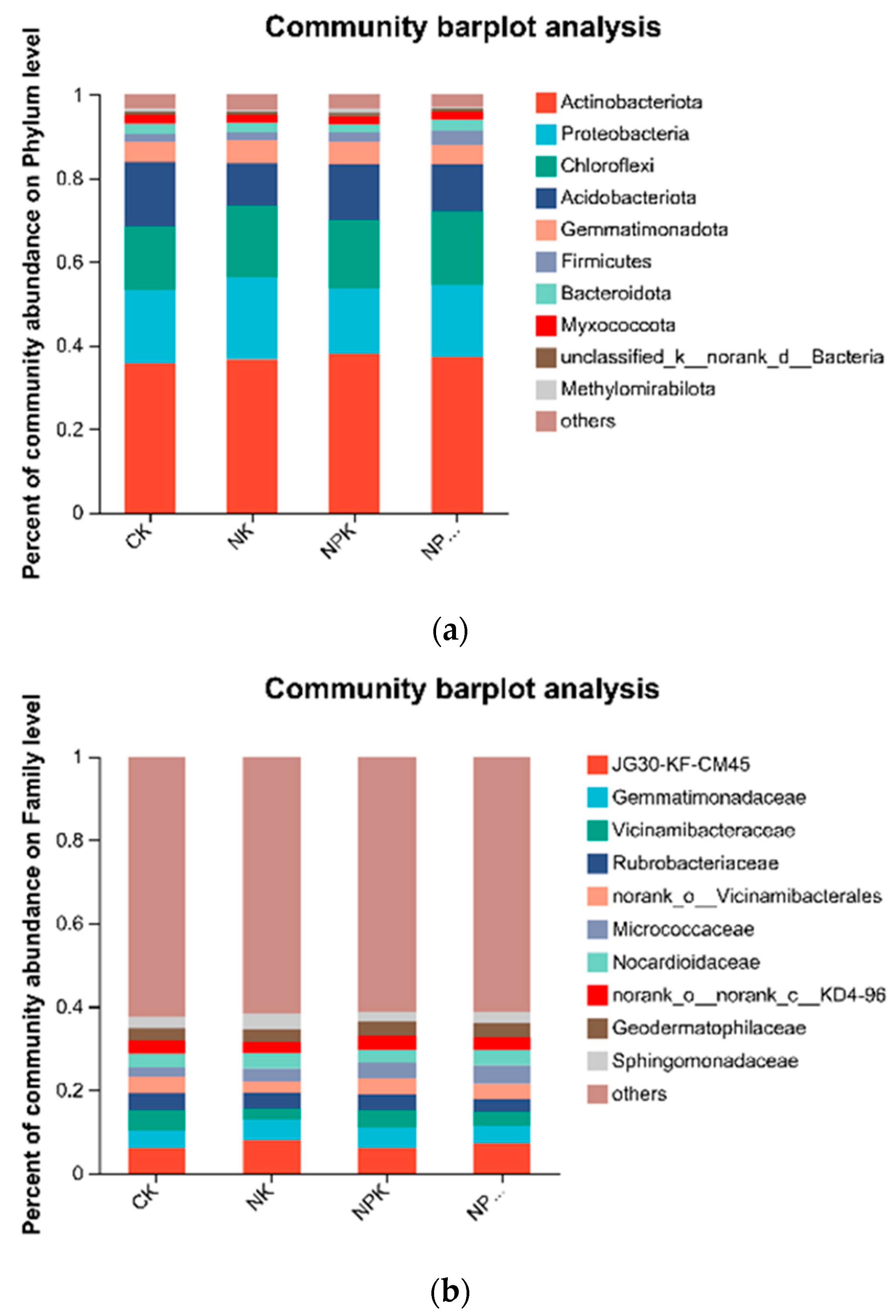
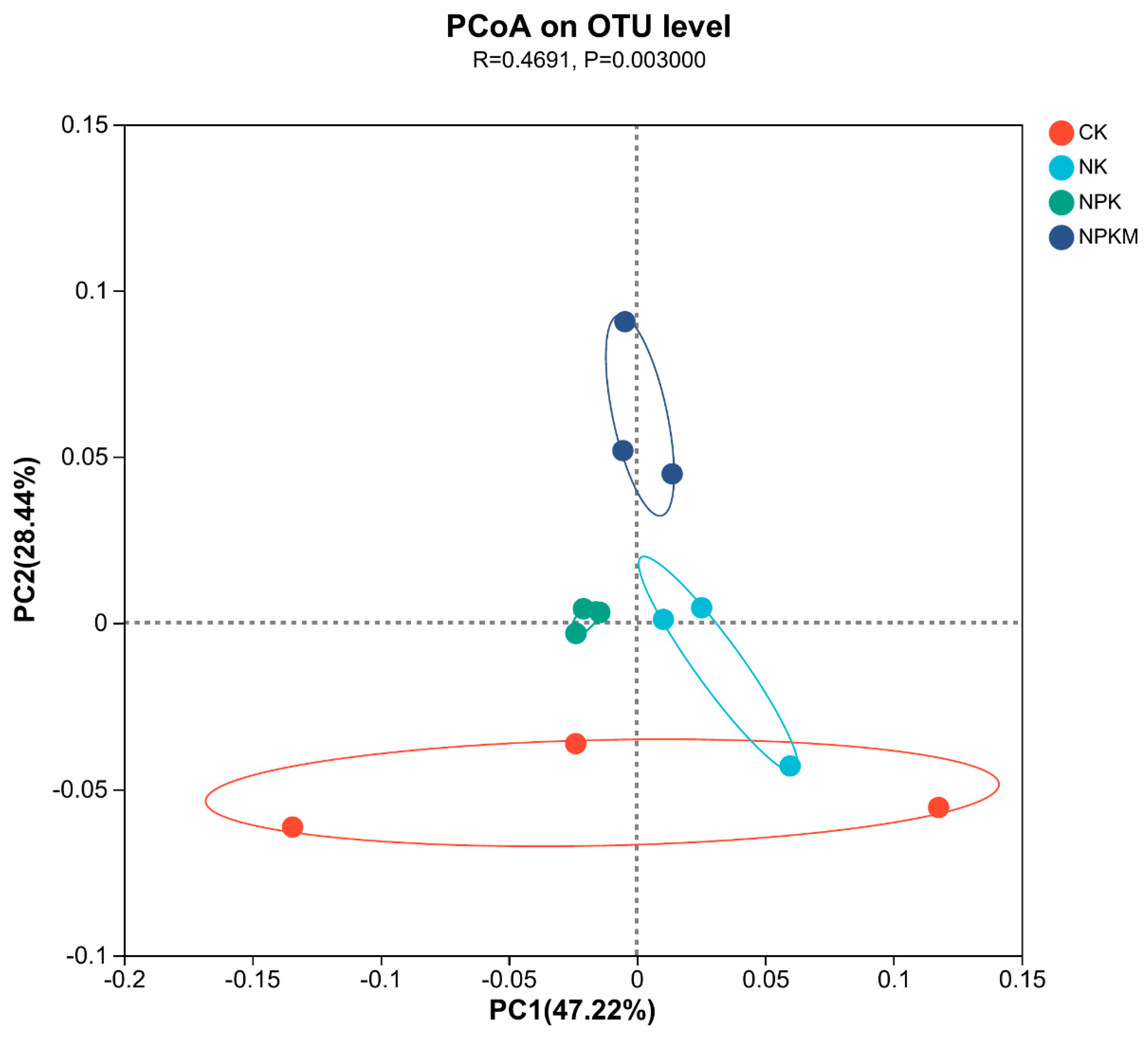
High-throughput sequencing results showed that Actinobacteriota, Proteobacteria, Chloroflexi, and Acidobacteriota were the dominant soil bacterial phyla, with relative abundance of 36.85%, 17.48%, 16.64%, and 12.61%, respectively (Figure 3a). The relative abundance of Acidobacteria significantly decreased (p < 0.05), whereas that of Actinobacteriota and Chloroflexi significantly increased (p < 0.05) under fertilizer treatments. The greatest increase of 7.89% was observed for Actinobacteriota in the NPK treatment compared with that in the CK treatment and for Chloroflexi in the NPKM treatment (7.89%). Chloroflexi had the highest increase of 13.36% in the treatment. JG30-KF-CM45, Gemmatimonadaceae, Vicinamibacteraceaei, Rubrobacteriaceae, norank_o_ Vicinamibacterales, and Micrococcaceae were the dominant soil bacterial families, and their relative abundance was 6.70%, 4.54%, 4.03%, 3.62%, 3.61%, and 3.35%, respectively (Figure 3b). The relative abundance of Vicinamibacteraceae and norank_o_Vicinamibacterales decreased significantly (p < 0.05) under different fertilizer treatments, whereas that of Micrococcaceae increased significantly (p < 0.05) and was highest in the NPKM treatment. Principal coordinate analysis (PCoA) revealed (Figure 4) that soil bacterial community composition differed significantly among the four organic fertilizer treatments (r2 = 0.52, p < 0.001), with the PCoA1 and PCoA2 axes explaining 47.22% and 28.44% of the variation in soil bacterial communities, respectively. The random forest model was used to assess the mean squared difference values of the increased contribution of environmental factors to the effect of bacterial community composition (Figure 5). The results showed that soil AN (9.61%) and TN (8.69%) were the main variables determining soil bacterial composition (p < 0.05).
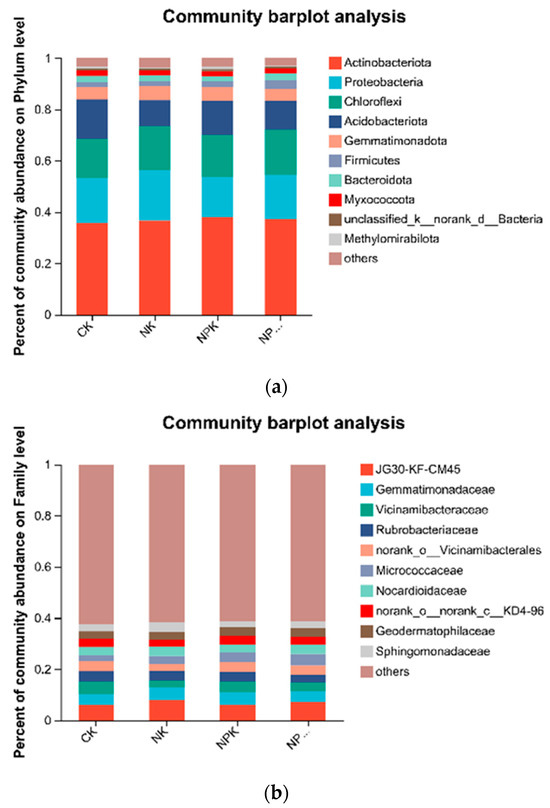
Figure 3.
Relative abundance of the dominant bacterial phylum and family under different fertilizer treatments, (a) Relative abundance of the dominant bacterial phylum under different fertilizer treatments; (b) Relative abundance of the dominant bacterial family under different fertilizer treatments.
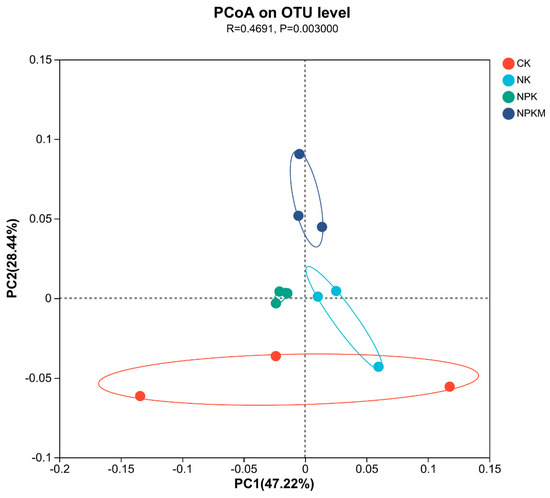
Figure 4.
Primary coordinate analysis (PCoA) of the soil bacterial community.
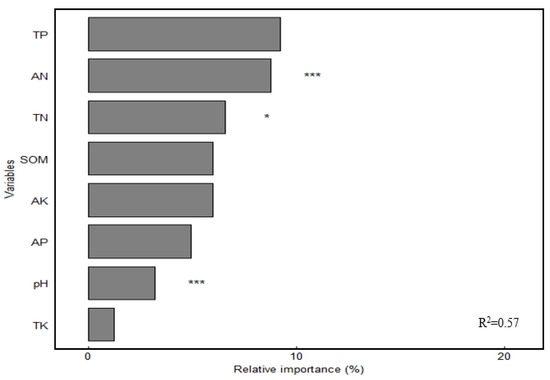
Figure 5.
Random forest modeling analysis of the soil bacterial community. Notes: * Significant correlation at the p < 0.05 level; *** significant correlation at the p < 0.001 level.
3.3. Relationships among Soil Chemical Properties, Bacterial Communities, and Potato Productivity
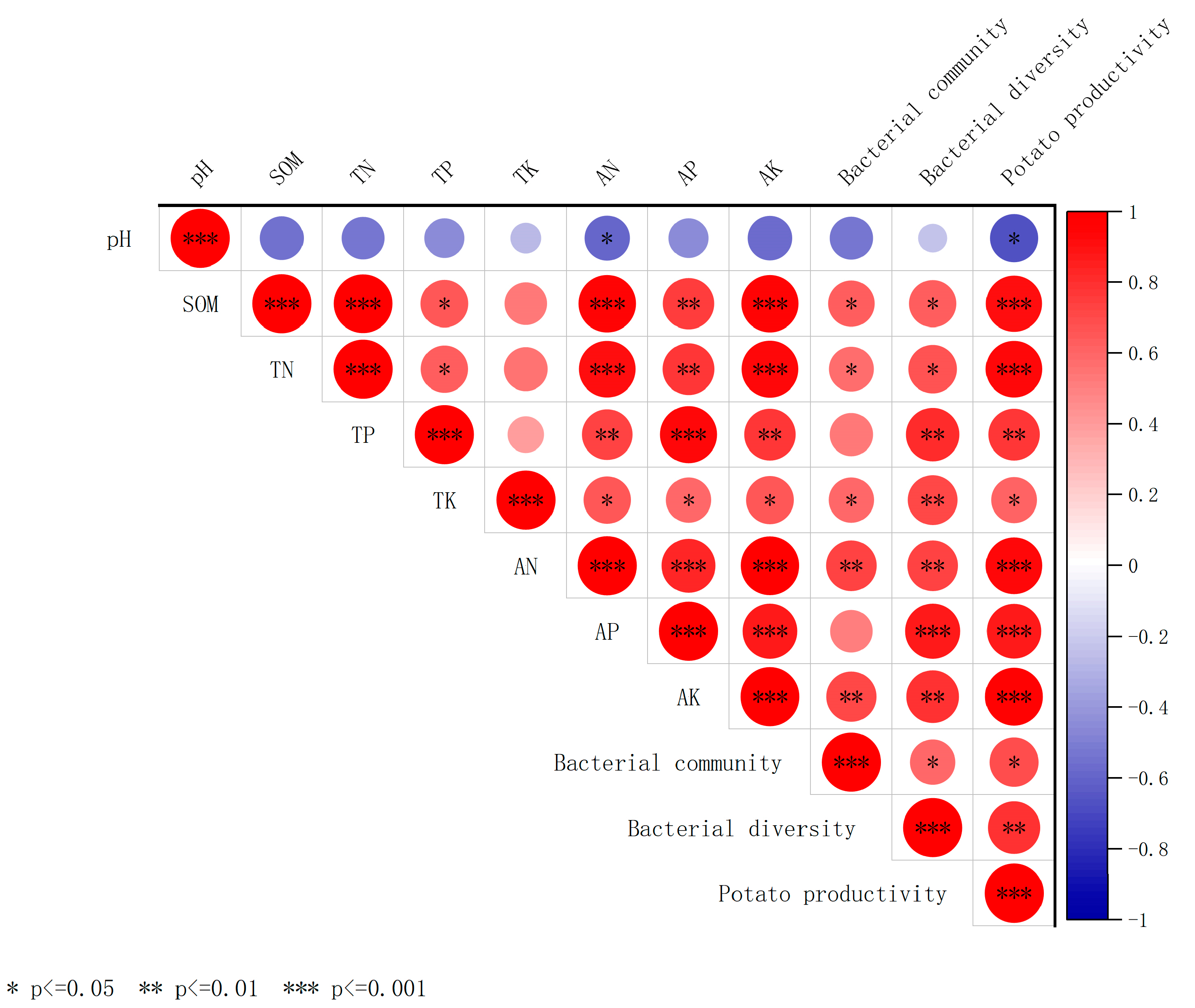
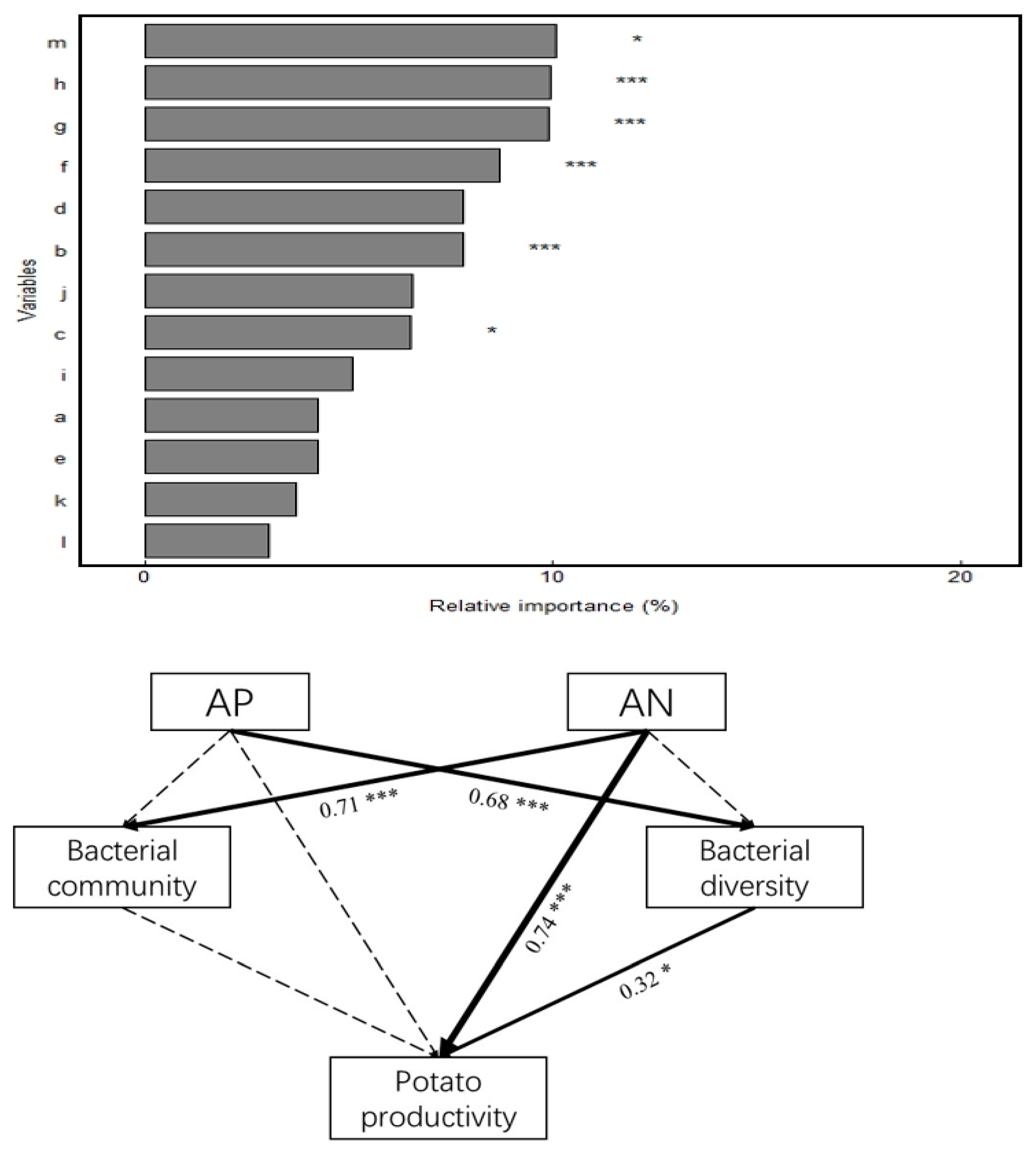
Correlation analysis (Figure 6) revealed that soil SOM, TN, pH, and TP were significantly or highly significantly positively correlated with bacterial community composition (r = 0.58–0.73, p < 0.01). In addition, soil bacterial diversity was positively correlated with SOM (r = 0.48) and TN (r = 0.36) at the p < 0.05 level; with TP, TK, AN, AP, and AK at the p < 0.01 level; and with AP at the p < 0.001 level. Potato productivity correlated positively and highly significantly (p < 0.001) with SOM, TN, AN, AP, and AK and positively significantly (p < 0.05) with TK and TP. Random forest analysis of the significance of potential factors affecting potato productivity (increased mean square value, Figure 7) showed a highly significant effect (p < 0.001) of SOM (9.14%), soil AN (9.46%), AP (10.15%), and AK (10.17%) on potato productivity. The bacterial diversity Chao1 index significantly affected potato productivity (p < 0.05). Soil physicochemical properties (AP and AN), bacterial community composition and diversity indices, and potato productivity were screened to construct an SEM using correlation and random forest analyses (Figure 7). The results showed that AN (path coefficient of 0.74, p < 0.001) had a direct positive effect on potato productivity, whereas it indirectly affected potato productivity by influencing soil bacterial diversity. Moreover, AN (path coefficient of 0.71, p < 0.001) had a significant positive effect on soil bacterial community structure. Furthermore, bacterial diversity (path coefficient of 0.32, p < 0.05) showed significant positive effects on potato productivity. Therefore, soil properties (AN and AP) can directly and indirectly affect potato productivity by changing soil bacterial diversity and community composition.
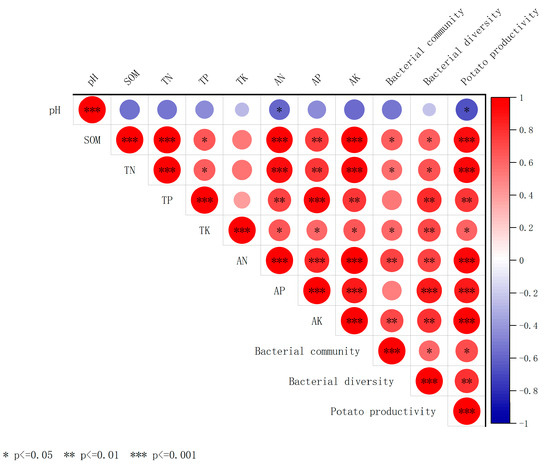
Figure 6.
Correlations among soil chemical properties, diversity, and composition of soil bacterial communities, and potato productivity. Notes: Red indicates positive correlation, blue indicates negative correlation, and the size of the circular area indicates the size of the correlation; * p < 0.05, ** p < 0.01, *** p < 0.001.
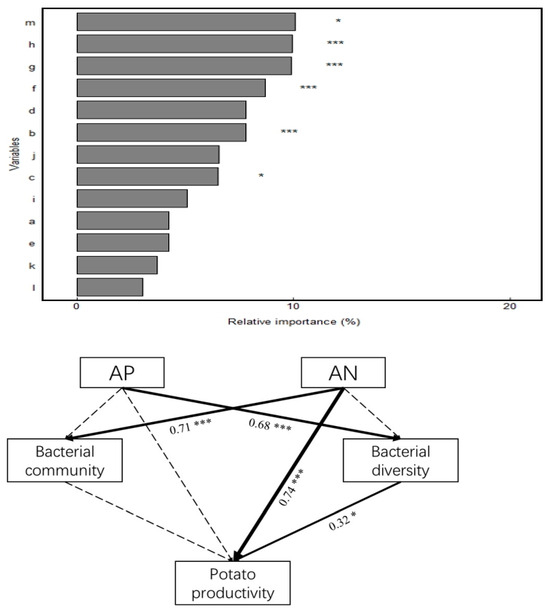
Figure 7.
Effects of soil chemical properties and bacterial communities on maize productivity based on random forest analysis and structural equation modeling. a. pH, b. SOM, c. TN, d. TP, e. TK, f. AN, g. AP, h. AK, i. Bacterial community, j. Sobs, k. Shannon, l. Ace, m. Chao1; the numbers next to the arrows indicate the path coefficients, and the thickness of the lines indicate the magnitude of the correlation, with a solid line indicating a significant correlation and a dashed line indicating an insignificant one. indicates nonsignificant. Notes: * Significant correlation at the p < 0.05 level; *** significant correlation at the p < 0.001 level.
4. Discussion
Organic fertilization can effectively improve soil fertility while stabilizing and increasing the productivity of cultivated land [15]. Different fertilization methods can change the soil’s nutrient content and pH value. For example, excessive use of chemical fertilizers leads to the accumulation of soil nitrogen, which aggravates the risk of soil acidification, a process that destroys the acid–base balance of the soil, inhibits the activity of soil microorganisms, and reduces the effectiveness of soil nutrients. This study found that 18 years of fertilizer use had decreased the soil pH significantly, corresponding to a decrease of 0.035 pH units per year compared to the start of the experiment in 2004. This decrease in pH value is beneficial, as it improves both the availability of soil nutrients and crop productivity in the region. Eighteen years of continuous fertilization has increased the content of SOM, TN, and available nutrients in the farmland soil of the region; moreover, it has improved the soil’s ability to supply nutrients to crops, resulting in high and stable yields. Among all fertilization methods, the combination of chemical and organic fertilizers has resulted in the most improved soil, leading to increased crop productivity. The organic fertilizer used in this study was sheep manure. Sheep manure is a relatively abundant organic fertilizer resource in northern China [16] and is rich in organic matter and nutrients, such as nitrogen, phosphorus, and potassium. Furthermore, it can effectively increase soil fertility, improve soil water-holding capacity [17], accelerate organic matter transformation, and promote crop yield increase [18]. In addition, the acidic substances produced by the decomposition of sheep manure play a role in balancing the soil pH [19], preventing soil from becoming too acidic or too alkaline, and promoting crop yield increase [20]. Bohoussou et al. found that the combined application of chemical fertilizers and organic fertilizers can increase the content of SOM and TN in the soil, but it needs to be optimized according to soil type and regional climate conditions [21]. The fertilization treatments in this study had a positive effect on soil fertility, and the combined application of chemical fertilizers and organic fertilizers had the best improvement effect, which may be due to the long-term fertilizer effect of organic fertilizers, which makes the soil nutrient supply sufficient for crop growth and yield stability [22]. Mubeen et al. pointed out that organic fertilizers can effectively increase the AP content in soil and significantly improve crop productivity [23], which is consistent with the results of this study. Furthermore, in calcareous soils of the north, where the AP content is low, the combined application of chemical and organic fertilizers can promote crop productivity by increasing the AP content. The content of available nitrogen (AN) in the soil also has a significant impact on crop productivity [24], but the mineralization rate of nitrogen in organic fertilizers is relatively slow. Long-term and continuous application of organic fertilizers can significantly increase the nitrogen level in the soil to support crop growth and development and increase crop yield [25].
The long-term and large-scale use of traditional chemical fertilizers can negatively impact soil bacterial diversity [26]. Although chemical fertilizers rapidly provide nutrients for crop growth, which is beneficial in the short term [27], these fertilizers disrupt the ecological balance of the soil in the long run, leading to a decrease in bacterial diversity. In contrast, organic fertilizers, which are rich in organic matter and nutrients, provide a suitable living environment for soil microorganisms, thereby promoting the increase in bacterial diversity [28,29]. In contrast to the results of previous studies, we found that both conventional chemical fertilization and the combination of chemical and organic fertilization can significantly increase the Sobs, Ace, and Chao indices of soil bacteria. However, under phosphorus deficiency (NK), soil bacterial alpha diversity was lower and not significantly different from that of the CK treatment. This may be due to the low soil phosphorus level, which tends to suppress soil bacterial diversity. Furthermore, low levels of soil fertility and phosphorus fertilizer input tend to intensify competition among soil bacteria, leading to the enrichment of some dominant bacterial populations in the soil, e.g., bacteria adapted to low-phosphorus environments [30]. These dominant populations can further reduce both the levels of the less competitive populations and the overall diversity of the soil bacterial community. Our results are partially consistent with those of previous reports that show that the addition of organic fertilizers significantly increases the TP content in the soil and the relative abundance of Chloroflexi. This group of bacteria participates in the decomposition of insoluble phosphorus in the soil, thereby improving its phosphorus supply capacity [31]. However, our results differed because they also showed that the soil phosphorus pool increased after 18 years of continuous application of organic fertilizers and its turnover was promoted, which stimulated the growth and reproduction of phosphorus-solubilizing bacteria. These bacteria convert insoluble organic phosphorus in the soil into soluble phosphate that can be absorbed by crops, thereby indirectly affecting potato productivity. In addition, we found that different fertilization treatments reshaped the soil bacterial community structure, and the combined application of chemical and organic fertilizers significantly increased the relative abundance of Actinobacteriota and Chloroflexi. These changes may be due to the significant increase in AP content in the soil and the change in C/P ratio after the addition of sheep manure, both of which promoted the changes in soil bacterial diversity [32]. The level of nitrogen in the dryland farmland of northern China is low, and the problem of nitrogen loss is serious [33]. The level of soil nitrogen is an important factor driving the changes in the composition of soil microbial communities in the farmlands of northern China [34]. These findings were consistent with our conclusion. However, our analysis also found that the AN content in the soil directly affects the soil bacterial community structure and crop productivity, although there is no direct relationship between the soil bacterial community structure and crop productivity.
5. Conclusions
Eighteen years’ continuous fertilization significantly increased the content of SOM, AN, and AP in the soil of the agricultural–pastoral interlaced belt in northern China. Among all fertilization treatments, the combination of chemical fertilizers with organic fertilizers (NPKM) demonstrated the best effect on improving soil fertility and potato productivity. The addition of organic fertilizers significantly affected the diversity and community structure of soil bacteria. AP and AN are the determining variables for soil bacterial diversity and community structure. Soil AP can significantly positively affect potato productivity by changing soil bacterial diversity. The findings of this study provide a scientific basis for improving soil fertility, establishing long-term management measures for organic fertilization, and achieving an increase in arable land productivity.
Author Contributions
Conceptualization, J.L. and X.S.; methodology, J.L.; software, T.Z.; validation, J.Z.; formal analysis, J.H.; investigation, D.H.; literature retrieval, H.L.; data curation, H.A.; writing—original draft preparation, J.L.; writing—review and editing, J.L. and P.Z.; visualization, X.S.; supervision, S.Z.; project administration, J.Z.; funding acquisition, J.L.; data acquisition, T.Z.; analysis/interpretation, H.A. and D.H.; charts, J.H.; data collection, H.L.; concept proposition, S.Z; research design, J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Inner Mongolia Agriculture and Animal Husbandry Innovation Youth Fund Project (2023QNJJN10), Inner Mongolia Natural Science Foundation Project (2020MS03087), Inner Mongolia Agricultural and Animal Husbandry Innovation Fund Project (2023CXJJN20), National Key Research and Development Plan Project (2023YFD1900505-03), National Soil Quality Wuchuan Observation Test Station, and Inner Mongolia Key Laboratory of Dryland Farming, Hohhot 010031, China.
Data Availability Statement
The data are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dang, H.; Rong, L.; Li, Y.; Zhao, M. Spatiotemporal evolution characteristics and influencing factors of production-living-ecological spaces in the farming-pastoral ecotone: Taking Hohhot of Inner Mongolia as an example. Arid Zone Res. 2023, 40, 1698–1706. [Google Scholar]
- Pan, Y.; Tang, H.; Fang, F.; Ma, Y.; Chen, Z. Is elemental stoichiometry (C, N, P) of soil and soil microbial biomass influenced by management modes and soil depth in the agro-pastoral transitional zone of northern China? J. Soils Sediments 2023, 23, 32–48. [Google Scholar] [CrossRef]
- Bai, Y.C.; Chang, Y.Y.; Hussain, M.; Lu, B.; Zhang, J.P.; Song, X.B.; Lei, X.S.; Pei, D. Soil Chemical and Microbiological Properties Are Changed by Long-Term Chemical Fertilizers That Limit Ecosystem Functioning. Microorganisms 2020, 8, 694. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Cotton, A.; Wei, Z.; Xia, Y.; Daniell, T.; Yan, X. How does partial substitution of chemical fertilizer with organic forms increase sustainability of agricultural production? Sci. Total Environ. 2022, 803, 149933. [Google Scholar] [CrossRef] [PubMed]
- Goyer, C.; Neupane, S.; Zebarth, B.J.; Burton, D.L.; Wilson, C.; Sennett, L. Diverse compost products influence soil bacterial and fungal community diversity in potato crop production systems. Appl. Soil Ecol. 2022, 169, 104247. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Rajput, V.D.; Kumari, A.; Espinosa-Saiz, D.; Menendez, E.; Minkina, T.; Dwivedi, P.; Mandzhieva, S. Plant growth-promoting rhizobacteria: A potential bio-asset for the restoration of degraded soil and crop productivity using sustainable emerging techniques. Environ. Geochem. Health 2023, 45, 9321–9344. [Google Scholar] [CrossRef]
- Shi, G.; Sun, H.; Calderón-Urrea, A.; Li, M.; Yang, H.; Wang, W.; Su, G. Bacterial communities as indicators of soil health in a continuous crop** system. Land Degrad. Dev. 2021, 32, 2393–2408. [Google Scholar] [CrossRef]
- Wu, L.; Jiang, Y.; Zhao, F.; He, X.; Liu, H.; Yu, K. Increased organic fertilizer application and reduced chemical fertilizer application affect the soil properties and bacterial communities of grape rhizosphere soil. Sci. Rep. 2020, 10, 9568. [Google Scholar] [CrossRef]
- Liu, J.; Shu, A.; Song, W.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S.; et al. Long-term organic fertilizer substitution increases rice yield by improving soil properties and regulating soil bacteria. Geoderma 2021, 404, 115287. [Google Scholar] [CrossRef]
- Jia, L.I.U.; Chen, X.F.; Li, W.T.; Jiang, C.Y.; Meng, W.U.; Ming Li, U.; Li, Z.P. Bacterial diversity and community composition changes in paddy soils with different parent materials and fertility levels. J. Integr. Agric. 2021, 20, 2797–2806. [Google Scholar]
- Gupta, A.; Singh, U.B.; Sahu, P.K.; Paul, S.; Kumar, A.; Malviya, D.; Singh, S.; Kuppusamy, P.; Singh, P.; Paul, D.; et al. Linking soil microbial diversity to modern agricultural practices: A review. Int. J. Environ. Res. Public Health 2022, 19, 3141. [Google Scholar] [CrossRef] [PubMed]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and soil microbial communities: A review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Han, J.; Dong, Y.; Zhang, M. Chemical fertilizer reduction with organic fertilizer effectively improves soil fertility and microbial community in newly cultivated land on the Loess Plateau of China. Appl. Soil Ecol. 2021, 165, 103966. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agrochemical Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Oyetunji, O.; Bolan, N.; Hancock, G. A comprehensive review on enhancing nutrient use efficiency and productivity of broadacre (arable) crops with the combined use of compost and fertilizers. J. Environ. Manag. 2022, 317, 115395. [Google Scholar] [CrossRef]
- Jia, X.; Wang, Y.; Zhang, Q.; Lin, S.; Zhang, Y.; Du, M.; Chen, M.; Ye, J.; Wu, Z.; Wang, H. Reasonable deep application of sheep manure fertilizer to alleviate soil acidification and improve tea yield and quality. Front. Plant Sci. 2023, 14, 1179960. [Google Scholar] [CrossRef]
- Lal, B.; Sharma, S.C.; Meena, R.L.; Sarkar, S.; Sahoo, A.; Balai, R.C.; Gautam, P.; Meena, B.P. Use of byproducts of sheep farming as organic fertilizer for improving soil health and productivity of barley forage. J. Environ. Manag. 2020, 269, 110765. [Google Scholar] [CrossRef]
- Yang, Q.; Zheng, F.; Jia, X.; Liu, P.; Dong, S.; Zhang, J.; Zhao, B. The combined application of organic and inorganic fertilizers increases soil organic matter and improves the soil microenvironment in wheat-maize fields. J. Soils Sediments 2020, 20, 2395–2404. [Google Scholar] [CrossRef]
- Esilaba, A.O. KCEP-CRAL Integrated Soil Fertility and Water Management Extension Manual; Kenya Agricultural and Livestock Research Organization: Nairobi, Kenya, 2021. [Google Scholar]
- Xu, T.; Yi, S.; Zhou, Y.; Li, Q.; Liu, Y. Temporal and Spatial Changes and Driving Forces of Soil Properties in Subtropical Mountainous Areas from 2017 to 2020: A Case Study of Baokang County, Hubei Province, China. Land 2022, 11, 1735. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Chen, L.; Liang, J.; Huang, R.; Tang, X.; Zhang, X.; Wang, C. Partial substitution of chemical fertilizer with organic fertilizer over seven years increases yields and restores soil bacterial community diversity in wheat–rice rotation. Eur. J. Agron. 2022, 133, 126445. [Google Scholar] [CrossRef]
- Vairavan, C.; Thiyageshwari, S.; Malarvizhi, P.; Saraswathi, T.; Teli, K.G.; Pugazenthi, K. Impact of tnau-water soluble fertilizers (tnau-wsf) on soil nutrient availability, nutrient content and bulb quality of small onion (allium cepa var. aggregatum). Int. J. Plant Soil Sci. 2023, 35, 446–454. [Google Scholar] [CrossRef]
- Verma, B.C.; Pramanik, P.; Bhaduri, D. Organic fertilizers for sustainable soil and environmental management. In Nutrient Dynamics for Sustainable Crop Production; Springer: Singapore, 2020. [Google Scholar]
- Kc, B.; Pandey, B.; Chand, H.; Bhusal, P.; Pandit, S.; Khanal, S. Promotion of organic and sustainable agriculture through the use of bio-fertilizers. J. Wastes Biomass Manag. 2020, 3, 1–8. [Google Scholar]
- Ma, L.; Zhang, J.; Li, Z.; Xin, X.; Guo, Z.; Wang, D.; Li, D.; Zhao, B. Long-term phosphorus deficiency decreased bacterial-fungal network complexity and efficiency across three soil types in china as revealed by network analysis. Appl. Soil Ecol. 2020, 148, 103506. [Google Scholar] [CrossRef]
- Jiang, H.; Li, S.; Wang, T.; Chi, X.; Qi, P.; Chen, G. Interaction between halotolerant phosphate-solubilizing bacteria (Providencia rettgeri Strain TPM23) and rock phosphate improves soil biochemical properties and peanut growth in saline soil. Front. Microbiol. 2021, 12, 777351. [Google Scholar]
- Wei, X.; Zhu, Z.; Liu, Y.; Luo, Y.; Deng, Y.; Xu, X.; Ge, T. C:N:P stoichiometry regulates soil organic carbon mineralization and concomitant shifts in microbial community composition in paddy soil. Biol. Fertil. Soils 2020, 56, 1093–1107. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Hu, T.; Gao, Y.; Stewart, B. Nitrogen in dryland soils of China and its management. Adv. Agron. 2009, 101, 123–181. [Google Scholar]
- Wang, X.; Feng, J.; Ao, G.; Qin, W.; Han, M.; Shen, Y.; Liu, M.; Chen, Y.; Zhu, B. Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil Biol. Biochem. 2023, 179, 108982. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).