Response of Soil Fungal Community in Coastal Saline Soil to Short-Term Water Management Combined with Bio-Organic Fertilizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Soil Example Collection and Analysis
2.4. Soil DNA Extraction and High-Throughput Sequencing Analysis
2.5. Statistic Analyses
3. Results
3.1. Soil Physico-Chemical Characteristics
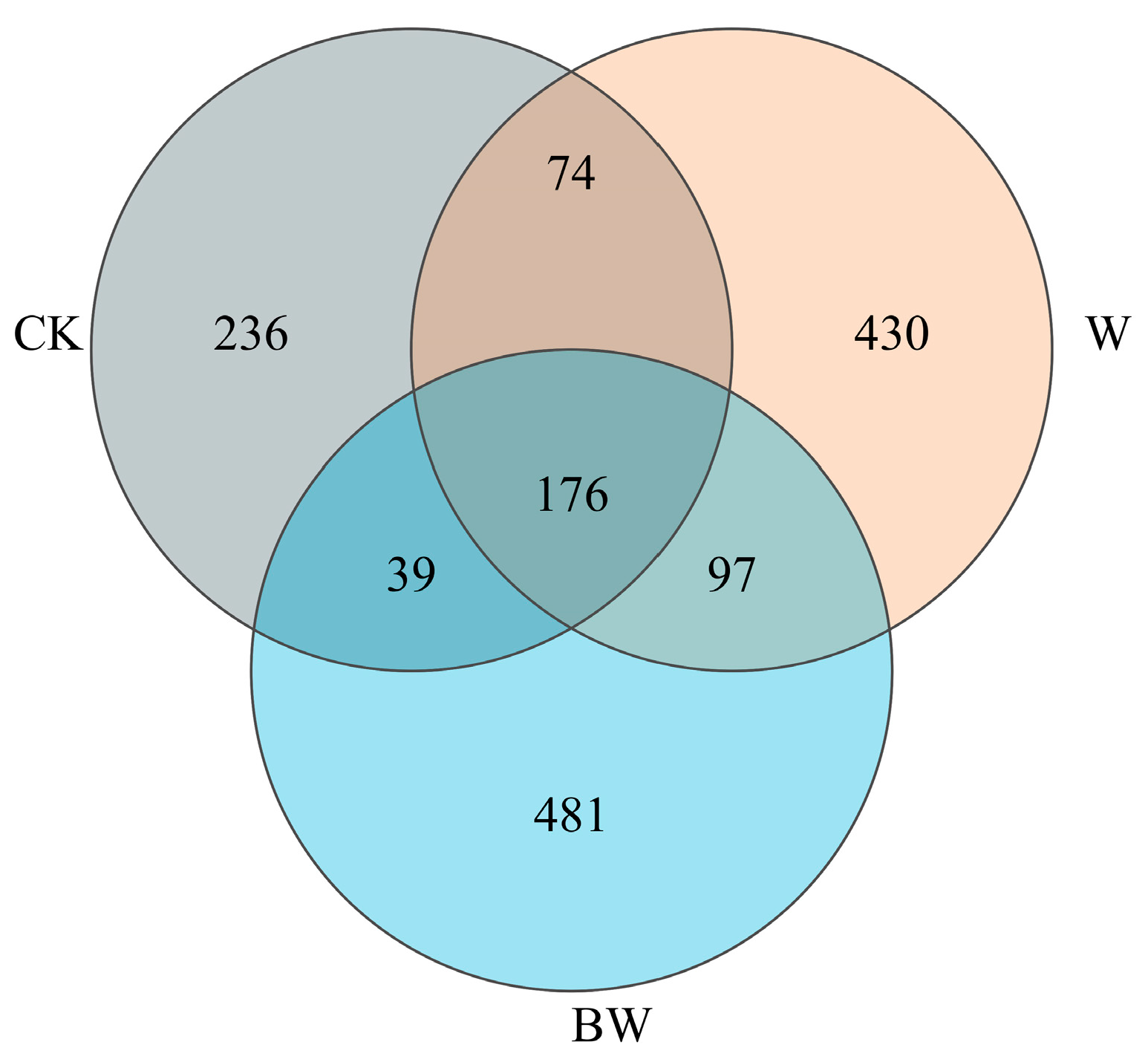
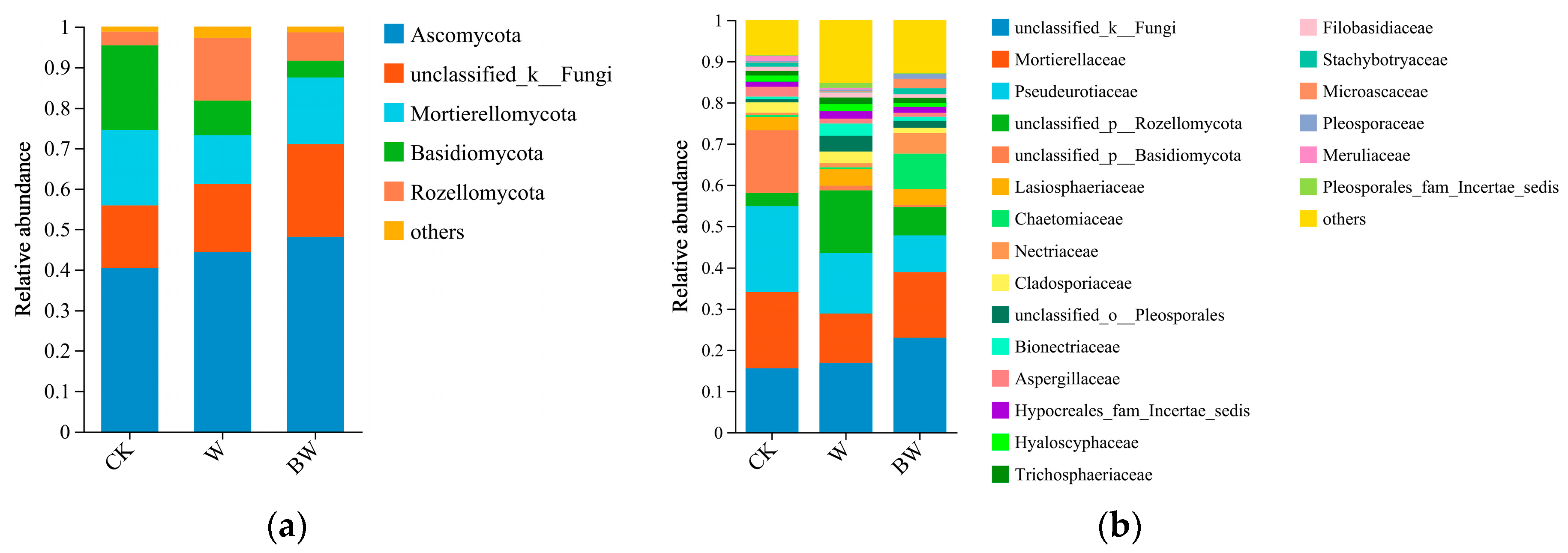
3.2. Soil Fungi Diversity and Community Structure
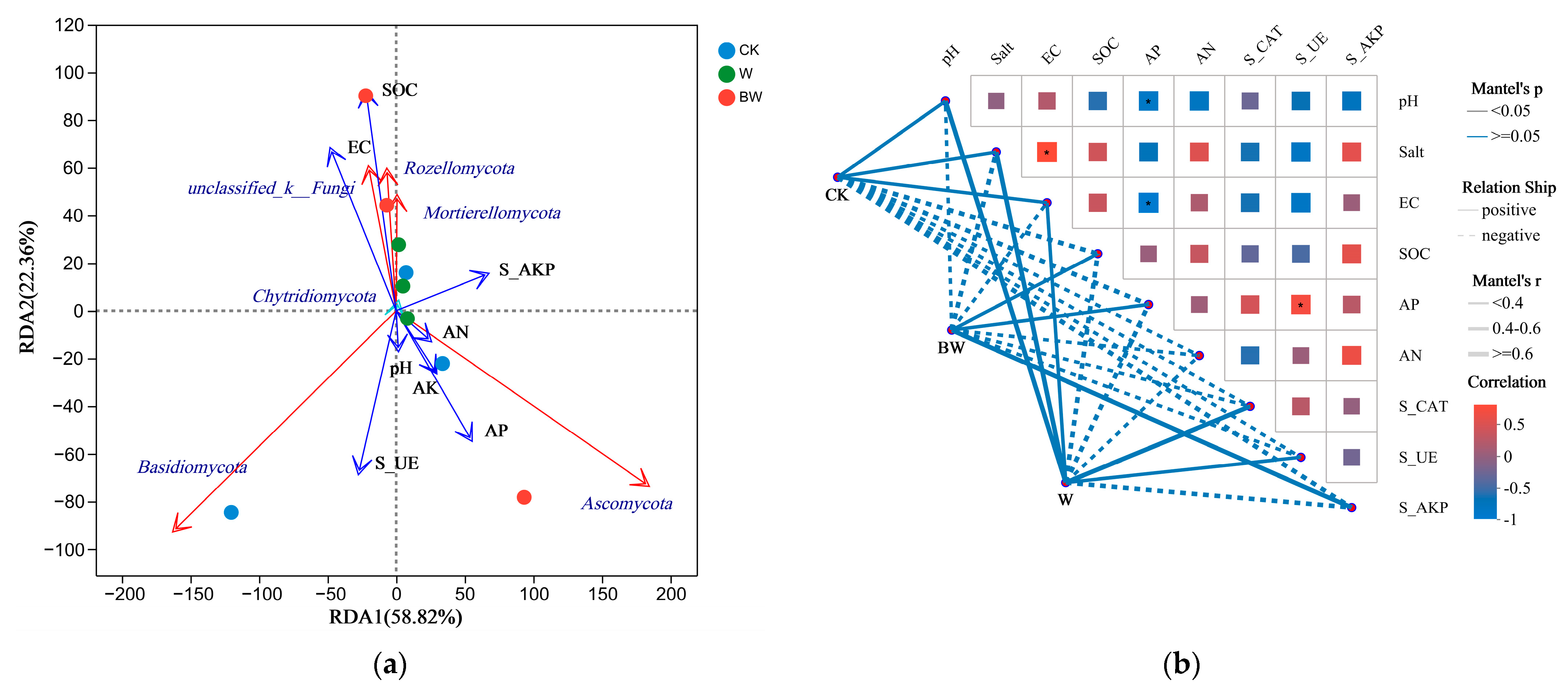
3.3. Relationship between Soil Fungi and Environment Factors
3.4. Functional Gene Prediction of Soil Fungi under Various Measures
4. Discussion
4.1. Effects of Drought to Water Combined with Biological Organic Fertilizer on Soil Physical and Chemical Properties
4.2. Responses of Soil Fungal Communities to Changes in Soil Environment
4.3. Prediction and Analysis of Key Factors and Functional Genes Affecting Soil Fungal Community
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Yang, Y.; Li, Y.; Gao, B.; Tang, Y.; Xie, J.; Zhao, H. Remediation of saline-sodic soil using organic and inorganic amendments: Physical, chemical, and enzyme activity properties. J. Soils Sediments 2019, 20, 1454–1467. [Google Scholar] [CrossRef]
- Yao, R.-J.; Li, H.-Q.; Yang, J.-S.; Wang, X.-P.; Xie, W.-P.; Zhang, X. Biochar Addition Inhibits Nitrification by Shifting Community Structure of Ammonia-Oxidizing Microorganisms in Salt-Affected Irrigation-Silting Soil. Microorganisms 2022, 10, 436. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, K.; Zhu, X.; Lu, T.; Wang, X. Effects of amendments on carbon and nitrogen fractions in agricultural soils of Yellow River Delta. Geosci. Lett. 2023, 10, 22. [Google Scholar] [CrossRef]
- Taher, D.; Nofal, E.; Hegazi, M.; El-Gaied, M.A.; El-Ramady, H.; Solberg, S.Ø. Response of Warm Season Turf Grasses to Combined Cold and Salinity Stress under Foliar Applying Organic and Inorganic Amendments. Horticulturae 2023, 9, 49. [Google Scholar] [CrossRef]
- Yao, R.; Yang, J.; Zhu, W.; Li, H.; Yin, C.; Jing, Y.; Wang, X.; Xie, W.; Zhang, X. Impact of crop cultivation, nitrogen and fulvic acid on soil fungal community structure in salt-affected alluvial fluvo-aquic soil. Plant Soil 2021, 464, 539–558. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, X.; Meng, Q.; Zeng, X.; Zhang, J.; Li, D.; Wang, J.; Du, W.; Ma, X. Additional application of aluminum sulfate with different fertilizers ameliorates saline-sodic soil of Songnen Plain in Northeast China. J. Soils Sediments 2019, 19, 3521–3533. [Google Scholar] [CrossRef]
- Yang, L.; Bian, X.; Yang, R.; Zhou, C.; Tang, B. Assessment of organic amendments for improving coastal saline soil. Land Degrad. Dev. 2018, 29, 3204–3211. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, J.; Li, H.; La, S.; Tian, Y.; Gao, L. Biochar addition combined with daily fertigation improves overall soil quality and enhances water-fertilizer productivity of cucumber in alkaline soils of a semi-arid region. Geoderma 2020, 363, 114170. [Google Scholar] [CrossRef]
- Farooqi, Z.U.R.; Qadir, A.A.; Alserae, H.; Raza, A.; Mohy-Ud-Din, W. Organic amendment–mediated reclamation and build-up of soil microbial diversity in salt-affected soils: Fostering soil biota for shaping rhizosphere to enhance soil health and crop productivity. Environ. Sci. Pollut. Res. 2023, 30, 109889–109920. [Google Scholar] [CrossRef]
- Gardner, T.G.; Frene, J.P.; Lawson, S.S.; Lue Sue, N.D.; Handy, J.; Crawford, R.H. The Impact of Tree Species on Microbial Community Structure and Soil Function on Forest Plantations in the Central Hardwoods Region (CHR). Forests 2023, 14, 859. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Ao, G.; Qin, W.; Han, M.; Shen, Y.; Liu, M.; Chen, Y.; Zhu, B. Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil Biol. Biochem. 2023, 179, 108982. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Zhang, J.; Zhang, C.; Ma, D.; Zhou, G.; Ning, Q. Organic amendments with high proportion of heterocyclic compounds promote soil microbiome shift and microbial use efficiency of straw-C. Front. Microbiol. 2023, 14, 1087709. [Google Scholar] [CrossRef]
- Peng, X.; Liu, J.; Duan, X.; Yang, H.; Huang, Y. Key Soil Physicochemical Properties Regulating Microbial Community Structure under Vegetation Restoration in a Karst Region of China. Ecosyst. Health Sustain. 2023, 9, 0031. [Google Scholar] [CrossRef]
- Edwards, K.R.; Bárta, J.; Mastný, J.; Picek, T. Multiple environmental factors, but not nutrient addition, directly affect wet grassland soil microbial community structure: A macrocosm study. FEMS Microbiol. Ecol. 2023, 99, fiad070. [Google Scholar] [CrossRef]
- Somenahally, A.C.; McLawrence, J.; Chaganti, V.N.; Ganjegunte, G.K.; Obayomi, O.; Brady, J.A. Response of soil microbial Communities, inorganic and organic soil carbon pools in arid saline soils to alternative land use practices. Ecol. Indic. 2023, 150, 110227. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Omidvar, N.; Liu, M.; Zou, D.; Zhang, M. Insight into functional mechanisms of percarbamide and nitrification inhibitors in degrading fungicide residues and shaping microbial communities in soil-plant systems. J. Environ. Manag. 2023, 345, 118687. [Google Scholar] [CrossRef]
- Han, L.; Qin, H.; Wang, J.; Yao, D.; Zhang, L.; Guo, J.; Zhu, B. Immediate response of paddy soil microbial community and structure to moisture changes and nitrogen fertilizer application. Front. Microbiol. 2023, 14, 1130298. [Google Scholar] [CrossRef]
- Zhang, H.; Xi, J.; Lv, Q.; Wang, J.; Yu, K.; Zhao, F. Effect of Aerated Irrigation on the Growth and Rhizosphere Soil Fungal Community Structure of Greenhouse Grape Seedlings. Sustainability 2022, 14, 12719. [Google Scholar] [CrossRef]
- Gao, S.; He, Q.; Huang, D.; Wang, Z.; Mao, J.; Xie, X.; Su, Y.; Qiu, Q.; Li, J.; Chen, Z. Responses of Fungal Community Structure and Functional Composition to Short-Term Fertilization and Dry Season Irrigation in Eucalyptus urophylla × Eucalyptus grandis Plantation Soils. Forests 2022, 13, 854. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Luo, Y.; Awasthi, M.K.; Yang, J.; Duan, Y.; Li, H.; Zhao, Z. Mulching practices alter the bacterial-fungal community and network in favor of soil quality in a semiarid orchard system. Sci. Total Environ. 2020, 725, 138527. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Zhang, Y.; Mao, Q.; Zhong, B.; Chen, W.; Mo, J.; Wang, F.; Lu, X. Consistent effects of nitrogen addition on soil microbial communities across three successional stages in tropical forest ecosystems. Catena 2023, 227, 107116. [Google Scholar] [CrossRef]
- Xiao, M.; Liu, G.; Jiang, S.; Guan, X.; Chen, J.; Yao, R.; Wang, X. Bio-Organic Fertilizer Combined with Different Amendments Improves Nutrient Enhancement and Salt Leaching in Saline Soil: A Soil Column Experiment. Water 2022, 14, 4084. [Google Scholar] [CrossRef]
- Ni, P.; Wang, S.; Liu, B.; Sun, H. Effects of Organic Manure and Biochar-Based Fertilizer Application on Soil Water and Salt Transport in Brackish Water Irrigated Soil Profile. J. Soil Sci. Plant Nutr. 2023, 23, 3120–3136. [Google Scholar] [CrossRef]
- Amer, M.M.; Aboelsoud, H.M.; Sakher, E.M.; Hashem, A.A. Effect of Gypsum, Compost, and Foliar Application of Some Nanoparticles in Improving Some Chemical and Physical Properties of Soil and the Yield and Water Productivity of Faba Beans in Salt-Affected Soils. Agronomy 2023, 13, 1052. [Google Scholar] [CrossRef]
- Suddarth, S.R.P.; Ferreira, J.F.S.; Cavalcante, L.F.; Fraga, V.S.; Anderson, R.G.; Halvorson, J.J.; Bezerra, F.T.C.; Medeiros, S.A.S.; Costa, C.R.G.; Dias, N.S. Can Humic Substances Improve Soil Fertility under Salt Stress and Drought Conditions? J. Environ. Qual. 2019, 48, 1605–1613. [Google Scholar] [CrossRef]
- Khan, I.; Mahmood, S.; Chattha, M.U.; Bilal Chattha, M.; Ahmad, S.; Awan, M.I.; Alqahtani, F.M.; Hashem, M.; Alhaithloul, H.A.S.; Qari, S.H.; et al. Organic Amendments Improved the Productivity and Bio-Fortification of Fine Rice by Improving Physiological Responses and Nutrient Homeostasis under Salinity Stress. Plants 2023, 12, 1644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Mi, W.; Xu, Y.; Zhou, W.; Bi, Y. Long-term integrated rice-crayfish culture disrupts the microbial communities in paddy soil. Aquac. Rep. 2023, 29, 101515. [Google Scholar] [CrossRef]
- Kalanaki, M.; Ritzema, H.; Bamshad, R.; Jones, E.; Fazilatnia, M. Application of bio-desalinization for reclamation of salt-affected soil under composted cow manure and deficit irrigation with saline water. Paddy Water Environ. 2020, 18, 469–479. [Google Scholar] [CrossRef]
- Guo, K.; Liu, X. Effect of initial soil water content and bulk density on the infiltration and desalination of melting saline ice water in coastal saline soil. Eur. J. Soil Sci. 2019, 70, 1249–1266. [Google Scholar] [CrossRef]
- Cao, J.; Zhao, P.; Wang, D.; Zhao, Y.; Wang, Z.; Zhong, N. Effects of a Nanonetwork-Structured Soil Conditioner on Microbial Community Structure. Biology 2023, 12, 668. [Google Scholar] [CrossRef]
- Chen, C.; Lv, Q.; Tang, Q. Impact of bio-organic fertilizer and reduced chemical fertilizer application on physical and hydraulic properties of cucumber continuous cropping soil. Biomass Convers. Biorefinery 2022, 14, 921–930. [Google Scholar] [CrossRef]
- Bello, S.K.; Alayafi, A.H.; Al-Solaimani, S.G.; Abo-Elyousr, K.A.M. Mitigating Soil Salinity Stress with Gypsum and Bio-Organic Amendments: A Review. Agronomy 2021, 11, 1735. [Google Scholar] [CrossRef]
- Sun, Y.P.; Yang, J.S.; Yao, R.J.; Chen, X.B.; Wang, X.P. Biochar and fulvic acid amendments mitigate negative effects of coastal saline soil and improve crop yields in a three year field trial. Sci. Rep. 2020, 10, 8946. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lin, M.; Du, C.; Hao, Y.; Zhang, Y.; Wang, Z. The use of manure shifts the response of α-diversity and network while not β-diversity of soil microbes to altered irrigation regimes. Appl. Soil Ecol. 2022, 174, 104423. [Google Scholar] [CrossRef]
- Lu, Y.; Lyu, M.; Xiong, X.; Deng, C.; Jiang, Y.; Zeng, M.; Xie, J. Understory ferns promote the restoration of soil microbial diversity and function in previously degraded lands. Sci. Total Environ. 2023, 870, 161934. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, C.; Guo, B.; Yu, J.; Ding, H.; Peng, S.; Sveen, T.R.; Zhang, Y. Effects of re vegetation restoration on soil bacterial community structure in degraded land insubtropical China. Eur. J. Soil Biol. 2020, 98, 103184. [Google Scholar] [CrossRef]
- Dong, W.Y.; Zhang, X.Y.; Dai, X.Q.; Fu, X.L.; Yang, F.T.; Liu, X.Y.; Sun, X.M.; Wen, X.F.; Schaeffer, S. Changes in soil microbial community composition in response to fer tilization of paddy soils in subtropical China. Appl. Soil Ecol. 2014, 84, 140–147. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, J.; Yang, L.; He, Q.; Su, Y.; Li, J.; Wang, G.; Qiu, Q. Soil Bacterial and Fungal Community Responses to Throughfall Reduction in a Eucalyptus Plantation in Southern China. Forests 2021, 13, 37. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Li, K. Responses of soil bacterial and fungal communities to the long-term monoculture of grapevine. Appl. Microbiol. Biotechnol. 2021, 105, 7035–7050. [Google Scholar] [CrossRef]
- Johnson, J.M.; Ludwig, A.; Furch, A.C.U. The beneficial root-colonizing fungus Mortierella hyaline promotes the aerial growth of Arabidopsis and activates calcium-dependent responses that restrict Alternaria brassicae-induced disease development in roots. Mol. Plant-Microbe Interact. 2019, 32, 351–363. [Google Scholar] [CrossRef]
- Zhu, X.; Mao, L.; Chen, B. Driving forces linking microbial community structure and functions to enhanced carbon stability in biochar-amended soil. Environ. Int. 2019, 133, 105211. [Google Scholar] [CrossRef]
- Zhang, W.W.; Wang, C.; Xue, R.; Wang, L.J. Effects of salinity on the soil microbial community and soil fertility. J. Integr. Agric. 2019, 18, 1360–1368. [Google Scholar] [CrossRef]
- Zou, L.; Bai, Y.P.; Huang, J.; Xiao, D.R.; Yang, G. Soil pH and dissolved organic carbon shape microbial communities in wetlands with two different vegetation types in Changdu area, Tibet. J. Mt. Sci. 2023, 20, 750–764. [Google Scholar] [CrossRef]
- Ji, C.; Huang, J.; Zhang, X.; Yang, G.; Xing, S.; Fu, W.; Hao, Z.; Chen, B.; Zhang, X. Response of soil fungal community to chromium contamination in agricultural soils with different physicochemical properties. Sci. Total Environ. 2023, 879, 163244. [Google Scholar] [CrossRef]

| Treatments | pH | EC us/cm | SOC g/kg | AK mg/kg | AP mg/kg | AN mg/kg | S-CAT (μmol/d/g) | S-UE (U/g) | S-AKP (U/g) |
|---|---|---|---|---|---|---|---|---|---|
| W | 8.40 ± 0.06 a | 352.66 ± 15.79 ab | 9.00 ± 0.37 ab | 259.66 ± 15.36 a | 25.75 ± 0.15 a | 59.53 ± 3.52 a | 117.19 ± 0.34 a | 209.66 ± 29.87 ab | 278.64 ± 36.78 b |
| BW | 8.17 ± 0.02 b | 393.00 ± 103.49 ab | 10.66 ± 1.51 a | 270.66 ± 20.43 a | 27.43 ± 2.92 a | 72.03 ± 8.14 a | 118.33 ± 1.72 a | 220.52 ± 29.72 a | 900.63 ± 137.19 a |
| CK | 8.51 ± 0.06 a | 417.00 ± 20.11 a | 9.10 ± 0.40 ab | 285.5 ± 2.04 a | 23.26 ± 1.12 a | 60.95 ± 0.43 a | 117.04 ± 0.09 a | 175.33 ± 5.01 b | 398.39 ± 79.15 b |
| Treatments | Sobs | ACE | Chao | Shannon | Simpson |
|---|---|---|---|---|---|
| W | 380.33 ± 70.53 a | 415.15 ± 26.57 a | 415 ± 26.44 a | 4.27 ± 0.23 a | 0.03 ± 0.01 b |
| BW | 315.66 ± 56.81 a | 318.26 ± 58.53 ab | 317.81 ± 57.94 ab | 3.89 ± 0.17 a | 0.06 ± 0.01 b |
| CK | 236 ± 53.09 a | 237.46 ± 54.52 b | 237.49 ± 54.79 b | 3.12 ± 0.25 b | 0.16 ± 0.03 a |
| Index | RDA1 | RDA2 | r2 | p-Value |
|---|---|---|---|---|
| pH | 0.0976 | −0.9952 | 0.0122 | 0.983 |
| EC | −0.576 | 0.8175 | 0.361 | 0.273 |
| SOC | −0.2327 | 0.9725 | 0.473 | 0.202 |
| AK | 0.7466 | −0.6653 | 0.074 | 0.764 |
| AP | 0.7108 | −0.7034 | 0.3095 | 0.281 |
| AN | 0.8926 | −0.4509 | 0.0355 | 0.83 |
| S-UE | −0.3725 | −0.928 | 0.2814 | 0.433 |
| S-AKP | 0.9743 | 0.2253 | 0.2358 | 0.423 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, M.; Chen, C.; Yao, R.; Wang, X.; Liu, G. Response of Soil Fungal Community in Coastal Saline Soil to Short-Term Water Management Combined with Bio-Organic Fertilizer. Agronomy 2024, 14, 1441. https://doi.org/10.3390/agronomy14071441
Xiao M, Chen C, Yao R, Wang X, Liu G. Response of Soil Fungal Community in Coastal Saline Soil to Short-Term Water Management Combined with Bio-Organic Fertilizer. Agronomy. 2024; 14(7):1441. https://doi.org/10.3390/agronomy14071441
Chicago/Turabian StyleXiao, Meng, Cheng Chen, Rongjiang Yao, Xiuping Wang, and Guangming Liu. 2024. "Response of Soil Fungal Community in Coastal Saline Soil to Short-Term Water Management Combined with Bio-Organic Fertilizer" Agronomy 14, no. 7: 1441. https://doi.org/10.3390/agronomy14071441
APA StyleXiao, M., Chen, C., Yao, R., Wang, X., & Liu, G. (2024). Response of Soil Fungal Community in Coastal Saline Soil to Short-Term Water Management Combined with Bio-Organic Fertilizer. Agronomy, 14(7), 1441. https://doi.org/10.3390/agronomy14071441






