Novel QTL Hotspots for Barley Flowering Time, Plant Architecture, and Grain Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Germplasm Material and Phenotypic Assessment
2.2. Statistical Analysis of Phenotyping Data
2.3. SNP Genotyping, Population Structure Analysis, and LD Decay
2.4. Genome-Wide Association Analyses
2.5. Candidate Gene/QTL Identification
3. Results
3.1. Phenotypic Traits Evaluation
3.2. Genotyping and Population Structure of Studied Barley Germplasm Collection
3.3. Genome-Wide Association Analysis
3.4. Candidate Gene Identification
3.5. Novel QTL Hotspots
4. Discussion
4.1. Performance of the Studied Barley Collection under Different Environmental Conditions
4.2. Population Structure
4.3. Comparison of Current QTLs with Barley Genes and QTLs from Previous Reports
4.4. Putative QTL Hotspots Associated with Variations in the Agronomic Traits of Barley
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sakuma, S.; Salomon, B.; Komatsuda, T. The domestication syndrome genes responsible for the major changes in plant form in the Triticeae crops. Plant Cell Physiol. 2011, 52, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Zohary, D.; Hopf, M.; Weiss, E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin, 4th ed.; Oxford University Press: Oxford, UK, 2012; pp. 1–12. [Google Scholar]
- Langridge, P. Economic and Academic Importance of Barley. In The Barley Genome. Compendium of Plant Genomes; Stein, N., Muehlbauer, G.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–10. [Google Scholar]
- Cockram, J.; Jones, H.; Leigh, F.J.; O’Sullivan, D.; Powell, W.; Laurie, D.A.; Greenland, A.J. Control of flowering time in temperate cereals: Genes, domestication, and sustainable productivity. J. Exp. Bot. 2007, 58, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calleja, M.; Casas, A.M.; Igartua, E. Major flowering time genes of barley: Allelic diversity, effects, and comparison with wheat. Theor. Appl. Genet. 2021, 134, 1867–1897. [Google Scholar] [CrossRef] [PubMed]
- Andres, F.; Coupland, G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 2012, 13, 627–639. [Google Scholar] [CrossRef]
- Turner, A.; Beales, J.; Faure, S.; Dunford, R.P.; Laurie, D.A. The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 2005, 310, 1031–1034. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Fu, D.; Li, C.; Blechl, A.; Tranquilli, G.; Bonafede, M.; Sanchez, A.; Valarik, M.; Yasuda, S.; Dubcovsky, J. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc. Natl. Acad. Sci. USA 2006, 103, 19581–19586. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Loukoianov, A.; Blechl, A.; Tranquilli, G.; Ramakrishna, W.; SanMiguel, P.; Bennetzen, J.L.; Echenique, V.; Dubcovsky, J. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 2004, 303, 1640–1644. [Google Scholar] [CrossRef]
- Yan, L.; Loukoianov, A.; Tranquilli, G.; Helguera, M.; Fahima, T.; Dubcovsky, J. Positional cloning of the wheat vernalization gene VRN1. Proc. Natl. Acad. Sci. USA 2003, 100, 6263–6268. [Google Scholar] [CrossRef] [PubMed]
- Comadran, J.; Kilian, B.; Russell, J.; Ramsay, L.; Stein, N.; Ganal, M.; Shaw, P.; Bayer, M.; Thomas, W.; Marshall, D.; et al. Natural variation in a homolog of Antirrhinum CENTRORADIALIS contributed to spring growth habit and environmental adaptation in cultivated barley. Nat. Genet. 2012, 44, 1388–1392. [Google Scholar] [CrossRef]
- Hyles, J.; Bloomfield, M.T.; Hunt, J.R.; Trethowan, R.M.; Trevaskis, B. Phenology and related traits for wheat adaptation. Heredity 2020, 125, 417–430. [Google Scholar] [CrossRef]
- Guo, T.; Mu, Q.; Wang, J.; Vanous, A.E.; Onogi, A.; Iwata, H.; Li, X.; Yu, J. Dynamic effects of interacting genes underlying rice flowering-time phenotypic plasticity and global adaptation. Genome Res. 2020, 30, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.M.; Sharma, R.; Pasam, R.K.; Graner, A.; Kilian, B.; Schnurbusch, T. Genetic Dissection of Photoperiod Response Based on GWAS of PreAnthesis Phase Duration in Spring Barley. PLoS ONE 2014, 9, e113120. [Google Scholar] [CrossRef]
- Maurer, A.; Draba, V.; Jiang, Y.; Schnaithmann, F.; Sharma, R.; Schumann, E.; Kilian, B.; Reif, J.C.; Pillen, K. Modelling the genetic architecture of flowering time control in barley through nested association mapping. BMC Genom. 2015, 16, 290. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, E.; Elbadry, N.; Tondelli, A.; Cattivelli, L.; Rossini, L. Genetic dissection of heading date and yield under Mediterranean dry climate in barley (Hordeum vulgare L.). Euphytica 2016, 212, 343–353. [Google Scholar] [CrossRef]
- He, T.; Hill, C.B.; Angessa, T.T.; Zhang, X.-Q.; Chen, K.; Moody, D.; Telfer, P.; Westcott, S.; Li, C. Gene-set association and epistatic analyses reveal complex gene interaction networks affecting flowering time in a worldwide barley collection. J. Exp. Bot. 2019, 70, 5603–5616. [Google Scholar] [CrossRef] [PubMed]
- Afsharyan, N.P.; Sannemann, W.; Léon, J.; Ballvora, A. Effect of epistasis and environment on flowering time in barley reveals a novel flowering-delaying QTL allele. J. Exp. Bot. 2020, 71, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ma, H.; Zhang, P.; Ren, L.; Yang, X.; Yao, G.; Zhang, P.; Zhou, M. Inheritance of stem strength and its correlations with culm morphological traits in wheat (Triticum aestivum L.). Can. J. Plant Sci. 2011, 91, 1065–1070. [Google Scholar] [CrossRef]
- Firoozabadi, Z.D.; Nikkhah, H.R.; Foruzesh, P. Study of relationship between morpho-physiological traits and grain yield under terminal drought stress conditions in barley genotypes. Cereal Res. Commun. 2023, 51, 207–216. [Google Scholar] [CrossRef]
- Zahn, S.; Schmutzer, T.; Pillen, K.; Maurer, A. Genomic Dissection of Peduncle Morphology in Barley through Nested Association Mapping. Plants 2021, 10, 10. [Google Scholar] [CrossRef]
- Nadolska-Orczyk, A.; Rajchel, I.K.; Orczyk, W.; Gasparis, S. Major genes determining yield-related traits in wheat and barley. Theor. Appl. Genet. 2017, 130, 1081–1098. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Lu, W.; Deng, D. Gibberellin in plant height control: Old player, new story. Plant Cell Rep. 2017, 36, 391–398. [Google Scholar] [CrossRef]
- Dockter, C.; Hansson, M. Improving barley culm robustness for secured crop yield in a changing climate. J. Exp. Bot. 2015, 66, 3499–3509. [Google Scholar] [CrossRef] [PubMed]
- Kuczynska, A.; Surma, M.; Adamski, T.; Mikolajczak, K.; Krystkowiak, K.; Ogrodowicz, P. Effects of the semi-dwarfing sdw1/denso gene in barley. J. Appl. Genet. 2013, 54, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.M.; Koppolu, R.; Wolde, G.M.; Graner, A.; Schnurbusch, T. The genetic architecture of barley plant stature. Front. Genet. 2016, 7, 203302. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, M.; Fakheri, B.A.; Aghnoum, R.; Mahdi Nezhad, N.; Ataei, R. GWAS analysis in spring barley (Hordeum vulgare L.) for morphological traits exposed to drought. PLoS ONE 2018, 13, e0204952. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghani, A.H.; Sharma, R.; Wabila, C.; Dhanagond, S.; Owais, S.J.; Duwayri, M.A.; Al-Dalain, S.A.; Klukas, C.; Chen, D.; Lübberstedt, T.; et al. Genome-wide association mapping in a diverse spring barley collection reveals the presence of QTL hotspots and candidate genes for root and shoot architecture traits at seedling stage. BMC Plant Biol. 2019, 19, 216. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, X.; Yao, X.; Yao, Y.; An, L.; Li, X.; Wang, Y.; Gao, X.; Jia, Y.; Guan, L.; et al. Genome wide association study of plant height and tiller number in hulless barley. PLoS ONE 2021, 16, e0260723. [Google Scholar] [CrossRef]
- Xu, X.; Sharma, R.; Tondelli, A.; Russell, J.; Comadran, J.; Schnaithmann, F.; Pillen, K.; Kilian, B.; Cattivelli, L.; Thomas, W.T.B.; et al. Genome-Wide Association Analysis of Grain Yield-Associated Traits in a Pan-European Barley Cultivar Collection. Plant Genome 2018, 11, 170073. [Google Scholar] [CrossRef] [PubMed]
- Francia, E.; Tondelli, A.; Rizza, F.; Badeck, F.W.; Nicosia, O.L.D.; Akar, T.; Grando, S.; Al-Yassin, A.; Benbelkacem, A.; Thomas, W.T.B.; et al. Determinants of barley grain yield in a wide range of Mediterranean environments. Field Crops Res. 2011, 120, 169–178. [Google Scholar] [CrossRef]
- Cammarano, D.; Ceccarelli, S.; Grando, S.; Romagosa, I.; Benbelkacem, A.; Akar, T.; Al-Yassin, A.; Pecchioni, N.; Francia, E.; Ronga, D. The impact of climate change on barley yield in the Mediterranean basin. Eur. J. Agron. 2019, 106, 1–11. [Google Scholar] [CrossRef]
- Cuesta-Marcos, A.; Casas, A.M.; Hayes, P.M.; Gracia, M.P.; Lasa, J.M.; Ciudad, F.; Codesal, P.; Molina-Cano, J.L.; Igartua, E. Yield QTL afected by heading date in Mediterranean grown barley. Plant Breed. 2019, 128, 46–53. [Google Scholar] [CrossRef]
- Börner, A.; Worland, A.J.; Plaschke, J.; Schumann, E.; Law, C.N. Pleiotropic effects of genes for reduced height (Rht) and day-length insensitivity (Ppd) on yield and its components for wheat grown in middle Europe. Plant Breed. 1993, 111, 204–216. [Google Scholar] [CrossRef]
- Waugh, R.; Jannink, J.-L.; Muehlbauer, G.J.; Ramsey, L. The emergence of whole genome association scans in barley. Curr. Opin. Plant Biol. 2009, 12, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Genievskaya, Y.; Almerekova, S.; Sariev, B.; Chudinov, V.; Tokhetova, L.; Sereda, G.; Ortaev, A.; Tsygankov, V.; Blake, T.; Chao, S.; et al. Marker-trait associations in two-rowed spring barley accessions from Kazakhstan and the USA. PLoS ONE 2018, 13, e0205421. [Google Scholar] [CrossRef] [PubMed]
- Almerekova, S.; Sariev, B.; Abugalieva, A.; Chudinov, V.; Sereda, G.; Tokhetova, L.; Ortaev, A.; Tsygankov, V.; Blake, T.; Chao, S.; et al. Association mapping for agronomic traits in six–rowed spring barley from the USA harvested in Kazakhstan. PLoS ONE 2019, 14, e0221064. [Google Scholar] [CrossRef] [PubMed]
- Genievskaya, Y.; Zatybekov, A.; Abugalieva, S.; Turuspekov, Y. Identification of Quantitative Trait Loci Associated with Powdery Mildew Resistance in Spring Barley under Conditions of Southeastern Kazakhstan. Plants 2023, 12, 2375. [Google Scholar] [CrossRef] [PubMed]
- Genievskaya, Y.; Almerekova, S.; Abugalieva, S.; Abugalieva, A.; Sato, K.; Turuspekov, Y. Identification of SNPs associated with grain quality traits in spring barley collection grown in southeastern Kazakhstan. Agronomy 2023, 13, 1560. [Google Scholar] [CrossRef]
- Dospekhov, B.A. Methodology of Filed Experiments, 4th ed.; Agropromizdat: Moscow, Russia, 1985; pp. 241–263. [Google Scholar]
- Villanueva, R.A.M.; Chen, Z.J. ggplot2: Elegant Graphics for Data Analysis (2nd ed.). Meas. Interdiscip. Res. Perspect. 2019, 17, 160–167. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R package ‘corrplot’: Visualization of a Correlation Matrix. (Version 0.92). Available online: https://github.com/taiyun/corrplot (accessed on 28 April 2024).
- Yu, G.; Hatta, A.; Periyannan, S.; Lagudah, E.; Wulff, B.B.H. Isolation of Wheat Genomic DNA for Gene Mapping and Cloning. In Wheat Rust Diseases. Methods in Molecular Biology; Periyannan, S., Ed.; Humana Press: New York, NY, USA, 2017; pp. 124–140. [Google Scholar]
- Bayer, M.M.; Rapazote-Flores, P.; Ganal, M.; Hedley, P.E.; Macaulay, M.; Plieske, J.; Ramsay, L.; Russell, J.; Shaw, P.D.; Thomas, W.; et al. Development and Evaluation of a Barley 50k iSelect SNP Array. Front. Plant Sci. 2017, 8, 1792. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. Clumpak: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, 78–82. [Google Scholar] [CrossRef]
- Segura, V.; Vilhjálmsson, B.; Platt, A.; Korte, A.; Seren, Ü.; Long, Q.; Nordborg, M. An efficient multi-locus mixed-model approach for genome-wide association studies in structured populations. Nat. Genet. 2012, 44, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, K.; Zhu, G.; Fang, W.; Chen, C.; Wang, X.; Zhao, P.; Guo, J.; Ding, T.; Guan, L.; et al. Genomic analyses of an extensive collection of wild and cultivated accessions provide new insights into peach breeding history. Genome Biol. 2019, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhang, H.; Tang, Z.; Xu, J.; Yin, D.; Zhang, Z.; Yuan, X.; Zhu, M.; Zhao, S.; Li, X.; et al. rMVP: A memory-efficient, visualization-enhanced, and parallel-accelerated tool for genome-wide association study. Genom. Proteom. Bioinform. 2021, 19, 619–628. [Google Scholar] [CrossRef]
- Szűcs, P.; Blake, V.C.; Bhat, P.R.; Chao, S.; Close, T.J.; Cuesta-Marcos, A.; Muehlbauer, G.J.; Ramsay, L.; Waugh, R.; Hayes, P.M. An integrated resource for barley linkage map and malting quality QTL alignment. Plant Genome 2009, 2, 134–140. [Google Scholar] [CrossRef]
- Bretani, G.; Shaaf, S.; Tondelli, A.; Cattivelli, L.; Delbono, S.; Waugh, R.; Thomas, W.; Russell, J.; Bull, H.; Igartua, E.; et al. Multi-environment genome-wide association mapping of culm morphology traits in barley. Front. Plant Sci. 2022, 13, 926277. [Google Scholar] [CrossRef]
- Dang, V.H.; Hill, C.B.; Zhang, X.Q.; Angessa, T.T.; McFawn, L.A.; Li, C. Multi-locus genome-wide association studies reveal novel alleles for flowering time under vernalisation and extended photoperiod in a barley MAGIC population. Theor. Appl. Genet. 2022, 135, 3087–3102. [Google Scholar] [CrossRef]
- Cosenza, F.; Shrestha, A.; Van Inghelandt, D.; Casale, F.A.; Wu, P.-Y.; Weisweiler, M.; Li, J.; Wespel, F.; Stich, B. Genetic mapping reveals new loci and alleles for flowering time and plant height using the double round-robin population of barley. J. Exp. Bot. 2024, 75, 2385–2402. [Google Scholar] [CrossRef]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Ensembl Plants: Barley. Available online: https://plants.ensembl.org/Hordeum_vulgare/Info/Index (accessed on 28 April 2024).
- Porter, J.R.; Gawith, M. Temperatures and the growth and development of wheat: A review. Eur. J. Agron. 1999, 10, 23–36. [Google Scholar] [CrossRef]
- Eliş, S.; Yıldırım, M. Dual-purpose ability of barley and wheat at drought and heat stress conditions. J. Arid. Agric. 2021, 7, 103–108. [Google Scholar] [CrossRef]
- Rollins, J.A.; Habte, E.; Templer, S.E.; Colby, T.; Schmidt, J.; von Korff, M. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). J. Exp. Bot. 2013, 64, 3201–3212. [Google Scholar] [CrossRef] [PubMed]
- Hemming, M.N.; Walford, S.A.; Fieg, S.; Dennis, E.S.; Trevaskis, B. Identification of high-temperature-responsive genes in cereals. Plant Physiol. 2012, 158, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, M.; von Korff, M. The genetic control of reproductive development under high ambient temperature. Plant Physiol. 2017, 173, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Wallwork, M.A.B.; Jenner, C.F.; Logue, S.J.; Sedgley, M. Effect of high temperature during grain-filling on the structure of developing and malted barley grains. Ann. Bot. 1998, 82, 587–599. [Google Scholar] [CrossRef]
- Sakata, T.; Takahashi, H.; Nishiyama, I.; Higashitani, A. Effects of high temperature on the development of pollen mother cells and microspores in barley Hordeum vulgare L. J. Plant Res. 2000, 113, 395–402. [Google Scholar] [CrossRef]
- Rezaei, E.E.; Webber, H.; Gaiser, T.; Naab, J.; Ewert, F. Heat stress in cereals: Mechanisms and modelling. Eur. J. Agron. 2015, 64, 98–113. [Google Scholar] [CrossRef]
- Malysheva-Otto, L.V.; Ganal, M.W.; Röder, M.S. Analysis of molecular diversity, population structure and linkage disequilibrium in a worldwide survey of cultivated barley germplasm (Hordeum vulgare L.). BMC Genet. 2006, 7, 6. [Google Scholar] [CrossRef]
- Hamblin, M.T.; Close, T.J.; Bhat, P.R.; Chao, S.; Kling, J.G.; Abraham, K.J.; Blake, T.; Brooks, W.S.; Cooper, B.; Griffey, C.A.; et al. Population structure and linkage disequilibrium in US barley germplasm: Implications for association mapping. Crop Sci. 2010, 50, 556–566. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, C.; Wang, D.; St. Amand, P.; Bernardo, A.; Li, W.; He, F.; Li, L.; Wang, L.; Yuan, X.; et al. High-resolution genome-wide association study identifies genomic regions and candidate genes for important agronomic traits in wheat. Mol. Plant. 2020, 13, 1311–1327. [Google Scholar] [CrossRef]
- Almerekova, S.; Genievskaya, Y.; Abugalieva, S.; Sato, K.; Turuspekov, Y. Population Structure and Genetic Diversity of Two-Rowed Barley Accessions from Kazakhstan Based on SNP Genotyping Data. Plants 2021, 10, 2025. [Google Scholar] [CrossRef] [PubMed]
- Turuspekov, Y.; Sariev, B.; Chudinov, V.; Sereda, G.; Tokhetova, L.; Ortaev, A.; Tsygankov, V.; Doszhanov, M.; Volis, S.; Abugalieva, S. Genotype×environment interaction patterns for grain yield of spring barley in different regions of Kazakhstan. Russ. J. Genet. 2013, 49, 196–205. [Google Scholar] [CrossRef]
- Karsai, I.; Szucs, P.; Meszaros, K.; Filichkina, T.; Hayes, P.M.; Skinner, J.S.; Láng, L.; Bedő, Z. The Vrn-H2 locus is a major determinant of flowering time in a facultative x winter growth habit barley (Hordeum vulgare L.) mapping population. Theor. Appl. Genet. 2005, 110, 1458–1466. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Kennedy, A.; Pan, S.; Jermiin, L.S.; Melzer, R. Genome-wide analysis of MIKC-type MADS-box genes in wheat: Pervasive duplications, functional conservation and putative neofunctionalization. New Phytol. 2020, 225, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Choi, D.-W.; Fenton, R.; Close, T.J. Expression of the barley dehydrin multigene family and the development of freezing tolerance. Mol. Gen. Genet. MGG 2000, 264, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Al-Baidhani, H.H.J.; Harris, J.; Riboni, M.; Li, Y.; Mazonka, I.; Bazanova, N.; Chirkova, L.; Hussain, S.S.; Hrmova, M.; et al. DREB/CBF expression in wheat and barley using the stress-inducible promoters of HD-Zip I genes: Impact on plant development, stress tolerance and yield. Plant Biotechnol. J. 2020, 18, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Pan, Z.; Liu, J.; Deng, G.; Long, H.; Zhang, H.; Liang, J.; Zeng, X.; Tang, Y.; Tashi, N.; et al. A mutation in Waxy gene affects amylose content, starch granules and kernel characteristics of barley (Hordeum vulgare). Plant Breed. 2019, 138, 513–523. [Google Scholar] [CrossRef]
- Breitling, R.; Li, Y.; Tesson, B.M.; Fu, J.; Wu, C.; Wiltshire, T.; Gerrits, A.; Bystrykh, L.V.; de Haan, G.; Su, A.I.; et al. Genetical genomics: Spotlight on QTL hotspots. PLoS Genet. 2008, 4, e1000232. [Google Scholar] [CrossRef]
- Jia, Y.; Westcott, S.; He, T.; McFawn, L.A.; Angessa, T.; Hill, C.; Tan, C.; Zhang, X.; Zhou, G.; Li, C. Genome-wide association studies reveal QTL hotspots for grain brightness and black point traits in barley. Crop J. 2021, 9, 154–167. [Google Scholar] [CrossRef]
- Kochevenko, A.; Jiang, Y.; Seiler, C.; Surdonja, K.; Kollers, S.; Reif, J.C.; Korzun, V.; Graner, A. Identification of QTL hot spots for malting quality in two elite breeding lines with distinct tolerance to abiotic stress. BMC Plant Biol. 2018, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, S.; Grando, S.; Baum, M.; Udupa, S.M. Breeding for drought resistance in a changing climate. Chall. Strateg. Dryland Agric. 2004, 32, 167–190. [Google Scholar]

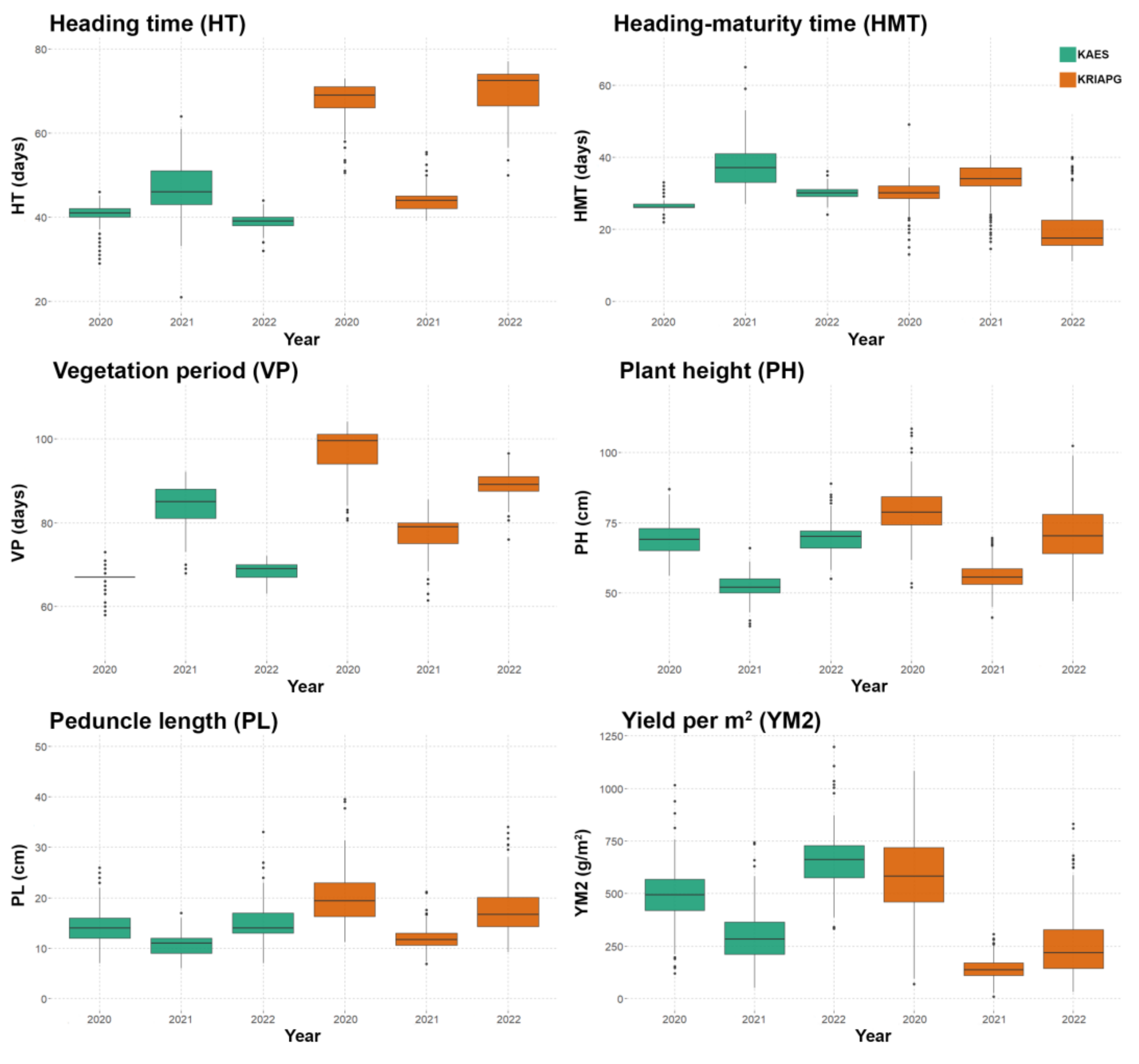

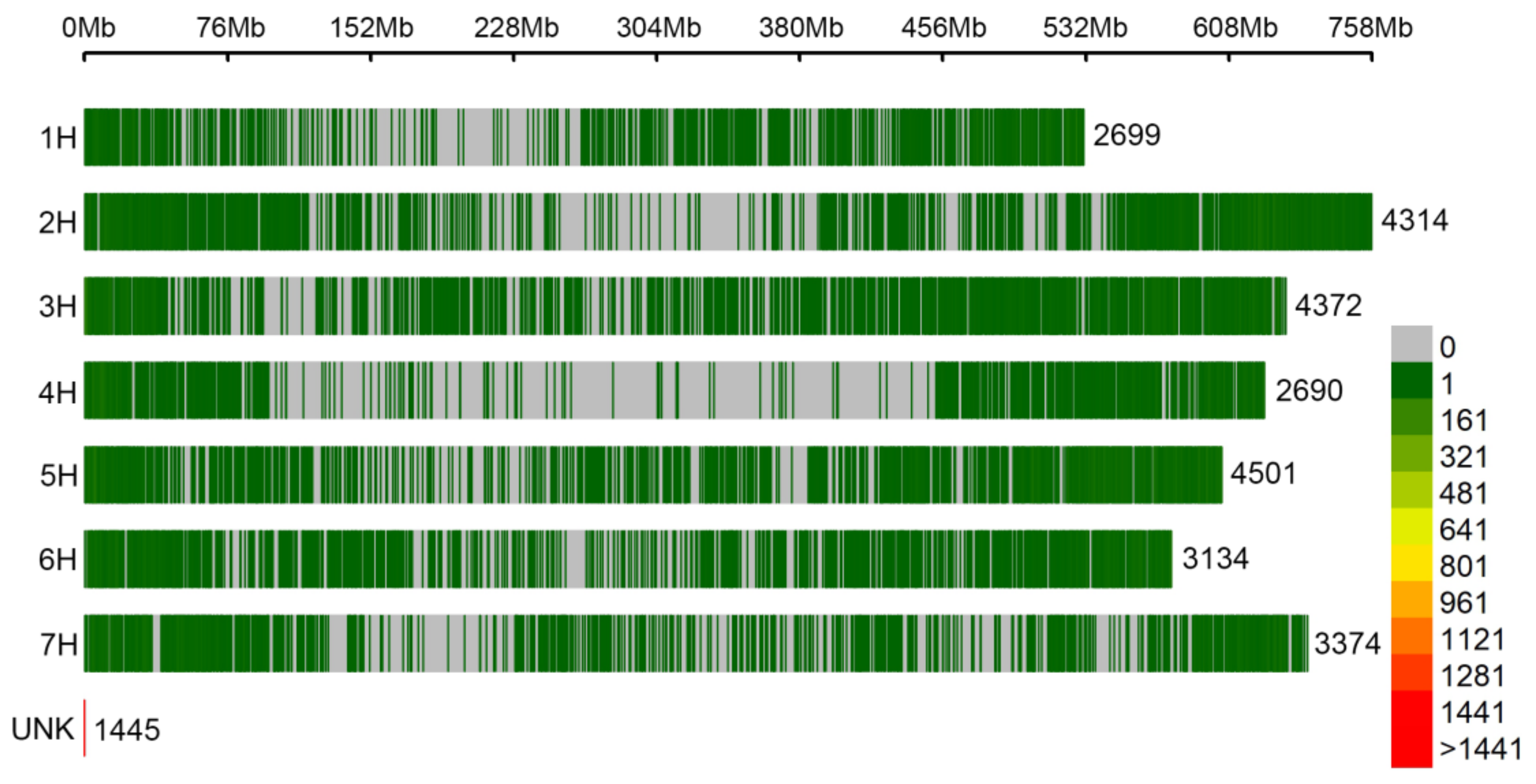
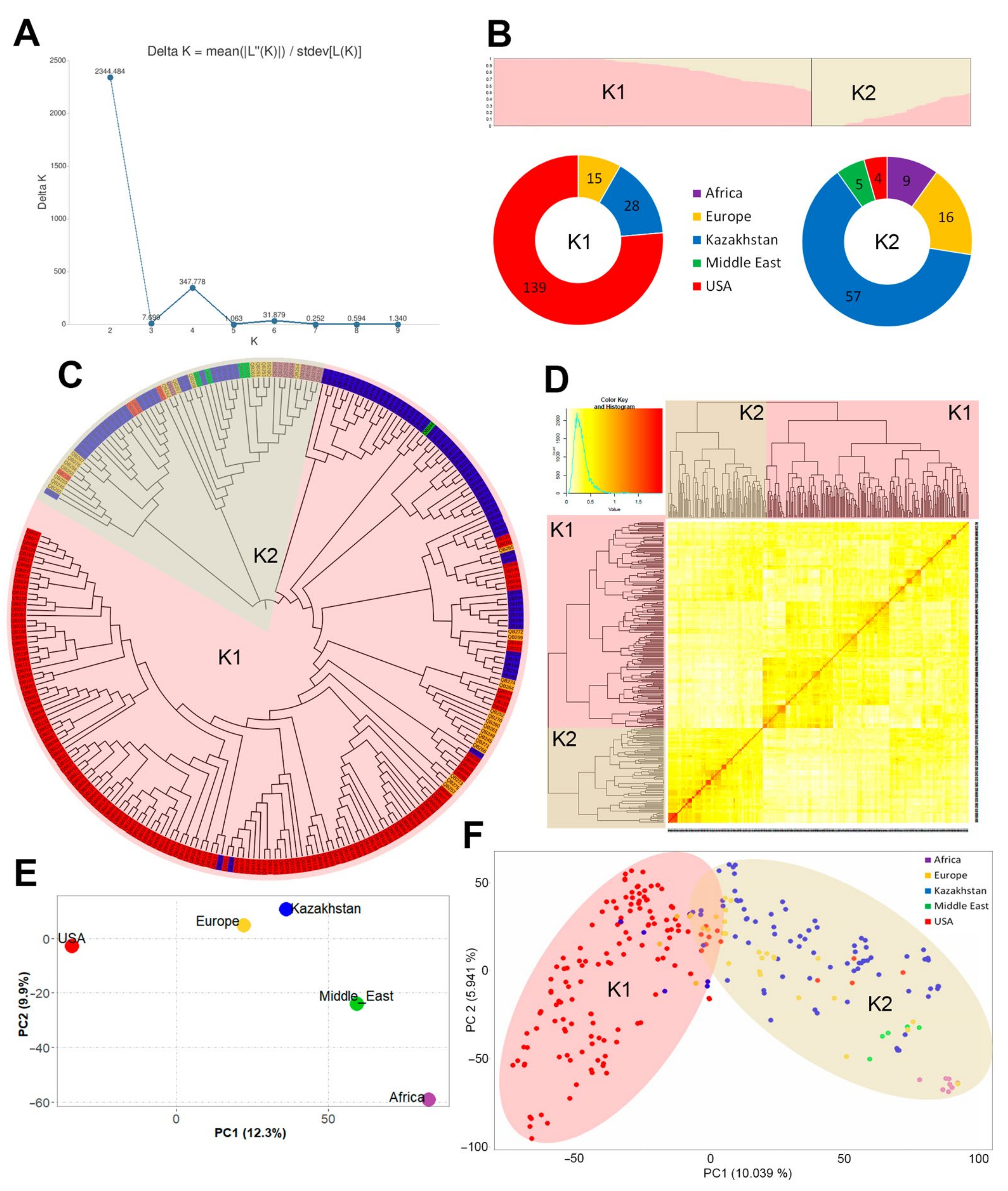


| Heading Time (HT, Days) | |||||||
|---|---|---|---|---|---|---|---|
| df | SS | MS | Variance | F-Value | p-Value | H2 (%) | |
| Genotype | 216 | 12,944 | 60 | 9.949 | 0.471 | 1.00 | 5.5 |
| Region | 1 | 109,479 | 109,479 | 84.149 | 860.343 | 2.11 × 10−21 | |
| Genotype × Region | 216 | 3901 | 18 | 2.998 | 0.142 | 1.00 | |
| Residuals | 867 | 110,327 | 127 | 84.801 | |||
| Total variance | 181.899 | ||||||
| Heading-Maturity Time (HMT, Days) | |||||||
| df | SS | MS | Variance | F-Value | p-Value | H2(%) | |
| Genotype | 216 | 3564 | 16 | 2.739 | 0.284 | 1.00 | 5.9 |
| Region | 1 | 3229 | 3229 | 2.481 | 55.633 | 2.12 × 10−13 | |
| Genotype × Region | 216 | 2967 | 14 | 2.280 | 0.237 | 1.00 | |
| Residuals | 867 | 50,329 | 58 | 38.684 | |||
| Total variance | 46.186 | ||||||
| Vegetation Period (VP, Days) | |||||||
| df | SS | MS | Variance | F-Value | p-Value | H2(%) | |
| Genotype | 216 | 7735 | 36 | 5.945 | 0.347 | 1.00 | 4.4 |
| Region | 1 | 75,103 | 75,103 | 57.727 | 727.885 | 4.22 × 10−20 | |
| Genotype × Region | 216 | 2288 | 11 | 1.758 | 0.103 | 1.00 | |
| Residuals | 867 | 89,456 | 103 | 68.759 | |||
| Total variance | 134.190 | ||||||
| Plant Height (PH, cm) | |||||||
| df | SS | MS | Variance | F-Value | p-Value | H2(%) | |
| Genotype | 216 | 22,167 | 103 | 17.038 | 0.729 | 0.998 | 13.6 |
| Region | 1 | 8100 | 8100 | 6.225 | 57.569 | 8.41 × 10−14 | |
| Genotype × Region | 216 | 10,913 | 51 | 8.388 | 0.359 | 1.00 | |
| Residuals | 867 | 121,991 | 141 | 93.767 | |||
| Total variance | 125.419 | ||||||
| Peduncle Length (PL, cm) | |||||||
| df | SS | MS | Variance | F-Value | p-Value | H2(%) | |
| Genotype | 216 | 8696 | 40.3 | 6.684 | 2.462 | 3.14 × 10−17 | 31.1 |
| Region | 1 | 3029 | 3028.8 | 2.328 | 185.199 | 2.99 × 10−17 | |
| Genotype × Region | 216 | 2045 | 9.5 | 1.571 | 0.579 | 1.00 | |
| Residuals | 867 | 14,179 | 16.4 | 10.898 | |||
| Total variance | 21.482 | ||||||
| Yield per m2 (YM2, g/m2) | |||||||
| df | SS | MS | Variance | F-Value | p-Value | H2(%) | |
| Genotype | 216 | 5,552,993 | 25,708 | 4268.251 | 0.42 | 1.00 | 8.2 |
| Region | 1 | 6,048,323 | 6,048,323 | 4648.982 | 98.732 | 1.55 × 10−17 | |
| Genotype × Region | 216 | 3,358,652 | 15,549 | 2581.593 | 0.254 | 1.00 | |
| Residuals | 867 | 52,867,291 | 61,260 | 40,635.890 | |||
| Total variance | 52,134.711 | ||||||
| QTL | Peak SNP | Chr. | Peak Pos. (bp) | Interval (bp) | MAF | Allele | p-Value | Effect 1 | Env. |
|---|---|---|---|---|---|---|---|---|---|
| QTL_VP_01 | JHI-Hv50k-2016-252385 | 1H | 13,354,477 | 8,586,441–21,024,505 | 0.121 | T | 1.89 × 10−4 | 1.8163 | 3 |
| QTL_YM2_01 | JHI-Hv50k-2016-12926 | 1H | 13,732,326 | 946,031–13,732,326 | 0.125 | G | 4.14 × 10−5 | 26.2907 | 5 |
| QTL_HMT_01 | JHI-Hv50k-2016-16625 | 1H | 24,658,016 | 24,658,016–38,812,600 | 0.151 | T | 1.79 × 10−4 | 1.8408 | 2 |
| QTL_HT_01 | JHI-Hv50k-2016-18574 | 1H | 41,433,228 | 41,433,228–59,789,397 | 0.155 | C | 9.44 × 10−5 | 1.3824 | 2 |
| QTL_VP_02 | JHI-Hv50k-2016-18606 | 1H | 41,441,320 | 35,971,057–41,441,320 | 0.151 | A | 3.78 × 10−4 | 1.1147 | 2 |
| QTL_VP_03 | JHI-Hv50k-2016-19669 | 1H | 71,853,559 | 69,604,346–71,853,559 | 0.214 | C | 1.35 × 10−4 | 1.4989 | 1 |
| QTL_HMT_02 | JHI-Hv50k-2016-23064 | 1H | 313,902,731 | 290,624,056–313,902,731 | 0.200 | C | 7.99 × 10−5 | 1.7896 | 1 |
| QTL_HMT_03 | JHI-Hv50k-2016-25561 | 1H | 351,780,525 | 340,908,307–351,780,525 | 0.109 | G | 3.66 × 10−5 | 2.3704 | 2 |
| QTL_PL_03 | JHI-Hv50k-2016-67512 | 2H | 15,345,002 | 15,345,002–19,619,940 | 0.296 | G | 6.14 × 10−4 | 0.6114 | 2 |
| QTL_HT_03 | JHI-Hv50k-2016-80632 | 2H | 67,504,945 | 47,473,212–67,722,920 | 0.162 | T | 1.80 × 10−5 | 1.3937 | 2 |
| QTL_HMT_05 | JHI-Hv50k-2016-80690 | 2H | 67,705,049 | 67,516,405–67,722,920 | 0.171 | A | 1.35 × 10−4 | 0.7800 | 1 |
| QTL_VP_05 | SCRI_RS_12492 | 2H | 543,450,750 | 532,288,289–546,728,206 | 0.170 | A | 4.83 × 10−5 | 2.2533 | 1 |
| QTL_PL_07 | JHI-Hv50k-2016–156361 | 3H | 16,388,921 | 15,509,320–16,388,921 | 0.375 | T | 1.51 × 10−4 | 0.5897 | 1 |
| QTL_YM2_06 | JHI-Hv50k-2016-182433 | 3H | 474,425,171 | 444,743,836–47,7051,144 | 0.172 | G | 3.03 × 10−4 | 75.4431 | 3 |
| QTL_VP_10 | JHI-Hv50k-2016-183028 | 3H | 487,517,028 | 482,733,343–487,517,028 | 0.196 | C | 1.48 × 10−4 | 1.2695 | 1 |
| QTL_HT_10 | BOPA1_8984-579 | 3H | 608,636,481 | 608,142,120–608,636,481 | 0.115 | C | 1.17 × 10−4 | 1.0528 | 1 |
| QTL_HMT_08 | JHI-Hv50k-2016-200877 | 3H | 611,779,208 | 605,213,263–611,857,914 | 0.105 | A | 9.97 × 10−5 | 2.9669 | 3 |
| QTL_VP_11 | JHI-Hv50k-2016-202703 | 3H | 621,964,596 | 604,674,979–622,000,270 | 0.283 | G | 1.38 × 10−4 | 1.5084 | 3 |
| QTL_PH_03 | JHI-Hv50k-2016-203453 | 3H | 624,910,313 | 598,829,887–625,131,677 | 0.285 | G | 2.35 × 10−4 | 2.4355 | 3 |
| QTL_PL_08 | JHI-Hv50k-2016-221388 | 3H | 684,467,546 | 684,258,655–705,215,146 | 0.398 | G | 5.24 × 10−5 | 0.7031 | 2 |
| QTL_HT_11 | JHI-Hv50k-2016-230933 | 4H | 16,473,681 | 9,847,402–16,473,681 | 0.347 | C | 3.70 × 10−4 | 0.8097 | 1 |
| QTL_VP_14 | JHI-Hv50k-2016-252402 | 4H | 540,116,318 | 522,567,921–541,553,980 | 0.121 | G | 1.89 × 10−4 | 1.8163 | 1 |
| QTL_HT_12 | JHI-Hv50k-2016-256960 | 4H | 57,4131,430 | 569,982,311–577,122,397 | 0.169 | C | 2.24 × 10−4 | 2.1270 | 1 |
| QTL_VP_15 | JHI-Hv50k-2016-278063 | 5H | 3,438,533 | 2,438,132–3,438,902 | 0.324 | C | 1.69 × 10−4 | 2.0597 | 2 |
| QTL_PL_10 | JHI-Hv50k-2016-304643 | 5H | 447,605,897 | 446,644,533–45,138,9893 | 0.102 | C | 2.94 × 10−4 | 1.0506 | 1 |
| QTL_HMT_12 | JHI-Hv50k-2016-309034 | 5H | 497,103,609 | 497,101,566–499,580,055 | 0.117 | G | 3.69 × 10−4 | 2.2463 | 1 |
| QTL_VP_16 | JHI-Hv50k-2016-309055 | 5H | 497,440,394 | 497,440,394–500,496,451 | 0.102 | C | 5.53 × 10−4 | 1.2983 | 2 |
| QTL_HMT_15 | JHI-Hv50k-2016-362302 | 5H | 659,419,522 | 659,417,722–659,422,073 | 0.117 | C | 2.67 × 10−4 | 1.7952 | 1 |
| QTL_PH_06 | JHI-Hv50k-2016-378195 | 6H | 18,912,150 | 11,583,956–38,574,610 | 0.174 | A | 2.27 × 10−4 | 3.1748 | 3 |
| QTL_PL_12 | JHI-Hv50k-2016-405071 | 6H | 420,155,207 | 417,602,169–420,155,207 | 0.151 | A | 2.37 × 10−4 | 1.5196 | 1 |
| QTL_VP_19 | JHI-Hv50k-2016-407075 | 6H | 460,162,406 | 460,088,561–460,162,406 | 0.137 | G | 1.86 × 10−4 | 1.2606 | 1 |
| QTL_YM2_10 | SCRI_RS_138188 | 6H | 553,764,030 | 552,273,422–559,065,543 | 0.426 | C | 1.56 × 10−4 | 36.0078 | 2 |
| QTL_HT_17 | JHI-Hv50k-2016–421541 | 6H | 554,873,294 | 552,901,600–555,717,271 | 0.143 | A | 1.61 × 10−4 | 2.1018 | 2 |
| QTL_PH_07 | JHI-Hv50k-2016–423251 | 6H | 559,845,481 | 546,897,277–569,259,569 | 0.324 | C | 8.67 × 10−5 | 1.4781 | 3 |
| QTL_VP_20 | JHI-Hv50k-2016–428246 | 6H | 572,790,688 | 569,743,557–582,529,873 | 0.100 | T | 2.06 × 10−4 | 2.4872 | 3 |
| QTL_YM2_12 | SCRI_RS_230487 | 7H | 59,806,703 | 59,719,498–68,752,632 | 0.354 | C | 1.06 × 10−4 | 34.7288 | 2 |
| QTL_PL_13 | JHI-Hv50k-2016-466975 | 7H | 65,594,444 | 65,594,444–65,632,392 | 0.199 | C | 1.56 × 10−4 | 0.8879 | 1 |
| QTL_HMT_20 | SCRI_RS_134640 | 7H | 575,017,311 | 575,017,311–584,089,113 | 0.188 | A | 1.58 × 10−4 | 1.8930 | 1 |
| Chr. | QTL Hotspot | Interval (bp) | Size (Mb) | Co-Localized QTLs | Number of Protein-Coding Genes [57] |
|---|---|---|---|---|---|
| 1H | qHv_FT_1H | 13,354,477–41,441,320 | 28.1 | QTL_VP_01 QTL_YM2_01 QTL_HMT_01 QTL_HT_01 QTL_VP_02 | 885 |
| 3H | qHv_FT_3H | 608,636,481–624,910,313 | 16.3 | QTL_HT_10 QTL_HMT_08 QTL_VP_11 QTL_PH_03 | 308 |
| 6H | qHv_FT_6H | 553,764,030–572,790,688 | 19.0 | QTL_YM2_10 QTL_HT_17 QTL_PH_07 QTL_VP_20 | 461 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genievskaya, Y.; Chudinov, V.; Abugalieva, S.; Turuspekov, Y. Novel QTL Hotspots for Barley Flowering Time, Plant Architecture, and Grain Yield. Agronomy 2024, 14, 1478. https://doi.org/10.3390/agronomy14071478
Genievskaya Y, Chudinov V, Abugalieva S, Turuspekov Y. Novel QTL Hotspots for Barley Flowering Time, Plant Architecture, and Grain Yield. Agronomy. 2024; 14(7):1478. https://doi.org/10.3390/agronomy14071478
Chicago/Turabian StyleGenievskaya, Yuliya, Vladimir Chudinov, Saule Abugalieva, and Yerlan Turuspekov. 2024. "Novel QTL Hotspots for Barley Flowering Time, Plant Architecture, and Grain Yield" Agronomy 14, no. 7: 1478. https://doi.org/10.3390/agronomy14071478





