The Isolation and Identification of a New Pathogen Causing Sunflower Disk Rot in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Culture
2.2. DNA Extraction, PCR Amplification, and DNA Sequencing
2.3. Pathogenicity Test
2.4. Re-Isolation of the Pathogens on Seeds
2.5. Fungicide Sensitivity Evaluation
| Fungicide Name | Dosage Form | Manufacturer Information |
|---|---|---|
| Tebuconazole · dimetachlone | 70% WG | Wilda Chemical Co., Ltd., Hangzhou, China |
| Pyraclostrobine | 25% EC | BASF (China) Co., Ltd., Shanghai, China |
| Iprodione | 500 g/L SC | Suzhou Fumeishi Plant Protector Co., Ltd., Suzhou, China |
| Hymexazol | 98% WP | Lvheng Biotechnology Co., Ltd., Jinan, China |
| Flutolanil | 42.4% SC | BASF (China) Co., Ltd., Nantong, China |
| Fludioxonil | 25 g/L FS | Syngenta Nantong Crop Protection Co., Ltd., Nantong, China |
3. Results
3.1. Symptoms of Sunflower Disk Rot (SDR)
3.2. Pathogen Isolation and Characterization
3.3. Pathogen Identification Molecularly
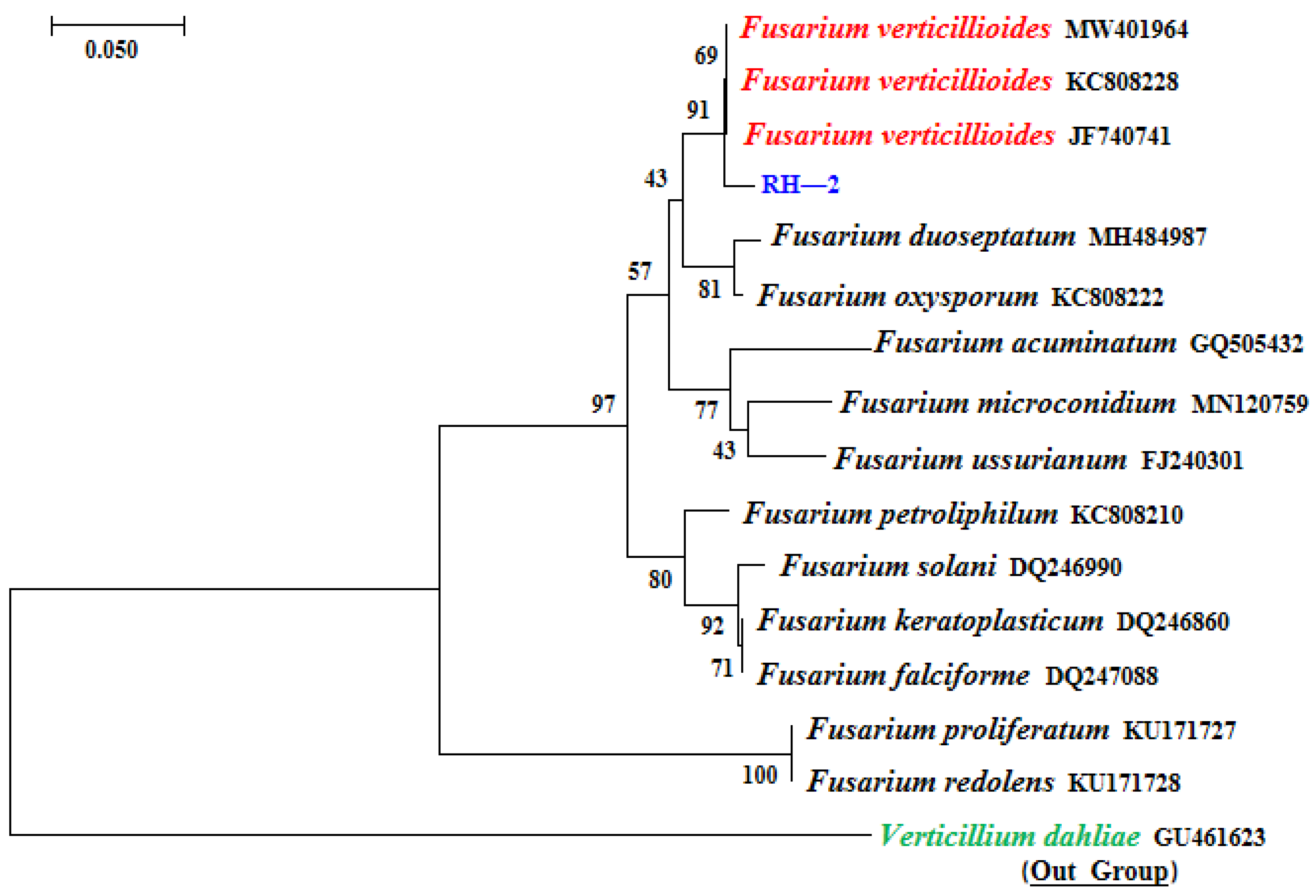
3.4. Phylogenetic Analyses
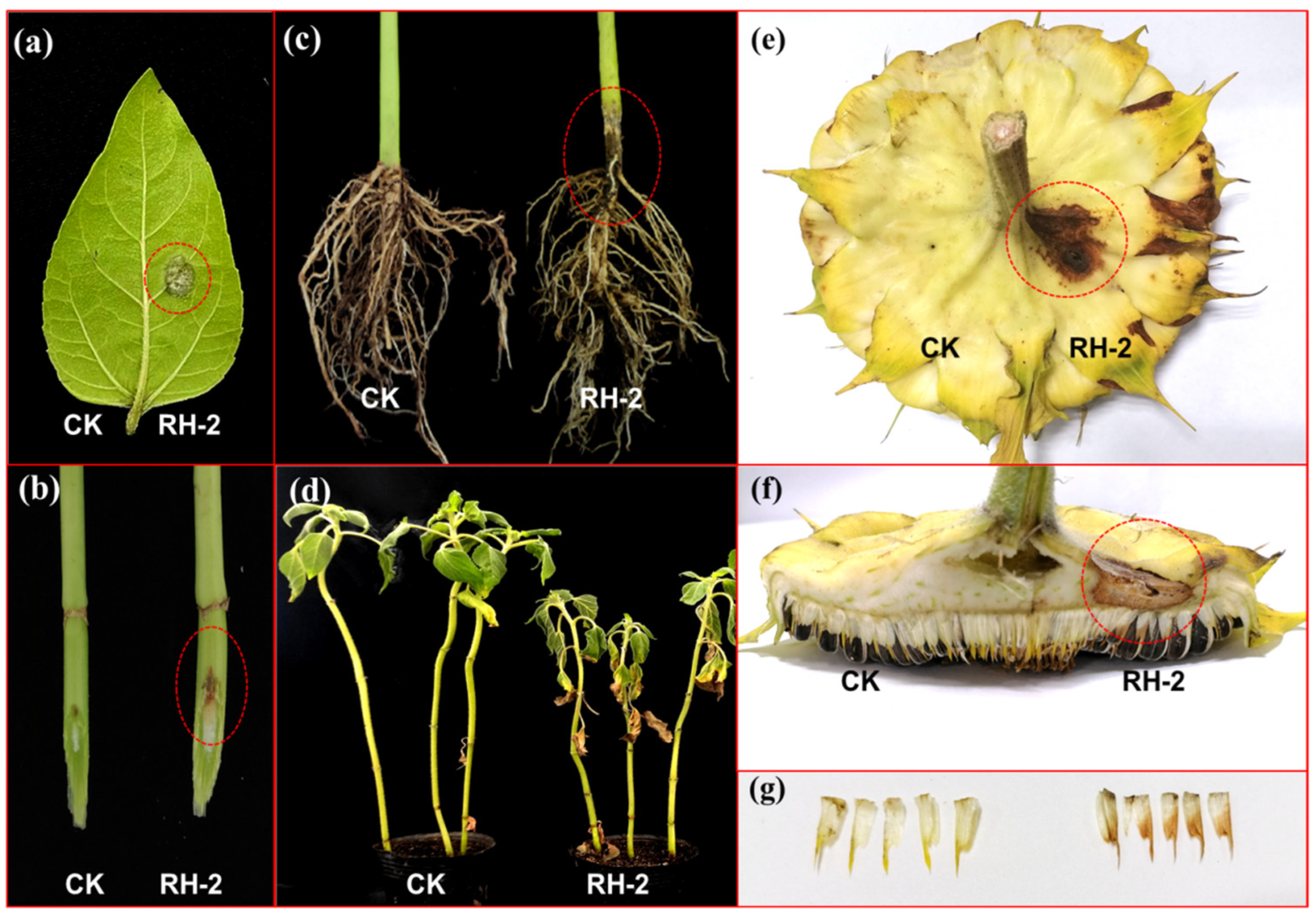
3.5. Pathogenicity Identification
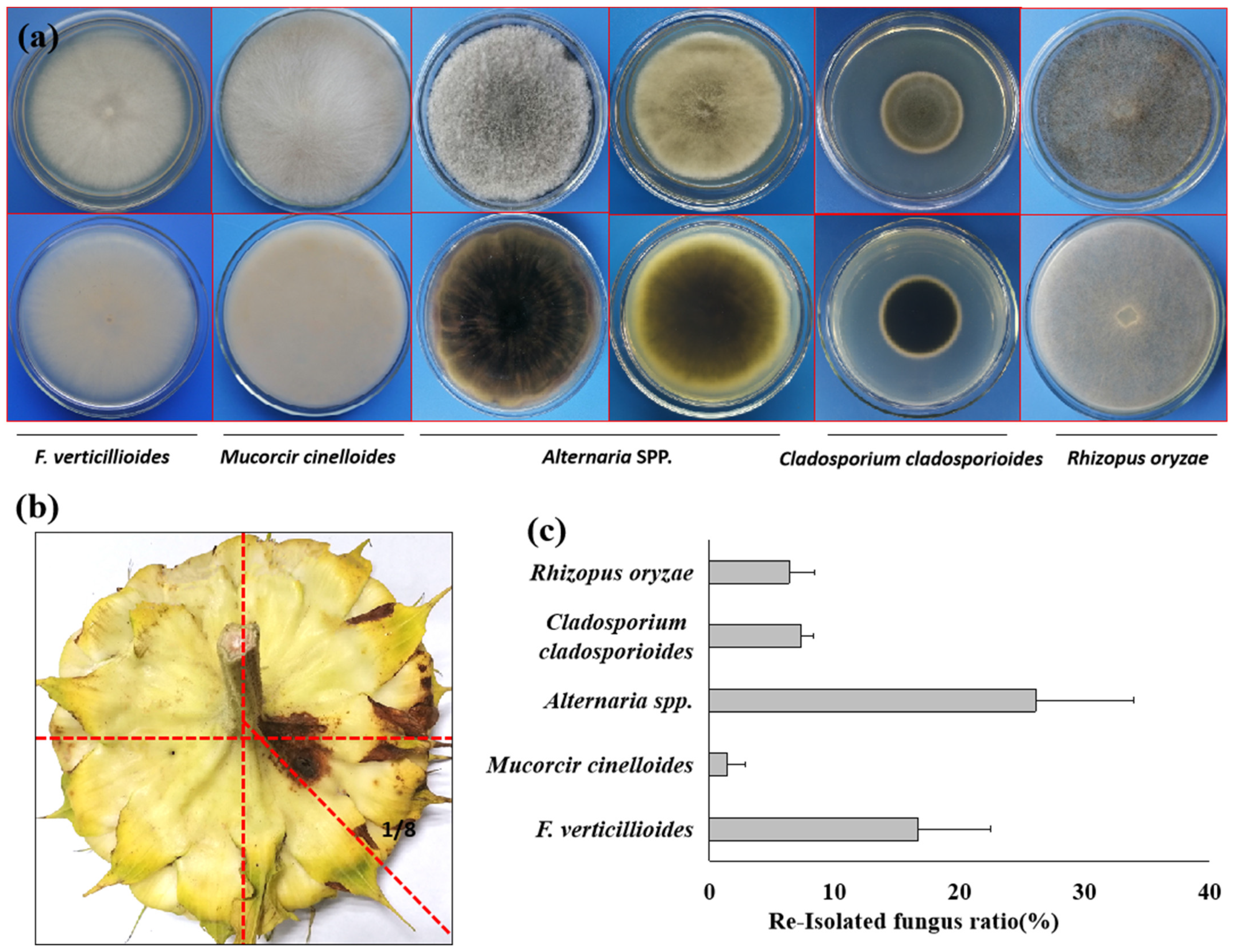
3.6. Pathogen Reisolation
3.7. Fungicides Sensitivity of F. verticillioides
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jockovic, M.; Cveji, S.; Joci, S.; Jeromela, A.M.; Radic, V. Evaluation of sunflower hybrids in multi-environment trial (met). Turk. J. Field Crops 2019, 24, 202–210. [Google Scholar] [CrossRef]
- Guo, S.C.; LI, S.P.; Sun, R.F. Analysis of the overall development of sunflower industry in the world and China. China Seed Ind. 2021, 10–13. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, K.Y.; Han, S.C.; Zhao, R.; Wen, Y.J.; Hu, H.C.; Qiao, Y.M.; Lu, J.F.; Cao, K.; Xu, Z.H.; Bao, H.Z.; et al. Current situation and prospects of sunflower production and consumption in China. Agric. Outlook 2023, 19, 64–71. (In Chinese) [Google Scholar]
- Kim, J.H.; Joen, Y.H.; Kim, S.G.; Kim, Y.H. First report on bacterial soft rot of graftcactus chamaecereus silvestrii caused by pectobacterium carotovorum subsp. carotovorum in korea. Plant Pathol. J. 2007, 23, 314–317. [Google Scholar] [CrossRef]
- Bauftauf, K.K.; Hekimhan, H.; Maden, S.; Tufr, M. First report of bacterial stalk and head rot disease caused by pectobacterium atrosepticum on sunflower in turkey. Plant Dis. 2009, 93, 1352. [Google Scholar]
- Rasera, K.; Osório, N.M.; Mitchell, D.A.; Krieger, N.; Ferreira-Dias, S. Interesterification of fat blends using a fermented solid with lipolytic activity. J. Mol. Catal. B Enzym. 2002, 76, 75–81. [Google Scholar] [CrossRef]
- Mathew, F.M.; Prasifka, J.R.; Gaimari, S.D.; Shi, L.; Gulya, T.J. Rhizopus oryzae associated with melanagromyza splendida and stem disease of sunflowers (helianthus annuus) in california. Plant Health Prog. 2015, 16, 39–42. [Google Scholar] [CrossRef]
- Gregoire, T.; Lamey, A.; Hofman, V. Sclerotinia head rot of sunflower. Magn. Reson. Imaging Clin. N. Am. 2010, 21, 6–18. [Google Scholar]
- Gai, X.T.; Jiang, N.; Lu, C.H.; Xia, Z.Y.; He, Y.S. First report of tobacco fusarium root rot caused by Fusarium verticillioides in china. Am. Phytopathol. Soc. 2021. [Google Scholar] [CrossRef]
- Chen, X.; Liu, D.; Zhang, Y.; Qin, Z.; Zhou, X. Isolation and identification of fusarium from cucumber wilt plants. J. Northeast Agric. Univ. 2010, 41, 37–44. [Google Scholar] [CrossRef]
- Moreira, S.I.; Melo, M.P.; Andrade, C.C.L.; Alves, E.; Pereira, O.L. Postharvest ginger rhizome rot caused by Fusarium verticillioides in brazil. Plant Dis. 2015, 99, 1177. [Google Scholar] [CrossRef]
- An, Y.N.; Murugesan, C.; Choi, H.; Kim, K.D.; Chun, S.C. Current Studies on Bakanae Disease in Rice: Host Range, Molecular Identification, and Disease Management. Mycobiology 2023, 51, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Borah, S.N.; Deka, S.; Sarma, H.K. First report of Fusarium verticillioides causing stalk rot of maize in assam, india. Plant Dis. 2016, 100, 1501. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Karlovsky, P. Systemic infection of maize, sorghum, rice, and beet seedlings with fumonisin-producing and nonproducing Fusarium verticillioides strains. Plant Pathol. J. 2015, 31, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Tahat, M.M.; Aldakil, H.; Alananbeh, K.; Salem, N.M. First Report of Fusarium verticillioides Causing Fusarium Ear Rot of Corn in Jordan. Plant Dis. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mehl, O.H.L. Fusarium hardlock associated with Lygus lineolaris (hemiptera: Miridae) injury in southeastern cotton. Plant Dis. 2021, 105, 53–59. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. lsolation of plant DNA from fresh tissue. Focus 1990, 12, 39–40. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protocl. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- Yan, M.G. Species diversity study and new species identification of endophytic Fusarium oxysporum in Longblood tree of Yunnan. Master’s Thesis, Yunnan University, Kunming, China, 2022. (In Chinese) [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Chen, C.; Zhou, M. Sensitivity of Fusarium graminearum to fungicide js399-19: In vitro determination of baseline sensitivity and the risk of developing fungicide resistance. Phytoparasitica 2008, 36, 326–337. [Google Scholar] [CrossRef]
- Addrah, M.E.; Zhang, Y.; Zhang, J.; Liu, L.; Zhou, H.; Chen, W.; Zhao, J. Fungicide Treatments to Control Seed-borne Fungi of Sunflower Seeds. Pathogens 2019, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.G.; Ye, Z.Y.; Liu, J.F. Progress of fungicide resistance. J. Nanjing Agric. Univ. 1994, 17, 33–41. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual Ⅱ A Brief History of Fusarium Taxonomy; Blackwell Publishing: Hoboken, NJ, USA, 2006. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.J.; Wang, C.B.; Tan, B.Y.; Luo, X.D. New and Antifungal Diterpenoids of Sunflower against Gray Mold. J. Agric. Food Chem. 2023, 71, 16647–16656. [Google Scholar] [CrossRef] [PubMed]
- Custers, J.H.; Harrison, S.J.; Sela-Buurlage, M.B.; van Deventer, E.; Lageweg, W.; Howe, P.W.; van der Meijs, P.J.; Ponstein, A.S.; Simons, B.H.; Melchers, L.S.; et al. Isolation and characterisation of a class of carbohydrate oxidases from higher plants, with a role in active defence. Plant J. 2004, 39, 147–160. [Google Scholar] [CrossRef]
- Dong, F.; Li, Y.; Chen, X.; Wu, J.; Wang, S.; Zhang, X.; Ma, G.; Lee, Y.W.; Mokoena, M.P.; Olaniran, A.O.; et al. Analysis of the Fusarium graminearum species complex from gramineous weeds near wheat fields in Jiangsu Province, China. Plant Dis. 2021, 105, 3269–3275. [Google Scholar] [CrossRef]
- Gai, X.T.; Xuan, Y.H.; Gao, Z.G. Diversity and pathogenicity of Fusarium graminearum species complex from maize stalk and ear rot strains in northeast China. Plant Pathol. 2017, 66, 1267–1275. [Google Scholar] [CrossRef]
- Chang, X.; Dai, H.; Wang, D.; Zhou, H.; He, W.; Fu, Y.; Ibrahim, F.; Zhou, Y.; Gong, G.; Shang, J.; et al. Identification of Fusarium species associated with soybean root rot in Sichuan Province, China. Eur. J. Plant Pathol. 2018, 151, 563–577. [Google Scholar] [CrossRef]
- Cao, S.; Yang, N.; Zhao, C.; Liu, J.; Han, C.; Wu, X. Diversity of Fusarium species associated with root rot of sugar beet in China. J. Gen. Plant Pathol. 2018, 84, 321–329. [Google Scholar] [CrossRef]
- El Mahjoub, M. Vascular fusarium wilt of sunflower in Tunisia caused by Fusarium oxysporum (Sn. et H.) F. sp. helianthi nov. sp. [French]. Ann. De L’institut Natl. De La Rech. Agron. De Tunis. 1975, 48, 3–11. [Google Scholar]
- Haggag, W.M.; Amin, A.W. Efficiency of trichoderma species on control of Fusarium rot, root knot and reniform nematodes disease complex on sunflower. Pak. J. Biol. Sci. 2001, 4, 679–683. [Google Scholar] [CrossRef]
- Pineda, P.J.B.; Avila, M.J.M. Management and control of sunflower disease in Portuguesa State. FONAIAP Divulg. 1991, 9, 6–8. [Google Scholar]
- Bhargava, S.N.; Shukla, D.N.; Singh, N. Wilt of sunflower caused by Fusarium moniliforme. Indian Phytopathol. 1978, 31, 382–383. [Google Scholar]
- Zazzerini, A. New sunflower disease caused by Fusarium tabacinum. Plant Dis. 1987, 71, 1043–1104. [Google Scholar] [CrossRef]
- Pozzi, C.R.; Braghini, R.; Arcaro, J.R.; Zorzete, P.; Israel, A.L.; Pozar, I.O.; Denucci, S.; Corrêa, B. Mycoflora and occurrence of alternariol and alternariol monomethyl ether in Brazilian sunflower from sowing to harvest. J. Agric. Food Chem. 2005, 53, 5824–5828. [Google Scholar] [CrossRef]


| Number | Taxon | Voucher Information | Genebank ID | |
|---|---|---|---|---|
| TEF-1a | RPB1 | |||
| 001 | Fusarium duoseptatum | CBS 102026 | MH484987 | MH484896 |
| 002 | Fusarium microconidium | CBS 119843 | MN120759 | MN120721 |
| 003 | Fusarium proliferatum | NRRL 62905 | KU171727 | KU171687 |
| 004 | Fusarium ussurianum | NRRL 45681 | FJ240301 | KM361648 |
| 005 | Fusarium redolens | NRRL 22901 | KU171728 | MT409432 |
| 006 | Fusarium verticillioides | NRRL 25115 | JF740741 | HM347183 |
| 007 | Fusarium verticillioides | NRRL 22172 | MW401964 | MW402497 |
| 008 | Fusarium verticillioides | NRRL 54997 | KC808228 | KC808308 |
| 009 | Fusarium oxysporum | NRRL 62545 | KC808222 | KC808305 |
| 010 | Fusarium acuminatum | NRRL 45994 | GQ505432 | KC808324 |
| 011 | Fusarium keratoplasticum | NRRL 25391 | DQ246860 | KC808311 |
| 012 | Fusarium petroliphilum | NRRL 54988 | KC808210 | KC808287 |
| 013 | Fusarium falciforme | NRRL 32778 | DQ247088 | KC808315 |
| 014 | Fusarium solani | NRRL 32492 | DQ246990 | EU329584 |
| 015 | Verticillium dahliae | Le1340 | GU461623 | — — |
| Sequencing Primers | Description | Scientific Name | Max Score | Total Score | Query Cover | Per. Ident | Acc.Len | Accession |
|---|---|---|---|---|---|---|---|---|
| Internal transcribed spacer 1 (ITS-1) gene | Fusarium verticillioides isolate CYQ007 | F. verticillioides | 922 | 922 | 99% | 98.84% | 522 | ON565434.1 |
| Gibberella fujikuroi isolate m8 | Gibberella. sp | 920 | 920 | 99% | 98.84% | 660 | MW405885.1 | |
| Fusarium verticillioides isolate s10 | F. verticillioides | 920 | 920 | 99% | 98.84% | 578 | MW405886.1 | |
| Fusarium verticillioides isolate MRRS1 | F. verticillioides | 918 | 918 | 97% | 99.22% | 517 | OQ363325.1 | |
| Fusarium verticillioides isolate ZC1-16 | F. verticillioides | 917 | 917 | 99% | 98.84% | 520 | OR884160.1 | |
| Translation glongation factor 1-alpha gene(Tef-1ɑ) | Fusarium verticillioides isolate FUG _6 | F. verticillioides | 904 | 904 | 99% | 98.63% | 712 | OQ957222.1 |
| Fusarium verticillioides isolate FB _8 | F. verticillioides | 904 | 904 | 100% | 98.44% | 614 | KU372144.1 | |
| Fusarium verticillioides isolate A293 | F. verticillioides | 904 | 904 | 100% | 98.44% | 631 | OQ948493.1 | |
| Fusarium verticillioides isolate CN125A2 | F. verticillioides | 904 | 904 | 100% | 98.44% | 593 | ON602014.1 | |
| Fusarium verticillioides isolate XC26 | F. verticillioides | 904 | 904 | 100% | 98.44% | 657 | ON649853.1 | |
| DNA-directed RNA polymerase II largest subunit (rpb1)gene | Fusarium verticillioides strain NRRL 22172 | F. verticillioides | 1749 | 1749 | 100% | 100% | 1555 | MN242935.1 |
| Fusarium verticillioides strain AmP10 | F. verticillioides | 1749 | 1749 | 100% | 100% | 1526 | OR841329.1 | |
| Fusarium verticillioides strain LY1306-6 | F. verticillioides | 1749 | 1749 | 100% | 100% | 1792 | OR178428.1 | |
| Fusarium verticillioides isolate ZCK-1 | F. verticillioides | 1749 | 1749 | 100% | 100% | 1616 | ON692996.1 | |
| Fusarium verticillioides strain LC13653 | F. verticillioides | 1749 | 1749 | 100% | 100% | 1563 | MW024492.1 |
| Drug Name | Treatment Concentration (μg/Ml) | Concentration Logarithm (x) | Inhibition Rate % | Probability Value (Y) | Virulence Regression Equation | EC50 (µg/mL) | R |
|---|---|---|---|---|---|---|---|
| Tebuconazole · dimetachlone | 280.00 | 2.45 | 86.54 | 6.10 | y = 1.3549x + 2.7613 | 45.23 | 0.9972 |
| 140.00 | 2.15 | 74.84 | 5.67 | ||||
| 70.00 | 1.85 | 58.21 | 5.21 | ||||
| 35.00 | 1.54 | 43.97 | 4.85 | ||||
| 17.50 | 1.24 | 30.02 | 4.48 | ||||
| Pyraclostrobine | 128.00 | 2.11 | 84.16 | 6.00 | y = 0.8295x + 4.2317 | 8.44 | 0.9907 |
| 64.00 | 1.81 | 75.77 | 5.70 | ||||
| 32.00 | 1.51 | 68.83 | 5.49 | ||||
| 16.00 | 1.20 | 58.61 | 5.22 | ||||
| 8.00 | 0.90 | 49.72 | 4.99 | ||||
| Iprodione | 500.00 | 2.70 | 59.11 | 5.23 | y =0.5142x + 3.8324 | 186.21 | 0.9919 |
| 250.00 | 2.40 | 52.35 | 5.06 | ||||
| 125.00 | 2.10 | 46.72 | 4.92 | ||||
| 62.50 | 1.80 | 39.02 | 4.72 | ||||
| 31.25 | 1.49 | 35.39 | 4.63 | ||||
| Hymexazol | 32.00 | 1.51 | 49.88 | 5.00 | y =0.7380x + 3.9027 | 30.68 | 0.9943 |
| 16.00 | 1.20 | 42.11 | 4.80 | ||||
| 8.00 | 0.90 | 33.33 | 4.57 | ||||
| 4.00 | 0.60 | 26.95 | 4.39 | ||||
| 2.00 | 0.30 | 18.24 | 4.09 | ||||
| Flutolanil | 160.00 | 2.20 | 76.39 | 5.72 | y =0.3252x + 5.0058 | 0.96 | 0.9964 |
| 80.00 | 1.90 | 73.60 | 5.63 | ||||
| 40.00 | 1.60 | 69.78 | 5.52 | ||||
| 20.00 | 1.30 | 67.07 | 5.44 | ||||
| 10.00 | 1.00 | 62.71 | 5.32 | ||||
| Fludioxonil | 5.00 | 0.70 | 76.39 | 5.72 | y =0.3519x + 5.4496 | 0.05 | 0.9825 |
| 1.00 | 0.00 | 65.75 | 5.41 | ||||
| 0.20 | −0.70 | 59.30 | 5.24 | ||||
| 0.05 | −1.30 | 48.74 | 4.97 | ||||
| 0.01 | −2.00 | 40.45 | 4.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wang, Y.; Shi, S.; Li, H.; Zhang, W.; Addrah, M.E.; Zhang, J.; Zhao, J. The Isolation and Identification of a New Pathogen Causing Sunflower Disk Rot in China. Agronomy 2024, 14, 1486. https://doi.org/10.3390/agronomy14071486
Yang J, Wang Y, Shi S, Li H, Zhang W, Addrah ME, Zhang J, Zhao J. The Isolation and Identification of a New Pathogen Causing Sunflower Disk Rot in China. Agronomy. 2024; 14(7):1486. https://doi.org/10.3390/agronomy14071486
Chicago/Turabian StyleYang, Jianfeng, Yujie Wang, Shenghua Shi, Haoyu Li, Wenbing Zhang, Mandela Elorm Addrah, Jian Zhang, and Jun Zhao. 2024. "The Isolation and Identification of a New Pathogen Causing Sunflower Disk Rot in China" Agronomy 14, no. 7: 1486. https://doi.org/10.3390/agronomy14071486





