Abstract
The fungal pathogen Setosphaeria turcica causes northern corn leaf blight (NCLB) in maize, sorghum, and related grasses. NCLB is a serious fungal foliar disease of cultivated maize that causes devastating yield losses. S. turcica infects maize leaves by means of a specialized cell, the appressorium, but the regulatory mechanisms that underlie appressorium-mediated infection remain largely unknown. Many regulatory pathways and a large number of genes have been described in S. turcica, and many of these genes have been cloned. Characterization of such disease-related genes is important for understanding the biological mechanisms of interaction between pathogen and host and can guide the development of strategies for disease control. There is a significant level of concern regarding the possible dissemination of the S. turcica pathogen to regions where NCLB is not presently prevalent. This scenario is of considerable concern and necessitates immediate research intervention. The present review brings together information on the epidemiology and infection mechanisms of S. turcica.
1. Introduction
Maize (Zea mays L.) is consumed as a staple food around the world. Over 4.5 billion people worldwide rely on maize for at least 30% of their food calories [1]. The worsening effects of plant disease outbreaks jeopardize maize production and have far-reaching consequences for food security. Setosphaeria turcica, also known as Exserohilum turcicum (formerly classified as Helminthosporium turcicum), is a fungal pathogen that mostly induces foliar disease in maize plants, commonly referred to as northern corn leaf blight (NCLB) [1]. Extensive research has been conducted so far, which demonstrates that NCLB reduces maize yield by up to 50% with serious outbreaks by promoting defoliation and thus halting absorption of photosynthetically active radiation during the grain-filling period.
S. turcica spreads by conidial propagation and infects plants using mature appressoria. The occurrence of NCLB depends on the successful invasion of the pathogen upon contact with the host surface to germinate the conidium and develop an appressorium to penetrate into plant epidermal cells. Initiation of infection includes such processes as conidial germination, germ tube formation, appressorium development, maturation, and penetration peg invasion [2]. Appressorium-mediated infection of maize by S. turcica is regulated by multiple pathways.
In summary, S. turcica-caused NCLB infection is a major threat to reducing maize yield worldwide. In this review, we explored the current knowledge on outbreaks of northern corn leaf blight. In addition, appressorium-mediated infection pathways such as S-phase checkpoint, the cyclic adenosine monophosphate (cAMP), mitogen activated protein kinase (MAPK), Ca2+ signal transduction, and guanine nucleotide-binding protein (G protein) pathways are discussed in detail [2,3,4,5]. However, in-depth research is needed to fully explore the mechanisms underlying appressorium development and pathogenicity of S. turcica.
2. Outbreaks of Northern Corn Leaf Blight
NCLB, also known as corn spot disease and turcicum leaf blight (TLB), is widely distributed in temperate climates throughout the world, as well as tropical and subtropical areas [6,7]. NCLB was first reported in New Jersey, USA, as early as 1878. The disease affects maize quality and yield by causing withering or even early shedding of leaves [8]. In recent years, sensitive temperate maize germplasm has been introduced to tropical environments, breaking quantitative resistance [9]. In addition, insufficient implementation of field cultivation management measures in China has led to large amounts of overwintering leaf spot pathogens in the field, causing NCLB to increase year after year and become the main disease in corn production.
NCLB mainly harms corn leaves, sometimes infects the leaf sheath and bract, but does not directly harm corn seeds; the disease gradually extends upward from the lower part of the plant [9]. Water-stained bluish-gray spots appear after the leaves are infected, then spread to both ends along the veins, forming oval spots with dark brown edges and light brown or bluish-gray centers, about 3–15 cm in length. Under wet conditions, large numbers of conidia accumulate and become grayish-black as the lesions mature. As disease development continues, the disease spots often crack longitudinally, and in severe cases, the spots fuse to form a large withered area that may encompass the entire leaf (Figure 1). Therefore, the disease mainly reduces the leaf area for light interception and lowers the chlorophyll content of the plants by either damaging or killing the leaf tissue, thereby affecting quality and yield.

Figure 1.
Northern corn leaf blight symptoms. (Photographed by Dong Jingao in September 2023 in Yu County, Hebei).
The effects of NCLB continue from the seedling period through the harvest period [8,10]. If the disease occurs at an early stage of corn growth, the leaves wither and die prematurely, thus causing the green stored corn to lose its nutritional value as feed. Seed yield and quality may also be affected, and seeds may show lower total sugar content, reduced germination capacity, and impaired starch formation. The extent of maize yield loss caused by NCLB is influenced by various factors, including environmental conditions, infection period, disease severity, and cultivar susceptibility. As the severity of leaf blight increases, the carbon dioxide emission from infected leaves decreases significantly, and leaf temperature and transpiration rate increase. When the weather is wet and rainy, spores make the diseased spots appear dark gray, olive, or black, and a gray-black mold layer appears on the spots [11]. Generally speaking, yield loss is highest, and ears and leaves are most heavily damaged when infection occurs before silking [11]. When the disease is serious, yield losses of early-maturing, sensitive corn varieties can be as high as 63%, and even as high as 90% in tropical corn-producing areas of India. Yield losses can reach 43% in middle-maturing varieties with quantitative resistance and 17% in late-maturing varieties with quantitative and qualitative resistance [12].
In the field, maize lesions grow 1.6–3.9 times faster at night than during the day. Shorter daylengths during the growing season thus enhance lesion growth, and this is one factor that makes NCLB so severe in tropical and subtropical regions [13]. Nonetheless, highly aggressive S. turcica isolates can compensate for suboptimal weather conditions, causing severe epidemics in temperate zones as well. In dead leaf tissue, sporulation commences under cloudy skies with a daylength of ~12 h, an extended period of high relative humidity (>90%), and a minimum dew period of 14 h, all of which promote higher spore production [13]. This secondary inoculum spreads to other maize leaves, thus continuing the infection cycle.
3. Signaling Pathways Regulating the Pathogenicity of S. turcica
The infection process of S. turcica relies on a specific infection structure, the appressorium, that develops through a number of stages. Early infection begins when a conidium of S. turcica lands on the maize leaf surface and germinates to form a highly polarized germ tube, which elongates and flattens against the plant surface. Growth at the germ tube tip then ceases, and the tip swells isotropically, resulting in the formation of a dome-shaped appressorium. The appressorium generates high turgor pressure, which is used by the fungus as a mechanical force for physical penetration of the plant cuticle and cell wall, enabling its entry into the underlying epidermal cells. Finally, the diseased spots produce new conidia to initiate a new disease cycle. From the perspective of cell biology, development of the appressorium is a typical coordinated process of cell division and differentiation [14].
Regulation of the cell cycle has become an important focus of research on pathogenic fungi in recent years. The latest research shows that S-phase checkpoint pathways control the formation, development, and invasion growth of appressoria in phytopathogenic fungi [2]. The S-phase checkpoint acting through the DNA damage response (DDR) signaling pathway inhibits the cell division cycle, and causes growth arrest of the appressorium in the G2 phase. Initiation of appressorium development depends upon DNA replication in the germinating conidium, which is therefore vulnerable to environmental genotoxic stresses that damage DNA or interfere with its replication. Because maintenance of genome integrity is critical for species reproduction, S. turcica has developed strategies to balance self-duplication and infection in the presence of genotoxic stress by blocking appressorium formation and subsequent maize infection. This regulatory mechanism requires the cell cycle S-phase checkpoint and the checkpoint effector kinase ataxia telangiectasia and Rad3 related (ATR): inhibition of ATR activity or knockdown of ATR leads to the recovery of appressorium formation under genotoxic stress. In addition to blocking appressorium formation, ATR also triggers other protective strategies, including melanin biosynthesis mediated by polyketide synthase (PKS) (Figure 2) and upregulation of autophagy-related gene expression. A number of genes related to secondary metabolites that function during infection were also downregulated in S. turcica during genotoxic stress. We therefore propose that S. turcica uses the ATR-dependent pathway to limit the transcription of genes that govern appressorium-mediated infection while inducing the transcription of genes related to autophagy. This process likely serves as a protective mechanism, in addition to the conserved function of the ATR-dependent pathway in eukaryotes [2].
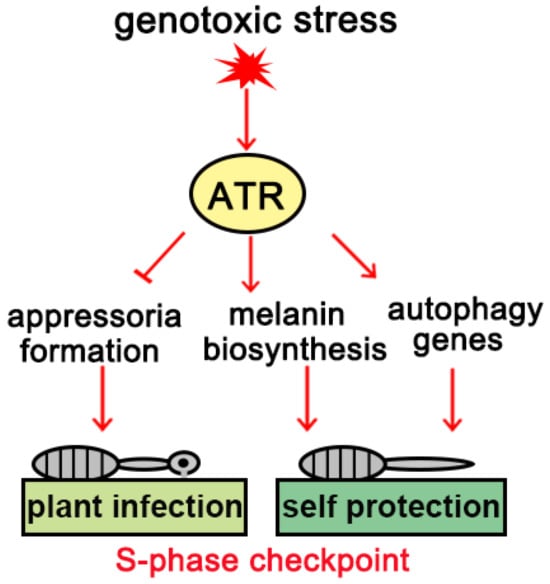
Figure 2.
Working model of cell cycle checkpoints.
The development of the appressorium plays a decisive role in the pathogenicity of S. turcica. Hyphal growth, conidiation, sexual reproduction, and penetration are promoted by the activation of multiple signal transduction pathways, including the cAMP, MAPK, and Ca2+ signaling pathways, which are mediated by G proteins [4,7,15,16,17,18,19,20]. The cAMP pathway plays an important regulatory role in the development and pathogenicity of filamentous fungi (Figure 3). cAMP-dependent protein kinase A (PKA) is a conserved element that acts downstream of cAMP. In response to extracellular stimuli, cAMP binds to the regulatory subunit of PKA (PKA-R), causing it to dissociate from the PKA holoenzyme and thus releasing the catalytic subunit (PKA-C) to phosphorylate downstream targets [21]. StPKA-C is an important regulator of pathogenesis during S. turcica–host interactions and regulates pathogenicity by modulating conidiogenesis, appressorial formation and penetration, melanin biosynthesis, and xylanase activity. Multiple isoforms of PKA-C, including PkaC1 and PkaC2, have been identified in S. turcica and shown to function in different ways [3]. Both StPkaC1 and StPkaC2 are necessary for conidiation and invasive growth, but only StPkaC2 negatively regulates filamentous growth. StPkaC2 interacts with the transcription factor StEfg1 to inhibit the transcription of StRAB1, which encodes a Rab GTPase homolog in S. turcica. By contrast, StPkaC1 interacts specifically with the transcriptional regulator StFlo8, preventing the transcriptional inhibition of StRAB1 by StEfg1. StRAB1 promotes the biosynthesis of chitin in hyphae, thereby promoting filamentous growth, but StPkaC2 inhibits chitin biosynthesis and thus mycelium development by promoting the Efg1-mediated inhibition of StRAB1 [3].
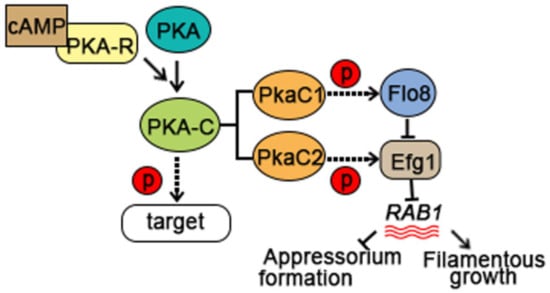
Figure 3.
The cAMP pathway contributes to S. turcica pathogenicity during the early stages of maize infection.
The MAPK family of serine/threonine protein kinases is involved in the transduction of various extracellular signals and regulation of multiple developmental processes in eukaryotic cells. MAP kinase cascades generally consist of three protein kinases that act in series: MAPK, MAPK kinase (MAPKK), and MAPKK kinase (MAPKKK). Sequential activation of the MAPK cascade eventually results in the activation of transcription factors and expression of a specific set of genes in response to environmental stimuli [22,23,24]. The MAPK kinase gene StPBS2 in S. turcica has an essential role in hyphal morphogenesis, condiogenesis, cell wall development, hypertonic stress response, and biosynthesis of secondary metabolites [4] (Figure 4A). StE12, which functions downstream of the Fus3/Kss1-homolog MAPK cascade in S. turcica, is a key transcription factor that is required for pathogenicity because of its roles in vegetative growth, conidiation, appressorium development, and penetration (Figure 4A). The activation of the HOG-MAPK pathway and the expression of its downstream genes are quickly upregulated at the transcriptional level to respond to osmotic stress. Importantly, it was demonstrated that osmotic stress affects the morphology of hypha, shortens the germination time of conidia, alters the structure of invasive hyphae, and enhances the pathogenicity of S. turcica. Genetic analysis revealed that StFPS1, a key gene downstream of the HOG-MAPK pathway, influences the development of appressorium and the penetration ability of maize [25].
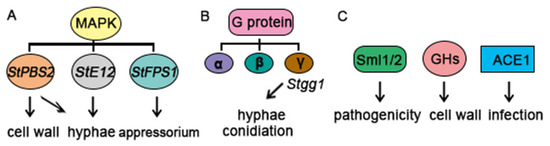
Figure 4.
Multiple pathways contribute to S. turcica pathogenicity in the early stages of maize infection. (A) StPBS2, StE12, and StFPS1 in the MAPK signaling pathway play important roles in the regulation of S. turcica. (B) G proteins are composed of separate genes that encode α, β, and γ subunits. Stgg1, a Gγ subunit, is involved in controlling conidiation and hyphal shape. (C) The cytoskeletal proteins Slm1/2, glycosyl hydrolase (GH) proteins, and effector (StACE1) have a significant impact on the pathogenicity, cell wall, and infection of S. turcica.
The evolutionarily conserved G protein signaling pathway is composed of G protein-coupled receptors (GPCRs, also known as seven-transmembrane receptors), heterotrimeric G proteins, and diverse downstream effectors [26]. GPCRs are the largest family of transmembrane receptor proteins and can transmit a variety of environmental signals, such as hormones, nutrients, light, odors, and chemoattractants, to the intracellular heterotrimeric G proteins [27,28]. These G proteins consist of α, β, and γ subunits encoded by independent genes. Our previous work in S. turcica demonstrated that the Gγ subunit Stgg1 is involved in the regulation of hyphal morphology and conidiation but is dispensable for colony growth (Figure 4B). Stgg1 is also required for the biogenesis of essential secondary metabolites, such as melanin and HT-toxin, that function in the pathogenesis of S. turcica [5].
The penetration peg produced by the differentiation of the appressorium pore pierces the plant epidermis and requires specific plant-cell-wall degrading enzymes, such as pectinase, cellulase, and hemicellulase, in addition to the physical and mechanical force caused by turgor pressure [29,30]. The synthesis of melanin also plays a vital role in the function of the appressorium and penetration peg [31,32]. Melanin is synthesized from intracellular acetic acid monomers by a series of enzymes that catalyze reduction, dehydration, oxidation, and polymerization reactions. Its main component is dihydroxynaphthalene (DHN), which is primarily distributed on the inner side of the cell wall and is necessary for the maintenance of turgor pressure [32,33,34,35]. However, the distribution of melanin in the appressorium cell wall is not uniform, and the melanin content is low in the portion of the wall that contacts the plant epidermis. Its main function is to facilitate rupture of the plant cell wall by the penetration peg in response to forces generated by high turgor pressure, enabling colonization of host tissue [2]. Melanin has been shown to be an important pathogenicity factor that can inhibit host defenses and aid fungal infection. Because it is necessary for the proper assembly of the fungal cell wall, melanin may also play an indirect role in pathogenicity [36]. In previous studies, the biosynthetic pathway of DHN melanin has been shown to comprise five key enzymes: polyketide synthase (PKS, catalyzing the synthesis of 1,3,6,8-THN), 1,3,6,8-tetra-HN reductase and 1,3,8-tri-HN reductase (4HNR and 3HNR, transforming 1,3,6,8-THN to scytalone and vermelone, respectively), scytalone dehydratase (SCD, transforming scytalone and vermelone to 1,3,8-THN and 1,8-DHN, respectively), and laccase (LAC, transforming 1,8-DHN to melanin) [37,38]. Studies have shown that fungal laccases participate in melanin biosynthesis, pathogenesis, and stress defense. A melanin regulation transcription factor (MR TF) has also been identified and shown to regulate the melanin biosynthetic pathway. For example, Amr1 in Alternaria brassicicola is involved in melanin biosynthesis and pathogenicity, and VdCmr1 is an important TF for melanin biosynthesis that affects survival under abiotic stress in Verticillium dahlia [39]. Targeted disruption of BMR1 in Bipolaris oryzae revealed that BMR1 also plays an important role in the biosynthesis of melanin [40]. The zinc finger protein StMr1 affects melanin synthesis in S. turcica by directly regulating the expression of genes in the DHN melanin synthesis pathway [41].
A recent RNA sequencing study of two S. turcica races at multiple infection timepoints revealed numerous genes and candidate effector proteins that function in the S. turcica–maize interaction [42]. Multiple quantitative trait loci associated with resistance to foliar diseases, including NCLB, have been identified in the maize genome [43,44], and the maize gene Htn1 has recently been shown to confer resistance to NCLB. However, our understanding of the essential genes that contribute to S. turcica pathogenesis during the early infection stages is still incomplete. A recent transcriptome study revealed three different strategies that contribute to S. turcica pathogenicity: cytoskeletal regulation, glycosyl hydrolase (GH)-mediated cell wall degradation, and secretion of effector proteins. Gene expression related to these essential processes was generally coupled to appressorium formation prior to host cell invasion. In particular, the cytoskeletal proteins Slm1 and Sml2 were required for pathogenicity and appeared to control the morphological transition during appressorium maturation. Three novel appressorium-related GH proteins were identified and appeared to contribute to host cell-wall degradation. An appressorium-coupled effector (StACE1) was also identified by systematic screening [2] (Figure 4C); it was found to induce cell death in Nicotiana benthamiana and was required for maize infection.
Plant pathogens also contain pathogenesis genes that function in additional processes, such as fungal cell wall integrity, host cell wall degradation, and nutrient acquisition. The cell wall of plant pathogens is a complex structure in which polysaccharides, proteins, and lipids interact, and its integrity is essential for fungal development, growth, and infection [2]. Chitin is the main component of many fungal cell walls, and chitin oligosaccharides released from hyphae during infection can activate the innate immune response in animals and plants [45]. Pathogenesis genes are also involved in the penetration of plant cell walls, and many pathogenic fungi produce cell-wall-degrading enzymes. A comparative genome analysis recently showed that gene families encoding such hydrolytic enzymes are expanded and more numerous in pathogenic fungi than in non-pathogenic fungi [45,46,47]. Pathogenic fungi must also obtain nutrition from the host during the invasion process, and some genes related to nutrition are essential for pathogenicity. For example, the high affinity sucrose transporter SRT1 in U. maydis can absorb sucrose from maize apoplasts. Srt1 is expressed only during U. maydis infection, and mutations of Srt1 result in loss of pathogenicity [48]. Additional genes with unknown functions related to host colonization also participate in the pathogenic process.
4. Conclusions and Perspective
The current study summarizes the NCLB disease outbreak and the molecular mechanisms of pathogens invading and infecting the host cellular machinery. The control of this devastating disease presents a great challenge to global sustainable agriculture, given that many agronomic crops, including maize, sorghum, and related grasses, are affected by the fungus S. turcica [49]. To provide a more intricate explanation, various studies have been carried out on key regulatory players, specifically cAMP, MAPK, Ca2+ signal transduction, and G protein-coupled receptor pathways. However, the regulatory network of the cell cycle is very complex and needs to be investigated comprehensively.
The control of blight disease presents a great challenge to global agriculture, given that many crops are affected by the fungus S. turcica. Some actions should be prioritized for the successful control of the NCLB. Through the study of the pathogenesis of the pathogen, we can select the appropriate target and design a fungicide to control the pathogen by studying its pathogenesis. For example, in terms of cell cycle regulation in the development of S. turcica, previous studies have found that the combination of cell cycle inhibitors and tricyclazole can effectively inhibit the infection efficiency of S. turcica [2], which has important application prospects for preventing tricyclazole durability. In addition, the development of cultivars with high levels of resistance to this disease is important to prevent the outbreak of S. turcica [50]. NCLB occurs frequently during corn planting and causes serious damage. Therefore, careful attention to prevention and control is important at this time and is critical to ensuring high corn yields. Based on the climate and soil conditions in the planting area, improved farming systems and a combination of prevention and treatment can effectively mitigate damage from NCLB. At the same time, it is necessary to continuously educate farmers on appropriate planting levels, management approaches, and disease prevention strategies to guarantee the stable production of corn. The current insights will provide new ideas for the prevention and control of the disease and will help to ensure food security by maintaining maize quality and yield.
Author Contributions
Conceptualization, P.L.; writing—original draft preparation, P.L. and A.S.; writing—review and editing, P.L., F.Z., Z.H. and J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Natural Science Foundation of Hebei Province (C2024204178).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Zeng, F.; Meng, Y.; Hao, Z.; Li, P.; Zhai, W.; Shen, S.; Cao, Z.; Dong, J. Setosphaeria turcica ATR turns off appressorium-mediated maize infection and triggers melanin-involved self-protection in response to genotoxic stress. Mol. Plant Pathol. 2020, 21, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, S.; Hao, Z.; Wang, Q.; Zhang, Y.; Zhao, Y.; Tong, Y.; Zeng, F.; Dong, J. Protein kinase a participates in hyphal and appressorial development by targeting Efg1-mediated transcription of a RabGTPase in Setosphaeria turcica. Mol. Plant Pathol. 2022, 23, 1608–1619. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Feng, S.; Zhao, J.; Tang, C.; Tian, L.; Fan, Y.; Cao, Z.; Hao, Z.; Jia, H.; Zang, J.; et al. StPBS2, a MAPK kinase gene, is involved in determining hyphal morphology, cell wall development, hypertonic stress reaction as well as the production of secondary metabolites in Northern Corn Leaf Blight pathogen Setosphaeria turcica. Microbiol. Res. 2017, 201, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, X.; Lin, Y.; Shen, S.; Zhao, Y.; Dong, J.; Hao, Z. The heterotrimeric G protein γ Stgg1 is required for conidiation, secondary metabolite production and pathogenicity of Setosphaeria turcica. Biotechnol. Biotechnol. Equip. 2018, 32, 929–935. [Google Scholar] [CrossRef]
- Renfro, B.L.; Ullstrup, A.J. A comparison of maize diseases in temperate and in tropical environments. Proc. Natl. Acad. Sci. USA 1976, 22, 491–498. [Google Scholar] [CrossRef]
- Chung, C.; Jamann, T.; Longfellow, J.; Nelson, R. Characterization and fine-mapping of a resistance locus for northern leaf blight in maize bin 8.06. Theor. Appl. Genet. 2010, 121, 205–227. [Google Scholar] [CrossRef] [PubMed]
- Shankara, K.; Pradhan, R.S.; Patole, S.P. Yield loss assessment due to turcicum leaf blight of maize caused by Exserohilum turcicum. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2888–2891. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, C.; Hussain, K.; Li, N.; Sun, Q.; Miao, Q.; Wu, S.; Lin, F. Pyramiding resistance genes to northern leaf blight and head smut in maize. Int. J. Agric. Biol. 2012, 14, 430–434. [Google Scholar]
- Chenulu, V.V.; Hora, T.S. Studies on losses due to Helminthosporium blight of maize. Indian Phytopathol. 1962, 15, 235–237. [Google Scholar]
- Pant, S.K.; Kumar, P.; Chauhan, V.S. Effect of turcicum leaf blight on photosynthesis in maize. Indian Phytopathol. 2001, 54, 252. [Google Scholar]
- Fajemisin, J.M.; Hooker, A.L. Predisposition to diplodia stalk rot in corn affected by three Helminthosporium leaf blights. Phytopathology 1974, 64, 1496–1499. [Google Scholar] [CrossRef]
- Leach, C.M.; Fullerton, R.A.; Young, K. Northern leaf blight of maize in New Zealand: Relationship of Dreschslera turcia airspora to factors influencing sporulation, conidium development, and chlamydospore formation. Phytopathology 1977, 67, 629–636. [Google Scholar] [CrossRef]
- Ryder, L.S.; Talbot, N.J. Regulation of appressorium development in pathogenic fungi. Curr. Opin. Plant Biol. 2015, 26, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Hamer, J.E. Divergent cAMP signaling pathways regulate growth and pathogenesis in the rice blast fungus Magnaporthe grisea. Plant Cell 1998, 10, 1361–1373. [Google Scholar] [CrossRef]
- Ebbole, D.J. Magnaporthe as a model for understanding host-pathogen interactions. Annu. Rev. Phytopathol. 2007, 45, 437–456. [Google Scholar] [CrossRef]
- Dohlman, H.G. G proteins and pheromone signaling. Annu. Rev. Physiol. 2002, 64, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Xu, J. MAP kinases in fungal pathogens. Fungal Genet. Biol. 2000, 31, 137–152. [Google Scholar] [CrossRef]
- Tucker, S.L.; Talbot, N.J. Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu. Rev. Phytopathol. 2001, 39, 385–417. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.K.; Dean, R.A. The cAMP-dependent protein kinase catalytic subunit is required for appressorium formation and pathogenesis by the rice blast pathogen Magnaporthe grisea. Plant Cell 1995, 7, 1869–1878. [Google Scholar]
- Doehlemann, G.; Berndt, P.; Hahn, M. Different signalling pathways involving a Galpha protein, cAMP and a MAP kinase control germination of Botrytis cinerea conidia. Mol. Microbiol. 2006, 59, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Kim, Y.; Park, G.; Xu, J. A mitogen-activated protein kinase cascade regulating infection-related morphogenesis in Magnaporthe grisea. Plant Cell 2005, 17, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Igbaria, A.; Lev, S.; Rose, M.S.; Lee, B.N.; Hadar, R.; Degani, O.; Horwitz, B.A. Distinct and combined roles of the MAP kinases of Cochliobolus heterostrophus in virulence and stress responses. Mol. Plant Microbe Interact. 2008, 21, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Mehrabi, R.; Xu, J. Mitogen-activated protein kinase pathways and fungal pathogenesis. Eukaryot. Cell 2007, 6, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, X.; Li, M.; Si, H.; Zhou, Q.; Liu, X.; Fan, Y.; Zhang, X.; Han, J.; Gu, S.; et al. Effect of osmotic stress on the growth, development and pathogenicity of Setosphaeria turcica. Front. Microbiol. 2021, 12, 706349. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Fisher, R.A. Introduction: G protein-coupled receptors and RGS proteins. Prog. Mol. Biol. Transl. 2015, 133, 1–11. [Google Scholar]
- Li, L.; Wright, S.J.; Krystofova, S.; Park, G.; Borkovich, K.A. Heterotrimeric G protein signaling in filamentous fung. Annu. Rev. Microbiol. 2007, 61, 423–452. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Hsueh, Y.; Heitman, J. Magnificent seven: Roles of G protein-coupled receptors in extracellular sensing in fungi. FEMS Microbiol. Rev. 2008, 32, 1010–1032. [Google Scholar] [CrossRef]
- Talbot, N.J. Living the sweet life: How does a plant pathogenic fungus acquire sugar from plants? PLoS Biol. 2010, 8, e1000308. [Google Scholar] [CrossRef]
- Meinhardt, L.W.; Costa, G.G.L.; Thomazella, D.P.; Teixeira, P.J.P.; Carazzolle, M.F.; Schuster, S.C.; Carlson, J.E.; Guiltinan, M.J.; Mieczkowski, P.; Farmer, T.R.J.C.A.; et al. Genome and secretome analysis of the hemibiotrophic fungal pathogen, Moniliophthora roreri, which causes frosty pod rot disease of cacao: Mechanisms of the biotrophic and necrotrophic phases. BMC Genom. 2014, 15, 164. [Google Scholar] [CrossRef]
- Howard, R.J.; Valent, B. Breaking and entering: Host penetration by the fungal rice blast pathogen Magnaporthe grisea. Annu. Rev. Microbiol. 1996, 50, 491–512. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.J.; Ferrari, M.A. Role of melanin in appressorium function. Exp. Mycol. 1989, 13, 403–418. [Google Scholar] [CrossRef]
- Talbot, N.J. On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 2003, 57, 177–202. [Google Scholar] [CrossRef]
- Ma, S.; Cao, K.; Liu, N.; Meng, C.; Cao, Z.; Dai, D.; Jia, H.; Zang, J.; Li, Z.; Hao, Z.; et al. The StLAC2 gene is required for cell wall integrity, DHN-melanin synthesis and the pathogenicity of Setosphaeria turcica. Fungal Biol. 2017, 121, 589–601. [Google Scholar] [CrossRef]
- Thompson, J.E.; Fahnestock, S.; Farrall, L.; Liao, D.; Valent, B.; Jordan, D.B. The second naphthol reductase of fungal melanin biosynthesis in Magnaporthe grisea. J. Biol. Chem. 2000, 275, 34867–34872. [Google Scholar] [CrossRef]
- Valiante, V.; Baldin, C.; Hortschansky, P.; Jain, R.; Thywißen, A.; Straßburger, M.; Shelest, E.; Heinekamp, T.; Brakhage, A.A. The Aspergillus fumigatus conidial melanin production is regulated by the bifunctional bHLH DevR and MADS-box RlmA transcription factors. Mol. Microbiol. 2016, 102, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.; Bandyopadhyay, S.; Free, S.J. Characterization of the Neurospora crassa DHN melanin biosynthetic pathway in developing ascospores and peridium cells. Fungal Biol. 2018, 123, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sone, Y.; Nakamura, S.; Sasaki, M.; Hasebe, F.; Kim, S.; Funa, N. Bacterial enzymes catalyzing the synthesis of 1,8-dihydroxynaphthalene, a key precursor of dihydroxynaphthalene melanin, from Sorangium cellulosum. Appl. Environ. Microbiol. 2018, 84, e218–e258. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Q.; Li, Y.; Qiao, L.; Pang, Q.; Huang, B. ITRAQ-based quantitative proteomic analysis of conidia and mycelium in the filamentous fungus Metarhizium robertsii. Fungal Biol. 2018, 122, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Kihara, J.; Moriwaki, A.; Tanaka, N.; Tanaka, C.; Ueno, M.; Arase, S. Characterization of the BMR1 gene encodinga transcription factor formelanin biosynthesis genes in the phytopathogenic fungus Bipolaris oryzae. FEMS Microbiol. Lett. 2008, 281, 221–227. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, H.; Liu, N.; Li, H.; Meng, Q.; Wu, N.; Cao, Z.; Dong, J. The zinc finger protein StMr1 affects the pathogenicity and melanin synthesis of Setosphaeria turcica and directly regulates the expression of DHN melanin synthesis pathway genes. Mol. Microbiol. 2022, 117, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Human, M.P.; Berger, D.K.; Crampton, B.G. Time-course RNAseq reveals Exserohilum turcicum effectors and pathogenicity determinants. Front. Microbiol. 2020, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Zuniga, L.O.; Wolters, P.; Davis, S.; Weldekidan, T.; Kolkman, J.M.; Nelson, R.; Hooda, K.S.; Rucker, E.; Thomason, W.; Wisser, R.; et al. Using maize chromosome segment substitution line populations for the identification of loci associated with multiple disease resistance. G3 Genes Genomes Genet. 2019, 9, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; He, Y.; Kabahuma, M.; Chaya, T.; Kelly, A.; Borrego, E.; Bian, Y.; El Kasmi, F.; Yang, L.; Teixeira, P.; et al. A gene encoding maize caffeoyl-CoA O-methyltransferase confers quantitative resistance to multiple pathogens. Nat. Genet. 2017, 49, 1364–1372. [Google Scholar] [CrossRef]
- de Jonge, R.; Thomma, B.P.H.J. Fungal LysM effectors: Extinguishers of host immunity? Trends Microbiol. 2009, 17, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; van der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Soanes, D.M.; Alam, I.; Cornell, M.; Wong, H.M.; Hedeler, C.; Paton, N.W.; Rattray, M.; Hubbard, S.J.; Oliver, S.G.; Talbot, N.J.; et al. Comparative genome analysis of filamentous fungi reveals gene family expansions associated with fungal pathogenesis. PLoS ONE 2008, 3, e2300. [Google Scholar] [CrossRef] [PubMed]
- Wahl, R.; Wippel, K.; Goos, S.; Kamper, J.; Sauer, N. A novel high-affinity sucrose transporter is required for virulence of the plant pathogen Ustilago maydis. PLoS Biol. 2010, 8, e1000303. [Google Scholar] [CrossRef]
- Perkins, J.M.; Pedersen, W.L. Disease development and yield losses associated with northern leaf blight on corn. Plant Dis. 1987, 71, 940–943. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, Z.; Luo, B.; Zhong, H.; Ma, P.; Zhang, H.; Hu, H.; Wang, Y.; Zhang, H.; Liu, D.; et al. Genetic architecture of maize yield traits dissected by QTL mapping and GWAS in maize. Crop J. 2022, 10, 436–446. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).