Monitoring and Occurrence Prediction of the Migration Population of Helicoverpa armigera (Hübner) Based on Adult Semiochemical Attractants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analysis of Reproductive Organ Development and Fecundity of Immigrant and Local Adult H. armigera Populations
2.1.1. Insects
2.1.2. Experimental Methods
2.2. Monitoring Methods for the Quantitative Dynamics and Reproductive Development State of H. armigera in the Field
2.2.1. Field Monitoring Materials
2.2.2. Field Monitoring Methods
2.3. Simulation Method for the Migration Trajectory of H. armigera
2.4. Prediction and Verification Method for the Fecundity of Trapped Adults in the Field
2.5. Statistical Analyses
3. Results
3.1. Construction of Adult Age Determination Model and Daily fecundity Model
3.2. Age Dynamics of H. armigera Population Trapped Using Semiochemical Attractants in the Field
3.3. Quantitative Dynamics and Trajectory of the Migration Population of H. armigera
3.4. Prediction and Verification of the Fecundity of Trapped Adults in the Field
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Riaz, S.; Johnson, J.B.; Ahmad, M.; Fitt, G.P.; Naiker, M. A review on biological interactions and management of the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Appl. Entomol. 2021, 145, 467–498. [Google Scholar] [CrossRef]
- Murúa, M.G.; Scalora, F.S.; Navarro, F.R.; Cazado, L.E.; Casmuz, A.; Villagrán, M.E.; Lobos, E.; Gastaminza, G. First record of Helicoverpa armigera (Lepidoptera: Noctuidae) in Argentina. Fla. Entomol. 2014, 97, 854–856. [Google Scholar] [CrossRef]
- Castiglioni, E.; Clérison, R.P.; Chiaravalle, W.; Jonas, A.; Ugalde, G.A.; Jerson, V.C.G. Primer registro de ocurrencia de Helicoverpa armigera (Hübner, 1808) (Lepidoptera: Noctuidae) En Soja, En Uruguay. Agrociencia 2016, 20, 31–35. [Google Scholar] [CrossRef]
- Arnemann, J.A.; James, W.J.; Walsh, T.K.; Guedes, J.V.C.; Smagghe, G.; Castiglioni, E.; Tay, W.T. Mitochondrial DNA COI characterization of Helicoverpa armigera (Lepidoptera: Noctuidae) from Paraguay and Uruguay. Genet. Mol. Res. 2016, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fitt, G.P. The ecology of Heliothis species in relation to agroecosystems. Annu. Rev. Entomol. 1989, 34, 17–53. [Google Scholar] [CrossRef]
- Arnemann, J.A.; Roxburgh, S.H.; Walsh, T.; Guedes, J.V.; Gordon, K.H.; Smagghe, G.; Tay, W.T. Multiple incursion pathways for Helicoverpa armigera in Brazil show its genetic diversity spreading in a connected world. Sci. Rep. 2019, 9, 19380. [Google Scholar] [CrossRef] [PubMed]
- Zalucki, M.; Murray, D.; Gregg, P.; Fitt, G.; Twine, P.; Jones, C. Ecology of Helicoverpa armigera (Hubner) and Heliothis punctigera (Wallengren) in the inland of Australia larval sampling and host plant pelationships during winter and spring. Aust. J. Zool. 1994, 42, 329–346. [Google Scholar] [CrossRef]
- Feng, H.Q.; Wu, X.F.; Wu, B.; Wu, K.M. Seasonal Migration of Helicoverpa armigera (Lepidoptera: Noctuidae) over the Bohai Sea. J. Econ. Entomol. 2009, 102, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Papanicolaou, A.; Mironidis, G.K.; Vontas, J.; Yang, Y.; Lim, K.S.; Oakeshott, J.G.; Bass, C.; Chapman, J.W. Genomewide transcriptional signatures of migratory flight activity in a globally invasive insect pest. Mol. Ecol. 2015, 24, 4901–4911. [Google Scholar] [CrossRef]
- Dalal, P.K.; Arora, R. Fecundity and life-table parameters of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) on tomato crop under alternating temperature regimes: Implications for pest monitoring in sub-tropical India. Int. J. Trop. Insect Sci. 2021, 41, 2851–2865. [Google Scholar] [CrossRef]
- Urúa, M.G.; Ogliata, S.V.; Errero, M.I.; Era, M.; Asmuz, A.S.; Omez, D. Biological and reproductive parameters of Helicoverpa armigera and Helicoverpa zea reared on artificial diet in Argentina. Bull. Insectology 2021, 74, 55–64. [Google Scholar]
- Specht, A.; Sosa-Gómez, D.R.; Rios, D.A.M.; Claudino, V.C.M.; Paula-Moraes, S.V.; Malaquias, J.V.; Silva, F.A.M.; Roque-Specht, V.F. Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) in Brazil: The big outbreak monitored by light traps. Neotrop. Entomol. 2021, 50, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Haile, F.; Nowatzki, T.; Storer, N. Overview of pest status, potential risk, and management considerations of Helicoverpa armigera (Lepidoptera: Noctuidae) for U.S. soybean production. J. Integr. Pest Manag. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Tay, W.T.; Soria, M.F.; Walsh, T.; Thomazoni, D.; Silvie, P.; Behere, G.T.; Anderson, C.; Downes, S. A brave new world for an old world pest: Helicoverpa armigera (Lepidoptera: Noctuidae) in Brazil. PLoS ONE 2013, 8, e80134. [Google Scholar] [CrossRef] [PubMed]
- Magor, J. Forecasting migrant insect pests. In Insect Migration: Tracking Resources through Space and Time; Drake, V.A., Gatehouse, A.G., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 399–426. [Google Scholar]
- Zhang, J.; Ma, J.H.; Xu, Y.C.; Wang, X.; Wang, P.L.; Omar, G.; Lv, Z.Z. Migration behavior of cotton bollworm in Xinjiang of Northwest China based on the ovarian development characteristics of adult females. Chin. J. Ecol. 2013, 32, 1428–1432. [Google Scholar]
- Sun, G.J.; Liu, S.H.; Luo, H.L.; Feng, Z.L.; Yang, B.J.; Luo, J.; Tang, J.; Yao, Q.; Xu, J.J. Intelligent monitoring system of migratory pests based on searchlight trap and machine vision. Front. Plant Sci. 2022, 13, 897739. [Google Scholar] [CrossRef] [PubMed]
- Keszthelyi, S.; Nowinszky, L.; Szeőke, K. Different catching series from light and pheromone trapping of Helicoverpa armigera (Lepidoptera: Noctuidae). Biologia 2016, 71, 818–823. [Google Scholar] [CrossRef]
- Thein, P.P.; Choi, S.W. Forest insect assemblages attracted to light trap on two high mountains (Mt. Jirisan and Mt. Hallasan) in South Korea. J. For. Res. 2016, 27, 1203–1210. [Google Scholar] [CrossRef]
- Cohnstaedt, L.W.; Gillen, J.I.; Munstermann, L.E. Light-emitting diode technology improves insect trapping. J. Am. Mosq. Control Assoc. 2008, 24, 331–334. [Google Scholar] [CrossRef]
- Yao, Q.; Feng, J.; Tang, J.; Xu, W.; Zhu, X.; Baojun, Y.; Lü, J.; Xie, Y.; Yao, B.; Wu, S.; et al. Development of an automatic monitoring system for rice light-trap pests based on machine vision. J. Integr. Agric. 2020, 19, 2500–2513. [Google Scholar] [CrossRef]
- He, W.; Wang, L.Y.; Lv, C.Y.; Ge, S.S.; Zhang, H.W.; Jiang, S.; Chu, B.; Yang, X.M.; Wyckhuys, K.A.G.; Wu, K.M. Use of food attractants to monitor and forecast Spodoptera frugiperda (J. E. Smith) Seasonal Abundance in Southern China. J. Pest Sci. 2023, 96, 1509–1521. [Google Scholar] [CrossRef]
- Gregg, P.C.; Del Socorro, A.P.; Henderson, G.S. Development of a synthetic plant volatile-based attracticide for female Noctuid moths. II. Bioassays of synthetic plant volatiles as attractants for the adults of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 2010, 49, 21–30. [Google Scholar] [CrossRef]
- Del Socorro, A.P.; Gregg, P.C.; Alter, D.; Moore, C.J. Development of a synthetic plant volatile-based attracticide for female Noctuid moths. I. Potential sources of volatiles attractive to Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 2010, 49, 10–20. [Google Scholar] [CrossRef]
- Gregg, P.C.; Del Socorro, A.P.; Hawes, A.J.; Binns, M.R. Developing bisexual attract-and-kill for polyphagous insects: Ecological rationale versus pragmatics. J. Chem. Ecol. 2016, 42, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Gregg, P.C.; Del Socorro, A.P.; Landolt, P.J. Advances in attract-and-kill for agricultural pests: Beyond pheromones. Annu. Rev. Entomol. 2018, 63, 453–470. [Google Scholar] [CrossRef]
- Justiniano, W.; Fernandes, M.G. Effect of food attractants and insecticide toxicity for the control of Spodoptera frugiperda (Lepidoptera: Noctuidae) adults. J. Agr. Sci. 2019, 12, 129. [Google Scholar] [CrossRef]
- He, W.; Zhao, X.C.; Ali, A.A.; Ge, S.S.; Zhang, H.W.; He, L.M.; Wu, K.M. Population dynamics and reproductive developmental analysis of Helicoverpa armigera (Lepidoptera: Noctuidae) trapped using food attractants in the Field. J. Econ. Entomol. 2021, 114, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.L.; Jiang, Y.Y.; Liu, J.; Hu, G.; Wu, K.M. Trajectory modeling revealed a southwest-northeast migration corridor for fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) emerging from the North China Plain. Insect Sci. 2021, 28, 649–661. [Google Scholar] [CrossRef]
- Queiroz-Santos, L.; Casagrande, M.M.; Specht, A. Morphological characterization of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae: Heliothinae). Neotrop. Entomol. 2018, 47, 517–542. [Google Scholar] [CrossRef]
- Zhou, S.H.; Li, J.; Yao, R.X.; Zhang, X.; Yuan, Z.M. Optimization of chemically defined diet for larvae of the cotton bollworm (Helicoverpa armigera) based on uniform design and support vector regression. Acta Entomol. Sin. 2012, 55, 124–132. [Google Scholar]
- Kong, D.S.; Sun, H.M.; Zhao, Y.L.; Xu, L.; Hui, X.H.; Qu, M.J.; Lu, X.T. Control effect and benefit analysis of sex attractant and biological food attractant on Helicoverpa armigera in peanut field. Shandong Agric. Sci. 2016, 48, 102–105. [Google Scholar]
- Stein, A.F.; Draxler, R.R.; Rolph, G.D.; Stunder, B.J.B.; Cohen, M.D.; Ngan, F. NOAA’s HYSPLIT atmospheric transport and dispersion modeling system. Bull. Am. Meteorol. Soc. 2015, 96, 2059–2077. [Google Scholar] [CrossRef]
- Gao, Y.B.; Zhai, B.P. Active temperature selection of flying Helicoverpa armigera ( Lepidoptera: Noctuidae) moths. Acta Entomol. Sin. 2010, 53, 540–548. [Google Scholar]
- Chen, H.X.; Yang, R.; Yang, W.; Zhang, L.; Camara, I.; Dong, X.H.; Liu, Y.; Shi, W.P. Efficacy of Bt maize producing the Cry1Ac protein against two important pests of corn in China. Environ. Sci. Pollut. Res. Int. 2016, 23, 21511–21516. [Google Scholar] [CrossRef]
- Pedigo, L.P. Entomology and Pest Management; Waveland Press: Long Grove, IL, USA, 1989. [Google Scholar]
- Feng, B.; Guo, Q.S.; Zhu, F.; Wang, X.; Liu, W.C.; Jiang, Y.Y.; Zhong, L.; Du, Y.J. Ovarian development and synthetic sex pheromone lure trapping of adults of the rice leaf folder, Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). Acta Entomol. Sin. 2017, 60, 211–221. [Google Scholar]
- Tigreros, N.; Davidowitz, G. Flight-fecundity tradeoffs in wing-monomorphic insects. Adv. Insect Physiol. 2019, 56, 1–41. [Google Scholar]
- Qi, G.J.; Lu, F.; Hu, G.; Wang, F.Y.; Gao, Y.; Lv, L.H. The application of ovarian dissection in the research on migratory insects in China. China Plant Prot. 2011, 31, 18–22. [Google Scholar]
- Zhang, X.X.; Lu, Z.Q.; Geng, J.G. Application of anatomy of female moth of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) in prediction. Insect Knowl. 1979, 3, 97–99. [Google Scholar]
- Cai, X.M.; Bian, L.; Xu, X.X.; Luo, Z.X.; Li, Z.Q.; Chen, Z.M. Field background odour should be taken into account when formulating a pest attractant based on plant volatiles. Sci. Rep. 2017, 7, 41818. [Google Scholar] [CrossRef]
- Wang, L.Y.; He, L.M.; Zhu, X.M.; Zhang, J.W.; Li, N.; Fan, J.F.; Li, H.F.; Sun, X.J.; Zhang, L.J.; Lin, Y.L.; et al. Large-area field application confirms the effectiveness of toxicant-infused bait for managing Helicoverpa armigera (Hübner) in maize fields. Pest Manag. Sci. 2023, 79, 5405–5417. [Google Scholar] [CrossRef]
- Ge, S.S.; Zhang, H.W.; Liu, D.Z.; Lv, C.Y.; Cang, X.Z.; Sun, X.X.; Song, Y.F.; He, W.; Chu, B.; Zhao, S.Y.; et al. Seasonal migratory activity of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) across China and Myanmar. Pest Manag. Sci. 2022, 78, 4975–4982. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.S.; Zhai, B.P.; Dong, B.X. Forecasting model for the oviposition peak day in the second generation of Helicoverpa armigera (Lepidoptera: Noctuidae) based on radial basis wavelet network. Acta Entomol. Sin. 2010, 53, 1429–1435. [Google Scholar]
- Li, Z.; Tang, B.S. Principal component analysis forecast model of Helicoverpa armigera based on precipitation index. Acta Agric. Boreali-Occident. Sin. 2017, 26, 1554–1558. [Google Scholar]
- Blum, M.; Nestel, D.; Cohen, Y.; Goldshtein, E.; Helman, D.; Lensky, I.M. Predicting Heliothis (Helicoverpa armigera) pest population dynamics with an age-structured insect population model driven by satellite data. Ecol. Model. 2018, 369, 1–12. [Google Scholar] [CrossRef]
- Feng, H.Q.; Gould, F.; Huang, Y.X.; Jiang, Y.Y.; Wu, K.M. Modeling the population dynamics of cotton bollworm Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) over a wide area in northern China. Ecol. Model. 2010, 221, 1819–1830. [Google Scholar] [CrossRef]
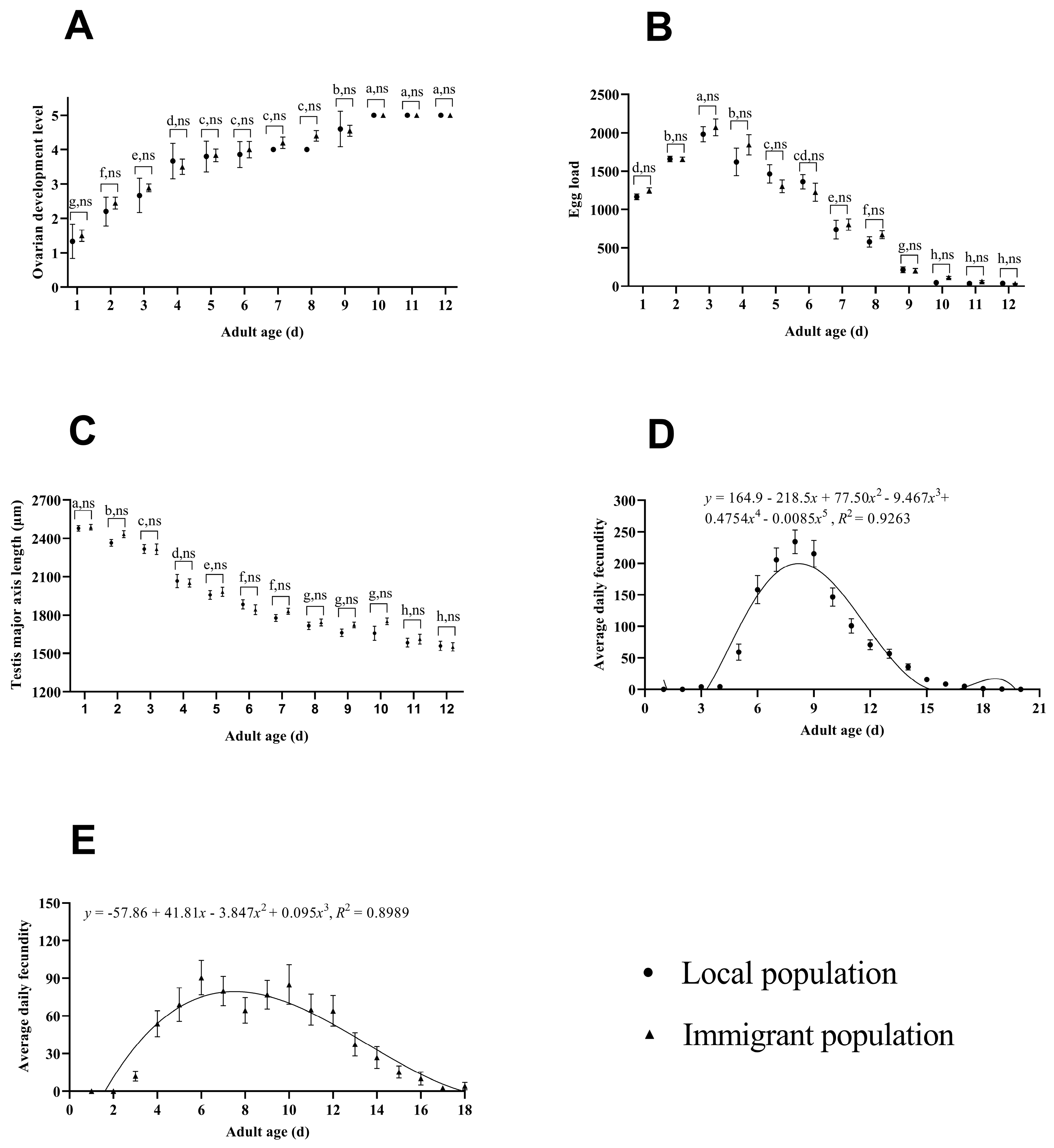
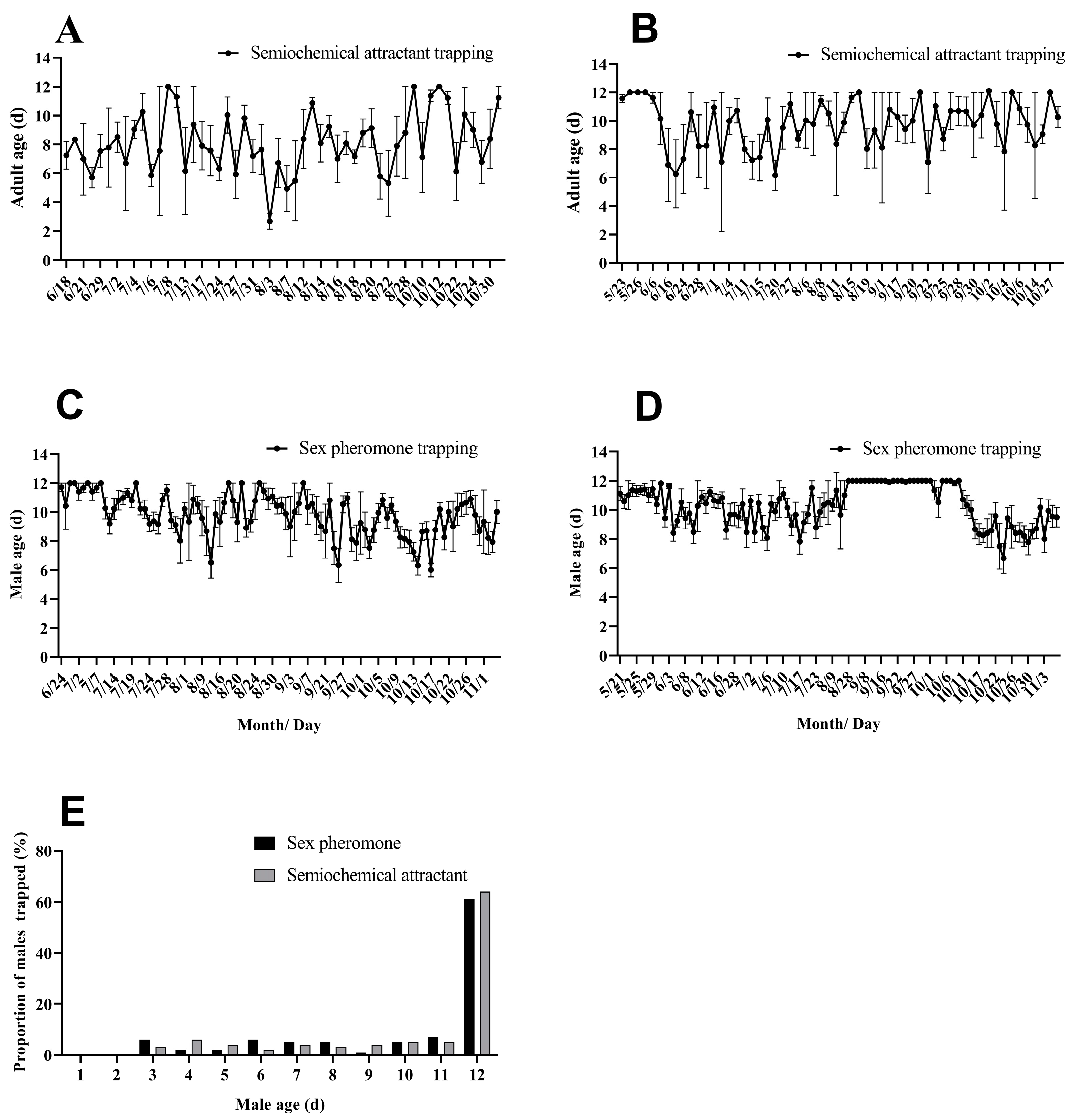


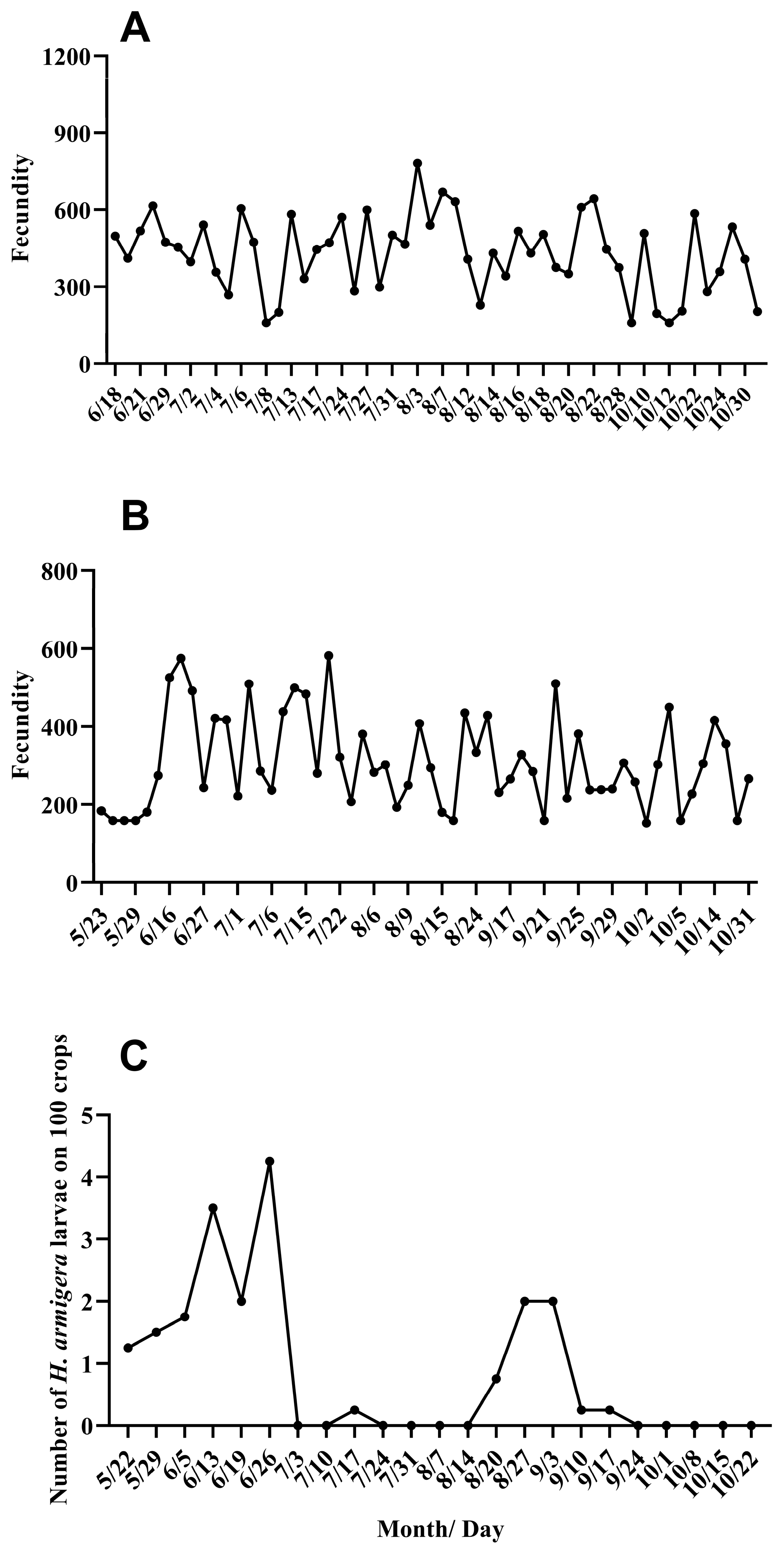
| Target Variable | Source | Type III SS | df | MS | F | p |
|---|---|---|---|---|---|---|
| Ovarian development level | Population | 0.111 | 1 | 0.111 | 0.892 | 0.346 |
| Age | 258.069 | 11 | 23.461 | 188.448 | <0.001 | |
| Population × Age | 0.846 | 11 | 0.077 | 0.618 | 0.812 | |
| Error | 21.662 | 216 | 0.124 | |||
| Total | 3101 | 239 | 34,613.09 | 0 | ||
| Egg load | Population | 34,613.090 | 1 | 8,858,698.115 | 0.975 | 0.325 |
| Age | 97,445,679.262 | 11 | 34,555.153 | 249.452 | <0.001 | |
| Population × Age | 380,106.688 | 11 | 35,512.664 | 0.973 | 0.473 | |
| Error | 6,179,203.543 | 216 | ||||
| Total | 254,492,221.000 | 239 | ||||
| Testis major axis length | Population | 42,970,736.200 | 1 | 65,792.325 | 2.812 | 0.094 |
| Age | 42,970,736.200 | 11 | 3,906,430.564 | 166.935 | <0.001 | |
| Population × Age | 159,955.295 | 11 | 14,541.390 | 0.621 | 0.811 | |
| Error | 10,483,613.697 | 457 | 23,400.923 | |||
| Total | 1,885,921,063.503 | 480 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Lv, C.; Zhang, H.; Cang, X.; Chu, B.; Yang, X.; Liang, G.; Wu, K. Monitoring and Occurrence Prediction of the Migration Population of Helicoverpa armigera (Hübner) Based on Adult Semiochemical Attractants. Agronomy 2024, 14, 1497. https://doi.org/10.3390/agronomy14071497
He W, Lv C, Zhang H, Cang X, Chu B, Yang X, Liang G, Wu K. Monitoring and Occurrence Prediction of the Migration Population of Helicoverpa armigera (Hübner) Based on Adult Semiochemical Attractants. Agronomy. 2024; 14(7):1497. https://doi.org/10.3390/agronomy14071497
Chicago/Turabian StyleHe, Wei, Chunyang Lv, Haowen Zhang, Xinzhu Cang, Bo Chu, Xianming Yang, Gemei Liang, and Kongming Wu. 2024. "Monitoring and Occurrence Prediction of the Migration Population of Helicoverpa armigera (Hübner) Based on Adult Semiochemical Attractants" Agronomy 14, no. 7: 1497. https://doi.org/10.3390/agronomy14071497





