Compatibility and Stability Analysis of Haploid Inducers under Different Source Germplasm and Seasons in Maize Using GGE Biplot
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials: Haploid Inducers and Source Germplasm
2.2. Experimental Design and Haploid Induction
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Combined Analysis of Variance
3.2. Effects of Season and Source Germplasm in In Vivo Haploid Induction
3.3. Stability of Haploid Inducer across Source Germplasm and Season
3.4. Compatibility of Haploid Inducer across Source Germplasm via GGE Biplot
3.5. Discriminating Ability of Source Germplasm against Haploid Inducers via GGE Biplot
4. Discussion
4.1. Haploid Inducer Plays Important Roles Affecting Pollination Rate in In Vivo Haploid Induction
4.2. The Importance of Source Germplasm on R1-nj Seed Set and R1-nj Expression as a Selectable Marker for Haploid Identification
4.3. GGE Biplot and Stability Indices Are Useful Tools for Dissecting the Complex Interaction between Season, Source Germplasm, and Haploid Inducer
4.4. Limitations of the Study and Potential Future Research Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaikam, V.; Molenaar, W.; Melchinger, A.E.; Prasanna, M.B. Doubled haploid technology for line development in maize: Technical advances and prospects. Theor. Appl. Genet. 2019, 132, 3227–3243. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; Jiaojiao, H.; Mei, M.; Frei, U.; Trampe, B.; Lübberstedt, T. Maize doubled haploids. Plant Breed. Rev. 2016, 40, 123–166. [Google Scholar] [CrossRef]
- Gilles, L.M.; Khaled, A.; Laffaire, J.B.; Chaignon, S.; Gendrot, G.; Laplaige, J.; Bergès, H.; Beydon, G.; Bayle, V.; Barret, P.; et al. Loss of pollen-specific phospholipase NOT LIKE DAD triggers gynogenesis in maize. EMBO J. 2017, 36, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, T.; Starr, D.; Richbourg, L.; Chintamanani, S.; Delzer, B.; Nuccio, M.L.; Green, J.; Chen, Z.; McCuiston, J.; Wang, W.; et al. MATRILINEAL, a sperm-specific phospholipase, triggers maize haploid induction. Nature 2017, 542, 105–109. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Meng, D.; Zhong, Y.; Chen, C.; Dong, X.; Xu, X.; Chen, B.; Li, W.; Li, L.; et al. A 4-bp insertion at ZmPLA1 encoding a putative phospholipase A generates haploid induction in maize. Mol. Plant 2017, 10, 520–522. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, C.; Qi, X.; Jiao, Y.; Wang, D.; Wang, Y.; Liu, Z.; Chen, C.; Chen, B.; Tian, X.; et al. Mutation of ZmDMP enhances haploid induction in maize. Nat. Plants 2019, 5, 575–580. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Yue, Y.; Zhao, H.; Fei, X.; Lizhu, E.; Liu, L.; Liu, C.; Chen, S.; Lai, J.; et al. Loss-of-function alleles of ZmPLD3 cause haploid induction in maize. Nat. Plants 2021, 7, 1579–1588. [Google Scholar] [CrossRef]
- Almeida, V.C.; Trentin, H.U.; Frei, U.K.; Lübberstedt, T. Genomic prediction of maternal haploid induction rate in maize. Plant Genome 2020, 13, e20014. [Google Scholar] [CrossRef]
- Dermail, A.; Lübberstedt, T.; Suwarno, W.B.; Chankaew, S.; Lertrat, K.; Ruanjaichon, V.; Suriharn, K. Combining ability of tropical× temperate maize inducers for haploid induction rate, R1-nj seed set, and agronomic traits. Front. Plant Sci. 2023, 14, 1154905. [Google Scholar] [CrossRef]
- Wu, P.; Li, H.; Ren, J.; Chen, S. Mapping of maternal QTLs for in vivo haploid induction rate in maize (Zea mays L.). Euphytica 2014, 196, 413–421. [Google Scholar] [CrossRef]
- De La Fuente, G.N.; Frei, U.K.; Trampe, B.; Nettleton, D.; Zhang, W.; Lübberstedt, T. A diallel analysis of a maize donor population response to in vivo maternal haploid induction: I. Inducibility. Crop Sci. 2018, 58, 1830–1837. [Google Scholar] [CrossRef]
- Kebede, A.Z.; Dhillon, B.S.; Schipprack, W.; Araus, J.L.; Bänziger, M.; Semagn, K.; Alvarado, G.; Melchinger, A.E. Effect of source germplasm and season on the in vivo haploid induction rate in tropical maize. Euphytica 2011, 180, 219–226. [Google Scholar] [CrossRef]
- Couto, E.G.d.O.; Cury, M.N.; E Souza, M.B.; Granato, Í.S.C.; Vidotti, M.S.; Garbuglio, D.D.; Crossa, J.; Burgueño, J.; Fritsche-Neto, R. Effect of F1 and F2 generations on genetic variability and working steps of doubled haploid production in maize. PLoS ONE 2019, 14, e0224631. [Google Scholar] [CrossRef] [PubMed]
- Prigge, V.; Sánchez, C.; Dhillon, B.S.; Schipprank, W.; Araus, J.L.; Bänziger, M.; Melchinger, A.E. Doubled haploids in tropical maize: I. Effect of inducers and source germplasm on in vivo haploid induction rates. Crop Sci. 2011, 51, 1498–1506. [Google Scholar] [CrossRef]
- Thawarorit, A.; Dermail, A.; Lertrat, K.; Chankaew, S.; Suriharn, K. Stratified haploid identification system through the R1-nj kernel and reduced seedling vigor in tropical maize germplasm. Biodiversitas 2023, 24, 4262–4268. [Google Scholar] [CrossRef]
- Trentin, H.U.; Batîru, G.; Frei, U.K.; Dutta, S.; Lübberstedt, T. Investigating the effect of the interaction of maize inducer and donor backgrounds on haploid induction rates. Plants 2022, 11, 1527. [Google Scholar] [CrossRef]
- Sintanaparadee, P.; Dermail, A.; Lübberstedt, T.; Lertrat, K.; Chankaew, S.; Ruanjaichon, V.; Phakamas, N.; Suriharn, K. Seasonal variation of tropical savanna altered agronomic adaptation of Stock-6-derived inducer lines. Plants 2022, 11, 2902. [Google Scholar] [CrossRef] [PubMed]
- Nanda, D.K.; Chase, S.S. An embryo marker for detecting monoploids of maize (Zea mays L.). Crop Sci. 1966, 6, 213–215. [Google Scholar] [CrossRef]
- Dermail, A.; Mitchell, M.; Foster, T.; Fakude, M.; Chen, Y.R.; Suriharn, K.; Frei, U.K.; Lübberstedt, T. Haploid identification in maize. Front. Plant Sci. 2024, 15, 1378421. [Google Scholar] [CrossRef]
- Chaikam, V.; Nair, S.K.; Babu, R.; Martinez, L.; Tejomurtula, J.; Prasanna, M.B. Analysis of effectiveness of R1-nj anthocyanin marker for in vivo haploid identification in maize and molecular markers for predicting the inhibition of R1-nj expression. Theor. Appl. Genet. 2015, 128, 159–171. [Google Scholar] [CrossRef]
- Yu, W.; Birchler, J.A. A green fluorescent protein-engineered haploid inducer line facilitates haploid mutant screens and doubled haploid breeding in maize. Mol. Breed. 2016, 36, 5. [Google Scholar] [CrossRef]
- Dang, N.C.; Munsch, M.; Aulinger, I.; Renlai, W.; Stamp, P. Inducer line generated double haploid seeds for combined waxy and opaque 2 grain quality in subtropical maize (Zea mays L.). Euphytica 2012, 183, 153–160. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar evaluation and mega-environment investigation based on the GGE biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Yan, W.; Tinker, N.A. Biplot analysis of multi-environment trial data: Principles and applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef]
- Dermail, A.; Chankaew, S.; Lertrat, K.; Lübberstedt, T.; Suriharn, K. Selection gain of maize haploid inducers for the tropical savanna environments. Plants 2021, 10, 2812. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, G.N. Improvement to the Maize (Zea mays L.) In Vivo Maternal Doubled Haploid System. Ph.D. Thesis, Iowa State University, Ames, Iowa, 2015. [Google Scholar]
- Thai Agricultural Practice. Available online: http://www.doa.go.th (accessed on 7 August 2022).
- Kang, M.S. Simultaneous selection for yield and stability in crop performance trials: Consequences for growers. Agron. J. 1993, 85, 754–757. [Google Scholar] [CrossRef]
- Francis, T.R.; Kannenberg, L.W. Yield stability studies in short-season maize. I. A descriptive method for grouping genotypes. Can. J. Plant Sci. 1978, 58, 1029–1034. [Google Scholar] [CrossRef]
- Finlay, K.W.; Wilkinson, G.N. The analysis of adaptation in a plant-breeding programme. Aust. J. Agric. Res. 1963, 14, 742–754. [Google Scholar] [CrossRef]
- STAR. Statistical Tool for Agricultural Research (STAR), version 2.0.1; Biometrics and Breeding Informatics, PBGB Division, International Rice Research Institute: Los Baños, Philippine, 2014.
- Pacheco, A.; Vargas, M.; Alvarado, G.; Rodríguez, F.; López, M.; Crossa, J.; Burgueño, J. GEA-R (Genotype x Environment Analysis with R for Windows.), version 4.1; International Maize and Wheat Improvement Center: El Batan, Mexico, 2016. [Google Scholar]
- Pfahler, P.L. Fertilization ability of maize pollen grains. I. Pollen sources. Genetics 1965, 52, 513. [Google Scholar] [CrossRef]
- Sprague, G.F. Pollen tube establishment and the deficiency of waxy seeds in certain maize crosses. Proc. Natl. Acad. Sci. USA 1933, 19, 838–841. [Google Scholar] [CrossRef]
- Westgate, M.E.; Lizaso, J.; Batchelor, W. Quantitative relationships between pollen shed density and grain yield in maize. Crop Sci. 2003, 43, 934–942. [Google Scholar] [CrossRef]
- Uribelarrea, M.; Cárcova, J.; Otegui, M.E.; Westgate, M.E. Pollen production, pollination dynamics, and kernel set in maize. Crop Sci. 2002, 42, 1910–1918. [Google Scholar] [CrossRef]
- Duvick, D.N. What is Yield: Developing Drought and Low N-Tolerant Maize; CIMMYT: El Batan, Mexico, 1997. [Google Scholar]
- Fonseca, A.E.; Westgate, M.E.; Grass, L.; Dornbos, D.L., Jr. Tassel morphology as an indicator of potential pollen production in maize. Crop Manag. 2003, 2, 1–15. [Google Scholar] [CrossRef]
- Bódi, Z.; Pepó, P.; Kovács, A. Morphology of tassel components and their relationship to some quantitative features in maize. Cereal Res. Commun. 2008, 36, 353–360. [Google Scholar] [CrossRef]
- Duvick, D.N.; Smith, J.S.C.; Cooper, M. Long-term selection in a commercial hybrid maize breeding program. Plant Breed. Rev. 2004, 24, 109–151. [Google Scholar] [CrossRef]
- Ci, X.; Li, M.; Xu, J.; Lu, Z.; Bai, P.; Ru, G.; Liang, X.; Zhang, D.; Li, X.; Bai, L.; et al. Trends of grain yield and plant traits in Chinese maize cultivars from the 1950s to the 2000s. Euphytica 2012, 185, 395–406. [Google Scholar] [CrossRef]
- Ma, D.L.; Xie, R.Z.; Yu, X.F.; Li, S.K.; Gao, J.L. Historical trends in maize morphology from the 1950s to the 2010s in China. J. Integr. Agric. 2022, 21, 2159–2167. [Google Scholar] [CrossRef]
- Qiu, F.; Liang, Y.; Li, Y.; Liu, Y.; Wang, L.; Zheng, Y. Morphological, cellular and molecular evidences of chromosome random elimination in vivo upon haploid induction in maize. Curr. Plant Biol. 2014, 1, 83–90. [Google Scholar] [CrossRef]
- Trentin, H.U.; Frei, U.K.; Lübberstedt, T. Breeding maize maternal haploid inducers. Plants 2020, 9, 614. [Google Scholar] [CrossRef]
- Trentin, H.U.; Yavuz, R.; Dermail, A.; Frei, U.K.; Dutta, S.; Lübberstedt, T. A comparison between inbred and hybrid maize haploid inducers. Plants 2023, 12, 1095. [Google Scholar] [CrossRef]
- Gain, N.; Chhabra, R.; Chandra, S.; Zunjare, R.U.; Dutta, S.; Chand, G.; Sarika, K.; Devi, E.L.; Kumar, A.; Madhavan, J.; et al. Variation in anthocyanin pigmentation by R1-navajo gene, development and validation of breeder-friendly markers specific to C1-inhibitor locus for in-vivo haploid production in maize. Mol. Biol. Rep. 2023, 50, 2221–2229. [Google Scholar] [CrossRef] [PubMed]
- Khulbe, R.K.; Pattanayak, A.; Panday, V. R1-nj expression in parental inbreds as a predictor of amenability of maize hybrids to R1-nj-based doubled haploid development. Indian J. Genet. Plant Breed. 2019, 79, 678–684. [Google Scholar] [CrossRef]
- Jampatong, C.; Jampatong, S.; Balla, C.; Grudloyma, P.; Jompuk, C.; Prodmatee, N. QTL Mapping for Downy Mildew (Peronosclerospora sorghi) Resistance in Maize. In Maize for Asia: Emerging Trends and Technologies. Proceeding of the 10th Asian Regional Maize Workshop, Makassar, Indonesia, 20–23 October 2008; Zaidi, P.H., Azrai, M., Pixley, K.V., Eds.; CIMMYT: Mexico City, Mexico, 2008; pp. 291–298. [Google Scholar]
- Mejaya, M.J.; Takdir, A.; Iriany, N.; Yasin, M.H.G. Development of Improved Maize Varieties in Indonesia. In Maize for Asia: Emerging Trends and Technologies. Proceeding of the 10th Asian Regional Maize Workshop, Makassar, Indonesia, 20–23 October 2008; Zaidi, P.H., Azrai, M., Pixley, K.V., Eds.; CIMMYT: Mexico City, Mexico, 2008; pp. 110–113. [Google Scholar]
- Stinard, P.S.; Sachs, M.M. The identification and characterization of two dominant r1 haplotype-specific inhibitors of aleurone color in Zea mays. J. Hered. 2002, 93, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Kang, M.S. GGE Biplot Analysis: A Graphical Tool for Breeders, Geneticists, and Agronomists; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Azrai, M.; Efendi, R.; Muliadi, A.; Aqil, M.; Suwarti; Zainuddin, B.; Syam, A.; Junaedi; Syah, U.T.; Dermail, A.; et al. Genotype by environment interaction on tropical maize hybrids under normal irrigation and waterlogging conditions. Front. Sustain. Food Syst. 2022, 6, 913211. [Google Scholar] [CrossRef]
- Singamsetti, A.; Shahi, J.P.; Zaidi, P.H.; Seetharam, K.; Vinayan, M.T.; Kumar, M.; Singla, S.; Shikha, K.; Madankar, K. Genotype× environment interaction and selection of maize (Zea mays L.) hybrids across moisture regimes. Field Crops Res. 2021, 270, 108224. [Google Scholar] [CrossRef]
- Mushayi, M.; Shimelis, H.; Derera, J.; Shayanowako, A.I.; Mathew, I. Multi-environmental evaluation of maize hybrids developed from tropical and temperate lines. Euphytica 2020, 216, 84. [Google Scholar] [CrossRef]
- Oyekunle, M.; Haruna, A.; Badu-Apraku, B.; Usman, I.S.; Mani, H.; Ado, S.G.; Olaoye, G.; Obeng-Antwi, K.; Abdulmalik, R.O.; Ahmed, H.O. Assessment of early-maturing maize hybrids and testing sites using GGE biplot analysis. Crop Sci. 2017, 57, 2942–2950. [Google Scholar] [CrossRef]
- Chen, C.; Liu, X.; Li, S.; Liu, C.; Zhang, Y.; Luo, L.; Miao, L.; Yang, W.; Xiao, Z.; Zhong, Y.; et al. Co-expression of transcription factors ZmC1 and ZmR2 establishes an efficient and accurate haploid embryo identification system in maize. Plant J. 2022, 111, 1296–1307. [Google Scholar] [CrossRef]
- Wang, D.; Zhong, Y.; Feng, B.; Qi, X.; Yan, T.; Liu, J.; Guo, S.; Wang, Y.; Liu, Z.; Cheng, D.; et al. The RUBY reporter enables efficient haploid identification in maize and tomato. Plant Biotechnol. J. 2023, 21, 1707–1715. [Google Scholar] [CrossRef]
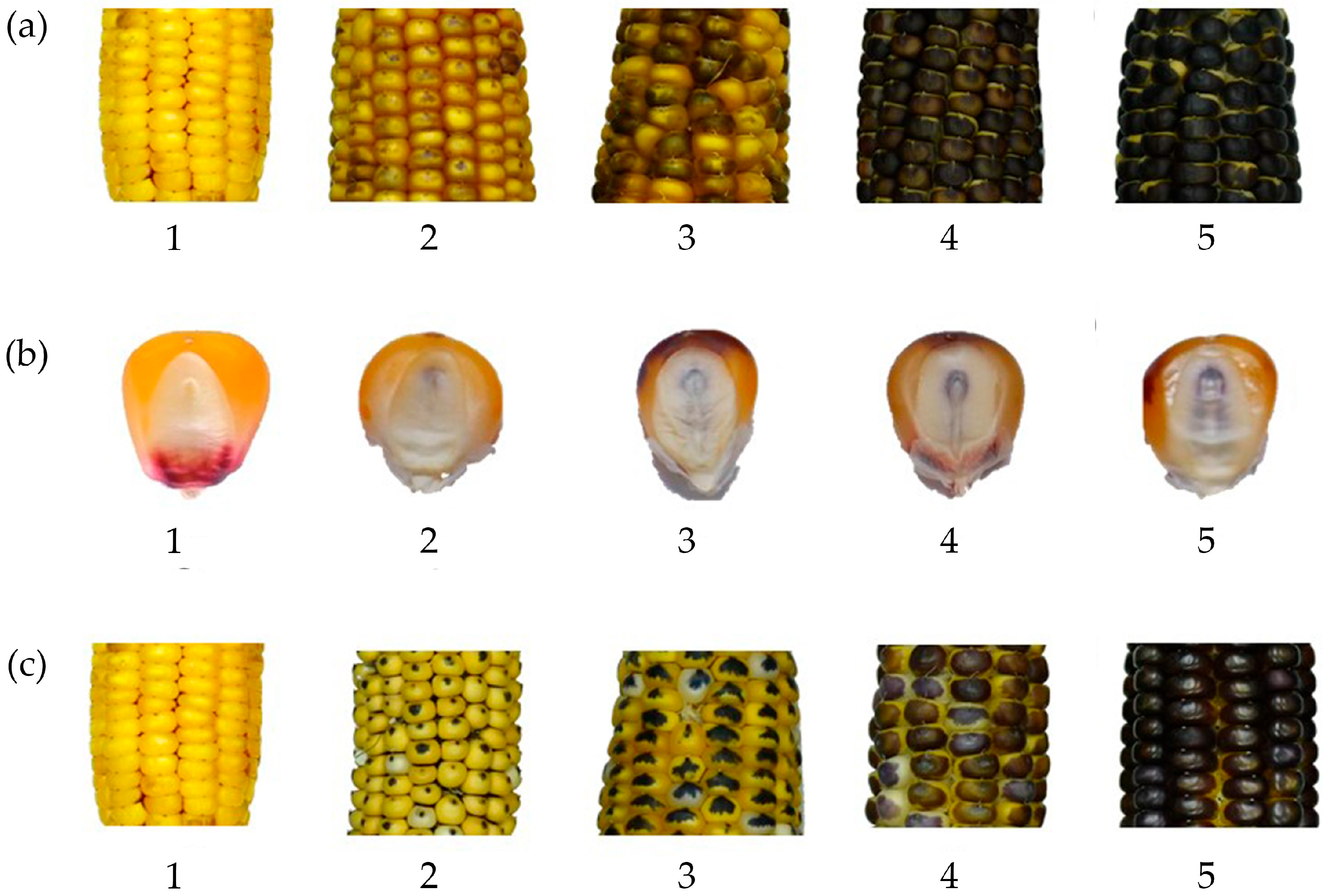





| Code | Haploid Inducer | Cultivar Type | Pedigree | Kernel Type | R1-nj Coverage | Haploid Induction Rate |
|---|---|---|---|---|---|---|
| 1 | KHI42 | Inbred | TL/Stock6-S(C6)-S3-IL1B-93-B-9 | Flint | Moderate | Low (4–7%) 1 |
| 2 | KHI54 | Inbred | WST/Stock6-S(C6)-S3-IL2A-34-1-14 | Flint | High | Low (4–78%) 1 |
| 3 | KHI64 | Inbred | NS3/Stock6-S(C6)-S3-IL2B-21-B-9 | Dent | Low | Low (2–78%) 1 |
| 4 | K7 | F3 population | TBL/Stock6-5B-B3 | Flint | Moderate | Poor (2–73%) 2 |
| 5 | BHI306 | Inbred | A632.75/(RWS/RWK-76)-PCPOP562-1-460-01 | Flint | High | High (11–714%) 3,4 |
| Code | Genotype | Cultivar Type | Recessive Allele (sh2, bt, su, se, and wx) | Kernel Type | Kernel Size | Kernel Color |
|---|---|---|---|---|---|---|
| Field maize | ||||||
| 1 | Nei9008 | Inbred | - | flint | medium | yellow |
| 2 | Takfa1 | Inbred | - | flint | medium | orange |
| 3 | Takfa7 | Inbred | - | dent | medium | orange |
| 4 | P789 | F1 hybrid | - | dent | big | orange |
| 5 | P789-S | F2 | - | semi-dent | big | orange |
| 6 | S7328 | F1 hybrid | - | semi-dent | big | orange |
| 7 | NS5-S | F2 | - | dent | big | orange |
| 8 | P789/NS5 | Double-cross hybrid | - | dent | big | orange |
| 9 | NS5/P789 | Double-cross hybrid | - | dent | big | orange |
| Waxy maize | ||||||
| 11 | 12C5-4 | Inbred | wx | flint | small | orange |
| 22 | Tein5-5-5 | Inbred | wx | flint | small | white-op |
| 18 | KKU WX-1 | Inbred | wx | flint | small | white-tr |
| 10 | Y.18W-6-4 | Inbred | wx | semi-flint | medium | white-op |
| 17 | RLW4 | Inbred | wx | semi-flint | large | white-op |
| 23 | Tein NS | OPV | wx | flint | small | yellow |
| 15 | CNW18178 | F1 hybrid | wx | dent | big | white-op |
| 16 | TSG1910 | F1 hybrid | bt and wx | dent-shrunken | big | white-op |
| Sweet maize | ||||||
| 21 | TSC/H3-1-8 | Inbred | sh2 | shrunken | extra small | orange |
| 24 | Pop. bt-5 | Inbred | bt | shrunken | small | pale white |
| 19 | W54/SQ | Inbred | su | sugary | small | white |
| 20 | W54/DEL | Inbred | su | sugary | small | orange |
| 25 | Pop. se-6 | Inbred | se | sugary | small | orange |
| 12 | Wan Dok Khun | OPV | sh2 | shrunken | big | light yellow |
| 13 | Jumbo Sweet | F1 hybrid | sh2 | shrunken | big | dark yellow |
| 14 | Jumbo Sweet-S | F2 | sh2 | shrunken | big | dark yellow |
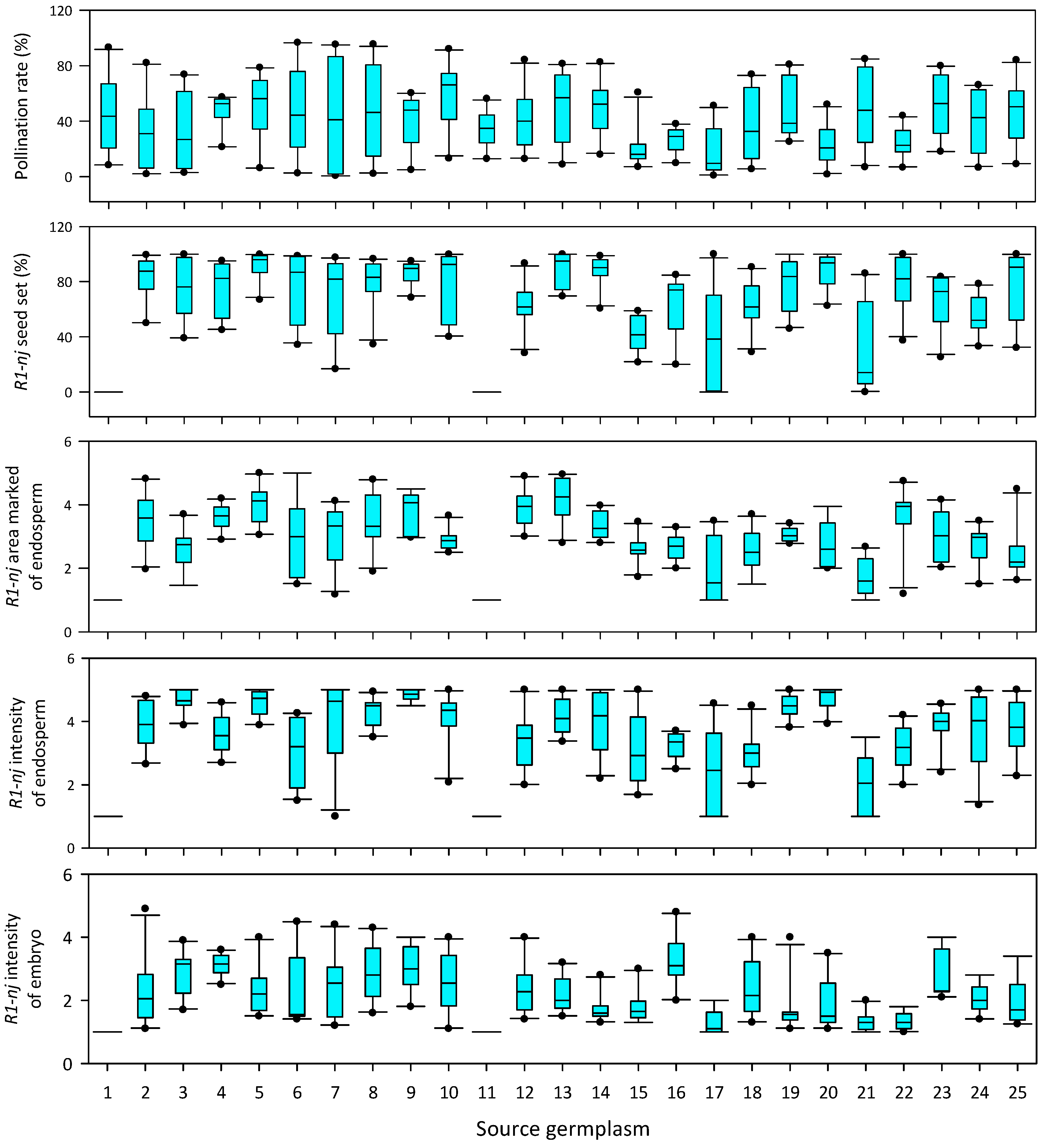
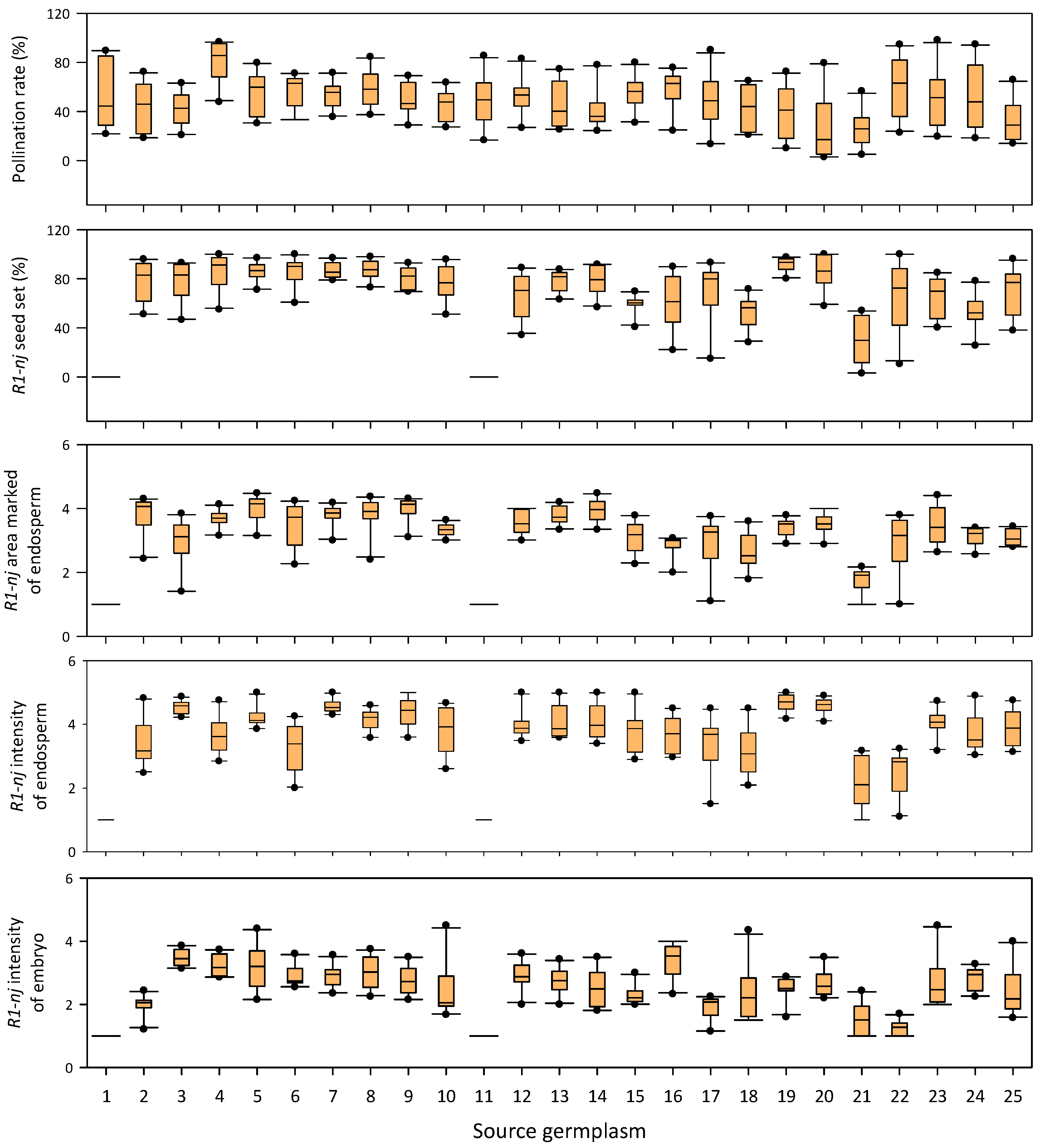
| Source of Variation | df | PR | RS | AED | IED | IEM |
|---|---|---|---|---|---|---|
| Season (S) | 1 | 3.3 ns | 0.04 ns | 1.6 ns | 0.06 ns | 3.0 * |
| Source germplasm (SG) | 24 | 10.6 ** | 64.82 ** | 61.5 ** | 67.39 ** | 48.8 ** |
| S × SG | 24 | 10.6 ** | 2.84 ** | 3.7 ** | 2.37 * | 3.8 ** |
| Pooled error (a) | 48 | 3.6 | 1.32 | 1.5 | 2.47 | 1.8 |
| Haploid inducer (HI) | 4 | 34.2 ** | 3.52 ** | 8.2 ** | 2.65 ** | 5.7 ** |
| SG × HI | 96 | 12.9 ** | 11.27 ** | 12.6 ** | 13.56 ** | 18.5 ** |
| S × HI | 4 | 0.8 * | 1.65 ** | 0.6 ** | 0.53 ** | 0.7 ** |
| S × SG × HI | 96 | 10.7 ** | 8.36 ** | 5.2 ** | 4.99 ** | 9.7 ** |
| Pooled error (b) | 200 | 11.8 | 6.15 | 4.9 | 5.53 | 7.9 |
| cv (a), % | 32.7 | 16.6 | 13.2 | 16.9 | 17.1 | |
| cv (b), % | 29.1 | 17.6 | 11.7 | 12.4 | 17.4 |
| HI | Pollination Rate (%) | |||||||
| Rainy | Dry | |||||||
| Yi | YSi | CVi | bi | Yi | YSi | CVi | bi | |
| KHI42 | 57.96 | 0 | 37.14 | 1.16 ** | 59.39 | −1 + | 32.18 | 1.48 ** |
| KHI54 | 43.02 | 4 + | 44.44 | 1.38 ** | 53.23 | −3 | 32.80 | 1.32 ** |
| KHI64 | 48.81 | 2 + | 39.30 | 1.22 ** | 61.68 | 0 + | 23.89 | 0.66 ** |
| K7 | 38.39 | 1 | 39.27 | 0.84 ** | 45.42 | −8 | 26.47 | 0.73 ** |
| BHI306 | 13.26 | −2 | 78.35 | 0.41 ** | 25.18 | −2 + | 41.38 | 0.80 ** |
| HI | R1-nj seed set (%) | |||||||
| Rainy | Dry | |||||||
| Yi | YSi | CVi | bi | Yi | YSi | CVi | bi | |
| KHI42 | 78.43 | 8 + | 35.84 | 1.03 ns | 70.80 | −2 + | 37.45 | 0.99 ns |
| KHI54 | 70.08 | 5 + | 39.63 | 1.01 ns | 71.51 | 7 + | 36.43 | 1.02 ns |
| KHI64 | 66.07 | 0 + | 43.42 | 1.01 ns | 62.21 | −8 | 41.10 | 0.91 ** |
| K7 | 60.70 | −8 | 53.97 | 1.12 ** | 61.69 | −10 | 41.81 | 0.91 ** |
| BHI306 | 52.89 | −10 | 48.77 | 0.82 ** | 67.81 | −4 | 51.19 | 1.17 ** |
| HI | R1-nj area marked of endosperm | |||||||
| Rainy | Dry | |||||||
| Yi | YSi | CVi | bi | Yi | YSi | CVi | bi | |
| KHI42 | 3.37 | 0 + | 32.68 | 1.13 ** | 3.46 | 0 + | 25.95 | 1.02 ns |
| KHI54 | 3.06 | −2 + | 30.18 | 0.97 ns | 3.35 | 7 + | 26.70 | 1.06 * |
| KHI64 | 2.87 | −6 | 35.23 | 1.06 * | 3.13 | −7 | 27.12 | 0.98 ns |
| K7 | 2.68 | −4 + | 34.49 | 0.98 ns | 3.20 | −4 | 26.38 | 0.97 ns |
| BHI306 | 2.49 | −10 | 36.26 | 0.85 ** | 2.61 | −10 | 38.42 | 0.97 ns |
| HI | R1-nj intensity of endosperm | |||||||
| Rainy | Dry | |||||||
| Yi | YSi | CVi | bi | Yi | YSi | CVi | bi | |
| KHI42 | 3.70 | 1 + | 29.40 | 0.92 ** | 3.51 | −6 | 27.04 | 0.89 ** |
| KHI54 | 3.63 | 4 + | 28.82 | 0.93 ** | 3.81 | −1 + | 26.33 | 0.94 * |
| KHI64 | 3.40 | −7 | 34.40 | 0.98 ns | 3.32 | −10 | 30.12 | 0.96 * |
| K7 | 3.16 | −9 | 38.47 | 1.02 ns | 3.45 | −8 | 28.53 | 0.93 ** |
| BHI306 | 3.74 | −1 + | 36.30 | 1.15 ** | 3.84 | 0 + | 38.29 | 1.28 ** |
| HI | R1-nj intensity of embryo | |||||||
| Rainy | Dry | |||||||
| Yi | YSi | CVi | bi | Yi | YSi | CVi | bi | |
| KHI42 | 2.44 | −1.5 + | 41.06 | 1.24 ** | 2.50 | 2 + | 30.37 | 1.02 ns |
| KHI54 | 1.92 | 0 + | 35.22 | 0.84 ** | 2.34 | −2 + | 29.71 | 0.92 ** |
| KHI64 | 2.27 | −4 | 46.32 | 1.37 ** | 2.64 | −2 + | 29.98 | 1.05 ns |
| K7 | 1.77 | −2 | 30.35 | 0.67 ** | 2.25 | −10 | 27.22 | 0.79 ** |
| BHI306 | 2.44 | −1.5 + | 36.49 | 0.87 ** | 2.68 | 0 + | 41.30 | 1.23 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dermail, A.; Lübberstedt, T.; Suwarno, W.B.; Chankaew, S.; Lertrat, K.; Ruanjaichon, V.; Suriharn, K. Compatibility and Stability Analysis of Haploid Inducers under Different Source Germplasm and Seasons in Maize Using GGE Biplot. Agronomy 2024, 14, 1505. https://doi.org/10.3390/agronomy14071505
Dermail A, Lübberstedt T, Suwarno WB, Chankaew S, Lertrat K, Ruanjaichon V, Suriharn K. Compatibility and Stability Analysis of Haploid Inducers under Different Source Germplasm and Seasons in Maize Using GGE Biplot. Agronomy. 2024; 14(7):1505. https://doi.org/10.3390/agronomy14071505
Chicago/Turabian StyleDermail, Abil, Thomas Lübberstedt, Willy Bayuardi Suwarno, Sompong Chankaew, Kamol Lertrat, Vinitchan Ruanjaichon, and Khundej Suriharn. 2024. "Compatibility and Stability Analysis of Haploid Inducers under Different Source Germplasm and Seasons in Maize Using GGE Biplot" Agronomy 14, no. 7: 1505. https://doi.org/10.3390/agronomy14071505
APA StyleDermail, A., Lübberstedt, T., Suwarno, W. B., Chankaew, S., Lertrat, K., Ruanjaichon, V., & Suriharn, K. (2024). Compatibility and Stability Analysis of Haploid Inducers under Different Source Germplasm and Seasons in Maize Using GGE Biplot. Agronomy, 14(7), 1505. https://doi.org/10.3390/agronomy14071505







