Screening of New Dendrobium officinale Strains Adapted to Karst Forest Environmental Stress Based on Electrophysiological Detection Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth and Treatment
2.2. Measurement of Real-Time Electrophysiological Parameters of Plant Leaves under Different Clamping Forces
2.3. Fitting Determination of Inherent Electrophysiological Parameters of Plant Leaves
2.4. Determination of Chlorophyll Fluorescence Parameters
2.5. Determination of Chlorophyll Content
2.6. Statistical Analysis
3. Results
3.1. Verification of Fitting Equation between Leaf Electrophysiological Parameters and Clamping Force
3.2. Differences in Electrophysiological Information, Intracellular Water Utilization, Nutrient Transport, and Metabolic Ability of Leaves of Different Strains
3.3. Differences in Leaf Growth of Different Strains
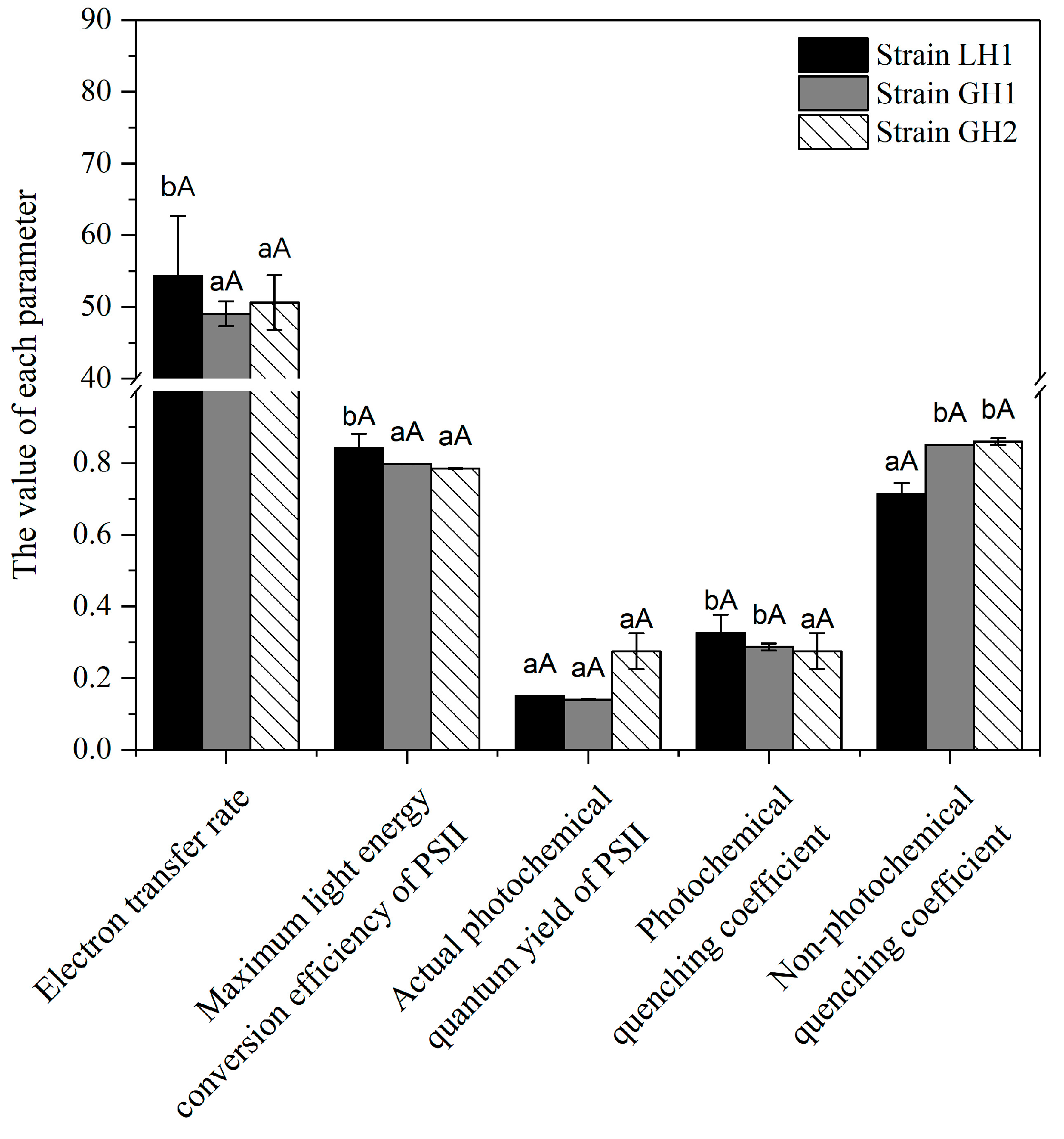
3.4. Differences in Chlorophyll Fluorescence in Leaves of Different Strains
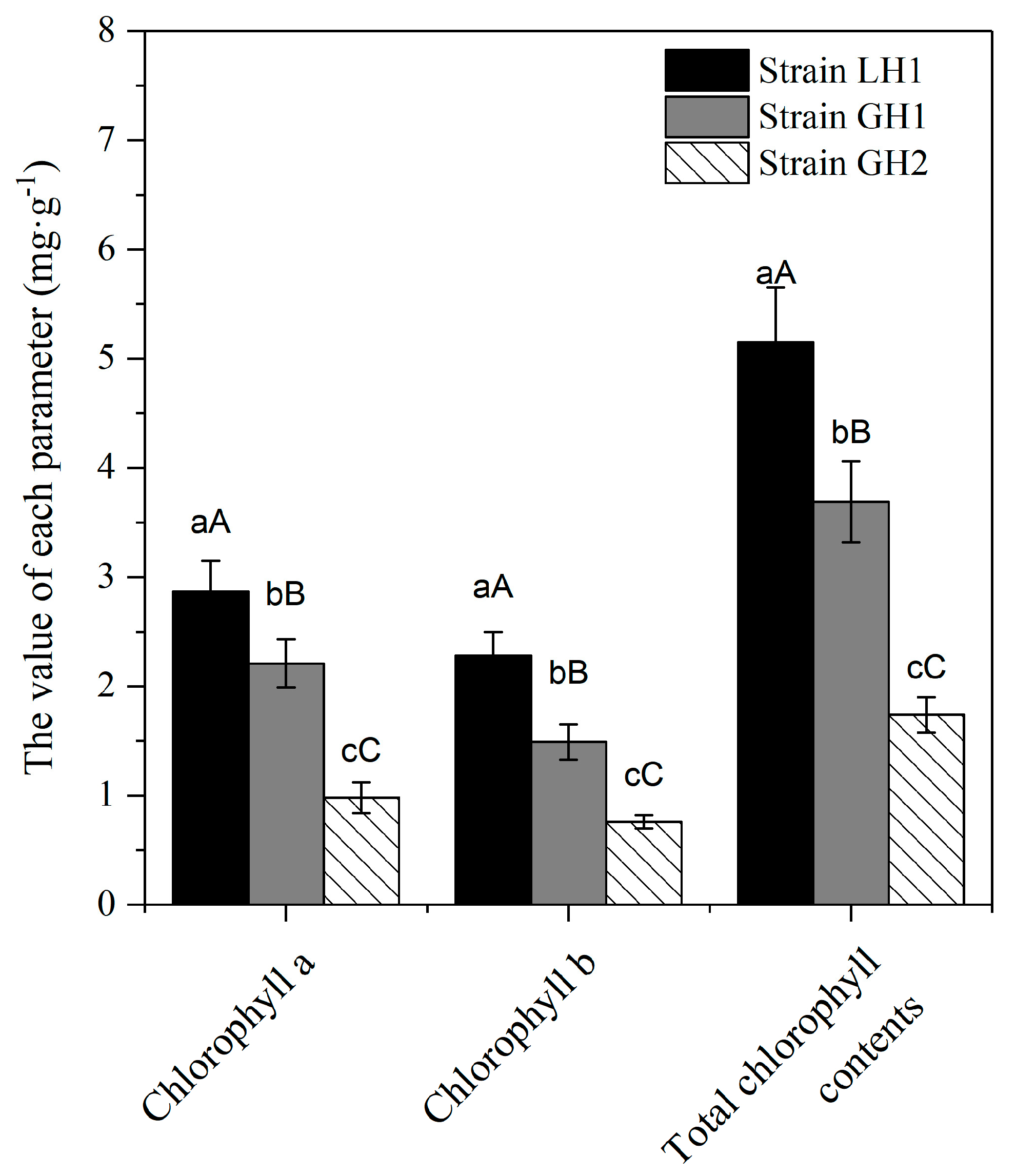
3.5. Differences in Chlorophyll Content in Leaves of Different Strains
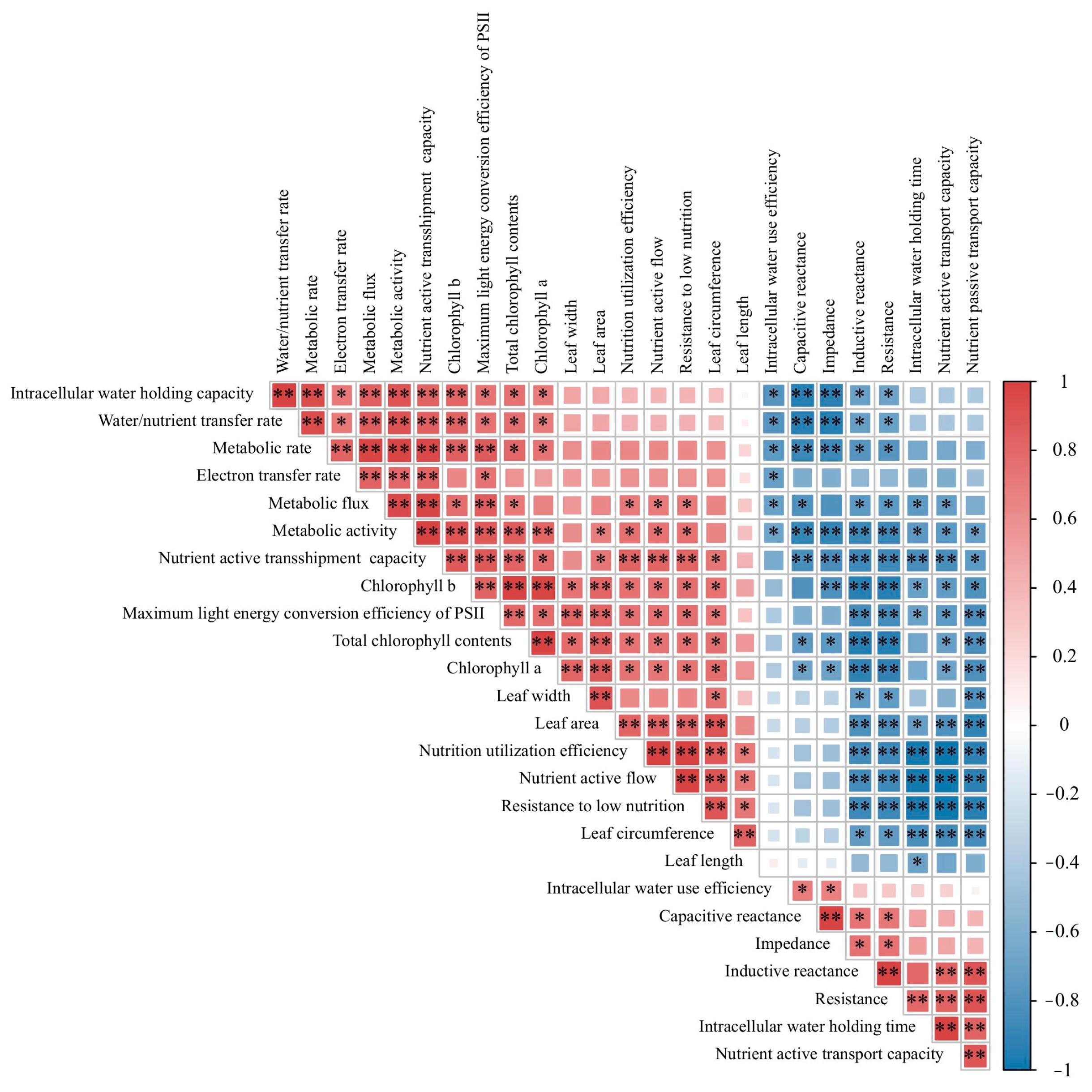
3.6. Correlation Analysis between Electrophysiological Parameters and Intracellular Water Utilization, Nutrient Transport, and Metabolic Parameters in Leaves of Different Strains
4. Discussion
4.1. Inherent Electrophysiological Information of Leaves of Different Strains of D. officinale
4.2. Differences in Intracellular Water and Nutrient Metabolism in Leaves of Different Strains of D. officinale Based on Electrophysiological Information
4.3. Effects of Drought Stress on Chlorophyll Fluorescence Parameters and Chlorophyll Content of Different Strains of D. officinale
4.4. Correlation among Electrophysiological Index, Growth Index, and Chlorophyll Index of D. officinale Leaves
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, R.C.; Shi, L.J.; Wu, Q.H.; Huang, Q.F.; Pan, L.M.; Nong, D.L.; Qin, F. Key Techniques of Dendrobium Officinale in Guangxi. Chin. J. Trop. Agric. 2018, 38, 43–45. [Google Scholar]
- Gu, H.Y.; Xie, K.P. Study on epiphytic cultivation techniques of Dendrobium officinale under forestin in Emei Mountain. South China For. Sci. 2021, 49, 40–43. [Google Scholar]
- Luo, Z.Q.; Long, Q.D.; Jang, Y.L.; Li, L. The current situation of Dendrobium industry in China and some thoughts on the development in Guizhou. Guizhou For. Sci. Technol. 2021, 49, 42–47. [Google Scholar]
- Acosta-Motos, J.R.; Álvarez, S.; Barba-Espín, G.; Hernández, J.A.; Sanchez-Blanco, M.J. Salts and nutrients present in regenerated waters induce changes in water relations, antioxidative metabolism, ion accumulation and restricted ion uptake in Myrtus communis L. plants. Plant Physiol. Biochem. 2014, 85, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New insights on plant salt tolerance mechanisms and their potential use for breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Jaleel, C.A.; Manivannan, P.; Panneerselvam, R.; Shao, M.A. Understanding water deficit stress-induced changes in the basic metabolism of higher plants—Biotechnologically and sustainably improving agriculture and the ecoenvironment in arid regions of the globe. Crit. Rev. Biotechnol. 2009, 29, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Wright, G.C.; Siddique, K. Adaptation of grain legumes (pulses) to water-limited environments. Adv. Agron. 2001, 71, 194–233. [Google Scholar] [CrossRef]
- Zeng, H.M.; Zhao, B. Relationship between Leaf Anatomical Structure and Drought Toleranee of 28 Hydrangea Cultivars. J. Northeast For. Univ. 2019, 47, 10–19. [Google Scholar]
- An, Y.Y.; Liang, Z.S. Staged strategy of plants in response to drought stress. Chin. J. Appl. Ecol. 2012, 23, 2907–2915. [Google Scholar]
- Wach, D.; Skowron, P. An overview of plant responses to the drought stress at morphological, physiological and biochemical levels. Pol. J. Agron. 2022, 50, 25–34. [Google Scholar] [CrossRef]
- Liu, S.; Fan, X.; Li, R.D.; Deng, J.; Dao, J.M.; Quan, Y.J.; Yang, S.L.; Zhang, Y.B. Physiological responses of four major sugarcane cultivars to drought stress. Chin. J. Trop. Crops. 2022, 43, 1812–1823. [Google Scholar]
- Zhu, W.; Zhang, X.B.; Gen, X.Y.; Chen, Y.L.; Dai, Q.G.; Zhou, G.S.; Wei, H.H.; Meng, T.Y. Research progresses on the mechanism of salinity and drought affecting root morpho-physiology and yield formation of rice. China Rice. 2023, 29, 34–40. [Google Scholar]
- Zhang, C. Application Ofelectrophysiological Information Inprediction and Control Ofsclerotinia in Brassica napus and Orychophragmus violaceus; Jiangsu University: Zhenjiang, China, 2021. [Google Scholar]
- Meteorological Bureau of Southwestern Guizhou. 2023. Available online: http://gz.cma.gov.cn/dsqxj/qxn/xwzx_39480/qxrd_39483/202305/t20230524_5526205.html (accessed on 24 May 2023).
- Wu, Y.Y.; Xie, J.J.; Xing, D.K.; Su, Y.; Zhang, C.; Tong, C.Y.; Zhou, Y.; Wang, S.J.; Liu, C.Q. A Detection Method for Plant Health Vitality Based on Electrophysiological Rhythms. China Patent CN113176301B, 30 April 2024. [Google Scholar]
- Wu, Y.Y.; Zhang, C.; Su, Y.; Wu, Y.S.; Fang, L.; Li, H.T.; Wu, M.K.; Wang, R. A Selection Method for Stress Resistant Crop Varieties Based on Electrophysiological Characteristics. China Patent CN110731191B, 13 July 2021. [Google Scholar]
- Wu, Y.Y.; Wu, Y.S.; Fang, L.; Wu, M.K.; Wang, R.; Su, Y.; Wang, S.J.; Liu, C.Q. A Method for Measuring Metabolic Energy of Plant Leaf Cells. China Patent CN109060886A, 21 December 2018. [Google Scholar]
- Zhang, M.M.; Wu, Y.Y.; Xing, D.K.; Zhao, K.; Yu, R. Rapid measurement of drought resistance in plants based on electrophysiological properties. Trans. ASABE. 2015, 58, 1441–1446. [Google Scholar]
- Javed, Q.; Wu, Y.Y.; Xing, D.K.; Azeem, A.; Ullah, I.; Zaman, M. Re-watering: An effective measure to recover growth and photosynthetic characteristics in salt-stressed Brassica napus L. Chil. J. Agric. Res. 2017, 77, 78–86. [Google Scholar] [CrossRef]
- Xing, D.; Chen, X.; Wu, Y.; Chen, Q.; Li, L.; Fu, W.; Shu, Y. Leaf stiffness of two Moraceae species based on leaf tensity determined by compressing different external gripping forces under dehydration stress. J. Plant Interact. 2019, 14, 610–616. [Google Scholar] [CrossRef]
- Zeng, W.J.; Qu, K.J.; Wan, C.; Li, P.; Xi, P.Y.; Li, L.J.; Wang, J.H. Effects of drought stress on photosynthesis and leaf tissue structure of Litsea coreana Levl. var. lanuginosa cutting seedlings. Guangdong Agric. Sci. 2021, 48, 7–14. [Google Scholar]
- He, J.; Bao, F.; Wu, B.; Yao, B.; Zhao, Y.M.; Liu, M.H. Response of chlorophyll fluorescence characteristics of a typical desert plant species nitraria tangutorumto simulated rainfall enhancement. J. Northwest For. Univ. 2020, 35, 55–63. [Google Scholar]
- Dghim, F.; Abdellaoui, R.; Boukhris, M.; Nefati, M.; Chaieb, M. Physiological and biochemical changes in Periploca angustifolinplants under withholding irrigation and rewatering conditions. S. Afr. J. Bot. 2018, 114, 241–249. [Google Scholar] [CrossRef]
- Tosin, R.; Pôças, I.; Novo, H.; Teixeira, J.; Fontes, N.; Graça, A.; Cunha, M. Assessing predawn leaf water potential based onhyperspectral data and pigment’s concentration of Vitis vinifera L. in the Douro Wine Region. Sci. Hortic. 2021, 278, 109860. [Google Scholar] [CrossRef]
- Guo, W.H.; Li, B.; Huang, Y.M.; Zhao, H.X.; Zhang, X.S. Effects of different water stresses on eco-physiological characteristics ofHippophae rhamnoides seedlings. Acta Bot. Sin. 2003, 45, 1238–1244. [Google Scholar]
- Zhang, C.; Wu, Y.Y.; Su, Y.; Xing, D.K.; Dai, Y.; Wu, Y.S.; Fang, L. A plant’s electrical parameters indicate its physiological state: Astudy of intracellular water metabolism. Plants. 2020, 9, 1256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, Y.Y.; Su, Y.; Li, H.; Fang, L.; Xing, D.K. Plant’s electrical information manifests the composition and nutrient transport characteristics of membrane proteins. Plant Signal. Behav. 2021, 16, 1918867. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.J.; Cheng, M.X.; Xie, S.Q.; Wang, X.S.; Hao, X.M.; Zhuang, L. Response of gowth characteristics and chlorophyll fluorescence characteristics of ephemeral pants to different soil types. Acta Agrestia Sin. 2022, 30, 3308–3316. [Google Scholar]
- Carvalho, L.B.; Alves, P.L.C.A.; Andrade, T. Content and fluorescence of chlorophyll in eucalypt exposed to glyphosate. Commun. Plant Sci. 2016, 6, 7–11. [Google Scholar] [CrossRef]
- Zhuang, Y.; Ge, J.X.; Wang, X.G.; Zan, J.Y.; Fu, G.Z.; Wang, X.D. Effect of rehydration after drought stress on the growth and physiological characteristics of flue-cured tobacco. Acta Tab. Sin. 2022, 28, 48–58. [Google Scholar]
- Qi, M.; Wang, J.; Zhang, M.; Ni, X.M.; Yang, M. Physiological response to drought stress and drought resistance evaluation of five Azalea varieties. Acta Agric. Jiangxi 2022, 34, 52–58. [Google Scholar]
- Zhang, P.; Zhang, H.; Liu, J.N.; Huan, X.J.; Wang, Q.C.; Li, L.; Liu, Y.J.; Qin, P. Effects of drought and re-watering treatment on physiological and biochemical indexes of different drought-resistant wheat varieties/lines at seedling stage. Northwest China J. Agric. Sci. 2020, 29, 1795–1802. [Google Scholar]
- Wu, Y.Y.; Zhang, C.; Su, Y.; Li, H.T.; Wu, Y.S.; Fang, L.; Tong, C.Y.; Zhou, Y.; Sun, T.; Wang, S.J.; et al. A Method for Quantifying Plant Nutritional Metabolism and Resource Plundering Capacity. China Patent CN112649474B, 1 March 2024. [Google Scholar]
- Wu, Y.Y.; Yu, R.; Zhang, C.; Su, Y.; Wu, Y.S.; Fang, L.; Wu, M.K.; Wang, R. A Quantitative Method for Assessing Plant Low Nutrient Tolerance and Nutrient Utilization Efficiency. China Patent CN110646469B, 19 April 2022. [Google Scholar]
- Wang, J.; Li, D.Q. The Accumulation of Plant Osmoticum and Activated Oxygen Metabolism under Stress. Chin. Bull. Bot. 2001, 18, 459–465. [Google Scholar]
- Paramasivam, M.; Cheruth, A.J.; Ramamurthy, S.; Rajaram, P. Osmoregulation and antioxidant metabolism in drought-stressed Helianthus annuus under triadimefon drenching. Comptes Rendus. Biologies. 2008, 331, 418–425. [Google Scholar] [CrossRef]
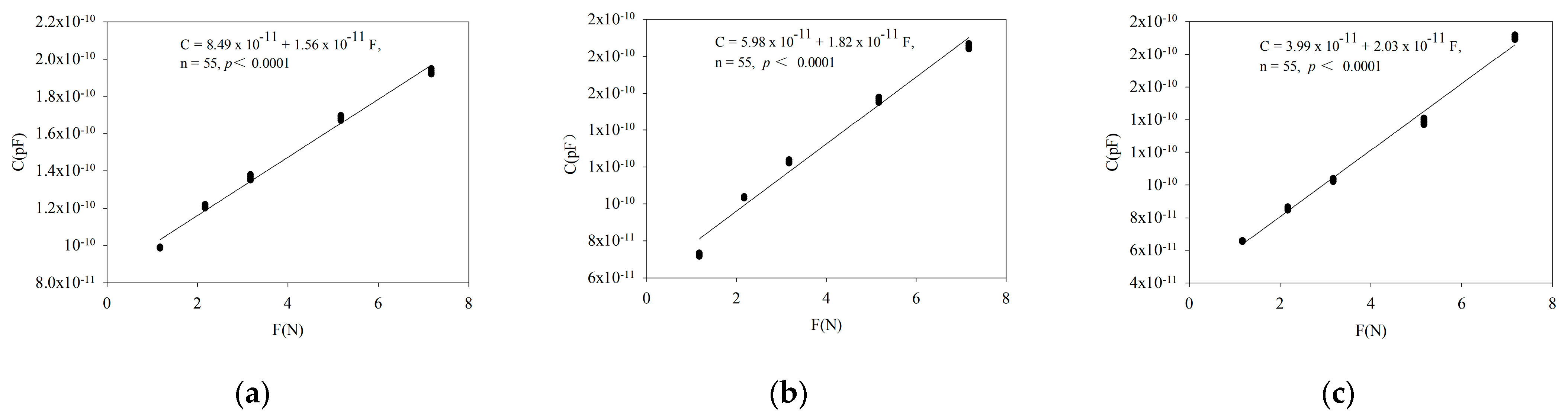
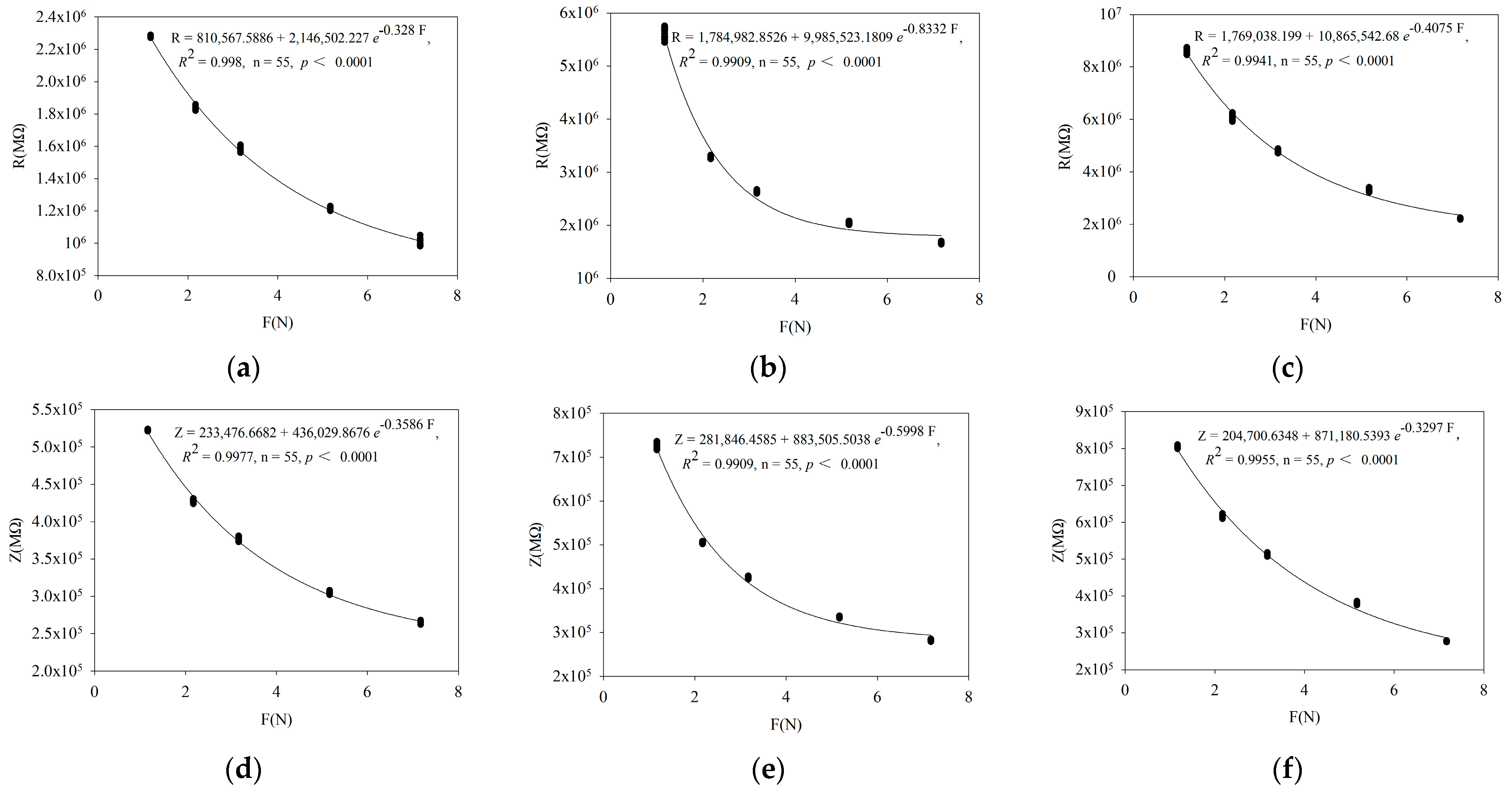
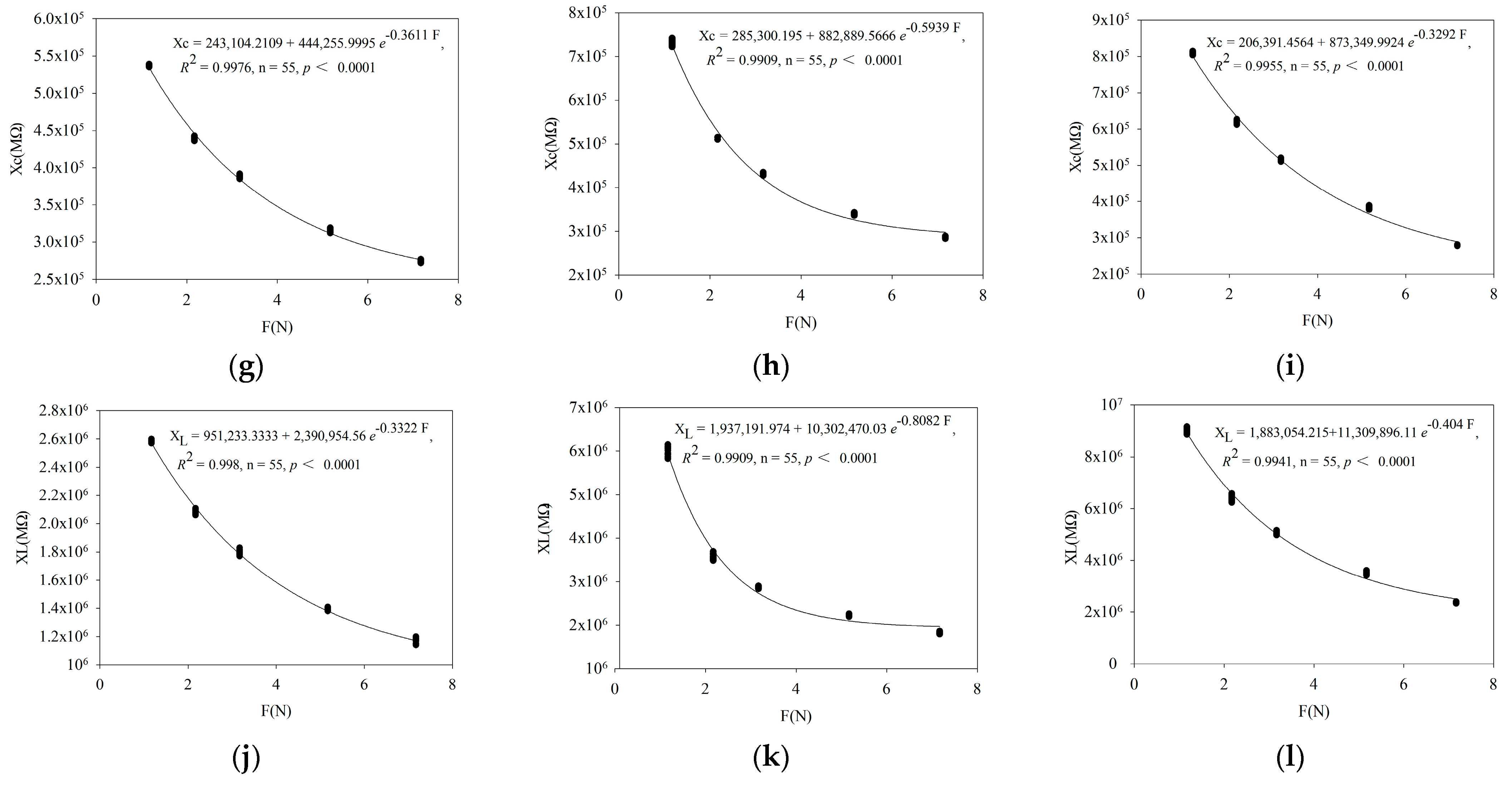
| Parameter | Fitting Equation |
|---|---|
| Resistance (R) | |
| Impedance (Z) | |
| Capacitive reactance (XC) | |
| Inductive reactance (XL) | |
| Capacitance (C) | |
| Effective thickness (d) | |
| Intracellular water-holding capacity (IWHC) | |
| Intracellular water-use efficiency (IWUE) | |
| Intracellular water-holding time (IWHT) | |
| Water/nutrient transfer rate (STR) | |
| Relative flux of nutrient unit (UNF) | |
| Nutrient transport rate (NTR) | |
| Nutrient transport capacity (NTC) | |
| Nutrient active flow (UAF) | |
| Nutrient active transshipment capacity (NAC) | |
| Nutrient active transport capacity (NAT) | |
| Nutrient passive transport capacity (NPT) | |
| Nutrition utilization efficiency (NUE) | |
| Resistance to low nutrition (RLN) | |
| Metabolic flux (MF) | |
| Metabolic rate (MR) | |
| Metabolic activity (MA) |
| Strains | Capacitance (C, pF) | Resistance (R, MΩ) | Impedance (Z, MΩ) | Capacitive Reactance (XC, MΩ) | Inductive Reactance (XL, MΩ) |
|---|---|---|---|---|---|
| LH1 | 78.35 ± 16.95 aA | 3.89 ± 0.82 cB | 0.69 ± 0.15 bA | 0.70 ± 0.15 bA | 4.28 ± 0.81 cB |
| GH1 | 49.01 ± 7.83 bA | 9.96 ± 2.33 bAB | 1.09 ± 0.16 aA | 1.10 ± 0.16 aA | 10.49 ± 2.23 bAB |
| GH2 | 44.44 ± 7.942 bA | 16.64 ± 3.63 aA | 1.22 ± 0.24 aA | 1.22 ± 0.24 aA | 17.30 ± 3.67 aA |
| Strains | Effective Thickness (d, m) | Intracellular Water-Holding Capacity (IWHC) | Intracellular Water-Use Efficiency (IWUE) | Relative Intracellular Water-Holding Time (IWHT) | Water/Nutrient Transfer Rate (STR) |
|---|---|---|---|---|---|
| LH1 | 17.92 ± 2.07 aA | 701.65 ± 225.99 aA | 0.028 ± 0.01 bA | 52.11 ± 0.50 bA | 13.44 ± 4.22 aA |
| GH1 | 18.67 ± 2.36 aA | 345.23 ± 83.98 bA | 0.06 ± 0.02 aA | 52.73 ± 0.35 abA | 6.54 ± 1.56 bA |
| GH2 | 15.08 ± 6.97 aA | 298.64 ± 77.25 bA | 0.05 ± 0.02 aA | 52.94 ± 0.07 aA | 5.64 ± 1.46 bA |
| Strains | Nutrient Passive Transport Capacity (NPT) | Nutrient Active Transport Capacity of (NAT) | Nutrition Utilization Efficiency (NUE) | Resistance to Low Nutrition (RLN) | Active Flow of Nutrient Unit (UAF) | Nutrient Active Transshipment Capacity (NAC) |
|---|---|---|---|---|---|---|
| LH1 | 5.79 ± 2.061 bA | 0.91 ± 0.03 bA | 15.81 ± 4.30 aA | 14.28 ± 3.51 aA | 167,811.39 ± 46,945.73 aA | 2,155,671.32 ± 500,151.86 aA |
| GH1 | 9.29 ± 2.96 abA | 0.95 ± 0.02 abA | 10.44 ± 3.49 abA | 9.82 ± 3.01 abA | 109,715.16 ± 37,627.76 abA | 689,626.06 ± 143,716.37 bB |
| GH2 | 13.85 ± 3.73 aA | 0.96 ± 0.01 aA | 7.01 ± 1.55 bA | 6.73 ± 1.43 bA | 72,323.58 ± 16,253.62 bA | 400,501.28 ± 115,524.01 bB |
| Strains | Metabolic Flux (MF) | Metabolic Rate (MR) | Metabolic Activity (MA) |
|---|---|---|---|
| LH1 | 147,451.78 ± 74,782.09 aA | 29,373,575.45 ± 11,514,173.85 aA | 125.34 ± 20.46 aA |
| GH1 | 8895.65 ± 3415.29bB | 4,488,409.55 ± 1,238,046.06 bB | 57.89 ± 6.54 bB |
| GH2 | 2887.13 ± 2009.22bB | 2,327,233.86 ± 1,102,590.90 bB | 42.29 ± 8.32 bB |
| Strains | Leaf Area (LA, mm2) | Leaf Circumference (LC, mm) | Leaf Length (LL, mm) | Leaf Width (LW, mm) |
|---|---|---|---|---|
| LH1 | 638.98 ± 14.02 aA | 127.10 ± 3.43 aA | 51.28 ± 1.37 aA | 16.90 ± 0.46 aA |
| GH1 | 580.37 ± 47.80 abA | 122.80 ± 4.49 abA | 51.28 ± 1.96 aA | 15.76 ± 0.60 abA |
| GH2 | 489.07 ± 73.54 bA | 117.31 ± 3.29 bA | 49.06 ± 1.14 aA | 13.89 ± 2.16 bA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, M.; Liu, X.; Wu, R.; Yang, P.; Yang, L.; Zhou, M.; Wu, M. Screening of New Dendrobium officinale Strains Adapted to Karst Forest Environmental Stress Based on Electrophysiological Detection Method. Agronomy 2024, 14, 1530. https://doi.org/10.3390/agronomy14071530
Luo M, Liu X, Wu R, Yang P, Yang L, Zhou M, Wu M. Screening of New Dendrobium officinale Strains Adapted to Karst Forest Environmental Stress Based on Electrophysiological Detection Method. Agronomy. 2024; 14(7):1530. https://doi.org/10.3390/agronomy14071530
Chicago/Turabian StyleLuo, Ming, Xiao Liu, Rongju Wu, Pingfei Yang, Lin Yang, Mei Zhou, and Mingkai Wu. 2024. "Screening of New Dendrobium officinale Strains Adapted to Karst Forest Environmental Stress Based on Electrophysiological Detection Method" Agronomy 14, no. 7: 1530. https://doi.org/10.3390/agronomy14071530
APA StyleLuo, M., Liu, X., Wu, R., Yang, P., Yang, L., Zhou, M., & Wu, M. (2024). Screening of New Dendrobium officinale Strains Adapted to Karst Forest Environmental Stress Based on Electrophysiological Detection Method. Agronomy, 14(7), 1530. https://doi.org/10.3390/agronomy14071530




