Abstract
To obtain effective biocontrol strains for downy mildew of grape, 38 endophytic bacteria were isolated from fruits, seeds, and old stems of six grape varieties. Using spot inoculation mixtures of sporangial suspensions of Plasmopara viticola and biocontrol bacterial suspension, this screen yielded three strains (G1, G5, and G9) with good antagonistic effects against P. viticola. The growth inhibition rate was 100%, which was comparable to the effect of the positive control Bacillus subtilis strain CN181. The enzyme activity and the metabolites of strain G1 were examined on casein hydrolysate medium, sodium carboxymethyl cellulose agar plates, and chrome azurol sulfonate (CAS) agar plates. The antifungal protein component was identified by liquid chromatography–mass spectrometry (LC–MS). The results showed that strain G1 was more effective against Plasmopara viticola after two field trials, and the inhibition rates of strain G1 on the seventh day of the two field trials were 47.5% and 36.9%, respectively. Strain G1 was identified as Bacillus amyloliquefaciens based on morphological examination and 16S rDNA sequence analysis. It produced proteases, cellulases, and siderophores. Crude protein of the strain mainly included the putative segregation protein SpoVG, which inhibited P. viticola.
1. Introduction
Downy mildew is a widespread and destructive disease that affects many species of plant including grapevines. It is caused by Plasmopara viticola, an obligate, parasitic, oomycete pathogen. The disease can result in serious yield reduction, or even lead to no yield in some years with excessive rainfall [1,2,3]. Therefore, new and more effective measures for the control of P. viticola are needed. At present, chemical control remains the main means of prevention and control of the disease, but the long-term use of chemical pesticides is associated with a number of problems, such as pesticide residue and the development of resistance, which in turn leads to a decreased quality of grape and wine. Biological control precisely reduces pesticide residues and pesticide resistance in grapes. Therefore, there is increasing research interest in the development of novel methods of biological control.
A number of beneficial endophytic bacteria have been found in plants, and these may both provide necessary nutrients and also enhance disease resistance. Such bacteria readily colonize host plants, interact directly with pathogens, and produce antibiotics and other beneficial substances, among other actions. Therefore, there is a great deal of research interest in utilizing such bacteria as biological controls of plant disease [4,5,6]. About 60 genera of endophytic bacteria have been isolated from corn, rice, tomato, wheat, and other plants. They have been used to control corn sheath blight (Rhizoctonia solani), corn leaf spot (Curvularia leaf spot of maize), tomato bacterial wilt (Fusarium oxysporum f. sp. lycopersici Snyder et Hansen), wheat sheath blight (Ceratobasidium cereale), and other commercially important diseases [7,8]. In recent years, attention has gradually begun to be paid to the control of grapevine downy mildew with biocontrol fungi. [9,10]. In this study, we isolated and selected endophytic bacteria from grape tissue to lay a foundation for the biological control of this pathogen in grape.
2. Materials and Methods
2.1. Materials
Old stems of Manicure Finger grapes Vitis labrusca × vinifera “Kyoho”, Vitis vinifera × Vitis labrusca “Boxin 1” and “Vitis vinifera Hongru” were obtained from Hebei Agricultural University’s nursery vineyard. The fruits of Kyoho grapes and red globe and centennial seedless grapes were purchased from the Hebei Agricultural University supermarket on 24 November 2015. Bacillus subtilis CN181 were provided by the Forest Pathology Laboratory of Hebei Agricultural University. The test media used in the experiments were nutrient agar (NA), casein medium, cellulase activity detection medium, and hydrophilic activity detection medium, which were provided by Beijing Tsingke Biotech Co., Ltd. (Beijing, China).
2.1.1. Isolation of Bacteria from Stems
The old stems of grape vines were soaked in 70% alcohol for 30 s, treated with 0.1% mercuric chloride solution for 3 min, and then washed three times with sterile water. The epidermis tissue was removed from the stem tissue and slices 2–5 mm thick were cut with a sterile scalpel. These were inoculated onto NA medium. A total of 100 μL sterile water was taken from the last cleaning and was used to coat the plate as a control. All samples were prepared in triplicate. Finally, the plates were cultured at 28 °C for 2–3 days, and the resulting colonies were picked and examined for morphology; bacteria were purified.
2.1.2. Separation of Bacteria from Fruits
Grape fruits were treated in the same way as stems. The grape peel was removed and the grapes were squeezed to extract juice. Aliquots of 100 μL juice were added to NA medium and spread evenly on plates. A total of 100 μL sterile water was taken from the last cleaning and used to coat the plate as a control. All samples were prepared in triplicate. Finally, the plates were cultured at 28 °C for 2–3 days, and the resulting colonies were picked and examined for morphology; bacteria were purified.
2.1.3. Separation of Bacteria from Seeds
Grape seeds were treated in the same way as stems. The seeds were homogenized in 2 mL sterile water with a mortar. Aliquots of 100 μL of the resulting solution were added to NA medium and spread evenly on plates. A total of 100 μL sterile water was taken from the last cleaning and used to coat the plates as a control. Finally, the plates were cultured at 28 °C for 2–3 days, and the resulting colonies were picked and examined for morphology; bacteria were purified.
2.2. Inhibition of Disease by Endogenous Bacteria
2.2.1. Preparation of Biocontrol Bacterial Suspension
Purified biocontrol bacteria were streaked on plates, and cultured for 1–2 days. The resulting colonies were scraped with an inoculation loop, placed in liquid medium, incubated at 28 °C, and placed in a 200 r/min shaker (provided by Ningbo Dongnan Instrument Co., Ningbo, China) for 2 days, before being prepared in a bacterial suspension of 2 × 108 cfu/mL with sterile water, stored in a refrigerator at 4 °C, and set aside [1,11].
2.2.2. Preparation of Grape Downy Mildew Sporangia
Leaves infected with downy mildew were rinsed with water, and the petioles were wrapped in absorbent cotton, placed in dishes with wet gauze, and cultured in an incubator (provided by Ningbo Dongnan Instrument Co.) for 1–2 days under a 12 h light (22 °C)/dark (20 °C) cycle to promote the production of new sporangia. Sporangia were brushed into sterile water with a brush, filtered with a single layer of lens paper, and prepared in a 1.5 × 106 sporangium/mL sporangia suspension stored in a 4 °C freezer for later use.
The biocontrol strain and the grape downy mildew sporangia suspensions were mixed at a 1:1 ratio and spotted onto the backs of fresh, healthy leaves of Kyoho grapes. Each leaf was spotted at 40 points (5 μL each), and the leaves were inoculated in three replications. The sporangia suspension of downy mildew in grapes was spotted and inoculated as a control. Specimens were cultured in an incubator for 8 days, and the incidence of infection was recorded to allow the calculation of the inhibition rate as follows:
incidence (%) = (infected points/total inoculated points) × 100%
inhibition rate (%) = ([control morbidity − treatment morbidity]/control morbidity) × 100%
2.3. Determination of Endophytic Antagonistic Bacteria in the Field
The strains that showed good inhibitory effects against grape downy mildew selected in the laboratory were cultured at 28 °C with shaking at 200 rpm for 2 days. The fermentation broth was diluted to 2 × 108 cfu/mL, sprayed onto fields of diseased plants (spraying from the onset of the disease), and sprayed with water, biocontrol bacteria CN181 fermentation broth and 1000 times the amount of mancozeb (80%) and 2000 times the amount of dimethylmorph (80%) on the fields of diseased plants as a control. The disease index of the plants was carried out prior to the treatment and on the third day and seventh day after the treatment, and the control rate was evaluated.
The disease index was graded according to the lesion area of diseased leaves: grade 0, lesion area 0%; grade 1, 1–5%; grade 3, 6–25%; grade 5, 26–50%; grade 7, 51–75%; and grade 9, ≥76% [12].
disease index = (Σ[number of leaves at each level × relative value]/[total number of surveys ×5]) × 100
Relative control rate (%) = [(Increase in control condition index − Increase in treatment condition index)/Increase in control condition index] × 100%.
2.4. Identification of Endogenous Antagonistic Bacteria
2.4.1. Morphological Analysis
The antagonistic strain was streaked on NA medium and incubated at 28 °C for 48 h to observe the morphology of the colony. The strain was examined for Gram reaction using 3% KOH Solution [13].
2.4.2. S rDNA Sequence Analysis
16S rDNA was amplified using the primers 27F and 1492R and sequenced by Beijing Sanboyuanzhi Biotechnology (Beijing, China). The sequences were compared to those in the GenBank database using BLAST, and a phylogenetic tree was constructed using the Neighbor Joining method with MEGA5.0.
2.5. Detection of Activity of Endogenous Antagonistic Enzymes and Secondary Metabolites
2.5.1. Detection of Protease Activity
The antagonistic strain was activated, isolated, and transferred onto casein medium with toothpicks, incubated at 28 °C for 2–3 days, and examined for the presence or absence of a transparent halo zone indicating protease activity. Sterile water was used as a blank negative control. All samples were prepared in triplicate.
2.5.2. Cellulase Activity Test
The antagonistic strain was inoculated onto cellulase plates according to a previous method [14]. The plates were incubated at 28 °C for 48 h, then stained with 1 g/L Congo red dye. After staining the resulting strain with 1 g/L Congo red for 10~15 min, the staining solution was poured off, and then the resulting strain was soaked in 1 mol/L NaCl for 15 min, and examined for the presence or absence of a transparent halo zone indicating cellulase activity. Sterile water was used as a blank negative control. All samples were prepared in triplicate.
2.5.3. Detection of Siderophore Activity
Siderophore production was examined according to the method of Shin et al. [15]. The bacterial antagonist strains obtained were inoculated by shaking them into an NA liquid medium and culturing at 28 °C with shaking at 200 rpm for 2 days. Nine wells of 5 mm in diameter were made in a siderophore activity medium on plates and inoculated with 35 μL bacterial suspension per well. The cultures were examined after 3 days of incubation for the presence or absence of orange halo zones indicating siderophore activity. Sterile water was used as a blank negative control. All samples were prepared in triplicate.
2.6. Extraction of Antifungal Crude Protein from Antagonistic Strain G1
The culture solution was centrifuged at 19,000 rpm for 10 min and the precipitate was discarded. Ammonium sulfate was added to the supernatant to different saturation levels (20%, 30%, 40%, 50%, 60%, 70%, 80%, and 90%) and precipitation was allowed to proceed at 4 °C overnight. The culture solution was centrifuged at 19,000 rpm for 10 min and the supernatant was discarded. The precipitate was dissolved with a small amount of phosphate buffer saline (pH 7.2), and the resulting solution was placed in a dialysis bag (with a molecular weight cutoff of 14 kDa) and then in a refrigerator at 4 °C for dialysis and desalination. The deionized water dialysate was replaced three times at 2–4 h, 6–8 h, and 10–14 h. After desalting, centrifugation was performed at 19,000 rpm for 10 min to collect the supernatant, which was purified with a bacterial filter (pore size: 0.22 μm) and then assayed for inhibitory activity to select the optimal ammonium sulfate saturation level.
2.7. Determination of Antibacterial Activity of Protein
A piece of Botrytis cinerea mycelial cake of 8 mm in diameter was added to a potato dextrose agar (PDA) medium with a filling volume of 50 mL protein concentration of bacillus solution per 250 mL triangular bottle. The extracted antifungal crude protein from antagonistic strain G1 was added to a flask and shaken for various numbers of days (see below) at 180 rpm at 28 °C. An equal volume of sterile water was used as a negative control. All samples were prepared in triplicate. Changes in the mycelia were observed under a microscope after 1, 2, 3, 4, and 5 days of incubation, and photographs were taken.
Determination of Bacterial Inhibitory Activity Using the Agar Well Diffusion Standoff Method
Four holes of about 6 mm in diameter were punched in a cross pattern at a distance of about 40 mm on plates 90 mm in diameter. Plugs of Botrytis cinerea cake 8 mm in diameter were placed in the center of the plates, and 60 µL of test supernatant was pipetted into each of the small holes. Then, the plates were sealed with film and incubated at 28 °C. After 5 days, the colony diameter of the indicator fungus was observed and measured. The same volume of PBS was applied as a negative control. Bacterial inhibitory activity was calculated as follows:
fungal inhibitory activity (%) = (control colony diameter − treatment colony diameter)/control colony diameter × 100
To suspensions of Botrytis cinerea conidia (1 × 106 cfu/mL) were added an equal volume of extracted crude protein solution. These were cultured on PDA plates at 28 °C for 12 h. Growth was examined regularly. Sterile water was used as a negative control. The inhibitory effects of extracted crude protein on downy mildew sporangia were determined as follows:
inhibition rate = [control spore germination number − treatment spore germination number]/control spore germination = 100%
2.8. Purification and Identification of Strain G1 Antifungal Protein
2.8.1. Q-Sepharose FF Anion Exchange Chromatography
The crude protein extract of strain G1 was dialyzed against 50 mM Tris-HCl (pH 8.0), and the dialysate was centrifuged at 4 °C for 10 min at 12,000 rpm. The resultant supernatant was mixed and incubated with Q-Sepharose FF ion exchange chromatography resin, subjected to anion-exchange high-performance liquid chromatography for 1.5 h (mobile phase A, 50 mmol/L Tris-HCl, pH = 8.0; mobile phase B, 50 mmol L−1 Tris-HCl buffer, pH = 8.0 containing 1 mol/L NaCl) at a flow rate of 2 mL min−1, and eluted with NaCl solutions of different concentrations (0.2, 0.4, 0.6, 0.8, and 1.0 mol/L NaCl in 50 mmol/L Tris-HCl buffer) for gradient elution. The absorbance at 280 nm (A280) of each component was determined, and the individual protein elution peaks were collected. The fractions of each absorption peak were purified by passing samples through a bacterial filter (pore size: 0.22 μm), and the fractions of each absorption peak were tested for their inhibitory effect on the mildew pathogens using the agar pore diffusion standoff method.
2.8.2. Identification of Proteins by LC-MS
Proteins were identified via liquid chromatography–mass spectrometry (LC–MS) at Nanjing Zhongding Biotechnology Co. (Nanjing, China).
2.9. Data Retrieval
Data for Bacillus amyloliquefaciens proteins were retrieved from the UniProt database (https://www.uniprot.org/taxonomy/1390) accessed on 1 January 2020.
3. Results
3.1. Isolation of Endophytic Bacteria from Grape Tissue
In total, 38 strains of endophytic bacteria were isolated from the grape varieties outlined above. Nineteen strains were isolated from stem tissue, 13 from fruit, and six from seeds (Table 1).

Table 1.
Grape Endogenous Bacteria Source Distribution.
3.2. Endogenous Strains Capable of Inhibiting Downy Mildew
Thirty-eight strains of endophytic bacteria were examined and had different effects on the mildew, with 30 showing an inhibitory effect, accounting for 78.9% of the total number isolated; nine of these showed >90% inhibition. These strains included G1, G5, and G9. Strains G1, G5, G9 showed the strongest inhibitory effect, showing 100% inhibition (Table 2).

Table 2.
Endogenous bacteria on the grape downy mildew inhibition rate.
3.3. Effects of Endophytic Antagonistic Strains on Downy Mildew in the Field
The results of field trials performed in August and September 2015 showed that the fermentation broth of three endophytic bacterial strains (G1, G5, and G9) had different antagonistic effects on the mildew (Figure 1A). For example, on day 3 of treatment the disease indexes were 13.7 and 14.2 for G5 and G9, respectively. These values were not significantly different from the negative control group but were significantly higher than the positive control group.
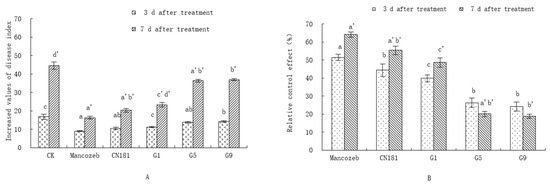
Figure 1.
Effect of endophytic antagonistic strains on the control of grape downy mildew on 19 August 2024. Note: (A) means disease index, (B) means relative control effect. The lowercase letters in Figure 1 show significant differences (p < 0.05). The letters a, b, and c represent the results 3 d after treatment, and a’, b’, and c’ represent the results after 7 d of treatment. The letter d’ represent the results 7 d after CK treatment.
On day 7, the values were 36.4 and 36.9, respectively—significantly lower than the negative controls but significantly higher than the positive controls. For G1, the values were 11.2 and 23.4, respectively—significantly lower than controls but significantly higher than the commonly used agricultural fungicide mancozeb (80%). However, there was no significant difference compared to treatment with CN181 fermentation broth (Figure 1A).
The effects also differed in terms of disease control, where the relative control rates of G5 and G9 were significantly lower than those of the two positive control groups on days 3 and 7, while G1 had relative control values of 39.9% and 48.7%, respectively. These levels were significantly lower than those of mancozeb (80%) at the corresponding time points. However, there was no significant difference in disease control after treatment with CN181 (Figure 1B). In addition, G1, CN181, and mancozeb (80), but not G5 and G9, showed the same control effect, with greater relative control on day 7 than on day 3.
In those trials, G1 showed a significant inhibitory effect on the disease index with a value of 26.3%—significantly lower than that of the sterile water negative control (44.1) and significantly higher than those of the two positive control groups (Figure 2A). On day 7, the relative control rate of G1 was 38.9%—significantly lower than in the two positive control groups (Figure 2B). However, the rate for G1 was higher on day 7 than day 3 after treatment, consistent with the performance of the control dimethomorph and CN181.

Figure 2.
Effect of endophytic antagonistic strains on grape downy mildew in Field 2 on 2 September 2024. Note: (A) means disease index, (B) means relative control effect. The lowercase letters in Figure 1 show significant differences (p < 0.05). The letters a, b, and c represent the results 3 d after treatment, and a’, b’, and c’; represent the results after 7 d of treatment. The letter d represents the results 3 d after Control treatment, and the letter d’ represents the results 7 d after Control treatment.
3.4. Identification of G1
3.4.1. Morphological Examination
The colonies of G1 cultured on an NA medium at 28 °C for 48 h were round, slightly elevated, and folded on the surface, with a diameter < 3 mm. The edges were regular and smooth, and the colonies were white and semitransparent. They showed a certain degree of stickiness, had a foul odor, and were translucent (Figure 3). The strain showed a positive Gram reaction.

Figure 3.
Colony morphology of endophyte G1.
3.4.2. 16S rDNA Sequence Analysis
The 16S rDNA sequence of endophyte G1 was compared to those in the NCBI database using Basic BLAST, which revealed >99.0% nucleotide sequence identity between G1 and multiple strains of B. amyloliquefaciens. A phylogenetic tree was constructed using the Neighbor Joining method in MEGA 5.0 (Figure 4). Combined with the results of morphological and 16S rDNA sequence analyses, G1 was identified as B. amyloliquefaciens.

Figure 4.
Phylogenetic tree of 16S rDNA of endophyte G1.
3.5. Enzyme Activity and Secondary Metabolites of G1
G1 produced a clear transparent zone in the casein culture medium, indicating the production and secretion of a molecule capable of decomposing casein during its growth process (Figure 5a). On a cellulose medium, it produced a clear zone around the colonies, indicating the production and secretion of an enzyme that decomposes cellulose (Figure 5b). On mesophyll medium, orange halo zones were observed around the colonies, indicating siderophore activity (Figure 5c).
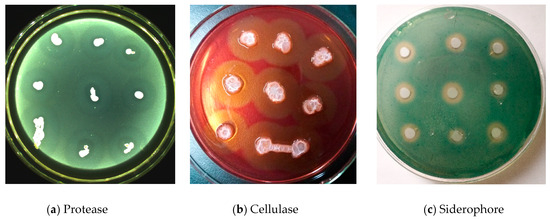
Figure 5.
Endogenous bacteria G1 Protease (a), Cellulase (b), and Siderophore (c) activity detection.
3.6. Antibacterial Activity of Crude Protein from G1
3.6.1. Extraction of Crude Protein
Experiments using the agar pore diffusion standoff method showed that the crude protein of strain G1 (precipitated with different saturation levels of ammonium sulfate) exhibited different degrees of fungal inhibition (Figure 6). Precipitation with 60% ammonium sulfate saturation showed the greatest inhibitory effect, with a colony diameter of only 27.9 mm in contrast to PBS control (diameter up to 64.5 mm) indicating an inhibition rate of 56.56%. From 20% to 40% saturation, the effect tended to increase with saturation; from 60% to 90%, it tended to decrease with saturation.

Figure 6.
Colony diameter and inhibition rate of Botrytis cinerea by salting-out of ammonium sulfate with different saturation. a~e representative colony diameter range.
3.6.2. Inhibitory Effects of G1 against Pathogenic Fungi
Experiments using the agar pore diffusion standoff method showed that G1 had a strong inhibitory effect against downy mildew of grape. In culture mixtures of mildew and crude protein, there were marked differences in the fungal mycelia on grapes treated with the biocontrol strain compared to controls under microscopy (400× objective). That is, the mycelia treated with the active protein extract were rough, deformed, and fractured with leakage of their contents. In contrast, the mycelia of negative controls had a smooth surface, uniform thickness, and evenly distributed internal material. Therefore, G1 inhibited mycelial growth and showed very good inhibition against fungal infection (Figure 7).
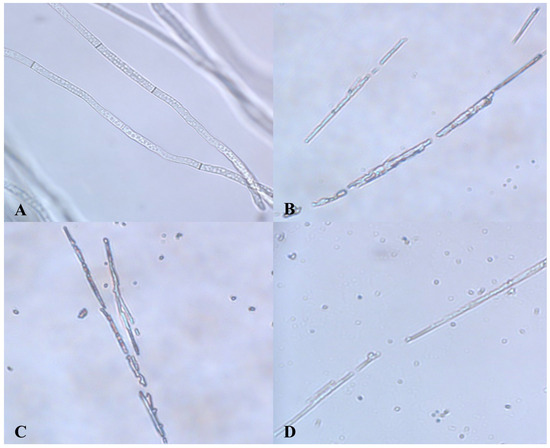
Figure 7.
Inhibitory ability of B. amyloliquefaciens G1 against B. cinerea. (A): Control hyphae; (B–D): Hyphae treated with crude protein extraction solution produced by G1.
3.6.3. Effects of G1 on Spore Germination
Spore suspensions treated with inhibitory protein extract were incubated in the dark at 28 °C for 12 h. G1 had an inhibitory effect on the germination of conidia on grape vines, and the rate of inhibition of spore germination increased gradually and significantly over time, showing the greatest inhibitory effect after 6 h (Table 3). The inhibitory rate remained at 64.82% after 12 h, so G1 had a strong inhibitory effect on spore germination.

Table 3.
Inhibitory effect of crude protein on spore germination of B. cinerea.
Grape leaves were inoculated with a 1:1 mixture of G1 crude protein extract and sporangium suspension (Table 4). The strain showed 70.8% inhibition of spore germination, lower than the effect of the original fermentation broth (100%). Therefore, the inhibitory effect of G1 was the result of synthesis of various antibacterial activities.

Table 4.
Inhibitory effect of crude protein on sporangium germination of downy mildew.
3.7. Purification and Identification of Antifungal Protein
3.7.1. Q-Sepharose FF Anion Exchange Chromatography
G1 was dialyzed against 50 mM Tris-HCl (pH 8.0) and centrifuged at 4 °C for 10 min at 12,000 rpm as described in the Methods section. The supernatant was mixed and incubated with Q-Sepharose FF ion exchange chromatography resin, subjected to anion-exchange high-performance chromatography for 1.5 h, and then eluted with solutions containing different concentrations of NaCl. Anion-exchange chromatography yielded five protein elution peaks designated as peaks I, II, III, IV, and V (Figure 8). The protein elutions at these peaks were collected and used for inhibition testing against the pathogen using the agar pore diffusion standoff method. Only the elution represented by peak II exhibited a significant inhibitory effect (Figure 9). Therefore, this was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE).

Figure 8.
Results of anion exchange chromatography.

Figure 9.
Antifungal test of five protein elution peaks. Note: I–V are protein agar blocks of five different NaCl concentrations.
3.7.2. Identification of Proteins by LC-MS
The protein corresponding to peak II was identified as the putative segregation protein stage V spore-forming protein G (SpoVG) by LC-MS and by searching the UniProt database. The molecular weight was 10.85 kDa, and the protein kurtosis index was 14.75. Other proteins represented by the peak included dihydrolipoic acid dehydrogenase lpdA (index = 7.83, molecular weight = 49.80 kDa), phosphate-binding protein DEU43_102129 (7.03, 31.87 kDa), polysucrose ribonucleotide nucleotidyl transferase pnp (6.56, 77.83 kDa), 60 kDa chaperone protein groL (6.44, 57.38 kDa), and alkyl hydroperoxide reductase C ahpC (6.1, 20.69 kDa).
4. Discussion and Conclusions
Endophytic Bacillus metabolites have roles in regulating the absorption of inorganic substances by host plants, and their endogenous hormones can enhance plant growth. In addition, their metabolites have antimicrobial and bacteriolytic effects, and confer disease resistance. Biocontrol strains are generally isolated from host tissue. The endophytic B. amyloliquefaciens strain G1 isolated from red grapes in the present study showed a good control effect against downy mildew of grape, with 100% control on leaves in vitro. Therefore, next, field trials were conducted with strain G1. In two field trials, the average disease index was 24.8% after 7 days of treatment, significantly lower than the mean value of 44.3% in control plants treated with water alone. The relative control rate was 43.8%—significantly lower than those of the two positive control groups. G1 was identified by morphological and 16S rDNA sequence analysis as B. amyloliquefaciens. A number of metabolites, including proteases, cellulases, and antifungal proteins, among others, were detected in this strain, which may be related to the antifungal mechanism of action against downy mildew. Such metabolites may play roles in inhibiting or killing pathogens [16,17,18,19]. Proteases of antagonistic strains can successfully inhibit the growth of pathogens. The production of siderophores can increase the competitive ability of biocontrol strains to iron resources, and compete with the mycelia of invasive fungal pathogens [20,21,22]. Cellulose is the main component of the cell walls of downy mildew. Our results indicate that G1 affects the normal growth of mycelia and inhibits the germination of spores, resulting in uneven growth and damage to the mycelia, and up to 91.96% spore germination inhibition. The component of the antibacterial fraction of G1 metabolites was SpoVG. Proteins produced by B. amyloliquefaciens RY3 can effectively inhibit the growth of Penicillium digitatum and Alternaria alternata, inhibit germination of P. digitatum conidia, and also lead to deformity and ablation of mycelia [23,24]. Antifungal proteins produced by both B. subtilis ZI-2 and Bacillus velezensis S3-1 also show good inhibitory effects against hyphal growth and spore germination of Fusarium graminearum and Botrytis cinerea [25,26]. Lipopeptides are key substances with strong antifungal activity [8]. They can damage the cell walls and cell membranes of Aspergillus niger, leading to intracellular nucleic acid leakage and cell death. The mechanism of action underlying the biocontrol of downy mildew may be related to cell wall-degrading enzymes secreted by the bacteria [22,27,28].
Endophytic B. amyloliquefaciens JK-JS3 shows lethality against Bursaphelenchus xylophilus by secreting a non-proteinaceous substance [29]. Endophytic B. amyloliquefaciens TF28 shows a strong inhibitory effect against rice bakanae disease in the rice seedling stage [30]. B. amyloliquefaciens PPCB004 has a strong inhibitory effect against mycelial extension of Penicillium species, especially Penicillium crustosum, with a rate of inhibition of 73.3% [31]. In addition, B. amyloliquefaciens has good antagonistic effects against Fusarium wilt of banana, eggplant pathogenic fungi, and Verticillium wilt of cotton [32,33,34]. However, there have been relatively few reports of the application of B. amyloliquefaciens for the biological control of downy mildew of grape.
In this study, field experiments were performed to examine this. G1 was less effective at controlling disease than the chemical agents mancozeb (80%) and dimethylmorph (80%) but was significantly more effective than water alone, which may have been because endogenous G1 did not colonize the tissue of grape leaves. In addition, the proteases, cellulases, siderophores, and antifungal protein SpoVG secreted by G1 may be important antimicrobial substances involved in the inhibition of the growth of this mildew. The high biosafety of these proteins makes G1 a candidate for further studies on the development and utilization of such metabolites for biocontrol.
Author Contributions
Investigation, W.Q., X.K., X.M., L.R. and Z.Z.; Software, W.Q., X.K., X.M., L.R. and Z.Z.; Formal analysis, W.Q., X.K., X.M., L.R. and Z.Z.; Validation, W.Q., X.K. and X.M.; Conceptualization, L.R. and Z.Z.; Data curation, L.R. and Z.Z.; Methodology, L.R. and Z.Z.; Writing—review & editing, L.R. and Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Du, X.; Li, Z.; Ji, X.; Ran, L. Long-term preservation methods for sporangia of Plasmopara viticola. Acta Bot. Sin. 2008, 27, 908–914. [Google Scholar]
- Wong, F.P.; Burr, H.N.; Wilcox, W.F. Heterothallism in Plasmopara viticola. Plant Pathol. 2001, 50, 427–432. [Google Scholar] [CrossRef]
- Chen, H.; Hu, L.; Tang, C.; Hu, J.; Shi, Z. Efficacy of the strain B-FS01 of Bacillus subtilis in suppression of the grape downy mildew caused by Plasmopara viticola. Plant Prot. 2011, 37, 194–197. [Google Scholar]
- Shi, J.; Yang, Z. Present Situation of The Study on Grapevine Downy Mildew. North Ningxia Agric. Coll. 2004, 25, 92–94. [Google Scholar]
- Sturz, A.V.; Christie, B.R.; Nowak, J. Bacterial endophytes: Potential role in developing sustainable systems of crop production. Crit. Rev. Plant Sci. 2000, 19, 1–30. [Google Scholar] [CrossRef]
- Jalgaonwala, R.E.; Mahajan, R.T. A review: Bacterial endophytes and their bioprospecting. J. Pharm. Res. 2011, 4, 795–799. [Google Scholar]
- Wang, F.; Chao, S.H.; Tsai, C.H.; Blanco, S.D.; Yang, Y.Y.; Lin, Y.H. Developing fermentation liquid of Bacillus amyloliquefaciens PMB04 to control bacterial leaf spot of sweet pepper. Agriculture 2023, 13, 1456. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, K.; Ye, C.; Zou, D.; Liu, D.; Wei, X. Enhancing biological control of apple rot: Unveiling the antifungal potential and mechanism of Bacillus amyloliquefaciens HZ-12′s lipopeptide. Sci. Hortic. 2024, 325, 112704. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Bois, B.; Zito, S.; Calonnec, A. Climate vs grapevine pests and diseases worldwide: The first results of a global survey. OENO One 2017, 51, 133–139. [Google Scholar] [CrossRef]
- Li, W. Screening and Control Effect of Antagonists and Antibiotics on Grapevine Downy Mildew. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2015. [Google Scholar]
- Xu, J.; Zhang, S.; Zhan, H.; Pu, Z. Research on the control of grape mildew by compounding potassium phosphite containing amino acid and manganese zinc diclofenac. Zhejiang Citrus 2020, 37, 29–32. [Google Scholar]
- Wang, Y. Biocontrol of Wheat Leaf Rust Caused by Puccinia triticina and Induction of Systemic Acquired Resistance by Salicylic Acid. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2011. [Google Scholar]
- Ghose, T.K. Measurement of cellulase activities. Int. Union Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Shin, S.H.; Lim, Y.; Lee, S.E.; Yang, N.W.; Rhee, J.H. CAS agar diffusion assay for the measurement of siderophores in biological fluids. J. Microbiol. Methods 2001, 44, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Long, M. Study on Bacillus amyloliquefaciens B10 Inhibiting Cloning and Expression of Growth Active Substance of Aspergillusflavus and its Efficacy. Anim. Nutr. 2023, 35, 8024–8035. [Google Scholar]
- Chen, L.H.; Zhang, A.X.; Zhu, T.; Lai, Z.B.; Wang, Z.H.; Chen, H.G. Identification and characterization of bacteria antagonistic to Rhizoctonia cerealis. Chin. J. Plant Pathol. 2008, 38, 88–95. [Google Scholar]
- Kamensky, M.; Ovadis, M.; Chet, I.; Chernin, L. Soil-borne strain IC14 of Serratia plymuthica with multiple mechanisms of antifungal activity provides biocontrol of Botrytis cinerea and Sclerotinia sclerotiorum diseases. Soil Biol. Biochem. 2003, 35, 323–331. [Google Scholar] [CrossRef]
- Xue, Q.Y.; Li, J.Q.; Zheng, Y.; Ding, X.Y.; Guo, J.H. Screening tomato-associated bacteria for biological control of grey mold on tomato. Biocontrol Sci. Technol. 2013, 23, 245–259. [Google Scholar] [CrossRef]
- Arora, N.K.; Kim, M.J.; Kang, S.C.; Maheshwari, D.K. Role of chitinase and β-1,3-glucanase activities produced by a fluorescent pseudomonad and in vitro inhibition of Phytophthora capsici and Rhizoctonia solani. Can. J. Microbiol. 2007, 53, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Picard, K.; Tirilly, Y.; Benhamou, N. Cytological effects of cellulases in the parasitism of Phytophthora parasitica by Pythium oligandrum. Appl. Environ. Microbiol. 2000, 66, 4305–4314. [Google Scholar] [CrossRef]
- Zang, C.; Zhao, K.; Liu, C.; Liang, C.; Liu, L.; Yu, S. Inhibitory Effect of SY286 Strain against Plasmopara viticola. J. Shenyang Agric. Univ. 2014, 45, 221–224. [Google Scholar]
- Cheng, Z.; Zhang, H.; Zhang, N.; Qiao, Y. Physicochemical property and inhibitory activity against Penicillium digitatum of antifungal crude protein produced by Bacillus am yloliquefaciens RY3. Food Mach. 2013, 29, 187–190. [Google Scholar]
- Zhang, B.; Zhang, J.; Han, J.; Liu, H.; Wang, J. Purification and characterization of an antifungal protein from endophytic Bacillus amyloliquefaciens LP-5. Chin. J. Plant Prot. 2010, 37, 143–147. [Google Scholar]
- Zheng, X.; Dong, C.; Niu, R.; Qi, X.; Sun, C.; Wang, X.; Liu, C.; Jia, G.; Chen, Z. Characterization and inhibition effects of antifungal protein from Bacillus subtilis Zl-2. J. Nat. Sci. Heilongjiang Univ. 2018, 35, 206–211. [Google Scholar]
- Yang, W. Preliminary Study on the Antimicrobial Protein Of The Bioprophylactic Bacillus cereus S3-1 and Optimization of the Fermentation Process. Master’s Thesis, Shanghai Normal University, Shanghai, China, 2021. [Google Scholar]
- Hu, J.; Wu, Y.; Li, J.; Wei, Y.; Chen, Q.; Yang, H. Study on the control effect of antagonistic Bacillus polymyxa bacillus PB-2 and its preparation on grape downy mildew. Shandong Sci. 2014, 27, 30–33. [Google Scholar]
- Xie, X.; Wang, Y.; Hu, J.; Yang, H.; Li, J. Screening and identification of antagonistic bacteria strain BMJBN02 and its effect on grape downy mildew. Shandong Sci. 2015, 28, 39–44, 73. [Google Scholar]
- Zhu, L.; Wu, X.; Chen, T.; Xu, X. Elementary Properties of the Nematicidal Crude Toxin Produced by Bacillus amyloliquefaciens JK-JS3. Chin. J. Biol. Control 2009, 25, 359–363. [Google Scholar]
- Tian, J.; Wang, Y.; Zhang, S. Field control efficacy of Bacillus amyloliquefaciens on rice bakanae disease. Heilongjiang Sci. 2010, 1, 10–11, 14. [Google Scholar]
- Arrebola, E.; Sivakumar, D.; Korsten, L. Effect of volatile compounds produced by Bacillus strains on postharvest decay in citrus. Biol. Control 2010, 53, 122–128. [Google Scholar] [CrossRef]
- Lu, J.; Xia, Q.; Gu, W. Isolation, identification of Bacillus amyloliquefaciens LX1 Strain Againsts Fusarium oxysporum f. sp. cubense and cloning of its antifungal protein gene. Acta Metall. Sin. 2013, 34, 117–124. [Google Scholar]
- Santhosh, C.R.; Mahadevakuamar, S.; Nuthan, B.R.; Chandranayak, S.; Satish, S. Eggplant (Solanum melongena L.) associated endophytic bacteria promote plant growth and counter soil-borne plant pathogenic fungi. Authorea Prepr. 2023. [Google Scholar] [CrossRef]
- Chen, Y.; Li, A.; Feng, L.; Meng, Q.; Li, Y.; Zhang, D.; Liu, D.; Chi, G. Identification and colonization of an antagonistic endophytic bacterium L-4-2 against cotton verticillium wilt. J. Northwest Agric. Univ. 2012, 21, 68–71. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).