Consortium of Phosphorus-Solubilizing Bacteria Promotes Maize Growth and Changes the Microbial Community Composition of Rhizosphere Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation, Purification, and Identification of PSB
2.2. Activity Determination of PSB
2.2.1. Determination of Phosphorus-Solubilizing Capacity of PSB on Various Insoluble Phosphorus Sources
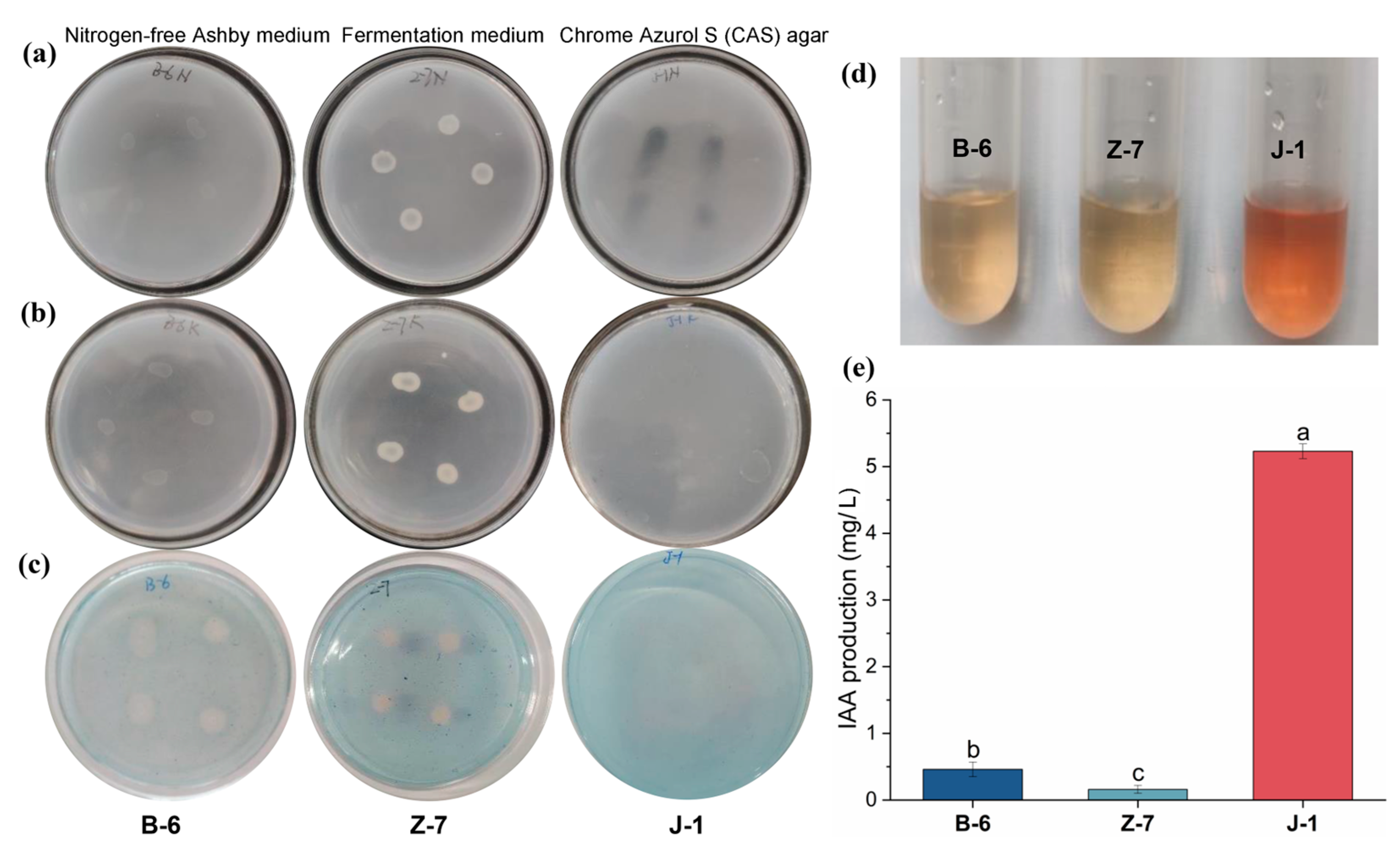
2.2.2. Determination of IAA Production
2.2.3. Determination of Nitrogen-Fixing Capacity, Potassium-Solubilizing Capacity, and Siderophore Production
2.3. The Plant Growth-Promoting Ability of PSB—A Pot Experiment
2.4. High-Throughput Sequencing and Data Analysis
3. Results and Discussion
3.1. Screening and Identification of PSB
3.2. Phosphorus-Solubilizing Characteristics and Growth-Promoting Function of PSB
3.3. Effect of PSB on Maize Seed Germination and Seedling Growth Promotion
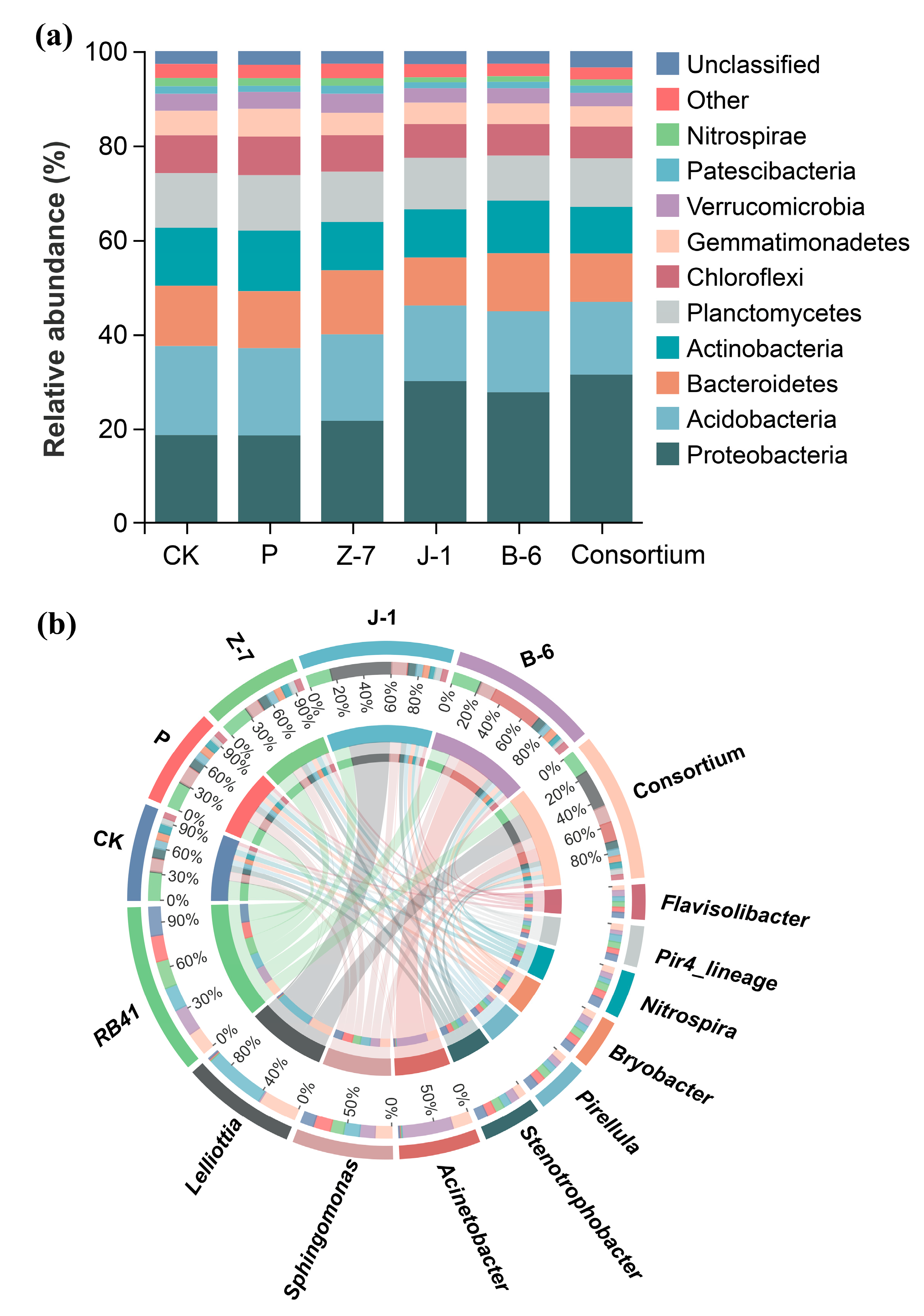
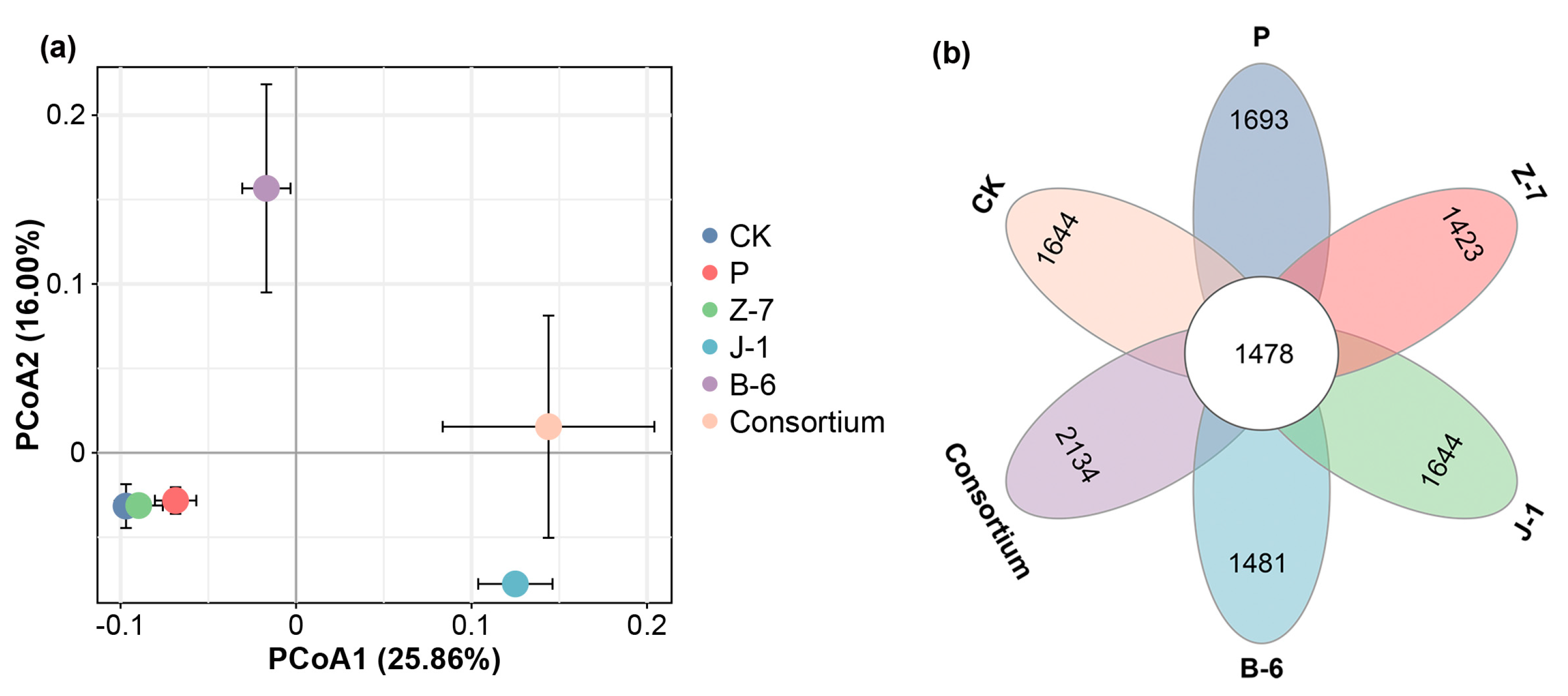
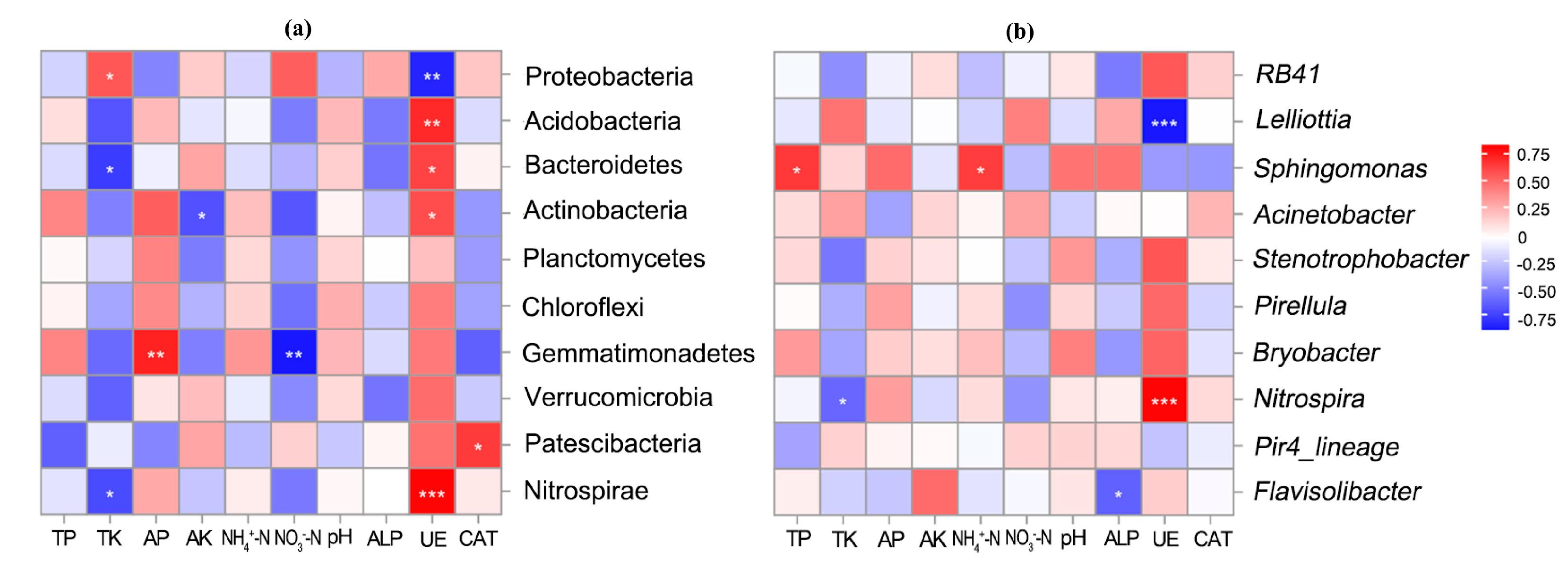
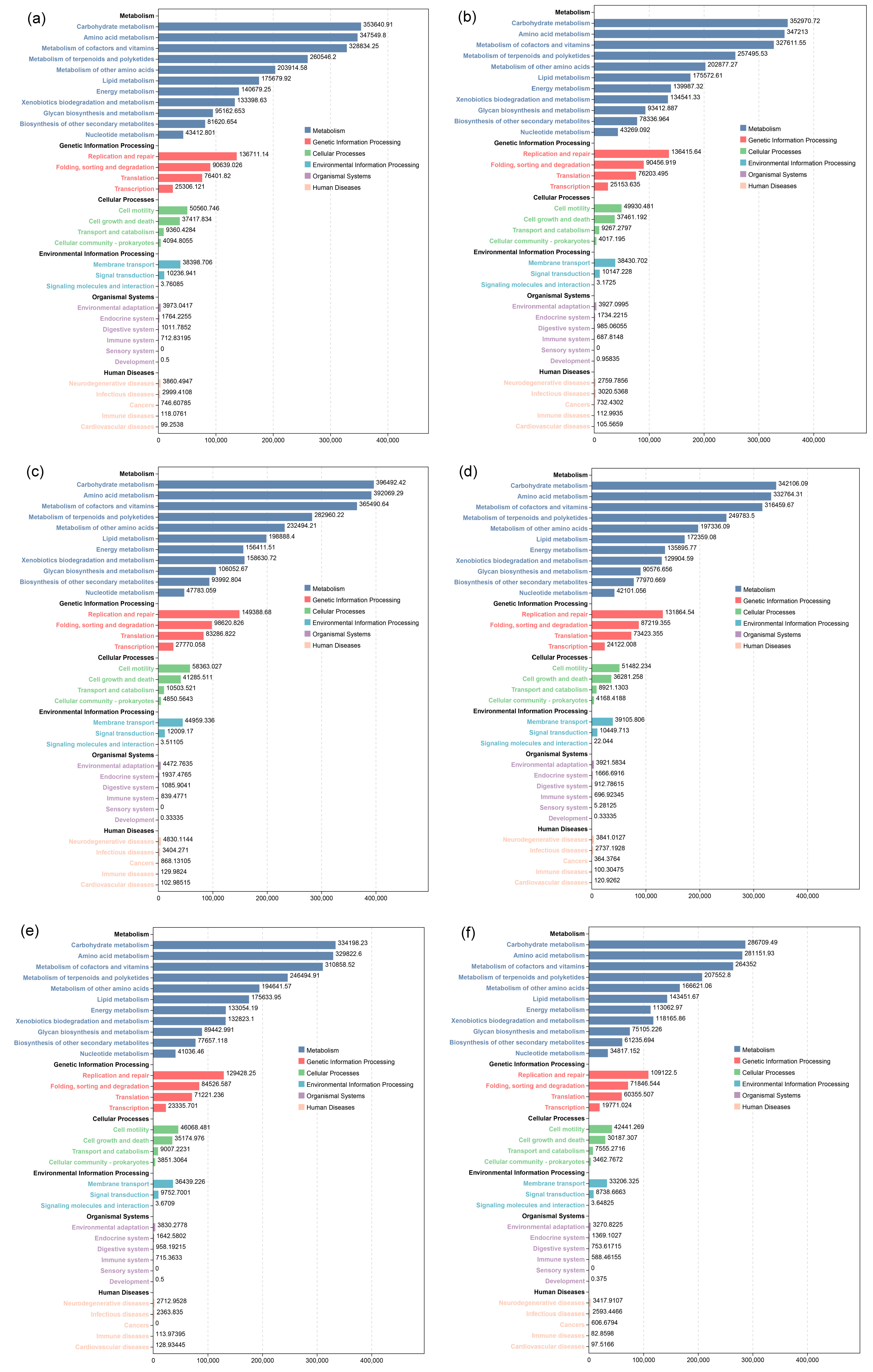
3.4. Effects of PSB on the Microbial Community Structure of Maize Rhizosphere Soil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oldroyd, G.E.D.; Leyser, O. A plant’s diet, surviving in a variable nutrient environment. Science 2020, 368, eaba0196. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Abdelfattah, A.; Norelli, J.; Burchard, E.; Schena, L.; Droby, S.; Wisniewski, M. Apple endophytic microbiota of different rootstock/scion combinations suggests a genotype-specific influence. Microbiome 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Zhang, X.; Davidson, E.A. Global trends of cropland phosphorus use and sustainability challenges. Nature 2022, 611, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Medici, A.; Szponarski, W.; Dangeville, P.; Safi, A.; Dissanayake, I.M.; Saenchai, C.; Emanuel, A.; Rubio, V.; Lacombe, B.; Ruffel, S.; et al. Identification of molecular integrators shows that nitrogen actively controls the phosphate starvation response in plants. Plant Cell 2019, 31, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Zhang, B.; Wang, Y.; Meng, J.; Gao, Y.; He, X.; Hu, X. Identification of phosphate-solubilizing microorganisms and determination of their phosphate-solubilizing activity and growth-promoting capability. Bioresources 2020, 15, 2560–2578. [Google Scholar] [CrossRef]
- López-Bucio, J.; De la Vega, O.M.; Guevara-García, A.; Herrera-Estrella, L. Enhanced phosphorus uptake in transgenic tobacco plants that overproduce citrate. Nat. Biotechnol. 2000, 18, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, J.; Wang, H.; Fang, Z.; Wang, X.; Feng, S.; Wang, Z.; Yuan, T.; Zhang, S.; Ou, S.; et al. A comprehensive synthesis unveils the mysteries of phosphate-solubilizing microbes. Biol. Rev. 2021, 96, 2771–2793. [Google Scholar] [CrossRef] [PubMed]
- Rafi, M.M.; Krishnaveni, M.S.; Charyulu, P.B.B.N. Chapter 17—Phosphate-Solubilizing Microorganisms and their emerging role in sustainable agriculture. In Recent Developments in Applied Microbiology and Biochemistry; Buddolla, V., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 223–233. [Google Scholar]
- Kour, D.; Kour, H.; Khan, S.S.; Khan, R.T.; Bhardwaj, M.; Kailoo, S.; Kumari, C.; Rasool, S.; Yadav, A.N.; Sharma, Y.P. Biodiversity and functional attributes of rhizospheric microbiomes: Potential tools for sustainable agriculture. Curr. Microbiol. 2023, 80, 192. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.; Ghosh, S.B.; D’Souza, S.F. Isolation of a starch utilizing, phosphate solubilizing fungus on buffered medium and its characterization. Bioresour. Technol. 2007, 98, 3408–3411. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, G.; Wei, Y.; Dong, Y.; Hou, L.; Jiao, R. Isolation and screening of multifunctional phosphate solubilizing bacteria and its growth-promoting effect on Chinese fir seedlings. Sci. Rep. 2021, 11, 9081. [Google Scholar] [CrossRef]
- Zahir, Z.A.; Akhtar, S.S.; Ahmad, M.; Saifullah; Nadeem, S.M. Comparative effectiveness of Enterobacter aerogenes and Pseudomonas fluorescens for mitigating the depressing effect of brackish water on maize. Int. J. Agric. Biol. 2012, 14, 337–344. [Google Scholar]
- Ijaz, M.; Sarfraz, M.; Nawaz, A.; Yasir, T.A.; Sher, A.; Sattar, A.; Wasaya, A. Diluted Sugar mill effluent application with PGPR improves the performance of maize (Zea mays L.) under an arid climate. Agronomy 2018, 8, 67. [Google Scholar]
- Upadhyay, S.K.; Chauhan, P.K. Optimization of eco-friendly amendments as sustainable asset for salt-tolerant plant growth-promoting bacteria mediated maize (Zea Mays L.) plant growth, Na uptake reduction and saline soil restoration. Environ. Res. 2022, 211, 113081. [Google Scholar] [CrossRef] [PubMed]
- Saadouli, I.; Mosbah, A.; Ferjani, R.; Stathopoulou, P.; Galiatsatos, I.; Asimakis, E.; Marasco, R.; Daffonchio, D.; Tsiamis, G.; Ouzari, H.-I. The impact of the inoculation of Phosphate-solubilizing bacteria Pantoea agglomerans on phosphorus availability and bacterial community dynamics of a semi-arid soil. Microorganisms 2021, 9, 1661. [Google Scholar] [CrossRef] [PubMed]
- Tagele, S.; Kim, S.; Lee, H.; Lee, Y. Potential of novel sequence type of Burkholderia cenocepacia for biological control of root rot of maize (Zea mays L.) caused by Fusarium Temp. Int. J. Mol. Sci. 2019, 20, 1005. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, H.; Rossolini, G.M.; Gonzalez, T.; Li, J.; Glick, B.R. Isolation of a gene from Burkholderia cepacia IS-16 encoding a protein that facilitates phosphatase activity. Curr. Microbiol. 2000, 40, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Sifuentes, G.; Hernandez-Montiel, L.G.; Saenz-Mata, J.; Fortis-Hernandez, M.; Blanco-Contreras, E.; Chiquito-Contreras, R.G.; Preciado-Rangel, P. Plant growth-promoting rhizobacteria improve growth and fruit quality of cucumber under greenhouse conditions. Plants 2022, 11, 1612. [Google Scholar] [CrossRef]
- Li, P.; Liu, J.; Saleem, M.; Li, G.; Luan, L.; Wu, M.; Li, Z. Reduced chemodiversity suppresses rhizosphere microbiome functioning in the mono-cropped agroecosystems. Microbiome 2022, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, R.; Huang, Y.; Sun, X.; Qin, W.; Wei, F.; Ye, Y. Effects of various phosphorus fertilizers on maize yield and phosphorus uptake in soils with different pH values. Arch. Agron. Soil. Sci. 2022, 68, 1746–1754. [Google Scholar] [CrossRef]
- Luo, D.; Meng, X.; Zheng, N.; Li, Y.; Yao, H.; Chapman, S.J. The anaerobic oxidation of methane in paddy soil by ferric iron and nitrate, and the microbial communities involved. Sci. Total Environ. 2021, 788, 147773. [Google Scholar] [CrossRef]
- Meador, T.B.; Schoffelen, N.; Ferdelman, T.G.; Rebello, O.; Khachikyan, A.; Könneke, M. Carbon recycling efficiency and phosphate turnover by marine nitrifying archaea. Sci. Adv. 2020, 6, eaba1799. [Google Scholar] [CrossRef] [PubMed]
- Barbaccia, P.; Gaglio, R.; Dazzi, C.; Miceli, C.; Bella, P.; Lo Papa, G.; Settanni, L. Plant growth-promoting activities of bacteria isolated from an anthropogenic soil located in Agrigento province. Microorganisms 2022, 10, 2167. [Google Scholar] [CrossRef] [PubMed]
- Kizilkaya, R. Yield response and nitrogen concentrations of spring wheat (Triticum aestivum) inoculated with Azotobacter Chroococcum strains. Ecol. Eng. 2008, 33, 150–156. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, H.; Shen, Z.; Ye, J. Whole-genome sequencing and potassium-solubilizing mechanism of Bacillus aryabhattai SK1-7. Front. Microbiol. 2022, 12, 722379. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, S.; Meyer, S.; Forquet, R.; Hommais, F.; Muskhelishvili, G.; Nasser, W. The nucleoid-associated protein IHF acts as a ‘transcriptional domainin’ protein coordinating the bacterial virulence traits with global transcription. Nucleic Acids Res. 2020, 49, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Gorelova, V.; De Lepeleire, J.; Van Daele, J.; Pluim, D.; Meï, C.; Cuypers, A.; Leroux, O.; Rébeillé, F.; Schellens, J.H.M.; Blancquaert, D.; et al. Dihydrofolate reductase/thymidylate synthase fine-tunes the folate status and controls redox homeostasis in plants. Plant Cell 2017, 29, 2831–2853. [Google Scholar] [CrossRef] [PubMed]
- Lazcano, C.; Gomez-Brandon, M.; Dominguez, J. Comparison of the effectiveness of composting and vermicomposting for the biological stabilization of cattle manure. Chemosphere 2008, 72, 1013–1019. [Google Scholar] [CrossRef]
- Crouch, S.R.; Malmstadt, H.V. Mechanistic investigation of molybdenum blue method for determination of phosphate. Anal. Chem. 1967, 39, 1084–1089. [Google Scholar] [CrossRef]
- Mani, V.; Bromley, S.K.; Äijö, T.; Mora-Buch, R.; Carrizosa, E.; Warner, R.D.; Hamze, M.; Sen, D.R.; Chasse, A.Y.; Lorant, A.; et al. Migratory DCs activate TGF-β to precondition naïve CD8+ T cells for tissue-resident memory fate. Science 2019, 366, eaav5728. [Google Scholar] [CrossRef]
- Zhang, H.; Phillip, F.O.; Wu, L.; Zhao, F.; Yu, S.; Yu, K. Effects of temperature and nitrogen application on carbon and nitrogen accumulation and bacterial community composition in apple rhizosphere soil. Front. Plant Sci. 2022, 13, 859395. [Google Scholar] [CrossRef]
- Cohen, G.; Dembiec, D.; Marcus, J. Measurement of catalase activity in tissue extracts. Anal. Biochem. 1970, 34, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Li, Y.; Yao, H.; Chapman, S.J. Effects of different carbon sources on methane production and the methanogenic communities in iron rich flooded paddy soil. Sci. Total Environ. 2022, 823, 153636. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wu, X.; Wang, J.; Ding, X. Phosphate Solubilization and gene expression of phosphate-solubilizing bacterium Burkholderia multivorans WS-FJ9 under different levels of soluble phosphate. J. Microbiol. Biotechnol. 2017, 27, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Park, G.; Cha, M.; Heo, M. Solubilization of insoluble inorganic phosphates by a novel salt- and pH-tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere. Bioresour. Technol. 2006, 97, 204–210. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Fang, S.; MacDonald, J.; Xu, J.; Yuan, Z. Isolation and characterization of Burkholderia cenocepacia CR318, a phosphate solubilizing bacterium promoting corn growth. Microbiol. Res. 2020, 233, 126395. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mou, L.; Yi, J.; Wang, J.; Liu, A.; Yu, J. The Eno Gene of Burkholderia cenocepacia strain 71-2 is involved in phosphate solubilization. Curr. Microbiol. 2019, 76, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Alves, V.; Marriel, I.; Gomes, E.; Scotti, M.; Carneiro, N.; Guimaraes, C.; Schaffert, R.; Sa, N. Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil. Biol. Biochem. 2009, 41, 1782–1787. [Google Scholar] [CrossRef]
- Espinosa, M.; Turner, B.L.; Haygarth, P.M. Preconcentration and separation of trace phosphorus compounds in soil leachate. J. Environ. Qual. 1999, 28, 1497–1504. [Google Scholar] [CrossRef]
- Jain, R.; Bhardwaj, P.; Pandey, S.S.; Kumar, S. Arnebia euchroma, a plant species of cold desert in the himalayas, harbors beneficial cultivable endophytes in roots and leaves. Front. Microbiol. 2021, 12, 696667. [Google Scholar] [CrossRef]
- Bible, A.N.; Fletcher, S.J.; Pelletier, D.A.; Schadt, C.W.; Jawdy, S.S.; Weston, D.J.; Engle, N.L.; Tschaplinski, T.; Masyuko, R.; Polisetti, S.; et al. A carotenoid-deficient mutant in Pantoea sp. YR343, a bacteria isolated from the rhizosphere of Populus deltoides, is defective in root colonization. Front. Microbiol. 2016, 7, 491. [Google Scholar] [CrossRef]
- Ke, X.; Feng, S.; Wang, J.; Lu, W.; Zhang, W.; Chen, M.; Lin, M. Effect of inoculation with nitrogen-fixing bacterium Pseudomonas stutzeri A1501 on maize plant growth and the microbiome indigenous to the rhizosphere. Syst. Appl. Microbiol. 2019, 42, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- Martínez-Aguilar, L.; Salazar-Salazar, C.; Méndez, R.D.; Caballero-Mellado, J.; Hirsch, A.M.; Vásquez-Murrieta, M.S.; Estrada-de los Santos, P. Burkholderia caballeronis sp. nov., a nitrogen fixing species isolated from tomato (Lycopersicon esculentum) with the ability to effectively nodulate Phaseolus vulgaris. Antonie Van. Leeuwenhoek 2013, 104, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wei, Z.; Shao, Z.; Friman, V.-P.; Cao, K.; Yang, T.; Kramer, J.; Wang, X.; Li, M.; Mei, X.; et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat. Microbiol. 2020, 5, 1002–1010. [Google Scholar] [CrossRef]
- Ong, K.S.; Cheow, Y.L.; Lee, S.M. The role of reactive oxygen species in the antimicrobial activity of pyochelin. J. Adv. Res. 2017, 8, 393–398. [Google Scholar] [CrossRef]
- Ghazy, N.; El-Nahrawy, S. Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant. Arch. Microbiol. 2021, 203, 1195–1209. [Google Scholar] [CrossRef] [PubMed]
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef]
- Majeed, A.; Abbasi, M.K.; Hameed, S.; Imran, A.; Rahim, N. Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front. Microbiol. 2015, 6, 198. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Hao, T.; Chen, S. Colonization and maize growth promotion induced by phosphate solubilizing bacterial isolates. Int. J. Mol. Sci. 2017, 18, 1253. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.; Dai, X.; Sun, Y.; Chen, F. Effect of phosphorus and temperature on chlorophyll a contents and cell sizes of Scenedesmus obliquus and Microcystis aeruginosa. Limnology 2011, 12, 187–192. [Google Scholar] [CrossRef]
- Jiang, H.; Qi, P.; Wang, T.; Wang, M.; Chen, M.; Chen, N.; Pan, L.; Chi, X. Isolation and characterization of halotolerant phosphate-solubilizing microorganisms from saline soils. 3 Biotech. 2018, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, S.; Wang, T.; Chi, X.; Qi, P.; Chen, G. Interaction between halotolerant phosphate-solubilizing bacteria (Providencia rettgeri Strain TPM23) and rock phosphate improves soil biochemical properties and peanut growth in saline soil. Front. Microbiol. 2021, 12, 777351. [Google Scholar] [CrossRef] [PubMed]
- Bashan, Y.; Kamnev, A.A.; de-Bashan, L.E. A proposal for isolating and testing phosphate-solubilizing bacteria that enhance plant growth. Biol. Fertil. Soils 2013, 49, 1–2. [Google Scholar] [CrossRef]
- Sheridan, C.; Depuydt, P.; De Ro, M.; Petit, C.; Van Gysegem, E.; Delaere, P.; Dixon, M.; Stasiak, M.; Aciksoz, S.B.; Frossard, E.; et al. Microbial community dynamics and response to plant growth-promoting microorganisms in the rhizosphere of four common food crops cultivated in hydroponics. Microb. Ecol. 2017, 73, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qin, Y.; Liu, X.; Feng, Z.; Zhu, H.; Yao, Q. Soil bacterial function associated with stylo (legume) and bahiagrass (grass) is affected more strongly by soil chemical property than by bacterial community composition. Front. Microbiol. 2019, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Gałązka, A.; Marzec-Grządziel, A.; Varsadiya, M.; Niedźwiecki, J.; Gawryjołek, K.; Furtak, K.; Przybyś, M.; Grządziel, J. Biodiversity and metabolic potential of bacteria in bulk soil from the peri-root zone of Black Alder (Alnus glutinosa), Silver Birch (Betula pendula) and Scots Pine (Pinus sylvestris). Int. J. Mol. Sci. 2022, 23, 2633. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Jiang, H.; Bu, J.; Adnan, M.; Gillani, S.W.; Zhang, M. An insight to rhizosphere bacterial community composition and structure of consecutive winter-initiated sugarcane ratoon crop in Southern China. BMC Plant Biol. 2022, 22, 74. [Google Scholar] [CrossRef] [PubMed]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef]
- He, T.; Xu, Z.; Wang, J.; Zhang, K.; Wang, F.; Li, W.; Tian, P.; Li, Q. Inoculation of Escherichia coli enriched the key functional bacteria that intensified cadmium accumulation by halophyte Suaeda salsa in saline soils. J. Hazard. Mater. 2023, 458, 131922. [Google Scholar] [CrossRef]
- Tsegaye, Z.; Alemu, T.; Desta, F.A.; Assefa, F. Plant growth-promoting rhizobacterial inoculation to improve growth, yield, and grain nutrient uptake of teff varieties. Front. Microbiol. 2022, 13, 896770. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, D.; Shi, J.; Li, M.; Chen, J.; Wang, T.; Zhang, Q.; Yang, L.; Zhu, N.; Wang, Y. Consortium of Phosphorus-Solubilizing Bacteria Promotes Maize Growth and Changes the Microbial Community Composition of Rhizosphere Soil. Agronomy 2024, 14, 1535. https://doi.org/10.3390/agronomy14071535
Luo D, Shi J, Li M, Chen J, Wang T, Zhang Q, Yang L, Zhu N, Wang Y. Consortium of Phosphorus-Solubilizing Bacteria Promotes Maize Growth and Changes the Microbial Community Composition of Rhizosphere Soil. Agronomy. 2024; 14(7):1535. https://doi.org/10.3390/agronomy14071535
Chicago/Turabian StyleLuo, Dan, Ju Shi, Mei Li, Jixiang Chen, Tianfeng Wang, Qingfang Zhang, Linhai Yang, Ning Zhu, and Yonggang Wang. 2024. "Consortium of Phosphorus-Solubilizing Bacteria Promotes Maize Growth and Changes the Microbial Community Composition of Rhizosphere Soil" Agronomy 14, no. 7: 1535. https://doi.org/10.3390/agronomy14071535




