Straw Returning Alleviates the Inhibition of Soil Nitrification Medicated by Ammonia-Oxidizing Archaea under Low Nitrogen Fertilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Straw Decomposition Proportion and the Activity of Cellulase and Xylanase Determination
2.3. Soil Properties and Potential Nitrification Rate Determination
2.4. Quantification of amoA Genes
2.5. Amplicon Sequencing
2.6. Data Analysis
3. Results
3.1. Straw Decomposition and Potential Nitrification Rate
3.2. Soil Properties
3.3. Quantification of amoA Gene
3.4. Amplicon Sequencing of Ammonia-Oxidizing Archaea
3.5. Impacts of Various Factors on Ammonia-Oxidizing Archaea and Potential Nitrification Rate
4. Discussion
4.1. Soil PNR and Nitrifying Microorganism Abundance under Long-Term N Fertilization and Straw Returning
4.2. Ammonia-Oxidizing Archaea Community under Long-Term Nitrogen Fertilization and Straw Returning
4.3. Influence of Environmental Factors on AOA Community and Soil Nitrification Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- van Kessel, M.A.H.J.; Speth, D.R.; Albertsen, M.; Nielsen, P.H.; Op den Camp, H.Z.M.; Kartal, B.; Jetten, M.S.M.; Lücker, S. Complete nitrification by a single microorganism. Nature 2015, 528, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Könneke, M.; Bernhard, A.; de la Torre, J.; Walker, C.B.; Waterbury, J.B.; Stahl, D.A. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 2005, 437, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Daims, H.; Lebedeva, E.V.; Pjevac, P.; Han, P.; Herbold, C.; Albertsen, M.; Jehmlich, N.; Palatinszky, M.; Vierheilig, J.; Bulaev, A.; et al. Complete nitrification by Nitrospira bacteria. Nature 2015, 528, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Peng, Y.; Liu, Y.; Peng, Y. Research progress and prospects of complete ammonia oxidizing bacteria in wastewater treatment. Front. Environ. Sci. Eng. 2022, 16, 123. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Srikanthasamy, T.; Barot, S.; Koffi, F.K.; Tambosco, K.; Marcangeli, Y.; Carmignac, D.; N’Dri, A.B.; Gervaix, J.; Leloup, J.; Roux, X.L.; et al. Effects of vegetation cover and season on soil nitrifiers in an African savanna: Evidence of archaeal nitrifier inhibition by grasses. Geoderma 2022, 416, 115775. [Google Scholar] [CrossRef]
- Li, C.Y.; Hu, H.W.; Chen, L.Q.; Chen, D.L.; He, J.Z. Comammox Nitrospira play an active role in nitrification of agricultural soils amended with nitrogen fertilizers. Soil Biol. Biochem. 2019, 138, 107609. [Google Scholar] [CrossRef]
- Wang, M.; Yang, M.; Fan, T.; Wang, D.; He, J.; Wu, H.; Si, D.; Wang, M.; Wu, S.; Zhou, D. Activating soil nitrification by co-application of peanut straw biochar and organic fertilizer in a rare earth mining soil. Sci. Total Environ. 2023, 866, 161506. [Google Scholar] [CrossRef] [PubMed]
- Elrys, A.S.; Wang, J.; Metwally, M.A.S.; Cheng, Y.; Zhang, J.B.; Cai, Z.C.; Chang, S.X.; Müller, C. Global gross nitrification rates are dominantly driven by soil carbon-to-nitrogen stoichiometry and total nitrogen. Glob. Change Biol. 2021, 27, 6512–6524. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, G.; Wu, M.; Wang, D.; Liu, Q. Straw return and low N addition modify the partitioning of dissimilatory nitrate reduction by increasing conversion to ammonium in paddy fields. Soil Biol. Biochem. 2021, 162, 108425. [Google Scholar] [CrossRef]
- Bai, X.; Hu, X.; Liu, J.; Wei, D.; Zhu, P.; Cui, X.; Zhou, B.; Chen, X.; Liu, J.; Jin, J.; et al. Ammonia oxidizing bacteria dominate soil nitrification under different fertilization regimes in black soils of northeast China. Eur. J. Soil Biol. 2022, 111, 103410. [Google Scholar] [CrossRef]
- Tao, R.; Wakelin, S.A.; Liang, Y.; Chu, G. Response of ammonia-oxidizing archaea and bacteria in calcareous soil to mineral and organic fertilizer application and their relative contribution to nitrification. Soil Biol. Biochem. 2017, 114, 20–30. [Google Scholar] [CrossRef]
- Tzanakakis, V.A.; Taylor, A.E.; Bottomley, P.J. Different contributions of ammonia oxidizing archaea and bacteria to nitrification in soils amended with equivalent amounts of either ammonium-N or organic-N. Appl. Soil Ecol. 2022, 175, 104451. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Q.; Zhang, Q.; Long, C.Y.; Jia, W.; Cheng, C.L. Keystone species affect the relationship between soil microbial diversity and ecosystem function under land use change in subtropical China. Funct. Ecol. 2021, 35, 1159–1170. [Google Scholar] [CrossRef]
- Jiao, S.; Chen, W.; Wang, J.; Du, N.; Li, Q.; Wei, G. Soil microbiomes with distinct assemblies through vertical soil profiles drive the cycling of multiple nutrients in reforested ecosystems. Microbiome 2018, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Levy-Booth, D.J.; Prescott, C.E.; Grayston, S.J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biol. Biochem. 2014, 75, 11–25. [Google Scholar] [CrossRef]
- Yin, C.; Tan, C.; Chen, H.; Ye, M.; Fan, X.; Zheng, W.; Gao, Z.; Peng, H.; Liang, Y. Insight into the role of competition in niche differentiation between ammonia-oxidizing archaea and bacteria in ammonium-rich alkaline soil: A network-based study. Soil Biol. Biochem. 2022, 168, 108638. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, G.; He, S.; Zhou, N.; Wang, M.; Dang, C.; Wang, J.; Zheng, M. Abundance and community composition of comammox bacteria in different ecosystems by a universal primer set. Sci. Total Environ. 2019, 691, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, B.; Li, Y.; Jiang, D.; Zhou, Y.; Ding, A.; Zong, Y.; Ling, X.; Zhang, S.; Lu, H. Ubiquity, diversity, and activity of comammox Nitrospira in agricultural soils. Sci. Total Environ. 2020, 706, 135684. [Google Scholar] [CrossRef]
- Gao, S.; Chang, D.; Zou, C.; Cao, W.; Gao, J.; Huang, J.; Bai, J.; Zeng, N.; Rees, R.M.; Thorup-Kristensen, K. Archaea are the predominant and responsive ammonia oxidizing prokaryotes in a red paddy soil receiving green manures. Eur. J. Soil Biol. 2018, 88, 27–35. [Google Scholar] [CrossRef]
- Li, Y.; Chapman, S.J.; Nicol, G.W.; Yao, H. Nitrification and nitrifiers in acidic soils. Soil Biol. Biochem. 2018, 116, 290–301. [Google Scholar] [CrossRef]
- Wessen, E.; Nyberg, K.; Jansson, J.K.; Hallin, S. Responses of bacterial and archaeal ammonia oxidizers to soil organic and fertilizer amendments under long-term management. Appl. Soil Ecol. 2010, 3, 193–200. [Google Scholar] [CrossRef]
- Clark, D.R.; Mckew, B.A.; Dong, L.F.; Leung, G.; Dumbrell, A.J.; Stott, A.; Grant, H.; Nedwell, D.B.; Trimmer, M.; Whitby, C. Mineralization and nitrification: Archaea dominate ammonia-oxidising communities in grassland soils. Soil Biol. Biochem. 2020, 140, 107725. [Google Scholar] [CrossRef]
- Yao, R.; Li, H.; Yang, J.; Zhu, W.; Yin, C.; Wang, X.; Xie, W.; Zhang, X. Combined application of biochar and N fertilizer shifted nitrification rate and amoA gene abundance of ammonia-oxidizing microorganisms in salt-affected anthropogenic-alluvial soil. Appl. Soil Ecol. 2022, 171, 104348. [Google Scholar] [CrossRef]
- Wang, F.; Liang, X.; Ma, S.; Liu, L.; Wang, J.K. Ammonia-oxidizing archaea are dominant over comammox in soil nitrification under long-term nitrogen fertilization. J. Soils Sediments 2021, 21, 1800–1814. [Google Scholar] [CrossRef]
- Thi, H.H.T.; Petra, M. Presence of wheat straw in soil influences nutrient availability and leaching in soil mulched with high or low C/N organic materials. Arch. Agron. Soil Sci. 2021, 3, 342–353. [Google Scholar]
- Zhao, C.; Zhang, Y.; Liu, X.; Ma, X.; Meng, Y.; Li, X.; Quan, X.; Shan, J.; Zhao, W.; Wang, H. Comparing the Effects of Biochar and Straw Amendment on Soil Carbon Pools and Bacterial Community Structure in Degraded Soil. J. Soil Sci. Plant Nutr. 2020, 20, 751–760. [Google Scholar] [CrossRef]
- Chu, X.; Bai, N.; Zheng, X.; Wang, Q.; Pan, X.; Li, S.; Zhang, J.; Zhang, H.; He, W.; Zhong, F.; et al. Effects of straw returning combined with earthworm addition on nitrification and ammonia oxidizers in paddy soil. Front. Microbiol. 2022, 13, 1069554. [Google Scholar] [CrossRef]
- Wang, S.; Zhai, L.; Guo, S.; Zhang, F.; Hua, L.; Liu, H. Returned straw reduces nitrogen runoff loss by influencing nitrification process through modulating soil C:N of different paddy systems. Agric. Ecosyst. Environ. 2023, 354, 108438. [Google Scholar] [CrossRef]
- Liu, X.; Tang, Z.; Zhang, Q.; Kong, W. The contrasting effects of biochar and straw on N2O emissions in the maize season in intensively farmed soil. Environ. Sci. Pollut. Res. 2021, 28, 29806–29819. [Google Scholar] [CrossRef]
- Chen, S.; Gao, R.; Xiang, X.; Yang, H.; Yu, Y. Straw mulching and nitrogen application altered ammonia oxidizers communities and improved soil quality in the alkaline purple soil of southwest China. AMB Expr. 2021, 11, 52. [Google Scholar] [CrossRef]
- Zheng, J.; Tao, L.; Dini-Andreote, F.; Luan, L.; Kong, P.; Xue, J.; Zhu, G.; Xu, Q.; Jiang, Y. Dynamic Responses of Ammonia-Oxidizing Archaea and Bacteria Populations to Organic Material Amendments Affect Soil Nitrification and Nitrogen Use Efficiency. Front. Microbiol. 2022, 13, 911799. [Google Scholar] [CrossRef]
- Fan, C.; Zhang, W.; Chen, X.; Li, N.; Li, W.; Wang, Q.; Duan, P.; Chen, M. Residual effects of four-year amendments of organic material on N2O production driven by ammonia-oxidizing archaea and bacteria in a tropical vegetable soil. Sci. Total Environ. 2021, 781, 146746. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.Y.; Liu, T.Q.; Jiang, S.S.; Cao, C.G.; Li, C.F.; Chen, B.; Liu, J.B. Combined effects of straw returning and chemical N fertilization on greenhouse gas emissions and yield from paddy fields in northwest Hubei province China. J. Soil Sci. Plant Nut. 2019, 16, 1–15. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; Natural Resources Conservation Service: Washington, DC, USA, 1999; p. 436.
- Han, Y.; Yao, S.; Jiang, H.; Ge, X.; Zhang, Y.; Mao, J.; Dou, S.; Zhang, B. Effects of mixing maize straw with soil and placement depths on decomposition rates and products at two cold sites in the mollisol region of China. Soil Till Res. 2020, 197, 104519. [Google Scholar] [CrossRef]
- Lise, K.G.; Charles, W.H.; Jack, D.D. Interferences, limitations and an improvement in the extraction and assessment of cellulase activity in soil. Soil Biol. Biochem. 1994, 26, 65–73. [Google Scholar]
- Božinović, M.; Sokač, T.; Šalić, A.; Dukarić, A.M.; Tišma, M.; Planinić, M.; Zelić, B. Standardization of 3, 5-dinitrosalicylic acid (DNS) assay for measuring xylanase activity: Detecting and solving problems. Croat. J. Food Sci. Technol. 2023, 15, 151–162. [Google Scholar] [CrossRef]
- Wang, F.; Liang, X.; Ding, F.; Ren, L.; Liang, M.; An, T.; Li, S.; Wang, J.; Liu, L. The active functional microbes contribute differently to soil nitrification and denitrification potential under long-term fertilizer regimes in North-East China. Front. Microbiol. 2022, 13, 1021080. [Google Scholar] [CrossRef] [PubMed]
- Rasche, F.; Knapp, D.; Kaiser, C.; Koranda, M.; Kitzler, B.; Zechmeister-Boltenstern, S.; Richter, A.; Sessitsch, A. Seasonality and resource availability control bacterial and archaeal communities in soils of a temperate beech forest. ISME J. 2011, 5, 389–402. [Google Scholar] [CrossRef]
- Pjevac, P.; Schauberger, C.; Poghosyan, L.; Herbold, C.W.; van Kessel, M.; Daebeler, A.; Steinberger, M.; Jetten, M.; Lücker, S.; Wagner, M.; et al. AmoA-targeted polymerase chain reaction primers for the specific detection and quantification of comammox Nitrospira in the environment. Front Micribiol 2017, 8, 1508. [Google Scholar] [CrossRef]
- Zhang, M.; Alves, R.J.E.; Zhang, D.; Han, L.; He, J.; Zhang, L. Time-dependent shifts in populations and activity of bacterial and archaeal ammonia oxidizers in response to liming in acidic soils. Soil Biol. Biochem. 2017, 112, 77–89. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, J.; Peres-Neto, P. Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca.hp R package. Methods Ecol. Evol. 2022, 13, 782–788. [Google Scholar] [CrossRef]
- Liao, H.; Lu, X.; Rensing, C.; Friman, V.P.; Geisen, S.; Chen, Z.; Yu, Z. Hyperthermophilic composting accelerates the removal of antibiotic resistance genes and mobile genetic elements in sewage sludge. Environ. Sci. Technol. 2018, 52, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, A.K.A.; Lourenço, K.S.; Pitombo, L.M.; Mendes, L.W.; Roesch, L.F.W.; Pijl, A.; Carmo, J.B.; Cantaralle, H.; Kuramae, E.E. Recycling organic residues in agriculture impacts soil-borne microbial community structure, function, and N2O emissions. Sci. Total Environ. 2018, 631, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Li, Z.; Niu, S. Global soil gross nitrogen transformation under increasing nitrogen deposition. Glob. Biogeochem. Cycles 2021, 35, e2020GB006711. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, J.; Han, L.; Zheng, Y.; He, J. Influence of rice straw amendment on mercury methylation and nitrification in paddy soils. Environ. Pollut. 2015, 209, 53–59. [Google Scholar] [CrossRef]
- Cheng, J.; Zhao, M.; Cong, J.; Qi, Q.; Xiao, Y.; Cong, W.; Deng, Y.; Zhou, J.; Zhang, Y. Soil pH exerts stronger impacts than vegetation type and plant diversity on soil bacterial community composition in subtropical broad-leaved forests. Plant Soil 2020, 450, 273–286. [Google Scholar] [CrossRef]
- Xu, P.; Jiang, M.; Khan, I.; Zhao, J.; Yang, T.; Tu, J.; Hu, R. Available nitrogen and ammonia-oxidizing archaea in soil regulated N2O emissions regardless of rice planting under a double rice cropping-fallow system. Agric. Ecosyst. Environ. 2022, 340, 108166. [Google Scholar] [CrossRef]
- Huang, L.; Chakrabarti, S.; Cooper, J.; Perez, A.; John, S.M.; Daroub, S.H.; Martens-Habbena, W. Ammonia-oxidizing archaea are integral to nitrogen cycling in a highly fertile agricultural soil. ISME Commun. 2021, 1, 19. [Google Scholar] [CrossRef]
- Kits, K.; Sedlacek, C.; Lebedeva, E.; Han, P.; Bulaev, A.; Pjrvac, P.; Daebeler, A.; Romano, S.; Albertsen, L.Y.; Daims, H.; et al. Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature 2017, 549, 269–272. [Google Scholar] [CrossRef]
- Osburn, E.D.; Barrett, J.E. Abundance and functional importance of complete ammonia-oxidizing bacteria (comammox) versus canonical nitrifiers in temperate forest soils. Soil Biol. Biochem. 2020, 145, 107801. [Google Scholar] [CrossRef]
- Li, C.Y.; Hu, H.W.; Chen, Q.L.; Yan, Z.Z.; Nguyen, B.A.T.; Chen, D.; He, J.Z. Niche specialization of comammox Nitrospira clade A in terrestrial ecosystems. Soil Biol. Biochem. 2021, 156, 108231. [Google Scholar] [CrossRef]
- Levicnik-Hofferle, S.; Nicol, G.W.; Ausec, L.; Mandic-Mulec, I.; Prosser, J.I. Stimulation of thaumarchaeal ammonia oxidation by ammonia derived from organic nitrogen but not added inorganic nitrogen. FEMS Microbiol. Ecol. 2012, 80, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Lehtovirta-Morley, L.E.; Ge, C.; Ross, J.; Yao, H.; Nicol, G.W.; Prosser, J.I. Characterisation of terrestrial acidophilic archaeal ammonia oxidisers and their inhibition and stimulation by organic compounds. FEMS Microbiol. Ecol. 2014, 89, 542–552. [Google Scholar] [PubMed]
- Tourna, M.; Stieglmeier, M.; Spang, A.; Konneke, M.; Schintlmeister, A.; Urich, T.; Engel, M.; Schloter, M.; Wagner, M.; Richter, A.; et al. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc. Natl. Acad. Sci. USA 2011, 108, 8420–8425. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Amin, S.A.; Martens-Habbena, W.; Walker, C.B.; Urakawa, H.; Devol, A.H.; Ingalls, A.E.; Moffett, J.W.; Armbrust, E.V.; Stahl, D.A. Marine ammonia-oxidizing archaeal isolates display obligate mixotrophy and wide ecotypic variation. Proc. Natl. Acad. Sci. USA 2014, 111, 12504–12509. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Park, S.J.; Sinninghe Damsté, J.S.; Schouten, S.; Rijpstra, W.I.C.; Jung, M.Y.; Kim, S.J.; Gwak, J.H.; Hong, H.; Si, O.J.; et al. Hydrogen peroxide detoxification is a key mechanism for growth of ammonia-oxidizing Archaea. Proc. Natl. Acad. Sci. USA 2016, 113, 7888–7893. [Google Scholar] [CrossRef]
- Bates, S.T.; Berg-Lyons, D.; Caporaso, J.G.; Walters, W.A.; Knight, R.; Fierer, N. Examining the global distribution of dominant archaeal populations in soil. ISME J. 2011, 5, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.; Wanek, W.; Zappe, A.; Richter, A.; Svenning, M.M.; Schleper, C.; Urich, T. Nitrification rates in Arctic soils are associated with functionally distinct populations of ammonia-oxidizing archaea. ISME J. 2013, 7, 1620–1631. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xia, H.; Jiang, C.; Riaz, M.; Yang, L.; Chen, Y.; Fan, X.; Xia, X. 14 year applications of chemical fertilizers and crop straw effects on soil labile organic carbon fractions, enzyme activities and microbial community in rice-wheat rotation of middle China. Sci. Total Environ. 2022, 841, 156608. [Google Scholar] [CrossRef]
- Zou, W.; Lang, M.; Zhang, L.; Liu, B.; Chen, X. Ammonia-oxidizing bacteria rather than ammonia-oxidizing archaea dominate nitrification in a nitrogen-fertilized calcareous soil. Sci. Total Environ. 2022, 811, 151402. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Xu, Y.; Zhang, J.; Lu, Y. Environmental filtering drives distinct continental atlases of soil archaea between dryland and wetland agricultural ecosystems. Microbiome 2019, 7, 15. [Google Scholar] [CrossRef] [PubMed]

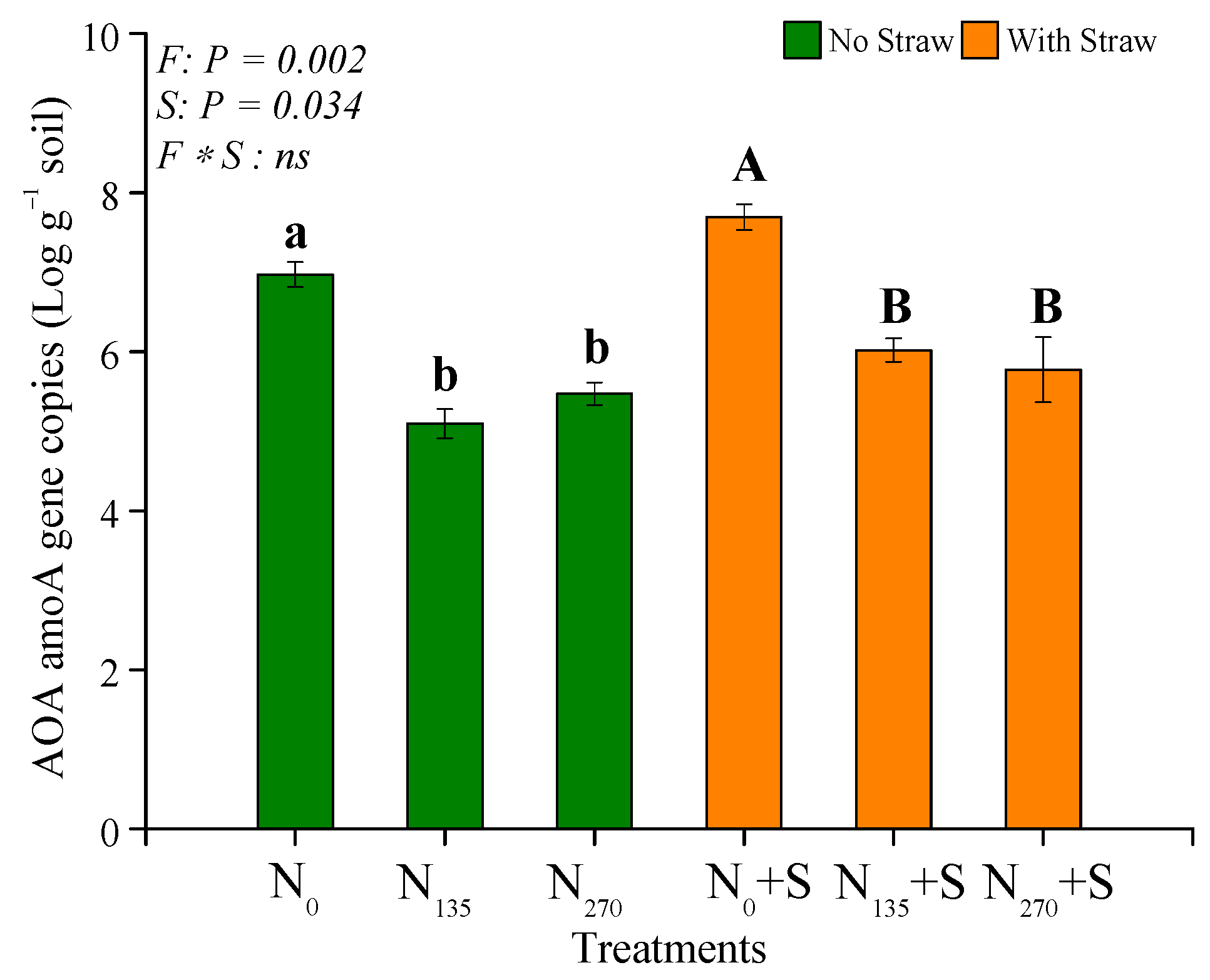
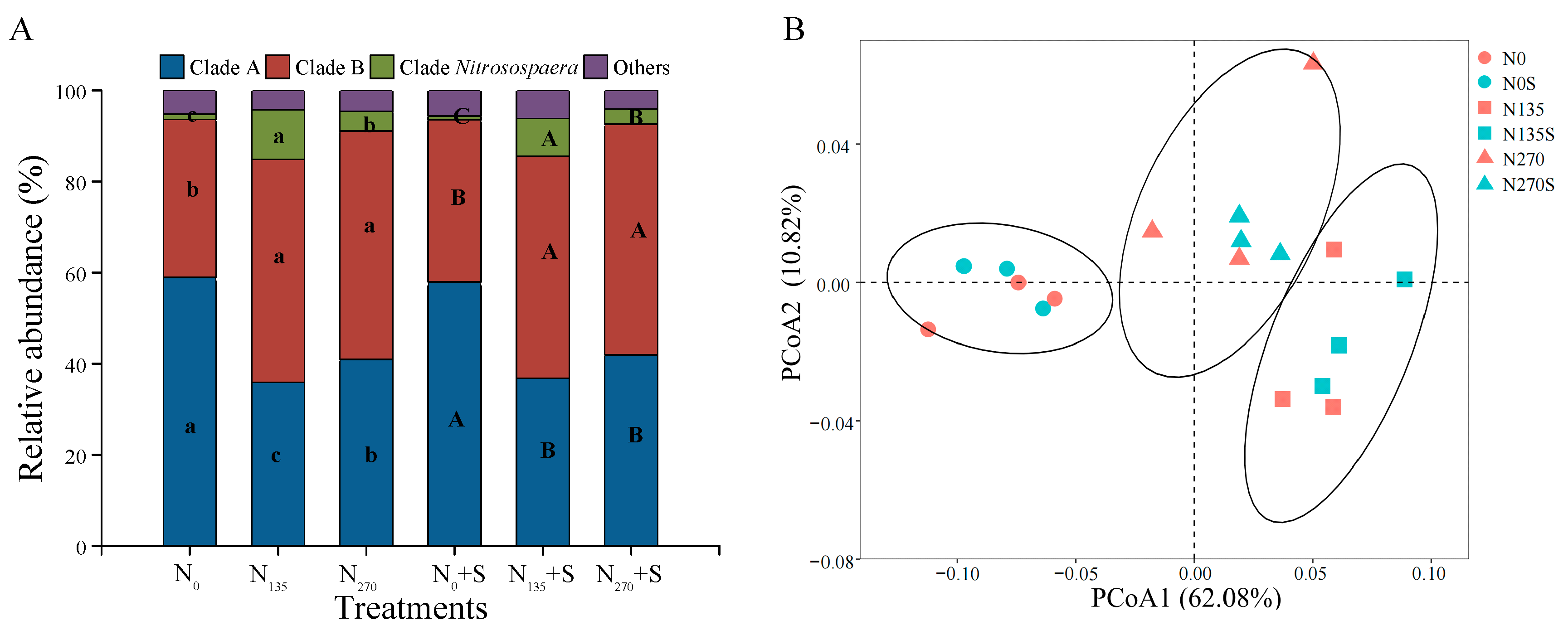

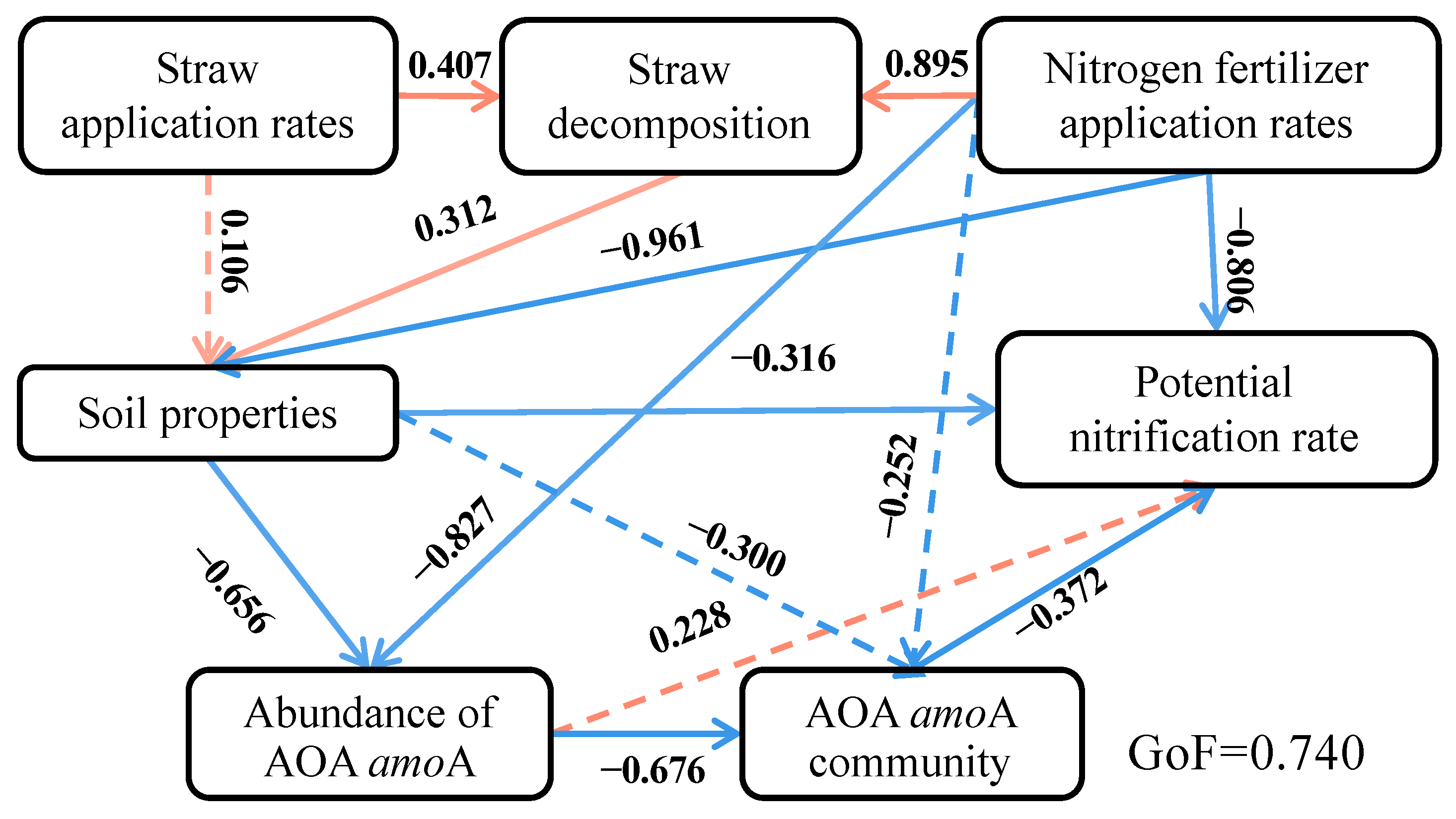
| SOC (g kg−1) | TN (g kg−1) | NH4+-N (mg kg−1) | NO3−-N (mg kg−1) | pH | |
|---|---|---|---|---|---|
| N0 | 9.08 | 0.74 | 3.97 | 27.39 | 6.69 |
| N135 | 8.80 | 0.70 | 2.63 | 17.46 | 6.12 |
| N270 | 8.48 | 0.72 | 19.48 | 38.16 | 4.64 |
| No Straw | Applied Straw | |||||
|---|---|---|---|---|---|---|
| N0 | N135 | N270 | N0 + S | N135 + S | N270 + S | |
| pH | 6.76 ± 0.02 a | 6.34 ± 0.03 b | 4.96 ± 0.02 c | 7.01 ± 0.04 A | 6.52 ± 0.01 B | 6.05 ± 0.06 C |
| SOC (g kg−1) | 8.71 ± 0.03 a | 8.44 ± 0.07 c | 8.66 ± 0.01 b | 9.52 ± 0.04 A | 9.22 ± 0.02 B | 9.02 ± 0.02 C |
| TN (g kg−1) | 0.75 ± 0.01 c | 0.78 ± 0.01 b | 0.86 ± 0.02 a | 0.76 ± 0.01 C | 0.88 ± 0.01 B | 0.97 ± 0.02 A |
| NH4+-N (mg kg−1) | 9.45 ± 0.25 c | 10.16 ± 0.06 b | 11.58 ± 0.23 a | 13.81 ± 0.14 C | 14.59 ± 0.09 B | 20.03 ± 0.51 A |
| NO3−-N (mg kg−1) | 0.75 ± 0.07 c | 1.95 ± 0.39 b | 6.22 ± 0.27 a | 1.57 ± 0.20 C | 5.10 ± 0.44 B | 7.34 ± 0.20 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Liang, X.; Liang, M.; Guo, B.; Li, S.; Liu, L.; Wang, J. Straw Returning Alleviates the Inhibition of Soil Nitrification Medicated by Ammonia-Oxidizing Archaea under Low Nitrogen Fertilization. Agronomy 2024, 14, 1550. https://doi.org/10.3390/agronomy14071550
Wang F, Liang X, Liang M, Guo B, Li S, Liu L, Wang J. Straw Returning Alleviates the Inhibition of Soil Nitrification Medicated by Ammonia-Oxidizing Archaea under Low Nitrogen Fertilization. Agronomy. 2024; 14(7):1550. https://doi.org/10.3390/agronomy14071550
Chicago/Turabian StyleWang, Feng, Xiaolong Liang, Minjie Liang, Bingqing Guo, Shuangyi Li, Lingzhi Liu, and Jingkuan Wang. 2024. "Straw Returning Alleviates the Inhibition of Soil Nitrification Medicated by Ammonia-Oxidizing Archaea under Low Nitrogen Fertilization" Agronomy 14, no. 7: 1550. https://doi.org/10.3390/agronomy14071550
APA StyleWang, F., Liang, X., Liang, M., Guo, B., Li, S., Liu, L., & Wang, J. (2024). Straw Returning Alleviates the Inhibition of Soil Nitrification Medicated by Ammonia-Oxidizing Archaea under Low Nitrogen Fertilization. Agronomy, 14(7), 1550. https://doi.org/10.3390/agronomy14071550






