Allelopathy and Allelochemicals in Chamaecyparis obtusa Leaves for the Development of Sustainable Agriculture
Abstract
:1. Introduction
2. Materials and Methods
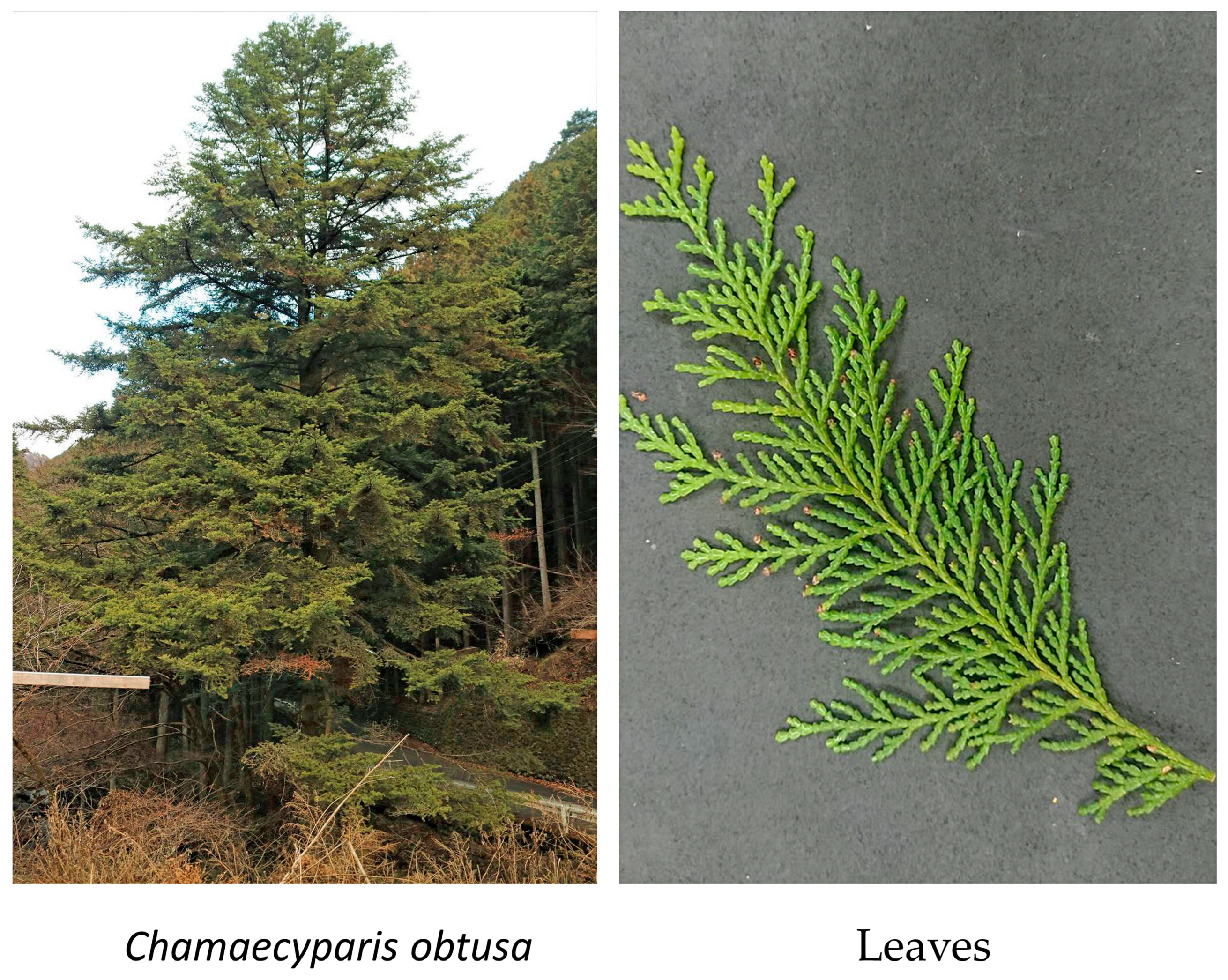
2.1. Plant Materials
2.2. Determination of Allelopathic Activity of C. obtusa Leaves under a Laboratory Condition
2.3. Allelopathic Activity of C. obtusa Leaves under Greenhouse Conditions
2.4. Isolation of Allelochemicals from C. obtusa Leaves
2.5. Isolation of an Allelochemical from Fraction 2 Obtained in Section 2.4
2.6. Isolation of an Allelochemical from Fraction 6 Obtained in Section 2.4
2.7. Spectrum Data for Isolated Compounds
2.8. Allelopathic Activity of the Isolated Compounds
2.9. Statistics
3. Results
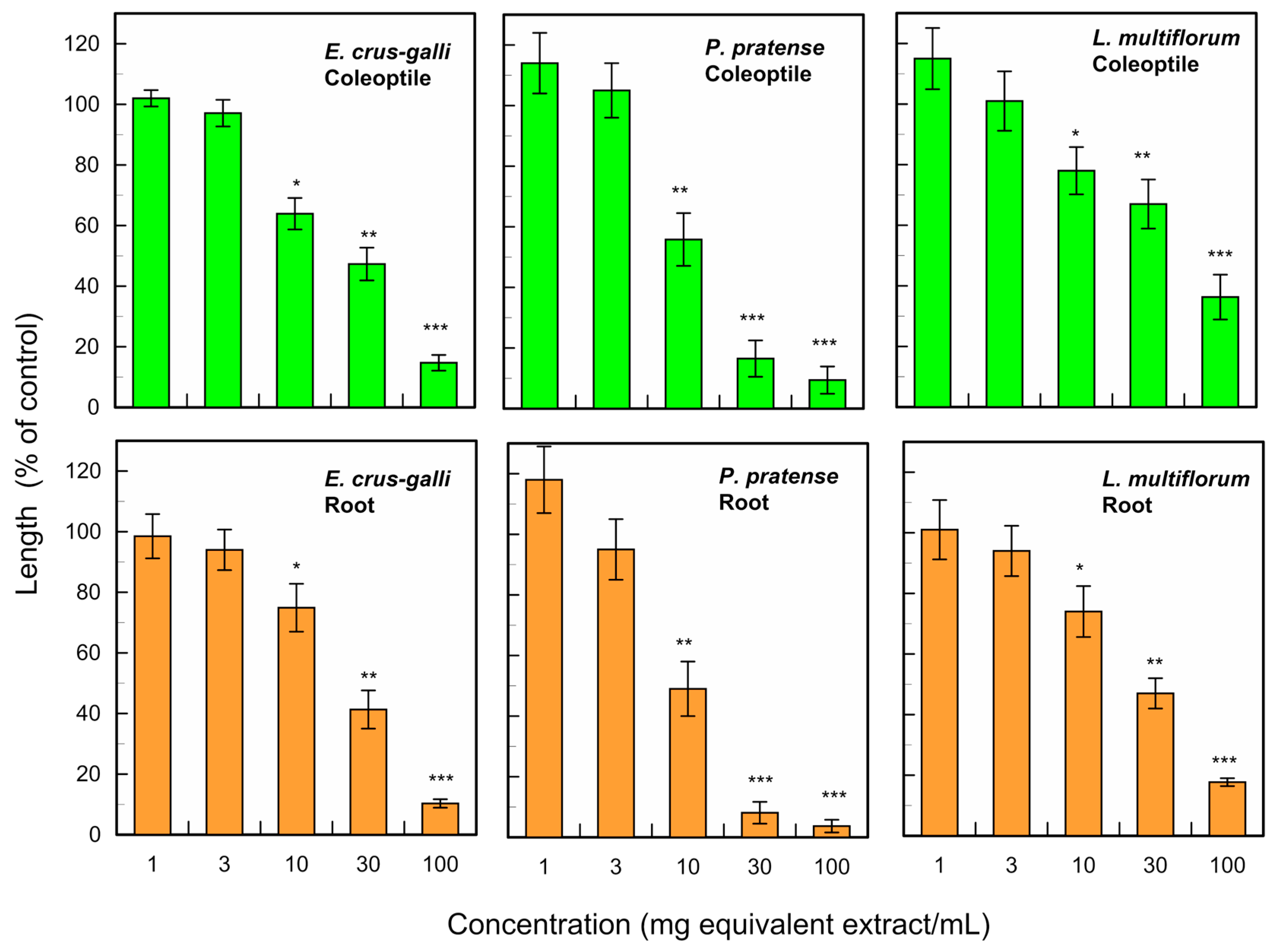
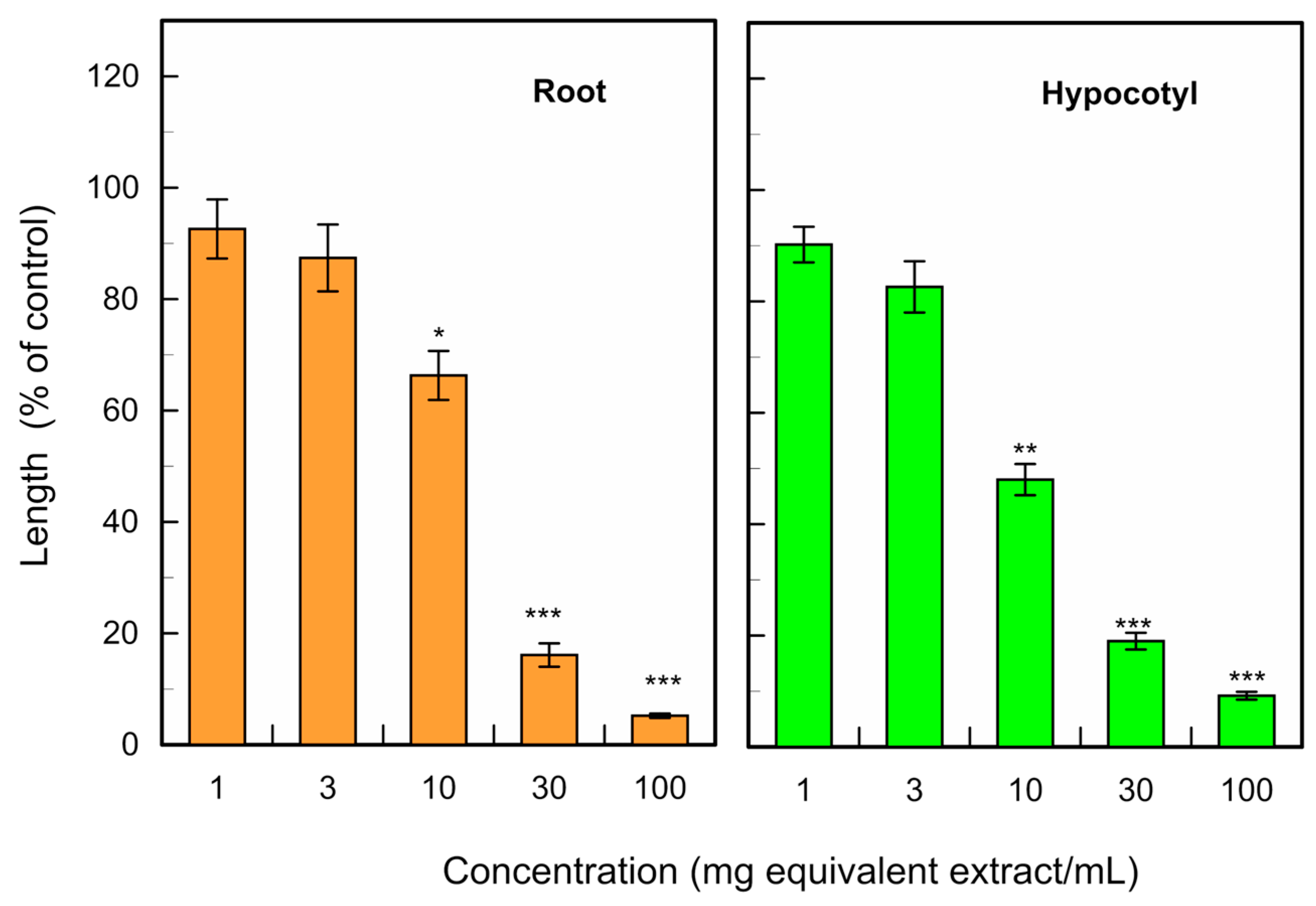
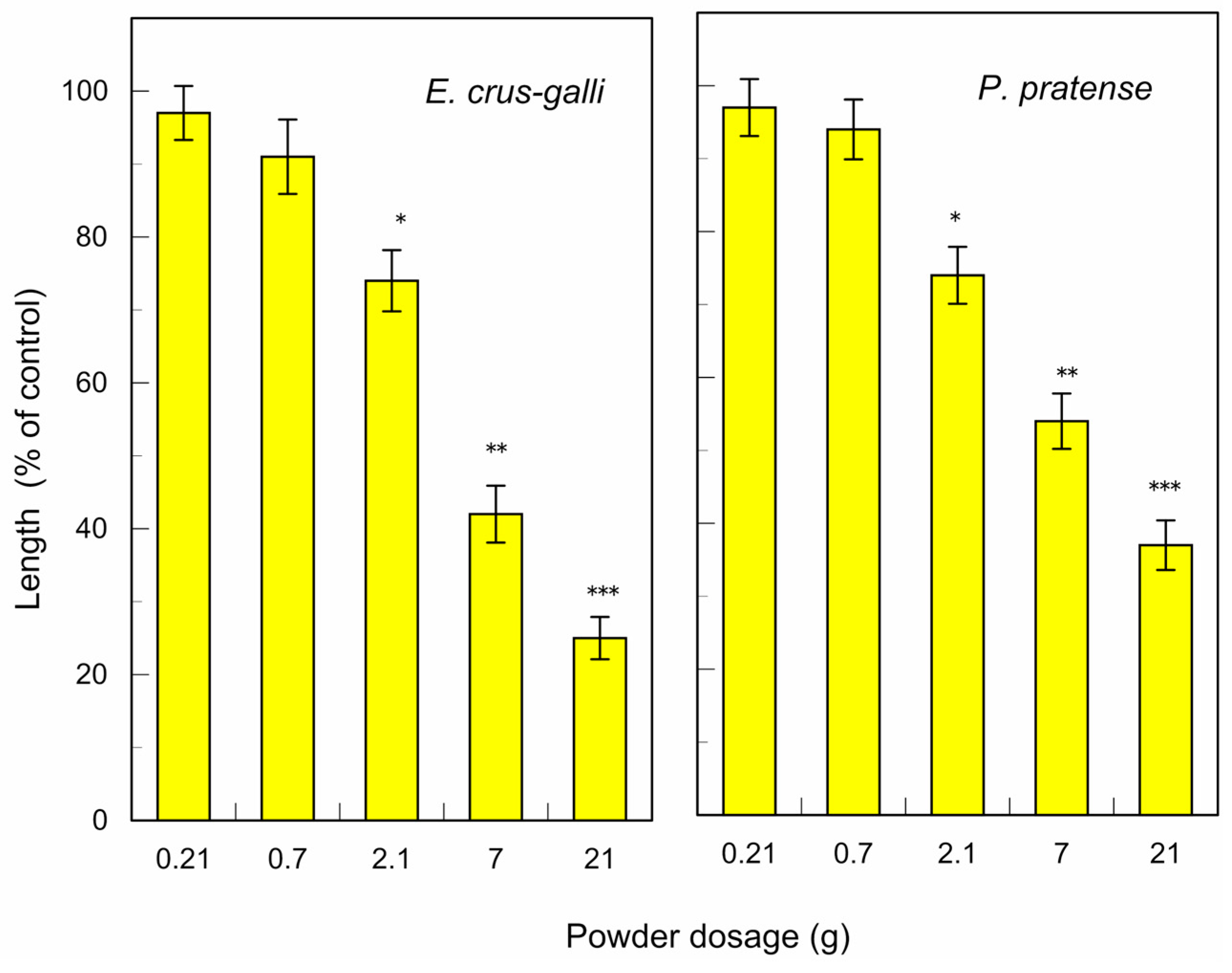
3.1. Allelopathic Activity of C. obtusa Leaves
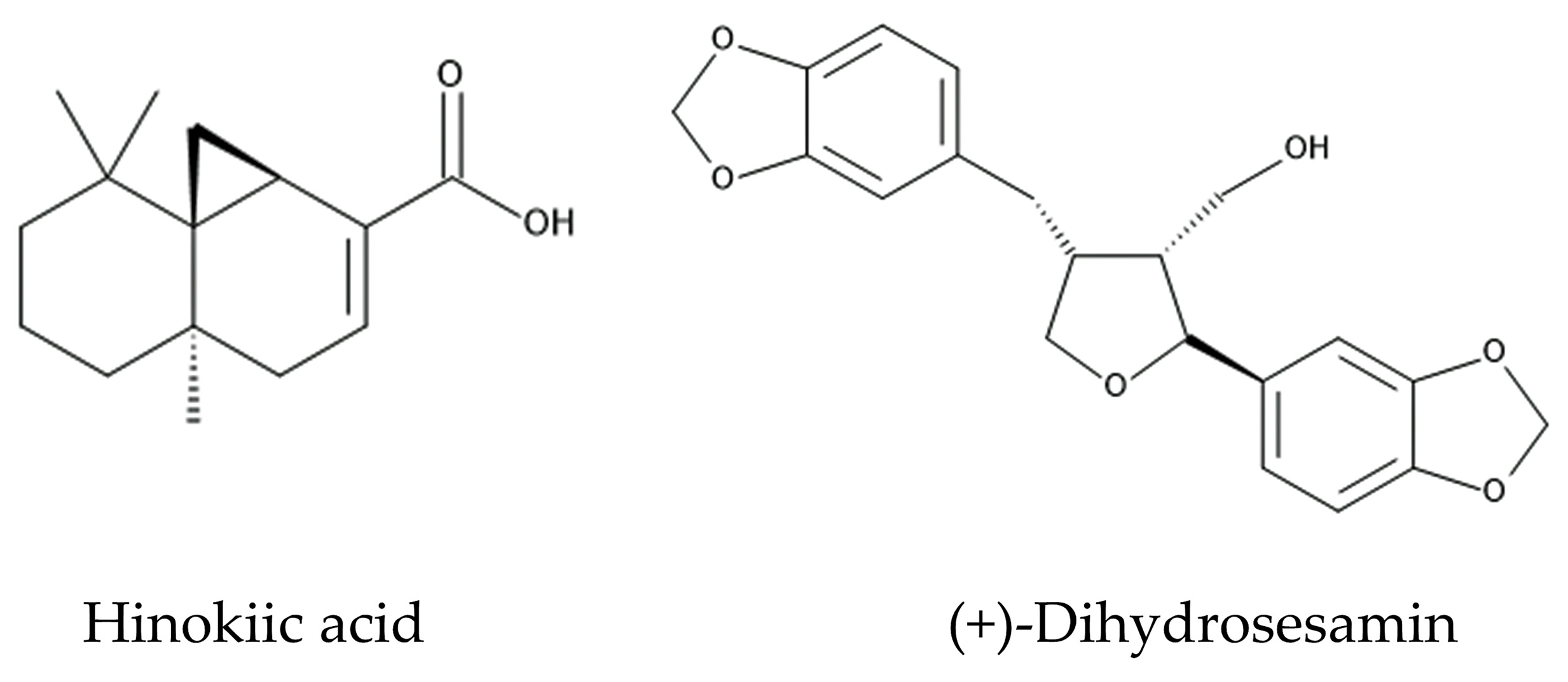
3.2. Isolation and Identification of Allelochemicals in C. obtusa Leaves
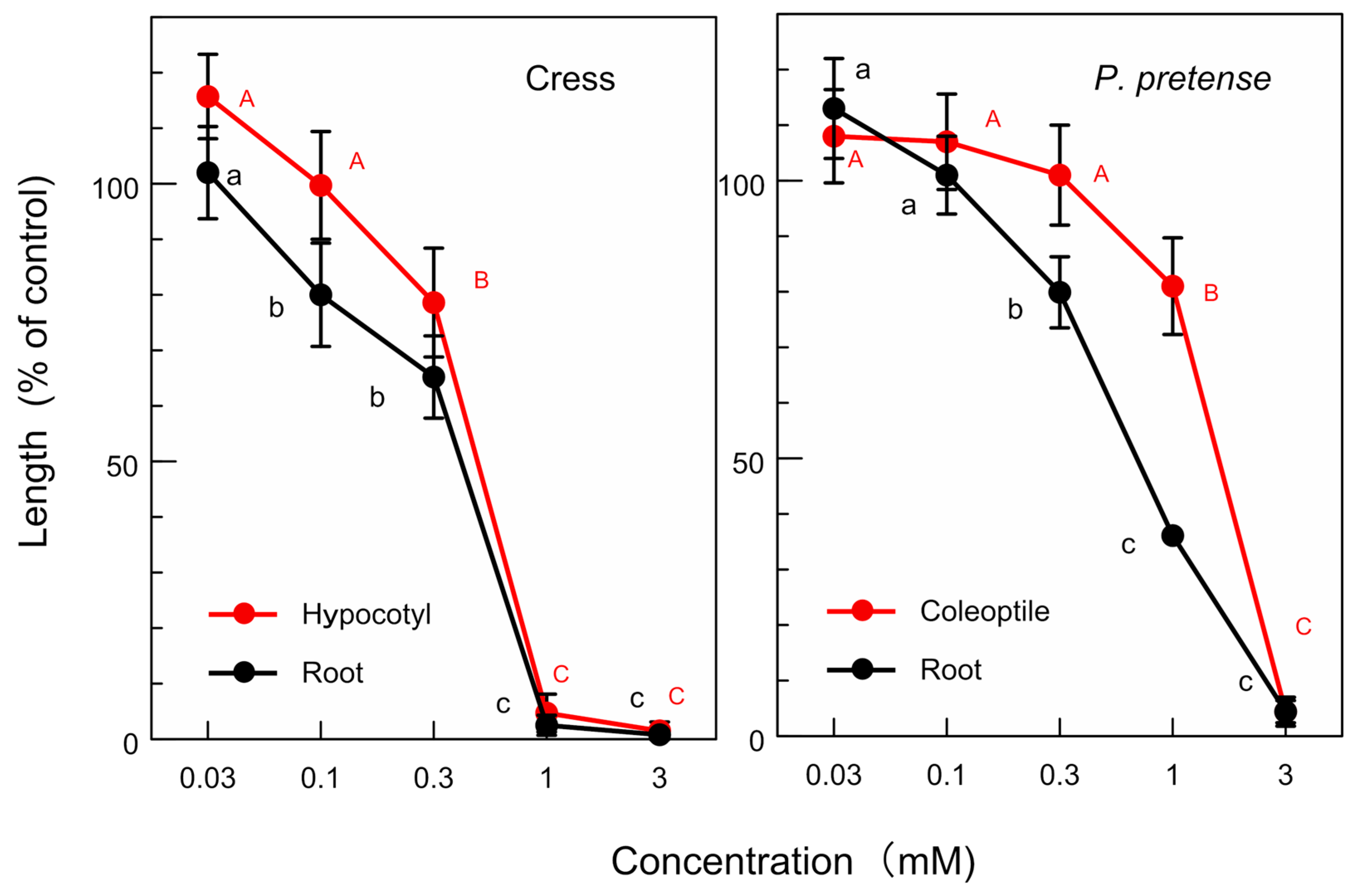
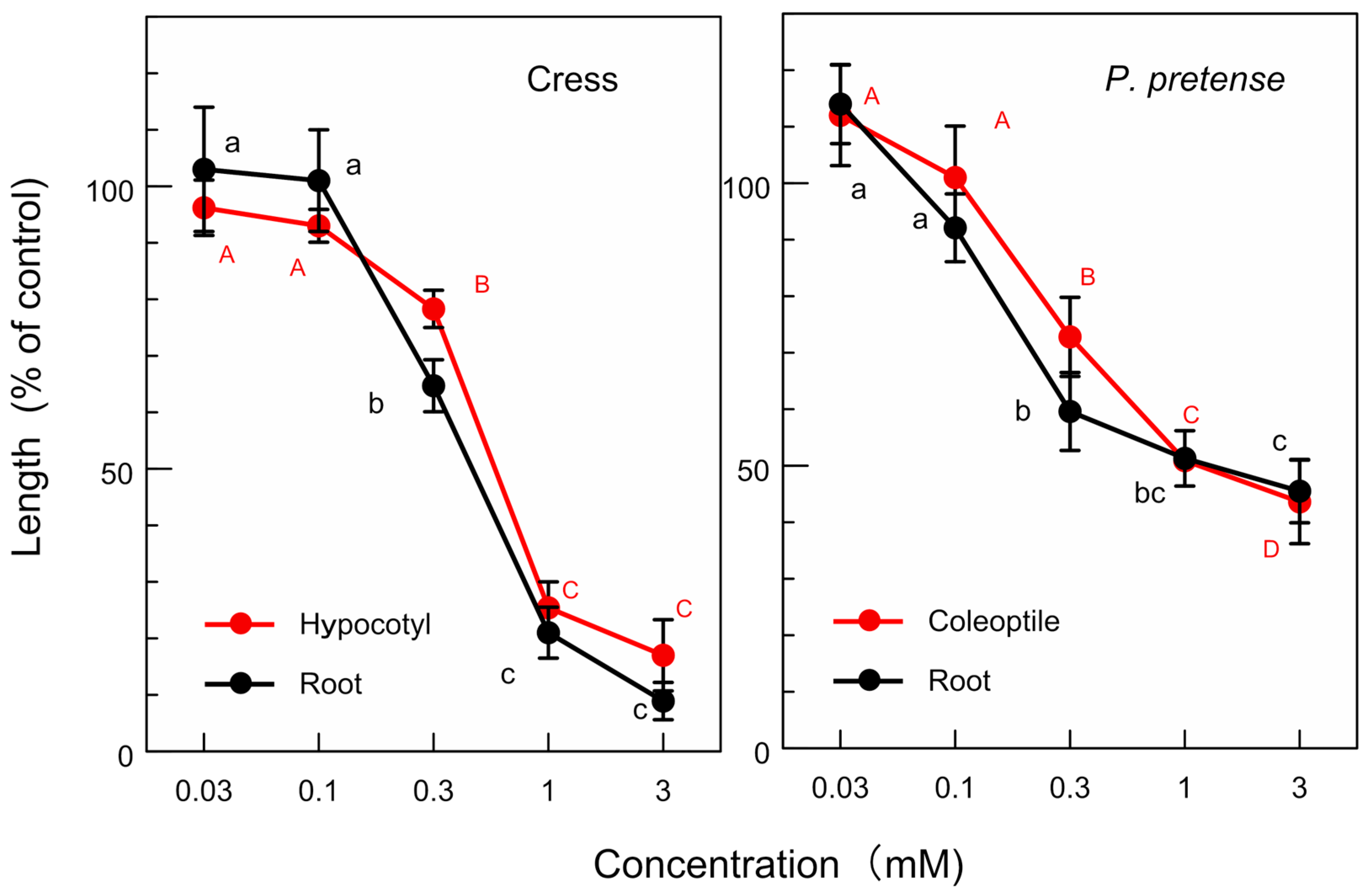
3.3. Allelopathic Activity of the Isolated Compounds
4. Discussion
4.1. Allelopathic Activity of C. obtusa Leaves
4.2. Allelochemicals in C. obtusa Leaves
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoshino, D.; Nishimura, N.; Yamamoto, S. Age, size structure and spatial pattern of major tree species in an old-growth Chamaecyparis obtusa forest, Central Japan. For. Ecol. Manag. 2001, 152, 31–43. [Google Scholar] [CrossRef]
- Kijidani, Y.; Kawasaki, Y.; Matsuda, D.; Nakazono, F.; Hayakawa, M.; Mutaguchi, H.; Sakagami, H. Tree heights in the ring-formed years affect microfibril angles in the rings from juvenile to mature wood at breast height in hinoki trees (Chamaecyparis obtusa). J. Wood Sci. 2014, 60, 381–388. [Google Scholar] [CrossRef]
- Kang, D.B.; Sung, J.W.; Lee, D.H. Effects of shading and fertilizer treatments on the growth characteristics of Chamaecyparis obtusa (S. et Z.) Endlicher seedlings. For. Sci. Technol. 2021, 17, 125–134. [Google Scholar] [CrossRef]
- Kew Royal Botanica Gardens, Chamaecyparis obtusa. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:261845-1 (accessed on 17 February 2024).
- Plant Finder, Chamaecyparis obtusa. Available online: https://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=c204 (accessed on 17 February 2024).
- Eshun, J.F.; Potting, J.; Leemans, R. Wood waste minimization in the timber sector of Ghana: A systems approach to reduce environmental impact. J. Clean. Prod. 2012, 26, 67–78. [Google Scholar] [CrossRef]
- Adhikari, S.; Ozarska, B. Minimizing environmental impacts of timber products through the production process “From Sawmill to Final Products”. Environ. Syst. Res. 2018, 7, 6. [Google Scholar] [CrossRef]
- Tseng, M.H.; Lai, W.R.; Hsieh, C.L.; Kuo, Y.H. Allelopathy on bark of downed logs of Chamaecyparis obtusa Sieb. and Zucc. var. formosana (Hayata) Rehder. J. Chem. Ecol. 2007, 33, 1283–1296. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Kadoya, K. Phytotoxic constituents in the bark and sawdust extracts of Chamaecyparis obtusa and Cryptomeria japonica and their effects on the growth of seedlings of trifoliate orange (Poncirus trifoliata Raf.) and rice (Oryza sativa L.). J. Jpn. Soc. Hort. Sci. 1993, 62, 285–294. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–422. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578. [Google Scholar] [CrossRef]
- Morikawa, T.; Ashitani, T.; Sekine, N.; Kusumoto, N.; Takahashi, K. Bioactivities of extracts from Chamaecyparis obtusa branch heartwood. J. Wood Sci. 2012, 58, 544–549. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Sharma, A.; Kim, S.H.; Baek, K.H. Phenolic content and antioxidant capacity of essential oil obtained from sawdust of Chamaecyparis obtusa by microwave-assisted hydrodistillation. Food Technol. Biotechnol. 2013, 51, 360–369. [Google Scholar]
- Chien, T.C.; Lo, S.F.; Ho, C.L. Chemical composition and anti-inflammatory activity of Chamaecyparis obtusa f. formosana wood essential oil from Taiwan. Nat. Prod. Commun. 2014, 9, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Eltayeb, L.M.; Yagi, S.; Mohamed, H.M.; Zengin, G.; Shariati, M.A.; Rebezov, M.; Uba, A.I.; Lorenzo, J.M. Essential oils composition and biological activity of Chamaecyparis obtusa, Chrysopogon nigritanus and Lavandula coronopifolia grown wild in Sudan. Molecules 2023, 28, 1005. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Woo, H.; Park, M.-J. Development of Pinaceae and Cupressaceae essential oils from forest waste in South Korea. Plants 2023, 12, 3409. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Choi, M.S.; Seo, W.T.; Rinker, D.L.; Han, S.W.; Cheong, G.W. Chemical composition and antimicrobial activity of Chamaecyparis obtusa leaf essential oil. Fitoterapia 2007, 78, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Yasutomi, R.; Anzawa, R.; Urakawa, M.; Usuki, T. Effective extraction of limonene and hibaene from hinoki (Chamaecyparis obtusa) using ionic liquid and deep eutectic solvent. Molecules 2021, 26, 4271. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Yeh, R.Y.; Cheng, S.S. Antifungal potential and chemical composition of Chamaecyparis formosensis and Chamaecyparis obtusa var. formosana essential oils in liquid and vapor phases against 4 fungal species. Nat. Prod. Commun. 2023, 18, 1–7. [Google Scholar] [CrossRef]
- Singh, H.P.; Kohli, R.K.; Batish, D.R.; Kaushal, P.S. Allelopathy of Gymnospermous trees. J. For. Res. 1999, 4, 245–254. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Takeshita, S.; Kimura, F.; Ohno, O.; Suenaga, K. A novel allelopathic active substance in Ginkgo biloba. J. Plant Physiol. 2013, 170, 1595–1599. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kimura, F.; Ohno, O.; Suenaga, K. Involvement of allelopathy in inhibition of understory growth in red pine forests. J. Plant Physiol. 2017, 218, 66–73. [Google Scholar] [CrossRef]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest. Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef] [PubMed]
- Macías, F.A.; Molinillo, J.M.G.; Varela, R.M.; Galindo, J.G.G. Allelopathy—A natural alternative for weed control. Pest Manag. Sci. Former. Pestic. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kato, M. Allelopathy and allelochemicals of Solidago canadensis L. and S. altissima L. for their naturalization. Plants 2022, 11, 3235. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kato, M. Evolution of the secondary metabolites in invasive plant species Chromolaena odorata for the defense and allelopathic functions. Plants 2023, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Kil, B.S. Allelopathic effects of volatile substances from Chamaecyparis obtusa. Korean J. Ecol. 2000, 23, 323–329. [Google Scholar]
- Hagihara, A.; Yokota, T.; Ogawa, K. Allometric relations in hinoki (Chamaecyparis obtusa (Sieb. et Zucc.) Endl.) trees. Bull Nagoya Univ. For. 1993, 12, 11–29. [Google Scholar]
- Ogawa, K. Size dependence of leaf area and the mass of component organs during a course of self-thinning in a hinoki (Chamaecyparis obtusa) seedling population. Ecol. Res. 2003, 18, 611–618. [Google Scholar] [CrossRef]
- Ogawa, K.; Adu-Bredu, S.; Yokota, T.; Hagihara, A. Leaf biomass changes with stand development in hinoki cypress (Chamaecyparis obtusa [Sieb. et Zucc.] Endl.). Plant Ecol. 2010, 211, 79–88. [Google Scholar] [CrossRef]
- Hossen, K.; Asato, Y.; Teruya, T.; Kato-Noguchi, H. Identification of four allelopathic compounds including a novel compound from Elaeocarpus floribundus Blume and determination of their allelopathic activity. J. Environ. Manag. 2023, 326, 116728. [Google Scholar] [CrossRef]
- Gao, H.Y.; Wu, L.J.; Muto, N.; Fuchino, H.; Nakane, T.; Shirota, O.; Sano, T.; Kuroyanagi, M. Beyerane derivatives and a sesquiterpene dimer from Japanese cypress (Chamaecyparis obtusa). Chem. Pharm. Bull. 2008, 56, 1030–1034. [Google Scholar] [CrossRef]
- Yamauchi, S.; Tanaka, T.; Kinoshita, Y. First highly stereoselective synthesis of (+)-dihydrosesamin, a trisubstituted tetrahydrofuran-type of lignan, by using highly erythro-selective aldol condensation. J. Chem. Soc. Perkin Trans. 2001, 1, 2158–2160. [Google Scholar] [CrossRef]
- Sun, Z.H.; Tan, N.H.; Zeng, G.Z.; Zhang, Y.M. Two new cinnamyl isovalerate derivatives from Sabina gaussenii. Molecules 2016, 21, 571. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.N.; Johnson, D.E.; Sivaprasad, B.; Ladha, J.K.; Mortimer, A.M. Weed management in direct-seeded rice. Adv. Agron. 2007, 93, 153–255. [Google Scholar]
- Narwal, S.S. Allelopathy in weed management. In Allelopathy Update; Basic and Applied Aspects; Narwal, S.S., Ed.; Science Publishers Inc.: Enfield, NH, USA, 1999; Volume 2, pp. 203–254. [Google Scholar]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and allelochemicals of Leucaena leucocephala as an invasive plant species. Plants 2022, 11, 1672. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Allelopathy and allelochemicals of Imperata cylindrica as an invasive plant species. Plants 2022, 11, 2551. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Allelopathy of knotweeds as invasive plants. Plants 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an invasive plant. Plants 2021, 10, 1028. [Google Scholar] [CrossRef] [PubMed]
- Heděnec, P.; Novotný, D.; Ust’ak, S.; Honzík, R.; Kovářová, M.; Šimáčková, H.; Frouz, J. Allelopathic effect of new introduced biofuel crops on the soil biota: A comparative study. Eur. J. Soil Biol. 2014, 63, 14–20. [Google Scholar] [CrossRef]
- Inderjit, S.; Dakshini, K.M.M. Investigations on some aspects of chemical ecology of cogongrass, Imperata cylindrica (L.) Beauv. J. Chem. Ecol. 1991, 17, 343–352. [Google Scholar] [CrossRef]
- Hui-Qiong, Z.; Ning, W.; Liu-Fa, W.; Ping, H.E. Effects of Lantana camara leaf extract on the activity of superoxide dismutase and accumulation of H2O2 in water hyacinth leaf. J. Plant Physiol. Mol. Biol. 2006, 32, 189–194. [Google Scholar]
- Poonpaiboonpipat, T.; Poolkum, S. Utilization of Bidens pilosa var. radiata (Sch. Bip.) Sherff integrated with water irrigation for paddy weed control and rice yield production. Weed Biol. Manag. 2019, 19, 31–38. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Brewin, N.J.; Tsyganov, V.E. Structure and development of the legume-rhizobial symbiotic interface in infection threads. Cells 2021, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Mathesius, U. Are legumes different? Origins and consequences of evolving nitrogen fixing symbioses. J. Plant Physiol. 2022, 276, 153765. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008; pp. 1–815. [Google Scholar]
- Diagne, N.; Ngom, M.; Djighaly, P.I.; Fall, D.; Hocher, V.; Svistoonoff, S. Roles of Arbuscular Mycorrhizal Fungi on Plant Growth and Performance: Importance in Biotic and Abiotic Stressed Regulation. Diversity 2020, 12, 370. [Google Scholar] [CrossRef]
- Tang, H.; Hassan, M.U.; Feng, L.; Nawaz, M.; Shah, A.N.; Qari, S.H.; Liu, Y.; Miao, J. The critical role of arbuscular mycorrhizal fungi to improve drought tolerance and nitrogen use efficiency in crops. Front. Plant Sci. 2022, 13, 919166. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Dayan, F.E.; Romagni, J.G.; Rimando, A.M. Natural products as sources of herbicide, current status and future trends. Weed Res. 2000, 40, 99–111. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Hamada, Y.; Kojima, M.; Kumagai, S.; Iwasaki, A.; Suenaga, K. Allelopathic substances of Osmanthus spp. for developing sustainable agriculture. Plants 2023, 12, 376. [Google Scholar] [CrossRef]
- Ohashi, H.; Asai, T.; Kawai, S. Screening of main Japanese conifers for antifungal leaf components, sesquiterpenes of Juniperus chinensis var. pyramidalis. Holzforschung 1994, 48, 193–198. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Shiu, L.l. Two new sesquiterpenes, 12-hydroxy-α-longipinene and 15-hydroxyacora-4 (14), 8-diene, from the heartwood of Juniperus chinensis LINN. var. tsukusiensis Masam. Chem. Pharm. Bull. 1996, 44, 1758–1760. [Google Scholar] [CrossRef]
- San Feliciano, A.; Del Corral, J.M.M.; Lopez, J.L.; De Pascual-Teresa, B. Lignans from polar extracts of Juniperus thurifera. Phytochemistry 1992, 31, 267–270. [Google Scholar] [CrossRef]
- Natural Product Activity and Species Source (NPASS). Available online: https://bidd.group/NPASS/compound.php?compoundID=NPC47181 (accessed on 17 February 2024).
- Takeda, H. A 5 year study of litter decomposition processes in a Chamaecyparis obtusa Endl. forest. Ecol. Res. 1995, 10, 95–104. [Google Scholar] [CrossRef]
- Adu-Bredu, S.; Yokota, T.; Ogawa, K.; Hagihara, A. Tree size dependence of litter production, and above-ground net production in a young hinoki (Chamaecyparis obtusa) stand. J. For. Res. 1997, 2, 31–37. [Google Scholar] [CrossRef]
- Inagaki, Y.; Kuramoto, S.; Torii, A.; Shinomiya, Y.; Fukata, H. Effects of thinning on leaf-fall and leaf-litter nitrogen concentration in hinoki cypress (Chamaecyparis obtusa Endlicher) plantation stands in Japan. For. Ecol. Manag. 2008, 255, 1859–1867. [Google Scholar] [CrossRef]
- Takahashi, T.; Toda, H.; Haibara, K. Changes in soil chemical and physical characteristics in Japanese cypress (Chamaecyparis obtusa Endl.) stands by mixture of deciduous broad-leaved trees in the northern Kanto region of Japan. J. For. Res. 1999, 4, 223–228. [Google Scholar] [CrossRef]
- Rees, R.; Chang, S.C.; Wang, C.P.; Matzner, E. Release of nutrients dissolved organic carbon during decomposition of Chamaecyparis obtusa var. formosana leaves in a mountain forest in Taiwan. J. Plant Nutr. Soil Sci. 2006, 169, 792–798. [Google Scholar] [CrossRef]


| Extract (mg of leaf equivalent extract per mL) | ||
| Test plant | Root growth | Coleoptile/hypocotyl growth |
| E. crus-galli | 20.6 | 24.5 |
| P. pratense | 11.5 | 12.1 |
| L. multiflorum | 53.4 | 81.2 |
| Cress | 15 | 10.9 |
| Leaf powder (g powder per pot) | ||
| Test plant | Germination | |
| E. crus-galli | 4.6 | |
| P. pratense | 6.7 | |
| (-)-Hinokiic (mM) | ||
| Test plant | Root growth | Coleoptile/hypocotyl growth |
| P. pratense | 0.6 | 1.6 |
| Cress | 0.4 | 0.4 |
| (+)-Dihydrosesamin (mM) | ||
| Test plant | Root growth | Coleoptile/hypocotyl growth |
| P. pratense | 1.3 | 1.5 |
| Cress | 0.5 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato-Noguchi, H.; Mori, K.; Iwasaki, A.; Suenaga, K. Allelopathy and Allelochemicals in Chamaecyparis obtusa Leaves for the Development of Sustainable Agriculture. Agronomy 2024, 14, 1557. https://doi.org/10.3390/agronomy14071557
Kato-Noguchi H, Mori K, Iwasaki A, Suenaga K. Allelopathy and Allelochemicals in Chamaecyparis obtusa Leaves for the Development of Sustainable Agriculture. Agronomy. 2024; 14(7):1557. https://doi.org/10.3390/agronomy14071557
Chicago/Turabian StyleKato-Noguchi, Hisashi, Kumpei Mori, Arihiro Iwasaki, and Kiyotake Suenaga. 2024. "Allelopathy and Allelochemicals in Chamaecyparis obtusa Leaves for the Development of Sustainable Agriculture" Agronomy 14, no. 7: 1557. https://doi.org/10.3390/agronomy14071557





