Self-Incompatibility and Pollination Efficiency in Coffea canephora Using Fluorescence Microscopy
Abstract
:1. Introduction
2. Material and Methods
2.1. Tester Plants and Breeding Population
2.2. In Vitro Pollination
2.3. Fluorescence Microscopy
2.4. Allelic and Genotypic Frequencies
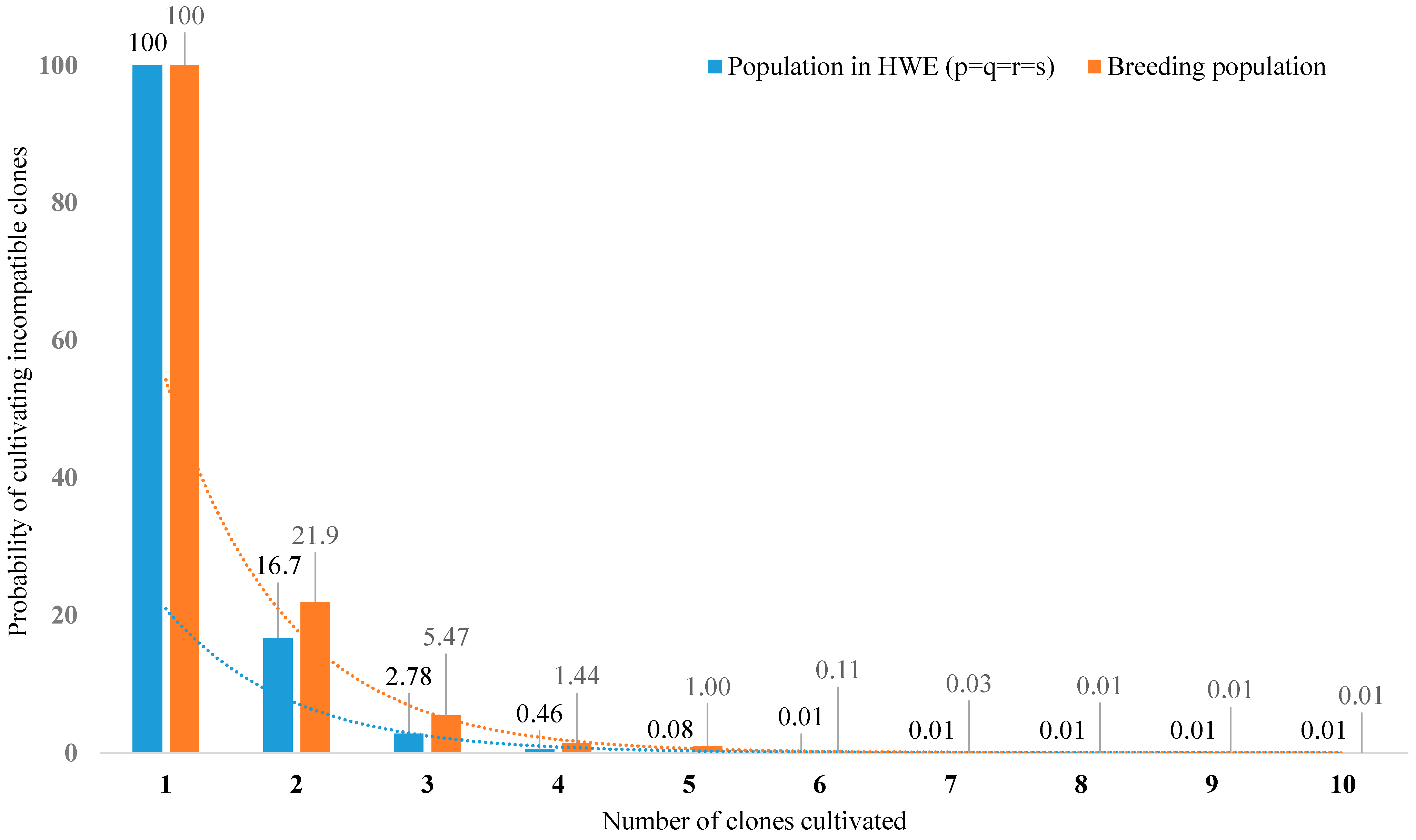
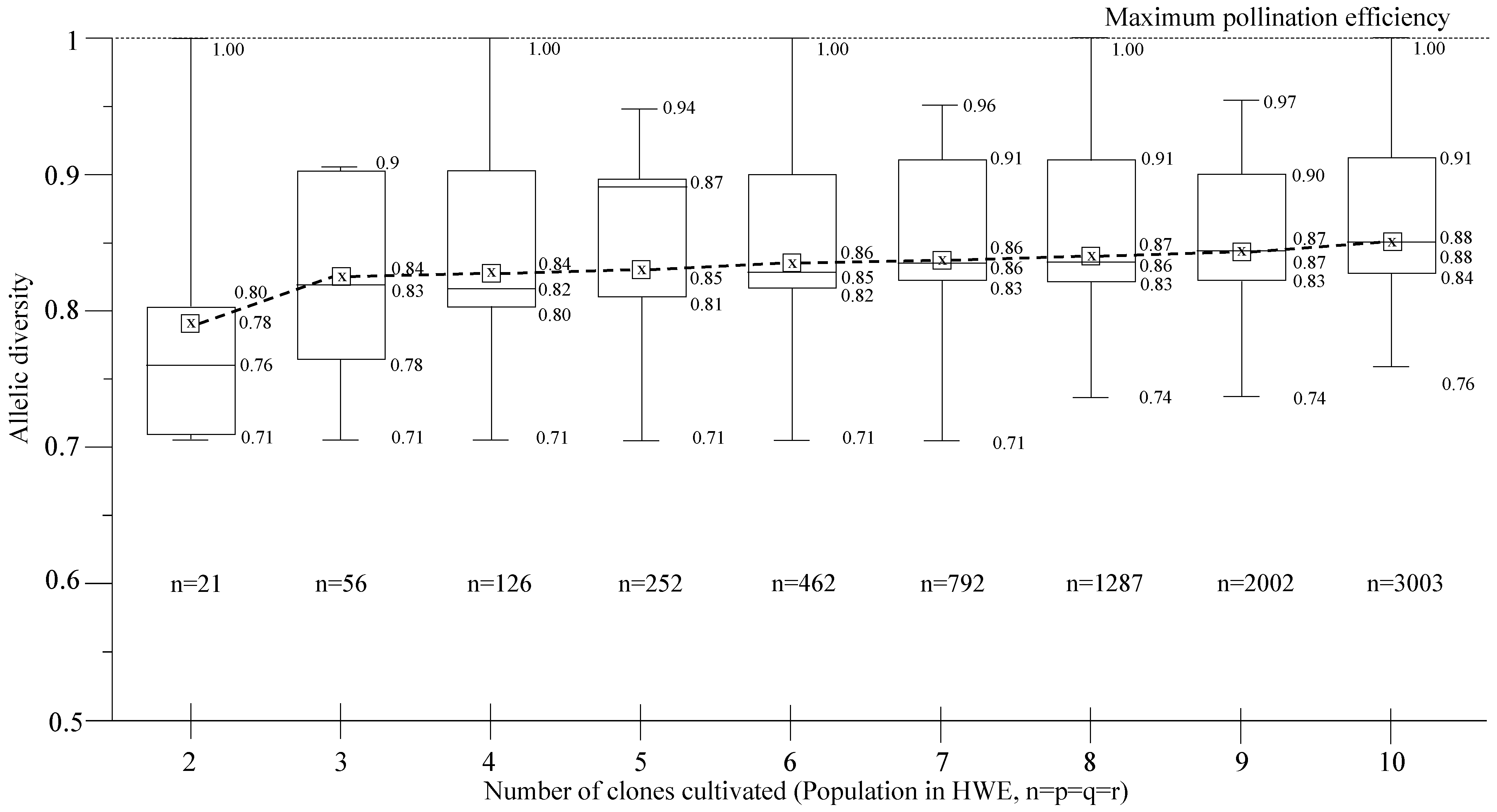
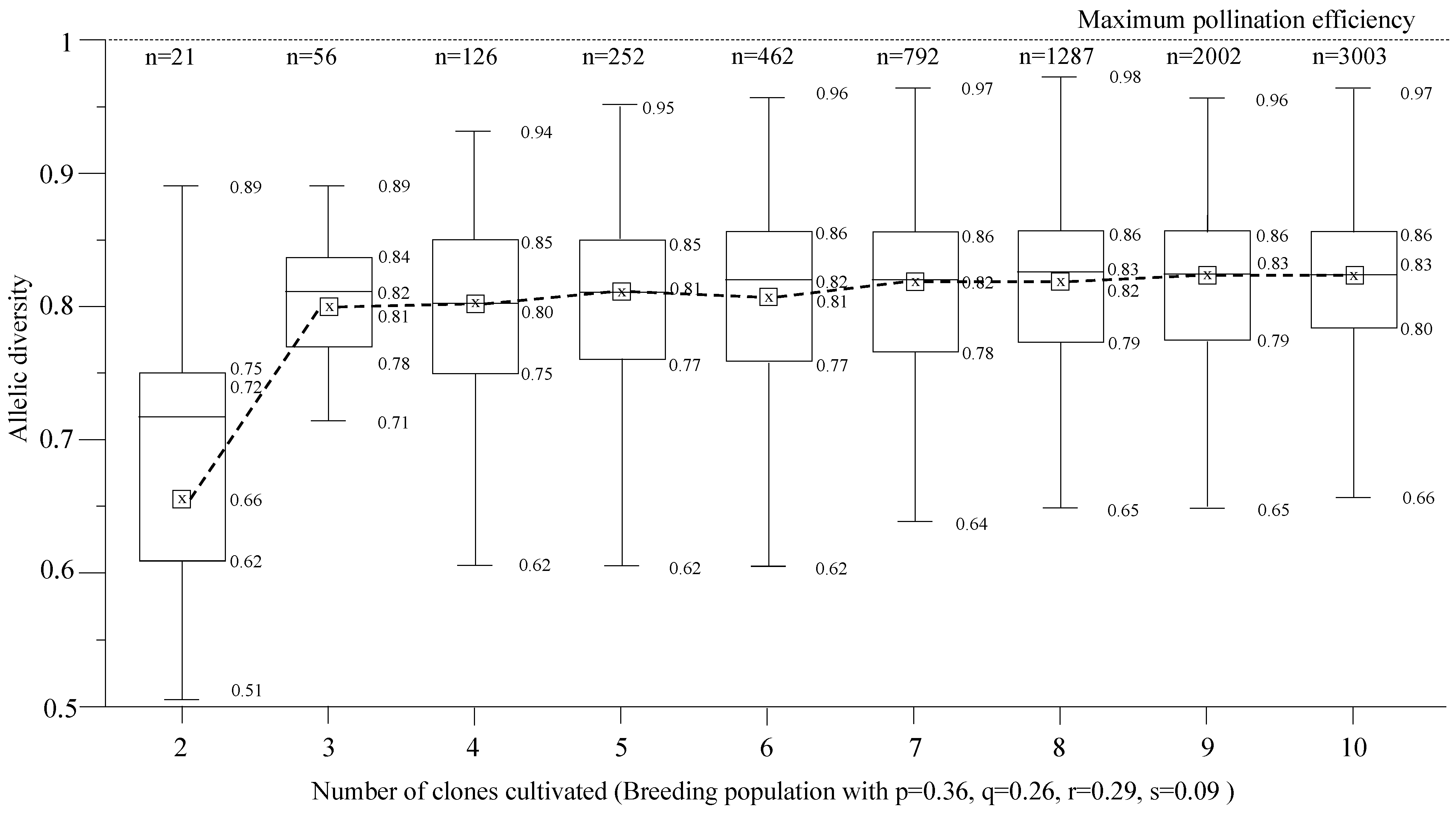
2.5. Pollination Efficiency
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akpertey, A.; Anim-Kwapong, E.; Adu-Gyamfi, P.K.K.; Ofori, A. Genetic variability for vigor and yield of robusta coffee (Coffea canephora) clones in Ghana. Heliyon 2022, 8, e10192. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P.; Rakotonasolo, F. Six new species of coffee (Coffea) from northern Madagascar. Kew Bull. 2021, 76, 497–511. [Google Scholar] [CrossRef]
- Salvador, H.P.; Berilli, A.P.C.G.; Rodrigues, W.P.; Mazzafera, P.; Partelli, F.L. A climate change perspective on the selection, development, and management of Coffea canephora genotypes. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2024; ISSN 0065-2296. [Google Scholar] [CrossRef]
- Charrier, A.; Berthaud, J. Principles and methods in coffee plant breeding: Coffea canephora Pierre. In Coffee: Agronomy; Clarke, R.J., Macrae, R., Eds.; Elsevier: London, UK, 1988; Volume 4, pp. 167–197. [Google Scholar]
- Partelli, F.L.; Silva, F.A.; Covre, A.M.; Oliosi, G.; Correa, C.C.G.; Viana, A.P. Adaptability and stability of Coffea canephora to dynamic environments using the Bayesian approach. Sci. Rep. 2022, 12, 11608. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.B.; Teixeira, A.L.; Ramalho, A.R.; Espindula, M.C.; Lunz, A.M.P.; Souza, F.F. Coffea canephora breeding: Estimated and achieved gains from selection in the Western Amazon, Brazil. Ciência Rural 2021, 51, e20200713. [Google Scholar] [CrossRef]
- Alkimin, E.R.; Caixeta, E.T.; Sousa, T.V.; Resende, M.D.V.; Silva, F.L.; Sakiyama, N.S.; Zambolim, L. Selective efficiency of genome-wide selection in Coffea canephora breeding. Tree Genet. Genomes 2020, 16, 41. [Google Scholar] [CrossRef]
- CONAB: Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira de Café. In Primeiro Levantamento—Safra 2024; CONAB: Companhia Nacional de Abastecimento: Porto Velho, RO, Brazil, 2024; Volume 1, pp. 1–60. [Google Scholar]
- Teixeira, A.L.; Rocha, R.B.; Espindula, M.C.; Ramalho, A.R.; Vieira Júnior, J.R.; Alves, E.A.; Lunz, A.M.P.; de França Souza, F.; Costa, J.N.M.; Fernandes, C.F. Amazonian Robustas—New Coffea canephora coffee cultivars for the Western Brazilian Amazon. Crop Breed. Appl. Biotechnol. 2020, 20, e323420318. [Google Scholar] [CrossRef]
- Montagnon, C.; Leroy, T.; Cilas, C.; Legnaté, H.; Charrier, A. Heterozygous genotypes are efficient testers for assessing between-population combining ability in the reciprocal recurrent selection of Coffea canephora. Euphytica 2008, 160, 101–110. [Google Scholar] [CrossRef]
- Berthaud, J.; Charrier, A. Genetic resources of Coffea. In Coffee—Agronomy; Clarck, R.J., Macrae, R., Eds.; Elsevier Applied Science: London, UK, 1985; pp. 1–40. [Google Scholar]
- Oliveira, L.N.L.; Rocha, R.B.; Ferreira, F.M.; Spinelli, V.M.; Ramalho, A.R.; Teixeira, A.L. Selection of Coffea canephora parents from the botanical varieties conilon and robusta for the production of intervarietal hybrids. Ciência Rural. 2018, 48, e20170444. [Google Scholar] [CrossRef]
- Espindula, M.C.; Dalazen, J.R.; Rocha, R.B.; Teixeira, A.L.; Diocleciano, J.M.; Dias, J.R.M.; Schmidt, R.; Lima, P.P.; Lima, G.M.; Gama, W. Robustas Amazônicos os cafeeiros cultivados em Rondônia, 1st ed.; Embrapa: Brasília, DF, Brazil, 2022; 144p. [Google Scholar]
- Devreux M, Vallaeys G, Pochet P, Gilles A Recherches sur l’autosterilité du caféier robusta (Coffea canephora Pierre). Série Sci. 1959, 78, 1–44.
- Conagin, C.H.T.M.; Mendes, A.J.T. Pesquisas citológicas e genéticas em três espécies de Coffea: Auto-incompatibilidade em Coffea canephora Pierre ex Froehner. Bragantia 1961, 20, 787–804. [Google Scholar] [CrossRef]
- Berthaud, J. L’incompatibilité chez Coffea canephora: Méthode de test et déterminisme génétique. Café Cacao Thé 1980, 24, 267–274. [Google Scholar]
- Lashermes, P.; Couturon, E.; Moreau, N.; Paillard, M.; Louarn, J. Inheritance and genetic mapping of self-incompatibility in Coffea canephora Pierre. Theor. Appl. Genet. 1996, 93, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.D.; Davis, A.P.; Anthony, F.; Yoder, A.D. Expression and trans-specific polymorphism of self-incompatibility RNases in Coffea (Rubiaceae). PLoS ONE 2011, 6, e21019. [Google Scholar] [CrossRef] [PubMed]
- De Franceschi, P.; Dondini, L.; Sanzol, J. Molecular bases and evolutionary dynamics of self-incompatibility in the Pyrinae (Rosaceae). J. Exp. Bot. 2012, 63, 4015–4032. [Google Scholar] [CrossRef] [PubMed]
- Asquini, E.; Gerdol, M.; Gasperini, D.; Igic, B.; Graziosi, G.; Pallavicini, A. S-RNase-like sequences in styles of Coffea (Rubiaceae): Evidence for S-RNase based gametophytic self-incompatibility? Trop. Plant Biol. 2011, 4, 237–249. [Google Scholar] [CrossRef]
- Vieira, J.; Pimenta, J.; Gomes, A.; Laia, J.; Rocha, S.; Heitzler, P.; Vieira, C.P. The identification of the Rosa S-locus and implications on the evolution of the Rosaceae gametophytic self-incompatibility systems. Sci. Rep. 2021, 11, 3710. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.S.; Teixeira, A.L.; Ramalho, A.R.; Espindula, M.C.; Ferrão, M.A.G.; Rocha, R.B. Characterization of gametophytic self-incompatibility of superior clones of Coffea canephora. Genet. Mol. Res. 2018, 17, gmr16039876. [Google Scholar] [CrossRef]
- Souza, C.A.; Rocha, R.B.; Santos, M.R.A.; Lopes, T.A.; Teixeira, A.L.; Espindula, M.C. In vitro pollination and fluorescence microscopy for characterization of gametophytic self-incompatibility of Coffea canephora Pierre ex A. Froehner. Crop Breed. Appl. Biotechnol. 2021, 21, e37692142. [Google Scholar] [CrossRef]
- Depolo, R.P.; Rocha, R.B.; Souza, C.A.; Santos, M.R.A.; Espindula, M.C.; Teixeira, A.L. Expression of self-incompatibility in Coffea canephora genotypes grown in the western Amazon. Pesqui. Agropecuária Bras. 2022, 57, e03031. [Google Scholar] [CrossRef]
- Silva, L.O.E.; Rodrigues, M.J.L.; Ferreira, M.F.S.; Almeida, R.N.; Ramalho, J.C.; Rakocevic, M.; Partelli, F.L. Modifications in floral morphology of Coffea spp. genotypes at two distinct elevations. Flora 2024, 310, 152443. [Google Scholar] [CrossRef]
- Santos, M.M.; Oliveira, M.G.; Cassol, D.; Rodrigues, W.P.; Falqueto, A.R.; Ramalho, J.C.; Partelli, F.L. Genotypic diversity of Coffea canephora cv. Conilon identified through leaf morpho and eco-physiological traits. Sci. Hortic. 2024, 324, 112603. [Google Scholar] [CrossRef]
- Ferrão, M.A.G.; Souza, E.M.R.; Fonseca, A.F.A.; Ferrão, R.G. Self-incompatibility and Sustainable Production of Conilon Coffee. In Conilon Coffee, 3rd ed.; Updated and Expanded; Ferrão, R.G., Fonseca, A.F.A., Ferrão, M.A.G., Muner, L.H., Eds.; Vitória, ES, Brazil, 2019; pp. 203–221. Available online: https://biblioteca.incaper.es.gov.br/ (accessed on 8 January 2024).
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longmans Green: Harlow, Essex, UK, 1996; 464p. [Google Scholar]

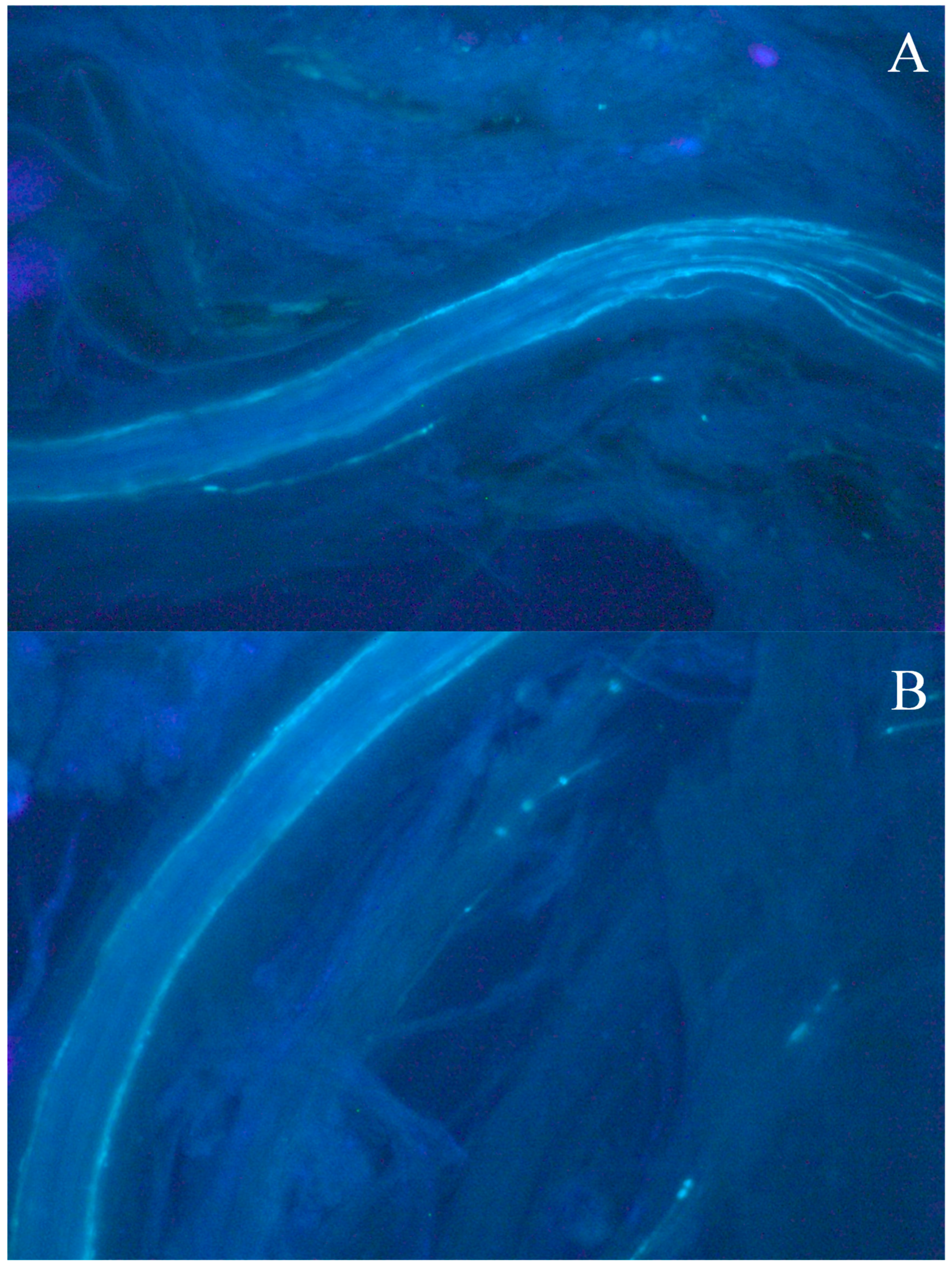

| n | Genotype | Origin | n | Genotype | Origin |
|---|---|---|---|---|---|
| 1 | AR106 | Aldinei Raasch 7 | 24 | LB10 | Laerte Braun 5 |
| 2 | AS1 | Ademar Schmidt 2 | 25 | LB12 | Laerte Braun 5 |
| 3 | AS10 | Ademar Schmidt 2 | 26 | LB15 | Laerte Braun 5 |
| 4 | AS12 | Ademar Schmidt 2 | 27 | LB160 | Laerte Braun 5 |
| 5 | AS2 | Ademar Schmidt 2 | 28 | LB20 | Laerte Braun 5 |
| 6 | AS5 | Ademar Schmidt 2 | 29 | LB33 | Laerte Braun 5 |
| 7 | AS7 | Ademar Schmidt 2 | 30 | LB68 | Laerte Braun 5 |
| 8 | BAG24 | Embrapa 1 | 31 | LB80 | Laerte Braun 5 |
| 9 | BAG27 | Embrapa 1 | 32 | LB88 | Laerte Braun 5 |
| 10 | BAG28 | Embrapa 1 | 33 | N13 | Nivaldo Ferreira 6 |
| 11 | BG180 | Adilson Berger 3 | 34 | N16 | Nivaldo Ferreira 6 |
| 12 | CA1 | Carlos Alves Silva 4 | 35 | N32 | Nivaldo Ferreira 6 |
| 13 | GB1 | Gilberto Boon 2 | 36 | N7 | Nivaldo Ferreira 6 |
| 14 | GB4 | Gilberto Boon 2 | 37 | N8[G8] | Nivaldo Ferreira 6 |
| 15 | GB7 | Gilberto Boon 2 | 38 | P50 | Valdecir Piske 2 |
| 16 | GJ2 | Geraldo Jacomini 5 | 39 | R152 | Ronaldo G Oliveira 2 |
| 17 | GJ20 | Geraldo Jacomini 5 | 40 | R22 | Ronaldo Vitoriano 2 |
| 18 | GJ25 | Geraldo Jacomini 5 | 41 | SK244 | Sergio Kalk 6 |
| 19 | GJ3 | Geraldo Jacomini 5 | 42 | SK41 | Sergio Kalk 6 |
| 20 | GJ30 | Geraldo Jacomini 5 | 43 | SK80 | Sergio Kalk 6 |
| 21 | GJ5 | Geraldo Jacomini 5 | 44 | VP156 | Valdecir Piske 2 |
| 22 | GJ8 | Geraldo Jacomini 5 | 45 | WP6 | Wanderley Peter 6 |
| 23 | L1 | Alcides Rosa 3 |
| n | Genotype | C | NC | FN | Group | p(FN) | n | Genotype | C | NC | FN | Group | p(FN) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AR106 | 5 | 1 | 2 | III | 0.05 | 24 | LB10 | 5 | 2 | 4 | III | <0.01 |
| 2 | AS1 | 5 | 2 | 1 | VI | <0.01 | 25 | LB12 | 5 | 1 | 3 | II | 0.05 |
| 3 | AS10 | 5 | 1 | 4 | VI | 0.05 | 26 | LB15 | 5 | 1 | 0 | III | 0.05 |
| 4 | AS12 | 5 | 1 | 0 | VI | 0.05 | 27 | LB160 | 5 | 1 | 4 | I | 0.05 |
| 5 | AS2 | 5 | 1 | 0 | III | 0.05 | 28 | LB20 | 5 | 1 | 2 | II | 0.05 |
| 6 | AS5 | 5 | 1 | 1 | II | 0.05 | 29 | LB33 | 5 | 1 | 1 | III | 0.05 |
| 7 | AS7 | 5 | 1 | 1 | II | 0.05 | 30 | LB68 | 5 | 1 | 2 | II | 0.05 |
| 8 | BAG24 | 5 | 1 | 2 | II | 0.05 | 31 | LB80 | 5 | 2 | 0 | II | <0.01 |
| 9 | BAG27 | 5 | 1 | 1 | I | 0.05 | 32 | LB88 | 5 | 1 | 0 | III | 0.05 |
| 10 | BAG28 | 5 | 1 | 0 | III | 0.05 | 33 | N13 | 5 | 1 | 1 | III | 0.05 |
| 11 | BG180 | 5 | 1 | 0 | VI | 0.05 | 34 | N16 | 5 | 1 | 0 | VI | 0.05 |
| 12 | CA1 | 5 | 3 | 1 | VI | <0.01 | 35 | N32 | 5 | 3 | 0 | I | <0.01 |
| 13 | GB1 | 5 | 1 | 1 | I | 0.05 | 36 | N7 | 5 | 2 | 1 | I | <0.01 |
| 14 | GB4 | 5 | 1 | 2 | II | 0.05 | 37 | N8[G8] | 5 | 1 | 0 | II | 0.05 |
| 15 | GB7 | 5 | 1 | 3 | I | 0.05 | 38 | P50 | 5 | 1 | 1 | II | 0.05 |
| 16 | GJ2 | 5 | 2 | 1 | I | <0.01 | 39 | R152 | 5 | 1 | 1 | III | 0.05 |
| 17 | GJ20 | 5 | 1 | 0 | III | 0.05 | 40 | R22 | 5 | 2 | 0 | IV | <0.01 |
| 18 | GJ25 | 5 | 1 | 0 | II | 0.05 | 41 | SK244 | 5 | 1 | 0 | I | 0.05 |
| 19 | GJ3 | 5 | 1 | 1 | V | 0.05 | 42 | SK41 | 5 | 1 | 2 | II | 0.05 |
| 20 | GJ30 | 5 | 2 | 0 | I | <0.01 | 43 | SK80 | 5 | 1 | 3 | II | 0.05 |
| 21 | GJ5 | 5 | 1 | 1 | II | 0.05 | 44 | VP156 | 5 | 1 | 1 | VI | 0.05 |
| 22 | GJ8 | 5 | 1 | 2 | I | 0.05 | 45 | WP6 | 5 | 2 | 2 | I | <0.01 |
| 23 | L1 | 5 | 1 | 1 | III | 0.05 |
| n | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| S1S2 | S1S3 | S2S3 | S(2,3)S4 | S(2,3)S4 | S1S4 | |
| 1 | BAG27 | BAG24 | AR106 | R22 | GJ3 | AS1 |
| 2 | GB1 | AS5 | AS2 | AS10 | ||
| 3 | GB7 | AS7 | BAG28 | AS12 | ||
| 4 | GJ2 | GB4 | GJ20 | BG180 | ||
| 5 | GJ8 | GJ5 | L1 | CA1 | ||
| 6 | GJ30 | GJ25 | LB10 | N16 | ||
| 7 | LB160 | LB12 | LB15 | VP156 | ||
| 8 | N7 | LB20 | LB33 | |||
| 9 | N32 | LB68 | LB88 | |||
| 10 | SK244 | LB80 | N13 | |||
| 11 | WP6 | N8[G8] | R152 | |||
| 12 | P50 | |||||
| 13 | SK41 | |||||
| 14 | SK80 | |||||
| Total | 11 | 14 | 11 | 1 | 1 | 7 |
| % | 24.44 | 31.11 | 24.44 | 2.22 | 2.22 | 15.57 |
| Alleles | Allelic Frequency | Groups | Genotypes | Genotypic Frequency | Expected Value |
|---|---|---|---|---|---|
| S1 | 0.36 | I | P(S1S2) | 0.25 | 11.44 |
| S2 | 0.26 | II | P(S1S3) | 0.29 | 12.93 |
| S3 | 0.29 | III | P(S2S3) | 0.21 | 9.30 |
| S4 | 0.10 | IV | P(S2S4) | 0.07 | 3.22 |
| V | P(S3S4) | 0.08 | 3.64 | ||
| VI | P(S1S4) | 0.10 | 4.48 | ||
| Groups | O | E | O-E | (O-E)2 | (O-E)2/E |
| I | 11 | 11.44 | −0.44 | 0.19 | 0.02 |
| II | 14 | 12.93 | 1.07 | 1.14 | 0.09 |
| III | 11 | 9.30 | 1.70 | 2.91 | 0.31 |
| IV | 1 | 3.22 | −2.22 | 4.92 | 1.53 |
| V | 1 | 3.64 | −2.64 | 6.96 | 1.91 |
| VI | 7 | 4.48 | 2.52 | 6.37 | 1.42 |
| 5.28 NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.N.R.; Rocha, R.B.; Teixeira, A.L.; Espindula, M.C.; Partelli, F.L.; Caixeta, E.T. Self-Incompatibility and Pollination Efficiency in Coffea canephora Using Fluorescence Microscopy. Agronomy 2024, 14, 1564. https://doi.org/10.3390/agronomy14071564
Silva ANR, Rocha RB, Teixeira AL, Espindula MC, Partelli FL, Caixeta ET. Self-Incompatibility and Pollination Efficiency in Coffea canephora Using Fluorescence Microscopy. Agronomy. 2024; 14(7):1564. https://doi.org/10.3390/agronomy14071564
Chicago/Turabian StyleSilva, Adriele Nunes Rodrigues, Rodrigo Barros Rocha, Alexsandro Lara Teixeira, Marcelo Curitiba Espindula, Fábio Luiz Partelli, and Eveline Teixeira Caixeta. 2024. "Self-Incompatibility and Pollination Efficiency in Coffea canephora Using Fluorescence Microscopy" Agronomy 14, no. 7: 1564. https://doi.org/10.3390/agronomy14071564







