Preparation and Evaluation of a Temperature-Sensitive Cuelure Nano-Controlled Release Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Source
2.2. Main Equipment
Reagents and Sources
2.3. Experimental Methods
2.3.1. Temperature Setting
2.3.2. Setting of Trapping Time
2.3.3. Evaluation of the Trapping Effect of Cuelure on Z. cucurbitae
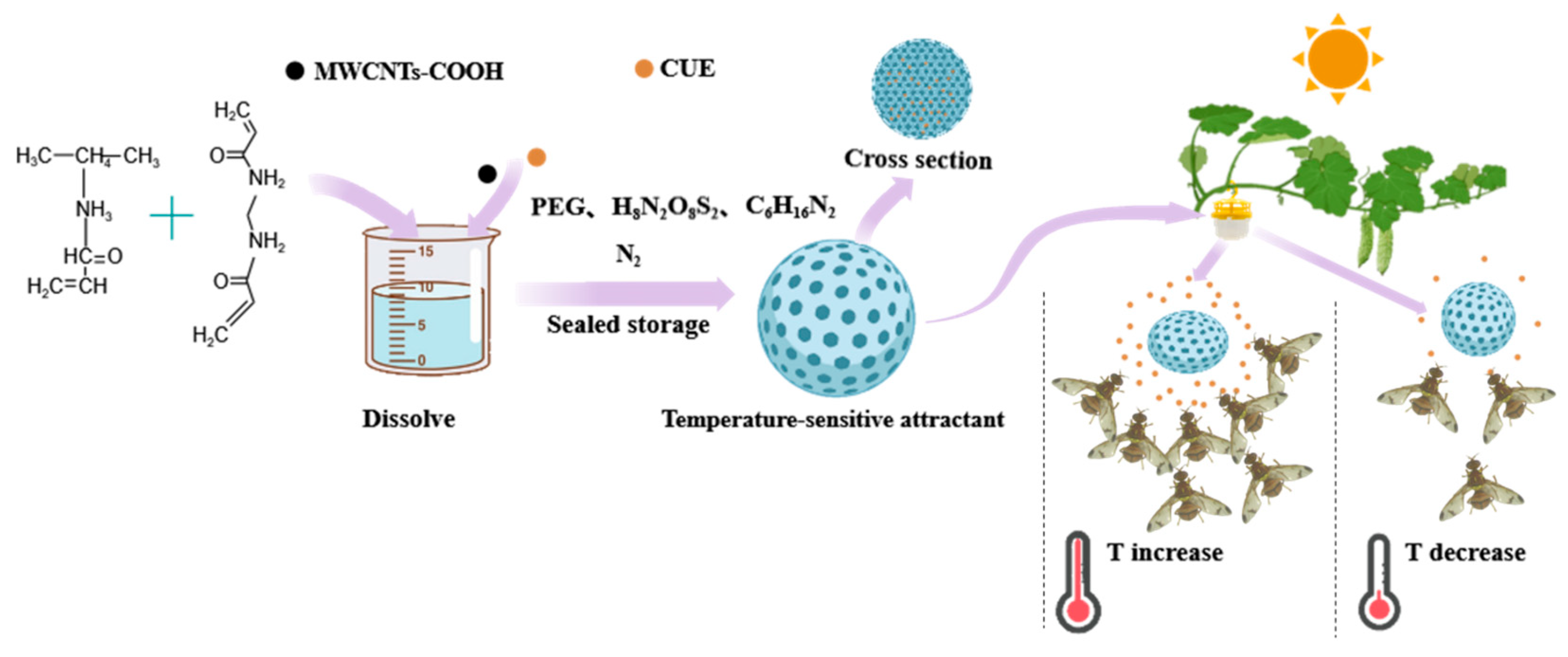
2.4. Preparation and Evaluation of Temperature-Sensitive Attractant
2.4.1. Preparation of Temperature-Sensitive Attractant
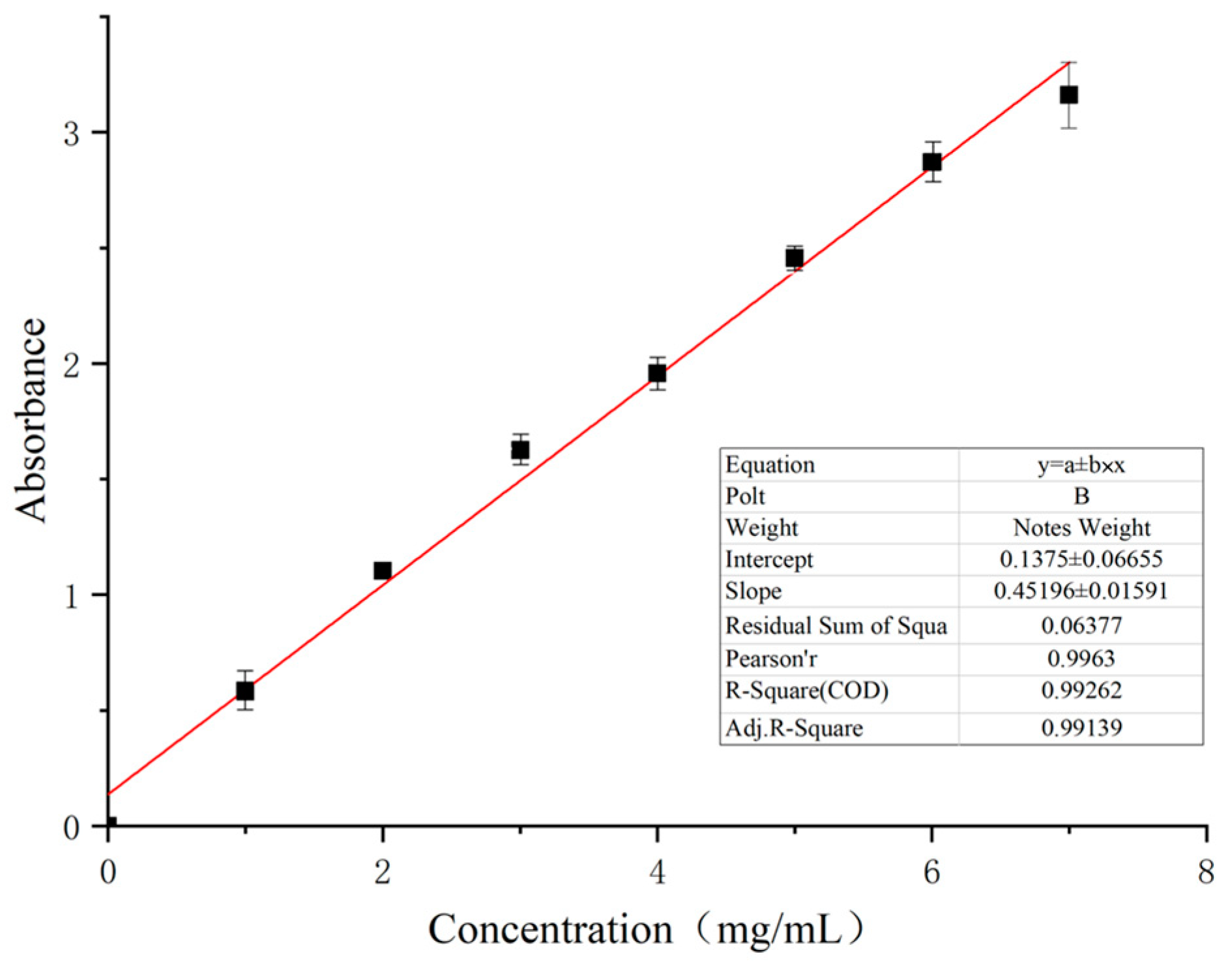
2.4.2. Plotting of Standard Curve of Cuelure Solution
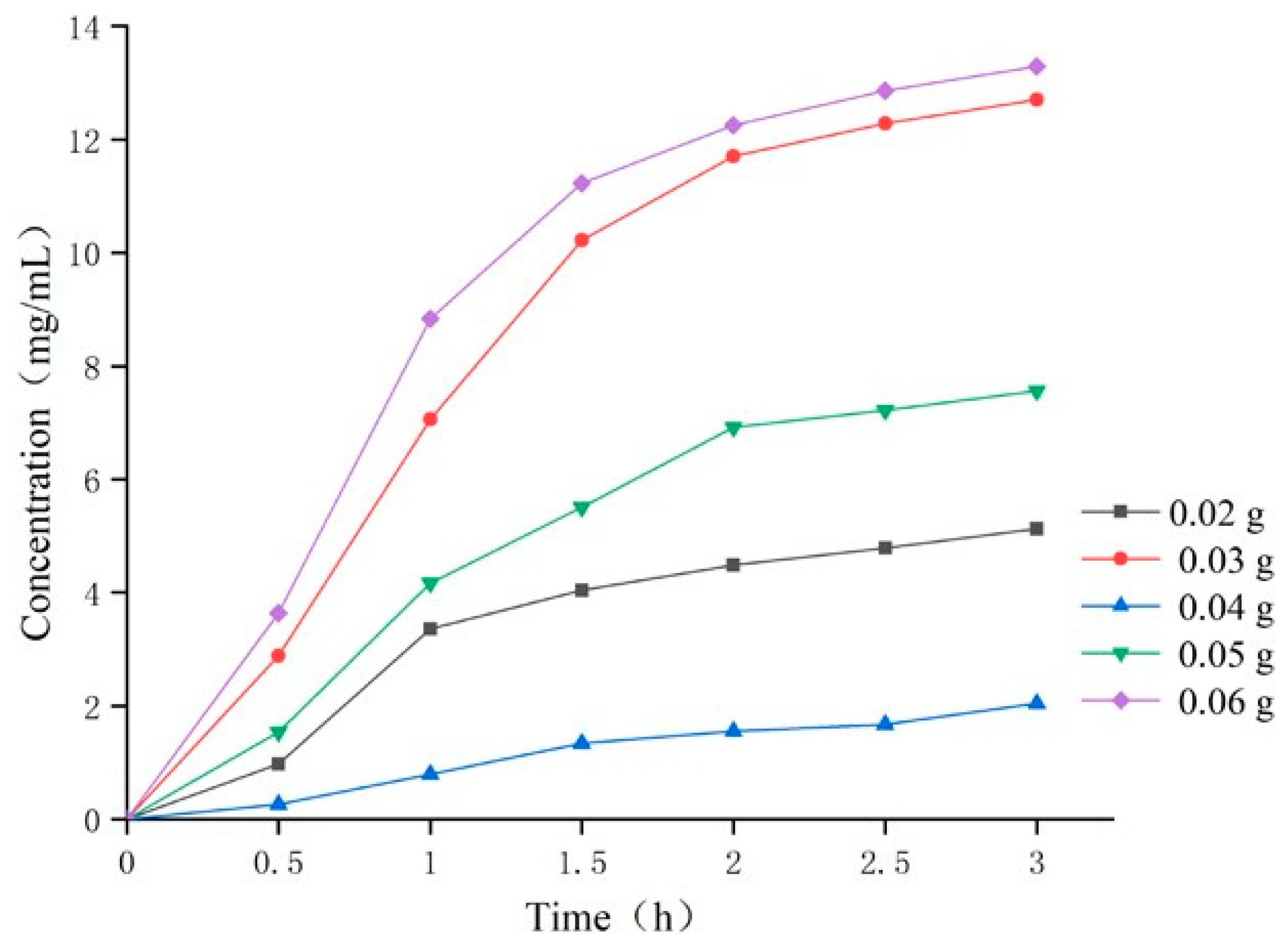
2.4.3. The Influence of Crosslinker Content on Temperature-Sensitive Attractants
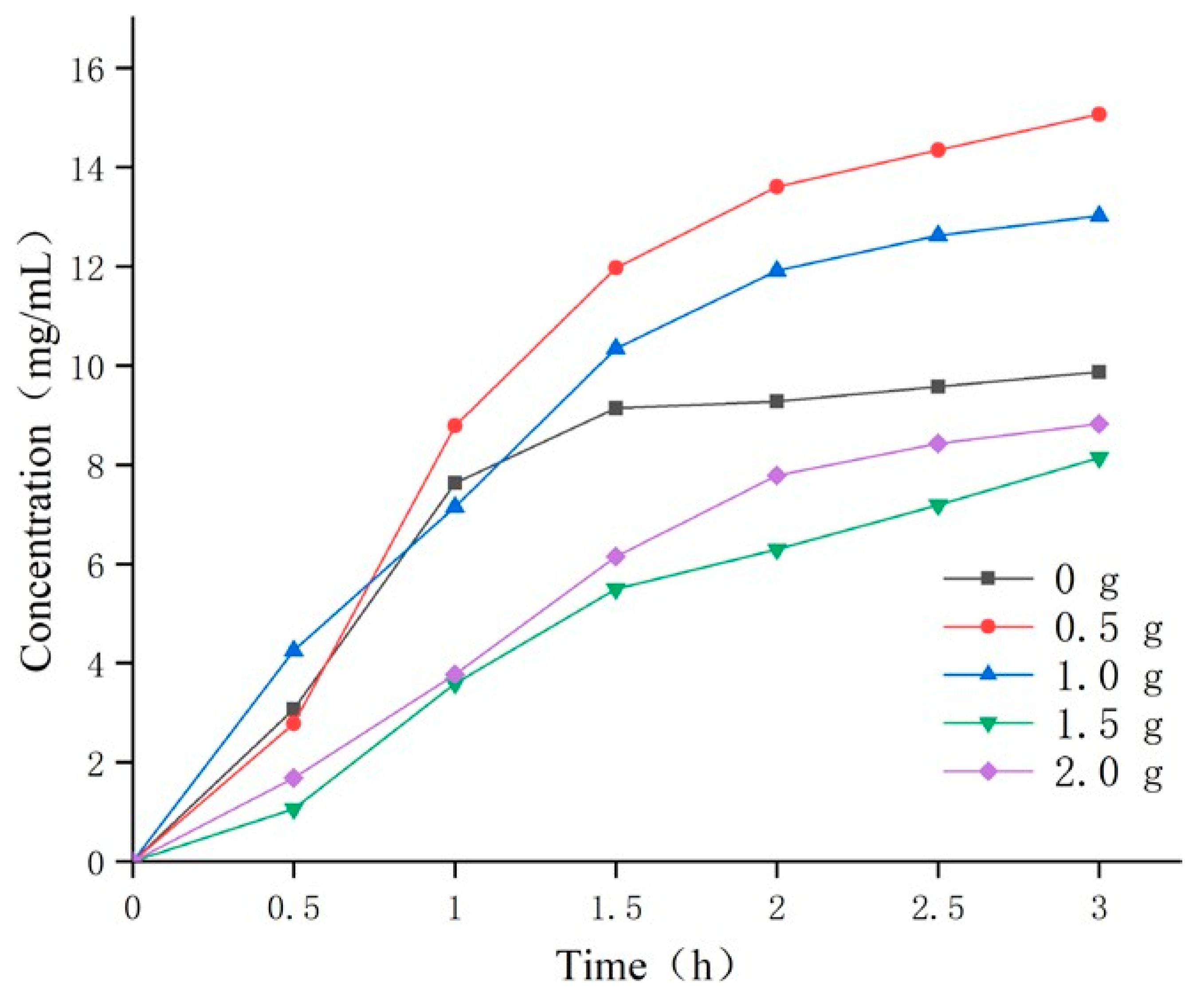
2.4.4. Effect of Porogen Content on Temperature Sensitivity of Temperature Sensitive Attractants
2.5. Scanning Electron Microscope
2.6. Performance Test of Temperature-Sensitive Attractant
2.6.1. Temperature Response Concentration Release Experiment
2.6.2. Determination of Entrapment Efficiency and Drug Loading
2.7. The Effect of Temperature-Sensitive Attractant on Trapping Z. cucurbitae Indoors
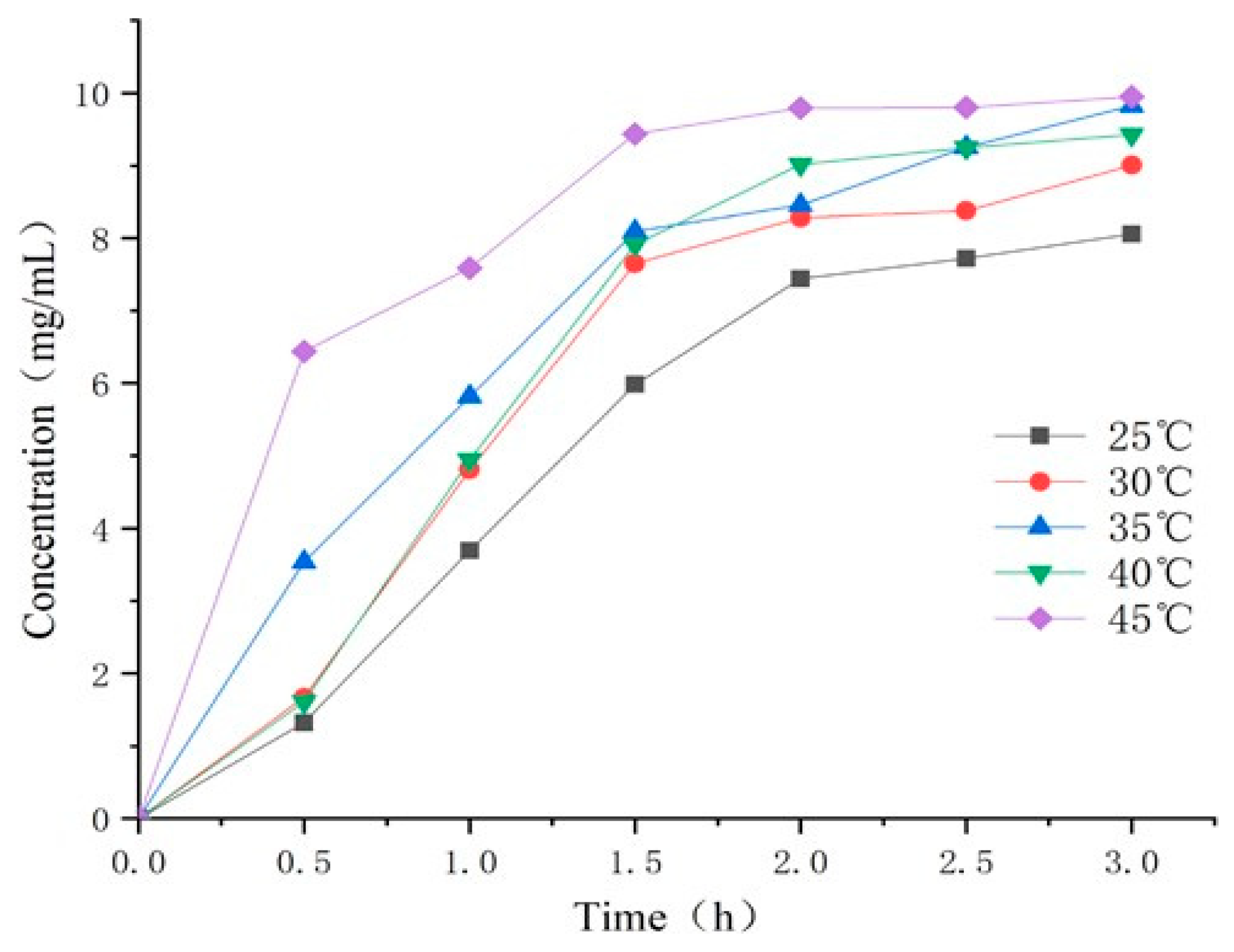
2.7.1. Evaluation of the Trapping Effect of Temperature-Sensitive Attractants on Z. cucurbitae under Different Temperature
2.7.2. Effect of the Dosage of Temperature-Sensitive Attractant on Trapping Z. cucurbitae in Different Temperature
2.7.3. High-Temperature Resistance Limit Test of Attractant
3. Results
3.1. Evaluation of the Trapping Effect of Cuelure on Z. cucurbitae
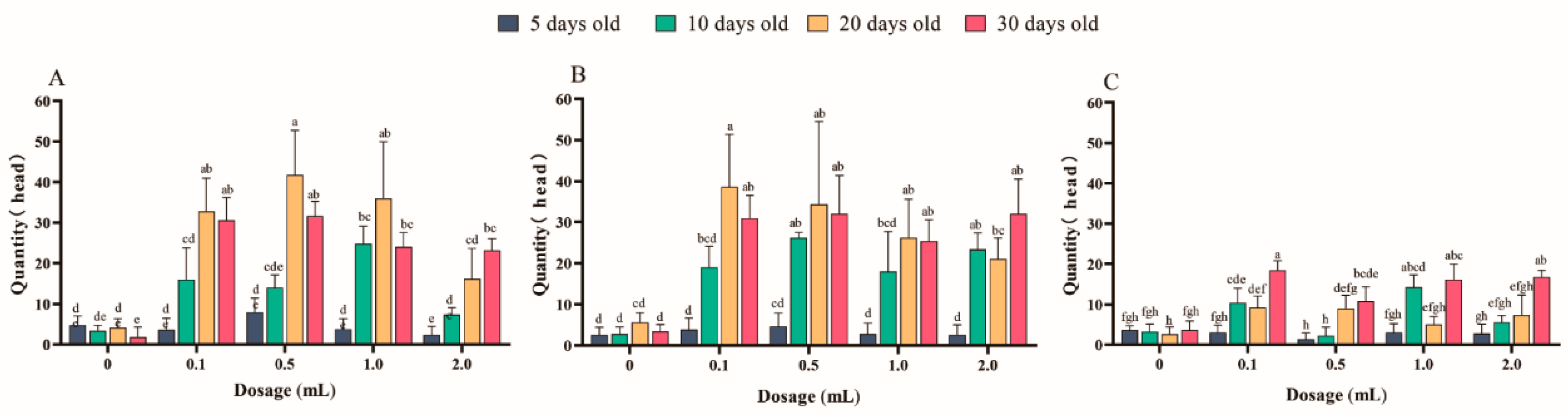
3.1.1. Study on the Optimum Day Age of Z. cucurbitae Trapped by Cuelure under Different Temperature
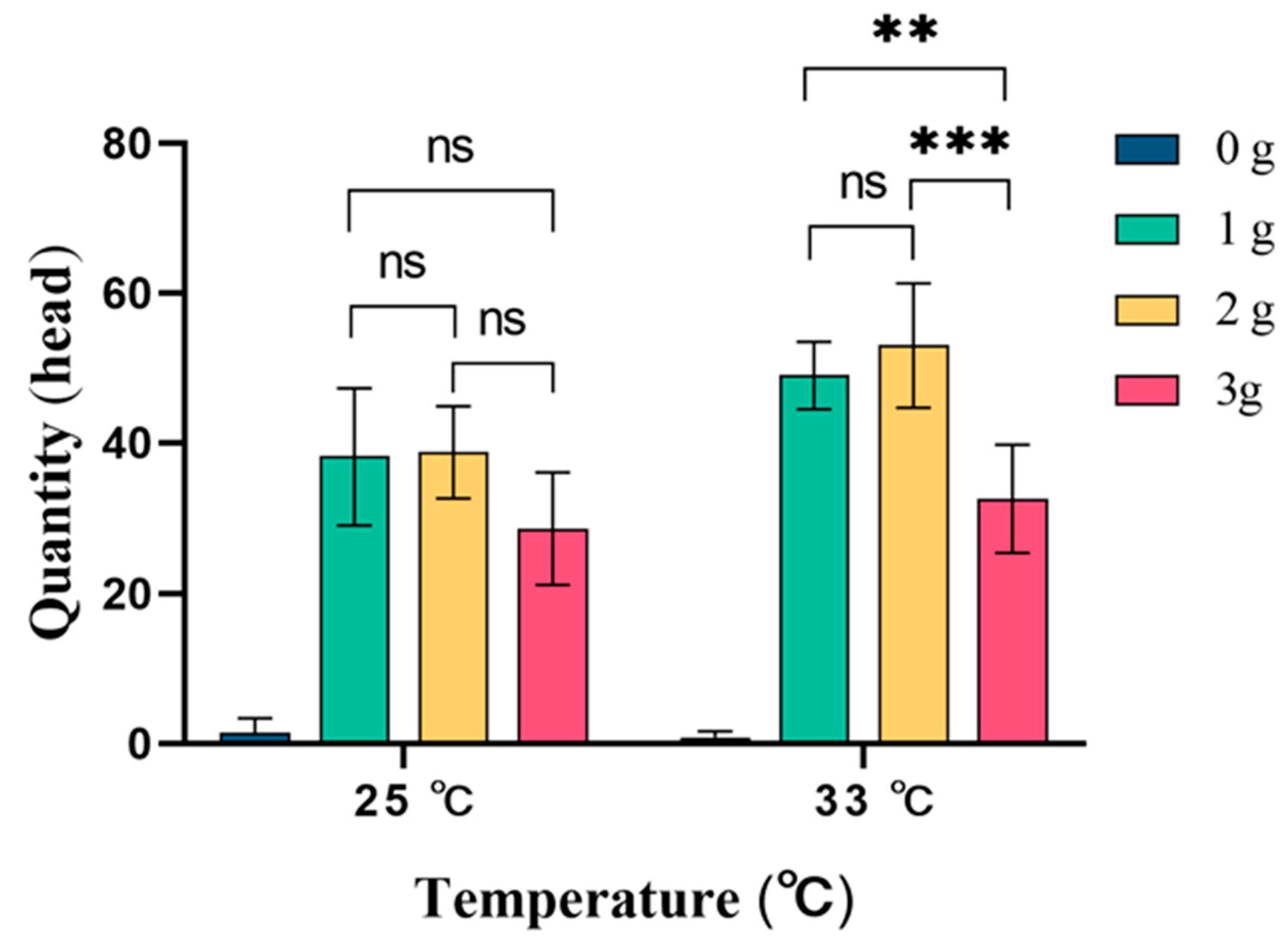
3.1.2. The Influence of the Dosage of Cuelure on the Trapping of Z. cucurbitae in Different Temperature
3.2. Preparation and Evaluation of Temperature-Sensitive Attractant
3.2.1. Drawing of Standard Curve of Cuelure
3.2.2. Effect of Crosslinking Agent Content on Temperature Sensitivity of Temperature-Sensitive Attractant
3.2.3. Effect of Pore-Forming Agent Content on Hydrogel Temperature Sensitivity
3.2.4. Morphology Characterization of Temperature-Sensitive Attractants
3.3. Performance Test of Temperature-Sensitive Attractant
3.3.1. Temperature Response Release Experiment of Temperature-Sensitive Trap
3.3.2. Determination of Entrapment Efficiency and Drug Loading
3.4. Indoor Effect Determination
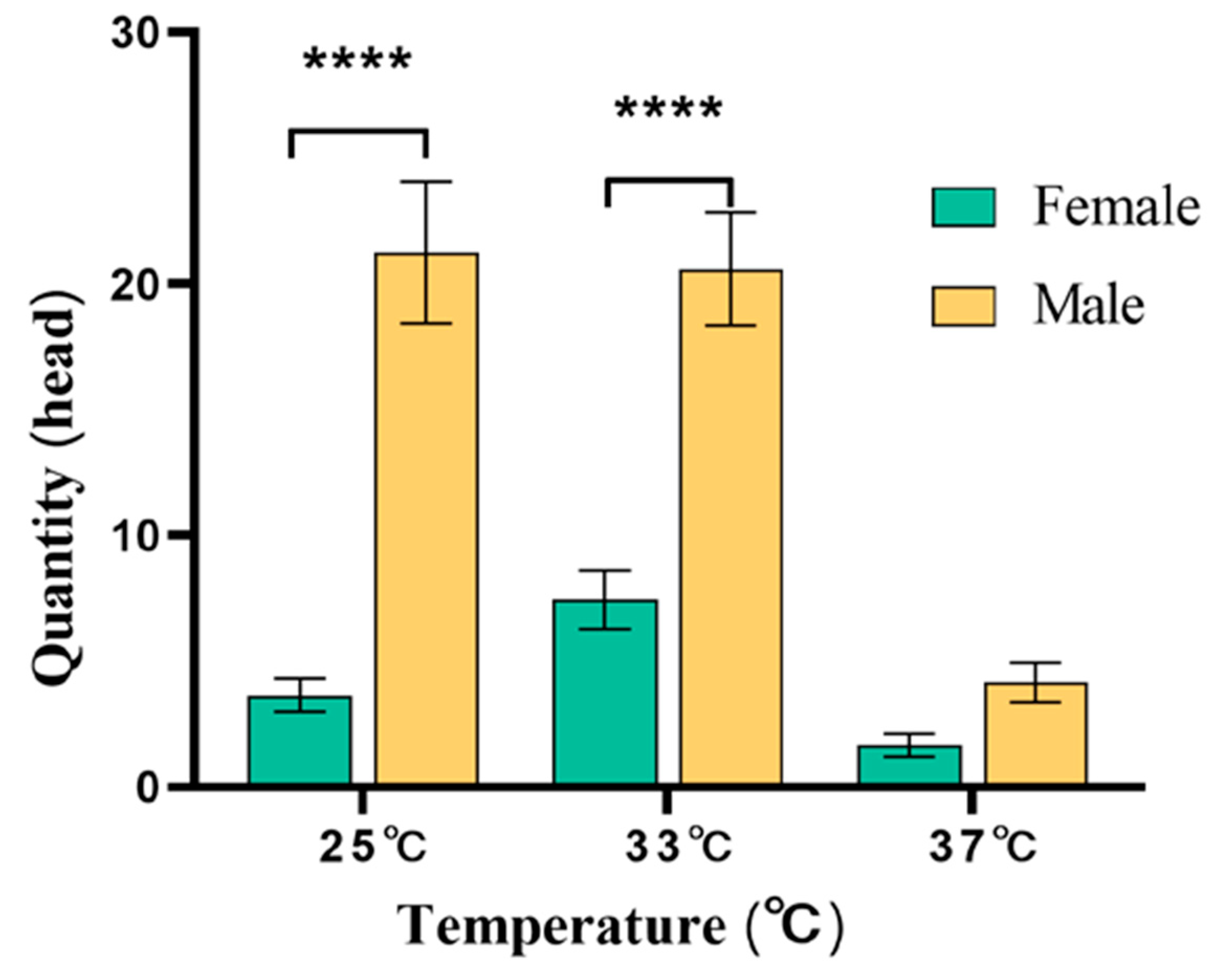
3.4.1. Evaluation of the Effect of Temperature-Sensitive Attractant on Trapping Z. cucurbitae
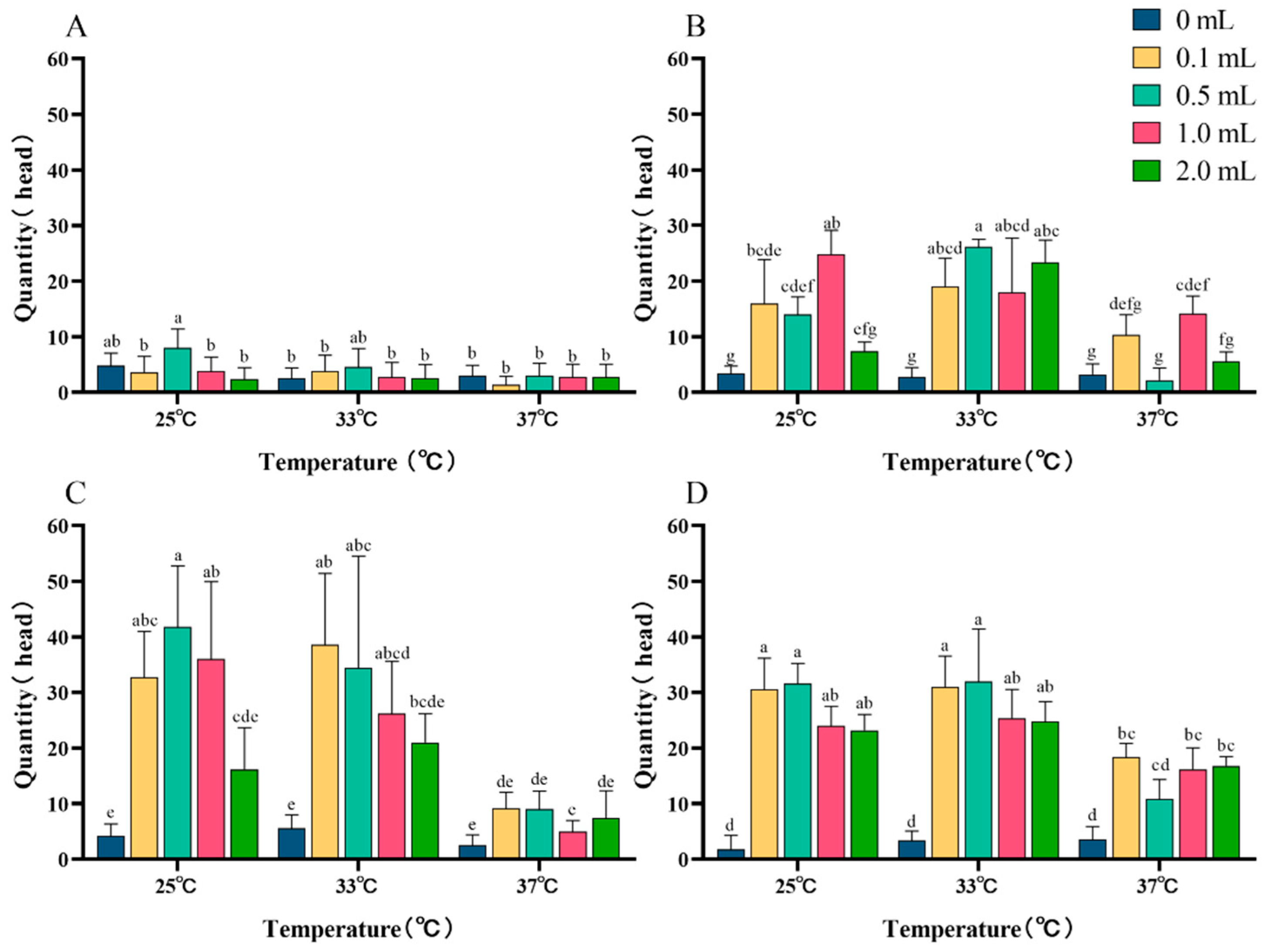
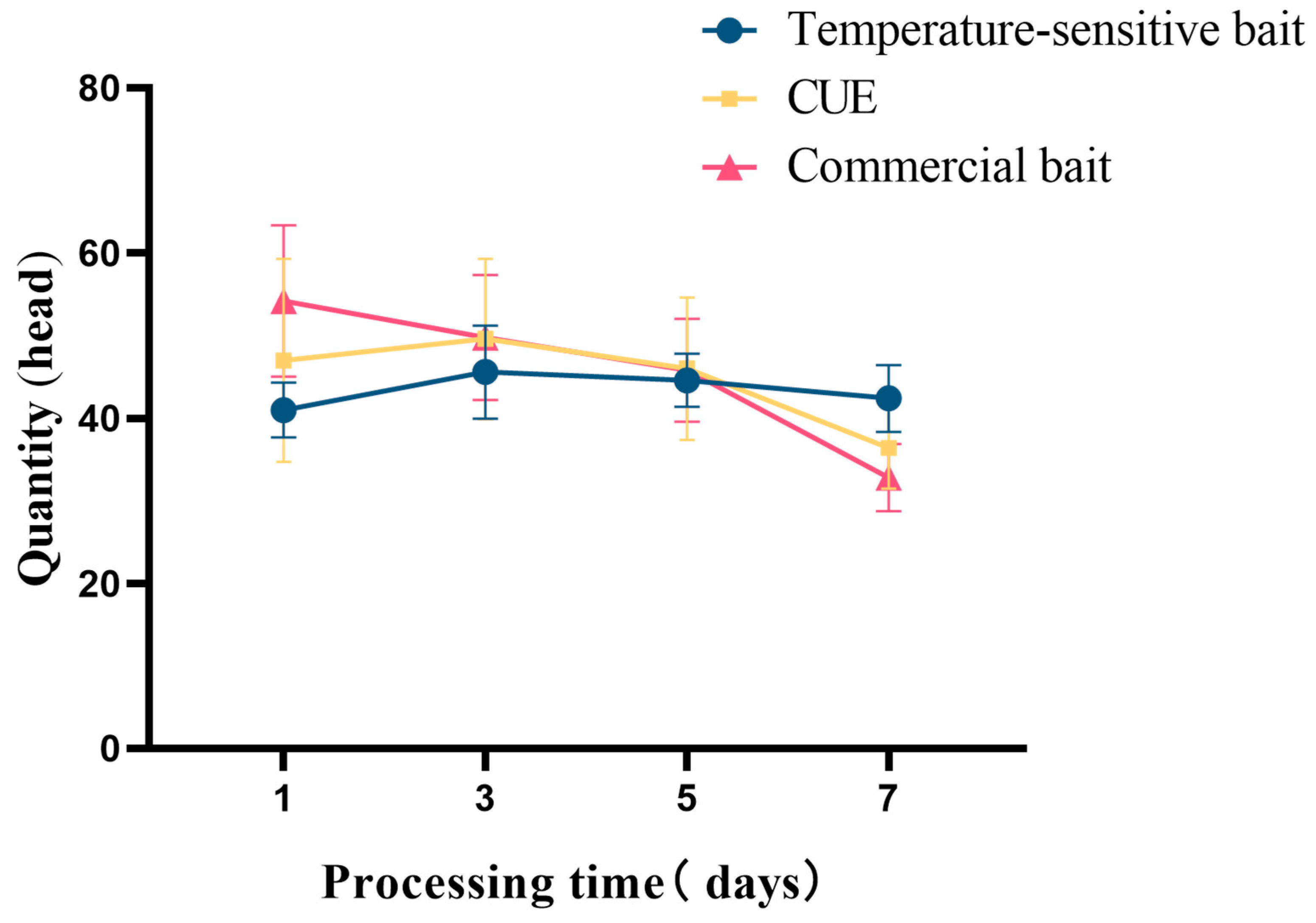
3.4.2. Effect of the Dosage of Temperature-Sensitive Attractant on Trapping Z. cucurbitae
3.4.3. High-Temperature Resistance Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Ma, C.S. Effect of global warming on insect: A literature review. Sheng Tai Xue Bao 2010, 30, 2159–2172. [Google Scholar]
- Ma, C.; Ma, G. The Impacts of Extreme High Temperature on Insect Populations under Climate Change: A Review. Sci. Sin. Vitae 2016, 46, 556–564. [Google Scholar] [CrossRef]
- Zhou, S.H. Studies on the Responses of Bactrocera cucurbitae to High Temperature Stress and Its Moelcular Basis. Ph.D. Thesis, Hainan University, Hainan, China, 2016. [Google Scholar]
- Maula, F.; Khan, A.A.; Ali, A.; Younus, M.; Israr, M.; Rauf, M.A.; Khan, I.A. Inamullah Evaluation of different traps and lures combinations for monitoring and eco-friendly management of fruit fly (Bactrocera spp.) in peach orchards. J. Entomol. Zool. Stud. 2022, 10, 105–110. [Google Scholar] [CrossRef]
- Witzgall, P.; Philipp, K.; Alan, C. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Shelly, T.; Epsky, N.; Jang, E.B.; Reyes-Flores, J.; Vargas, R. Trapping and the Detection, Control, and Regulation of Tephritid Fruit Flies: Lures, Area-Wide Programs, and Trade Implications; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Kong, W.; Wang, Y.; Guo, Y.; Chai, X.; Li, J.; Ma, R. Behavioral effects of different attractants on adult male and female oriental fruit moths, Grapholita molesta. Pest Manag. Sci. 2020, 76, 3225–3235. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Hossain, J.; Mainali, B.; Inskeep, J.R.; Gaire, S.K.; Cross, D.; Stringer, L.D.; Taylor, P.W.; Rempoulakis, P. Extended holding period and yeast hydrolysate in pre-release diet increase abundance of mature sterile Queensland fruit fly males in the field. J. Pest Sci. 2022, 95, 291–301. [Google Scholar] [CrossRef]
- Beroza, M.; Alexander, B.H.; Steiner, L.F.; Mitchell, W.C.; Miyashita, D.H. New Synthetic Lures for the Male Melon Fly. Science 1960, 131, 1044–1045. [Google Scholar] [CrossRef] [PubMed]
- Guptaa, A.; Regmib, R. Efficacy of different homemade and commercial baits in monitoring of fruit flies at Maranthana, Pyuthan, Nepal. Malays. J. Sustain. Agric. 2022, 6, 101–109. [Google Scholar] [CrossRef]
- Kumaran, N.; Hayes, R.A.; Clarke, A.R. Cuelure but not zingerone make the sex pheromone of male Bactrocera tryoni (Tephritidae: Diptera) more attractive to females. J. Insect Physiol. 2014, 68, 36–43. [Google Scholar] [CrossRef]
- Arya, V.; Srinivasa, N.; Tyagi, S.; Raju, S.V.S. A guide to prepare Cue-Lure for Bactrocera cucurbitae (Coquillett) man-agement in cucurbits. Indian Entomol. 2022, 3, 45–47. [Google Scholar]
- Yazdani, M. Developing Lines of Queensland Fruit Flies with Different Levels of Response to a Kairomone Lure. Insects 2022, 13, 666. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wei, X.M.; Li, j.; Wei, S.M.; Zhang, J.L.; Chen, G.H.; Zhang, X.M. Effect of short-term exposure to high temperatures on the reproductive behavior and physiological enzyme activities in the fruit fly Zeugodacus tau (Walker). Front. Physiol. 2023, 14, 1036397. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-T.; Zeng, K.; Fuhrmann, B.; Woelk, C.; Zhang, K.; Groth, T. Engineering of Stable Cross-Linked Multilayers Based on Thermo-Responsive PNIPAM-Grafted-Chitosan/Heparin to Tailor Their Physiochemical Properties and Biocompatibility. ACS Appl. Mater. Interfaces 2022, 14, 29550–29562. [Google Scholar] [CrossRef] [PubMed]
- Majstorović, N.; Agarwal, S. Strong, Stretchable, Dual-Responsive PNIPAM Nanogel Cross-Linked UCST-type Macrogels for Biomedical Applications. ACS Appl. Polym. Mater. 2022, 4, 5996–6005. [Google Scholar] [CrossRef]
- Xiao, Q.; Cui, Y.; Meng, Y.; Guo, F.; Ruan, X.; He, G.; Jiang, X. PNIPAm hydrogel composite membrane for high-throughput adsorption of biological macromolecules. Sep. Purif. Technol. 2022, 294, 121224. [Google Scholar] [CrossRef]
- Wu, J.Y.; Liu, S.Q.; Heng, P.W.S.; Yang, Y.Y. Evaluating proteins release from, and their interactions with, thermosen-sitive poly (N-isopropylacrylamide) hydrogels. J. Control. Release 2005, 102, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Baydin, A.; Tay, F.; Fan, J.; Manjappa, M.; Gao, W.; Kono, J. Carbon Nanotube Devices for Quantum Technology. Materials 2022, 15, 1535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wen, Q.N.; Wu, G.Y.; Shi, Y.; Xu, P. Reliability of ultrafiltration centrifugation in the evaluation of entrapment efficiency: A case study of Panax notoginseng saponins chitosan nanoparticles. Chin. Pharm. J. 2022, 57, 1367–1373. [Google Scholar]
- Zhang, X.; Meng, L.; Lu, Q.; Fei, Z.; Dyson, P.J. Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes. Biomaterials 2009, 30, 6041–6047. [Google Scholar] [CrossRef]
- Wang, Q. Synthesis and Properties of Thermo-Responsive Copolymers Based on N-Sopropylacrylamide. Master’s Thesis, Shenzhen University, Shenzhen, China, 2017. [Google Scholar]
- Chen, X.; Feng, C.H.; Shen, Y.L.; Lin, D.Q.; Feng, B.; He, Z.Q.; Du, Y.J. Chemical synthesis of cuelure and its attractive activities to Bactrocera cucurbitae. China Plant Prot. 2011, 31, 9–13. [Google Scholar]
- Pan, S.P.; Tao, Q.; Wang, Y.T. Study on the preparations and properties of temperature-responsive injectable hydrogel based on poly (N-isopropylacrylamide). Mod. Chem. Res. 2021, 22, 7–9. [Google Scholar]
- Zhang, X.; Zhang, W.; Dai, J.; Sun, M.; Zhao, J.; Ji, L.; Chen, L.; Zeng, F.; Yang, F.; Huang, B.; et al. Carboxylated carbon nanotubes with high electrocatalytic activity for oxygen evolution in acidic conditions. InfoMat 2021, 4, e12273. [Google Scholar] [CrossRef]
- Singh, A.; Kar, A.K.; Singh, D.; Verma, R.; Shraogi, N.; Zehra, A.; Gautam, K.; Anbumani, S.; Ghosh, D.; Patnaik, S. PH-responsive eco-friendly chitosan modified cenosphere/alginate composite hydrogel beads as carrier for controlled release of Imidacloprid towards sustainable pest control. Chem. Eng. J. 2022, 427, 131215. [Google Scholar] [CrossRef]
- Lim, G.-P.; Ahmad, M.S. Development of Ca-alginate-chitosan microcapsules for encapsulation and controlled release of imidacloprid to control dengue outbreaks. J. Ind. Eng. Chem. 2017, 56, 382–393. [Google Scholar] [CrossRef]
- Yuan, S.Y.; Kong, Q.; Li, Z.Y.; Xiao, Z.Y.; Chen, B.; Zhang, D.G. Study on biology of Bactrocera (Zeugodacus) cucurbitae. Acta Agric. Boreali-Occident. Sin. 2005, 3, 43–45+67. [Google Scholar]
- Shelly, T.E.; Villalobos, E.M. Cue Lure and the Mating Behavior of Male Melon Flies (Diptera: Tephritidae). Fla. Entomol. 1995, 78, 473. [Google Scholar] [CrossRef]
- Gheysoori, P.; Paydayesh, A.; Jafari, M.; Peidayesh, H. Thermoresponsive nanocomposite hydrogels based on Gela-tin/poly (N–isopropylacrylamide) (PNIPAM) for controlled drug delivery. Eur. Polym. J. 2023, 186, 111846. [Google Scholar] [CrossRef]
- Feng, J.C.; Dou, J.P.; Zhang, Y.Z.; Wu, Z.D.; Yin, D.X.; Wu, W.F. Thermosensitive hydrogel for encapsulation and con-trolled release of biocontrol agents to prevent peanut aflatoxin contamination. Polymers 2020, 12, 547. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Bai, B.; Wang, H.; Suo, Y. A Near-Infrared and Temperature-Responsive Pesticide Release Platform through Core–Shell Polydopamine@PNIPAm Nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 6424–6432. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Chu, X.; Feng, W.; Li, J.; Huang, X.; Zhou, N.; Shen, J. A new temperature-responsive controlled-release pesticide formulation—poly(N-isopropylacrylamide) modified graphene oxide as the nanocarrier for lambda-cyhalothrin delivery and their application in pesticide transportation. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 612, 125987. [Google Scholar] [CrossRef]


| Name | Treatment | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|---|
| Reagent | |||||||
| Thermosensitive monomer (g) | NIPAM | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| Crosslinking agent (g) | Methylene-Bis-Acrylamide | 0.02 | 0.03 | 0.04 | 0.05 | 0.06 | |
| Porogen (g) | PEG 10000 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| Accelerating agent (μL) | TMEDA | 50 | 50 | 50 | 50 | 50 | |
| Initiator (g) | Ammonium persulphate | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | |
| Solvent (mL) | 60% acetonitrile aqueous solution | 10 | 10 | 10 | 10 | 10 | |
| Name | Treatment | 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|---|---|
| Reagent | |||||||
| Thermosensitive monomer (g) | NIPAM | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| Crosslinking agent (g) | Methylene-Bis-Acrylamide | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | |
| Porogen (g) | PEG 10000 | 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| Accelerating agent (μL) | TMEDA | 50 | 50 | 50 | 50 | 50 | |
| Initiator (g) | Ammonium persulphate | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | |
| Solvent (mL) | 60% acetonitrile aqueous solution | 10 | 10 | 10 | 10 | 10 | |
| Sample | Absorbance | Concentration (mg/mL) | Entrapment Efficiency (%) | Drug Loading (%) |
|---|---|---|---|---|
| 1 | 1.186 ± 0.01 | 2.32 ± 0.01 | 75.53 ± 0.01 | 3.46 ± 0.02 |
| 2 | 1.174 ± 0.01 | 2.29 ± 0.02 | 74.83 ± 0.01 | 3.43 ± 0.03 |
| 3 | 1.182 ± 0.01 | 2.31 ± 0.03 | 73.34 ± 0.01 | 3.36 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Peng, S.; Zeng, B.; Lian, Y.; Jia, J.; Zhang, Q.; Wu, Q.; Zhou, S. Preparation and Evaluation of a Temperature-Sensitive Cuelure Nano-Controlled Release Agent. Agronomy 2024, 14, 1578. https://doi.org/10.3390/agronomy14071578
Wang A, Peng S, Zeng B, Lian Y, Jia J, Zhang Q, Wu Q, Zhou S. Preparation and Evaluation of a Temperature-Sensitive Cuelure Nano-Controlled Release Agent. Agronomy. 2024; 14(7):1578. https://doi.org/10.3390/agronomy14071578
Chicago/Turabian StyleWang, Aqiang, Sihua Peng, Bei Zeng, Yuyang Lian, Jingjing Jia, Qiongkuan Zhang, Qianxing Wu, and Shihao Zhou. 2024. "Preparation and Evaluation of a Temperature-Sensitive Cuelure Nano-Controlled Release Agent" Agronomy 14, no. 7: 1578. https://doi.org/10.3390/agronomy14071578





