Multidimensional Quality Characteristics of Sichuan South-Road Dark Tea and Its Chemical Prediction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Quantification of Tea Quality Chemicals
2.3. NIR Spectral Analysis of Dried SSDT
2.3.1. NIR Spectral Acquisition
2.3.2. Pretreatment of Raw Spectra and Establishment of Prediction Models
2.4. Analytical Procedure for Fluorescent EEM of SSDT Brew
2.5. Statistical Analysis
3. Results
3.1. Typical Quality Chemical Profile of SSDT
3.2. NIR Spectral Characteristics of Dried SSDT and the Chemical Prediction
3.2.1. Overview of NIR Spectrum
3.2.2. Optimal Pretreatment of NIR Spectra
3.2.3. SiPLS Models for Chemical Prediction
3.3. Fluorescence Spectral and Chromatic Characteristics of SSDT Brew
3.3.1. The Distinctive Color and Fluorescence Signal of SSDT Brew
3.3.2. Identification of KZ and JJ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, Y.; He, J.; Tang, M.; Chen, H.; Wei, T.; Zhang, B.; Liang, D.; Nie, X. Preventive effect of Ya’an Tibetan tea on obesity in rats fed with a hypercaloric high-fat diet revealed by gut microbiology and metabolomics studies. Food Res. Int. 2023, 165, 112520. [Google Scholar] [CrossRef]
- Zou, Y.; Yuan, Y.; Liu, M.; Li, X.; Lai, Y.; Liu, X.; Tan, L.; Tang, Q.; Chen, W.; Li, D.; et al. Metagenomics reveal the role of microorganism and GH genes contribute to Sichuan south-road dark tea quality formation during pile fermentation. LWT 2023, 178, 114618. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.Y.; Yang, X.; Zhu, F.; Wu, D.T.; Li, H.B.; Gan, R.Y. Green extraction, chemical composition, and in vitro antioxidant activity of theabrownins from Kangzhuan dark tea. Curr. Res. Food Sci. 2022, 5, 1944–1954. [Google Scholar] [CrossRef] [PubMed]
- Beć, K.B.; Grabska, J.; Huck, C.W. Miniaturized NIR spectroscopy in food analysis and quality control: Promises, challenges, and perspectives. Foods 2022, 11, 1465. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qu, F.; Shen, B.; Huang, Z.; Li, X.; Weng, H.; Ye, D.; Wu, R. Rapid detection of tea polyphenols in fresh tea leaves based on fusion of visible/short-wave and long-wave near infrared spectroscopy and its device development. Appl. Sci. 2023, 13, 1739. [Google Scholar] [CrossRef]
- Nelum, K.G.; Piyasena, P.; Ranatunga, M.A.B.; Jayawardhane, S.A.D.P.S.; Edirisinghe, E.N.U.; Tharangika, H.B.; Ghouse, A.S.; Abayarathne, A.A.B.; Jayasinghe, W.S.; Abeysinghe, I.S.B.; et al. Prediction of glucose and sucrose values of black tea samples using NIR spectroscopy and chemometrics. Food Humanit. 2023, 1, 1482–1493. [Google Scholar] [CrossRef]
- Jiang, Y.; Zareef, M.; Liu, L.; Ouyang, Q. Monitoring of the carotenoids changes during the matcha drying process using a portable developed spectral analytical system. J. Food Compos. Anal. 2023, 125, 105849. [Google Scholar] [CrossRef]
- Wu, Z.; Li, C.; Liu, H.; Lin, T.; Yi, L.; Ren, D.; Gu, Y.; Wang, S. Quantification of caffeine and catechins and evaluation of bitterness and astringency of Pu-erh ripen tea based on portable near-infrared spectroscopy. J. Food Compos. Anal. 2024, 125, 105793. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Liu, Y.; Wang, Y.; Lu, C.; Li, T.; Wei, Y.; Ning, J. Monitoring green tea fixation quality by intelligent sensors: Comparison of image and spectral information. J. Sci. Food Agric. 2023, 103, 3093–3101. [Google Scholar] [CrossRef]
- Qu, F.; Zhu, X.; Ai, Z.; Ai, Y.; Qiu, F.; Ni, D. Effect of different drying methods on the sensory quality and chemical components of black tea. LWT 2019, 99, 112–118. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, Z.; He, L.; Lu, X.; Tang, J.; Laghi, L. The longer the storage time, the higher the price, the better the quality? A 1H-NMR based metabolomic investigation of aged Ya’an Tibetan tea (Camellia sinensis). Foods 2022, 11, 2986. [Google Scholar] [CrossRef]
- Quatela, A.; Gilmore, A.M.; Gall, K.E.S.; Sandros, M.; Csatorday, K.; Siemiarczuk, A.; Yang, B.B.; Camenen, L. A-TEEMTM, a new molecular fingerprinting technique: Simultaneous absorbance-transmission and fluorescence excitation-emission matrix method. Methods Appl. Fluoresc. 2018, 6, 027002. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, J.N.; Jiang, Y.; Ma, X.M.; Ma, F.L.; Ma, X.L.; Zhang, Y.; Tang, L.H.; Wang, W.X.; Ma, G.M.; et al. Identification of oak-barrel and stainless steel tanks with oak chips aged wines in Ningxia based on three-dimensional fluorescence spectroscopy combined with chemometrics. Molecules 2023, 28, 3688. [Google Scholar] [CrossRef]
- Ranaweera, R.K.; Gilmore, A.M.; Capone, D.L.; Bastian, S.E.; Jeffery, D.W. Spectrofluorometric analysis combined with machine learning for geographical and varietal authentication, and prediction of phenolic compound concentrations in red wine. Food Chem. 2021, 361, 130149. [Google Scholar] [CrossRef] [PubMed]
- Ranaweera, R.K.; Gilmore, A.M.; Capone, D.L.; Bastian, S.E.; Jeffery, D.W. Authentication of the geographical origin of Australian Cabernet Sauvignon wines using spectrofluorometric and multi-element analyses with multivariate statistical modelling. Food Chem. 2021, 335, 127592. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, X.; Lei, W. Identifying the producer and grade of matcha tea through three-dimensional fluorescence spectroscopy analysis and distance discrimination. Foods 2023, 12, 3614. [Google Scholar] [CrossRef]
- Jiang, J.; Tan, X.; Zhang, L.; Zhu, Q.; Li, H.; Qiu, B. Hybrid N-way partial least squares and random forest model for brick tea identification based on excitation–emission matrix fluorescence spectroscopy. Food Bioproc. Technol. 2023, 16, 1335–1342. [Google Scholar] [CrossRef]
- Samokhvalov, A. Analysis of various solid samples by synchronous fluorescence spectroscopy and related methods: A review. Talanta 2020, 216, 120944. [Google Scholar] [CrossRef]
- GB/T 8305-2013; Tea-Determination of Water Extracts Content. Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- GB/T 8314-2013; Tea-Determination of Free Amino Acids Content. Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- Zhao, C.N.; Tang, G.Y.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Liu, Q.; Mao, Q.Q.; Shang, A.; Li, H.B. Phenolic profiles and antioxidant activities of 30 tea infusions from green, black, oolong, white, yellow and dark teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, W.; He, C.; Tao, M.; Liu, Z. Exploring the quality and application potential of the remaining tea stems after the postharvest tea leaves: The example of Lu’an Guapian tea (Camellia sinensis L.). Foods 2022, 11, 2357. [Google Scholar] [CrossRef]
- GB/T 8313-2018; Determination of Total Polyphenols and Catechins Content in Tea. Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- He, S.; Deng, X.; Han, Y.; Gong, Z.; Wang, J.; Tao, X.; Tong, H.; Chen, Y. Metabolites and metagenomic analysis reveals the quality of Pu-erh “tea head”. Food Chem. 2023, 429, 136992. [Google Scholar] [CrossRef]
- Obanda, M.; Owuor, P.O.; Mang’oka, R. Changes in the chemical and sensory quality parameters of black tea due to variations of fermentation time and temperature. Food Chem. 2001, 75, 395–404. [Google Scholar] [CrossRef]
- GB/T 23776-2018; Methodology for sensory evaluation of tea. Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- Ríos-Reina, R.; Ocaña, J.A.; Azcarate, S.M.; Pérez-Bernal, J.L.; Villar-Navarro, M.; Callejón, R.M. Excitation-emission fluorescence as a tool to assess the presence of grape-must caramel in PDO wine vinegars. Food Chem. 2019, 287, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wei, Y.; Peng, L.; Wei, K.; Liu, Z.; Wei, X. State-of-the-art review of theabrownins: From preparation, structural characterization to health-promoting benefits. Crit. Rev. Food Sci. Nutr. 2023, 1–20. [Google Scholar] [CrossRef]
- Tan, J.; De Bruijn, W.J.; Van Zadelhoff, A.; Lin, Z.; Vincken, J.P. Browning of epicatechin (EC) and epigallocatechin (EGC) by auto-oxidation. J. Agric. Food Chem. 2020, 68, 13879–13887. [Google Scholar] [CrossRef]
- Li, X.; Sun, C.; Luo, L.; He, Y. Determination of tea polyphenols content by infrared spectroscopy coupled with iPLS and random frog techniques. Comput. Electron. Agric. 2015, 112, 28–35. [Google Scholar] [CrossRef]
- Li, C.; Zong, B.; Guo, H.; Luo, Z.; He, P.; Gong, S.; Fan, F. Discrimination of white teas produced from fresh leaves with different maturity by near-infrared spectroscopy. Spectroc.Acta Pt. A-Mol. Biomolec. 2020, 227, 117697. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xie, Y.; Chen, J.; Yuan, T.; Leng, T.; Chen, Y.; Xie, J.; Yu, Q. NIR spectrometric approach for geographical origin identification and taste related compounds content prediction of lushan yunwu tea. Foods 2022, 11, 2976. [Google Scholar] [CrossRef]
- Li, C.; Guo, H.; Zong, B.; He, P.; Fan, F.; Gong, S. Rapid and non-destructive discrimination of special-grade flat green tea using Near-infrared spectroscopy. Spectroc.Acta Pt. A-Mol. Biomolec. Spectr. 2019, 206, 254–262. [Google Scholar] [CrossRef]
- Li, T.; Wei, Y.; Feng, W.; Lu, M.; Ke, H.; Li, Y.; Shao, A.; Dai, Q.; Ning, J. Exploring the mysterious effect of piling fermentation on Pu-erh tea quality formation: Microbial action and moist-heat action. LWT 2023, 185, 115132. [Google Scholar] [CrossRef]
- Upadhyay, R.; Gupta, A.; Mishra, H.N.; Bhat, S.N. At-line quality assurance of deep-fried instant noodles using pilot scale visible-NIR spectroscopy combined with deep-learning algorithms. Food Control 2022, 133, 108580. [Google Scholar] [CrossRef]
- Guo, Z.; Barimah, A.O.; Yin, L.; Chen, Q.; Shi, J.; El-Seedi, H.R.; Zou, X. Intelligent evaluation of taste constituents and polyphenols-to-amino acids ratio in matcha tea powder using near infrared spectroscopy. Food Chem. 2021, 353, 129372. [Google Scholar] [CrossRef] [PubMed]
- Ur Rehman, N.; Al-Harrasi, A.; Boqué, R.; Mabood, F.; Al-Broumi, M.; Hussain, J.; Alameri, S. FT-NIRS coupled with PLS regression as a complement to HPLC routine analysis of caffeine in tea samples. Foods 2020, 9, 827. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Dong, W.; Sanaeifar, A.; Wang, X.; Luo, W.; Zhan, B.; Liu, X.; Li, R.; Zhang, H.; Li, X. Development of simple identification models for four main catechins and caffeine in fresh green tea leaf based on visible and near-infrared spectroscopy. Comput. Electron. Agric. 2020, 173, 105388. [Google Scholar] [CrossRef]
- Radotić, K.; Stanković, M.; Bartolić, D.; Natić, M. Intrinsic fluorescence markers for food characteristics, shelf life, and safety estimation: Advanced analytical approach. Foods 2023, 12, 3023. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, L.; Zhang, Y. Three dimensional fluorescence spectral characteristics of tea polyphenols. J. Instrum. Anal. 2013, 32, 302–307. (In Chinese) [Google Scholar]
- Turgut, S.S.; Entrenas, J.A.; Taşkın, E.; Garrido-Varo, A.; Pérez-Marín, D. Estimation of the sensory properties of black tea samples using non-destructive near-infrared spectroscopy sensors. Food Control 2022, 142, 109260. [Google Scholar] [CrossRef]
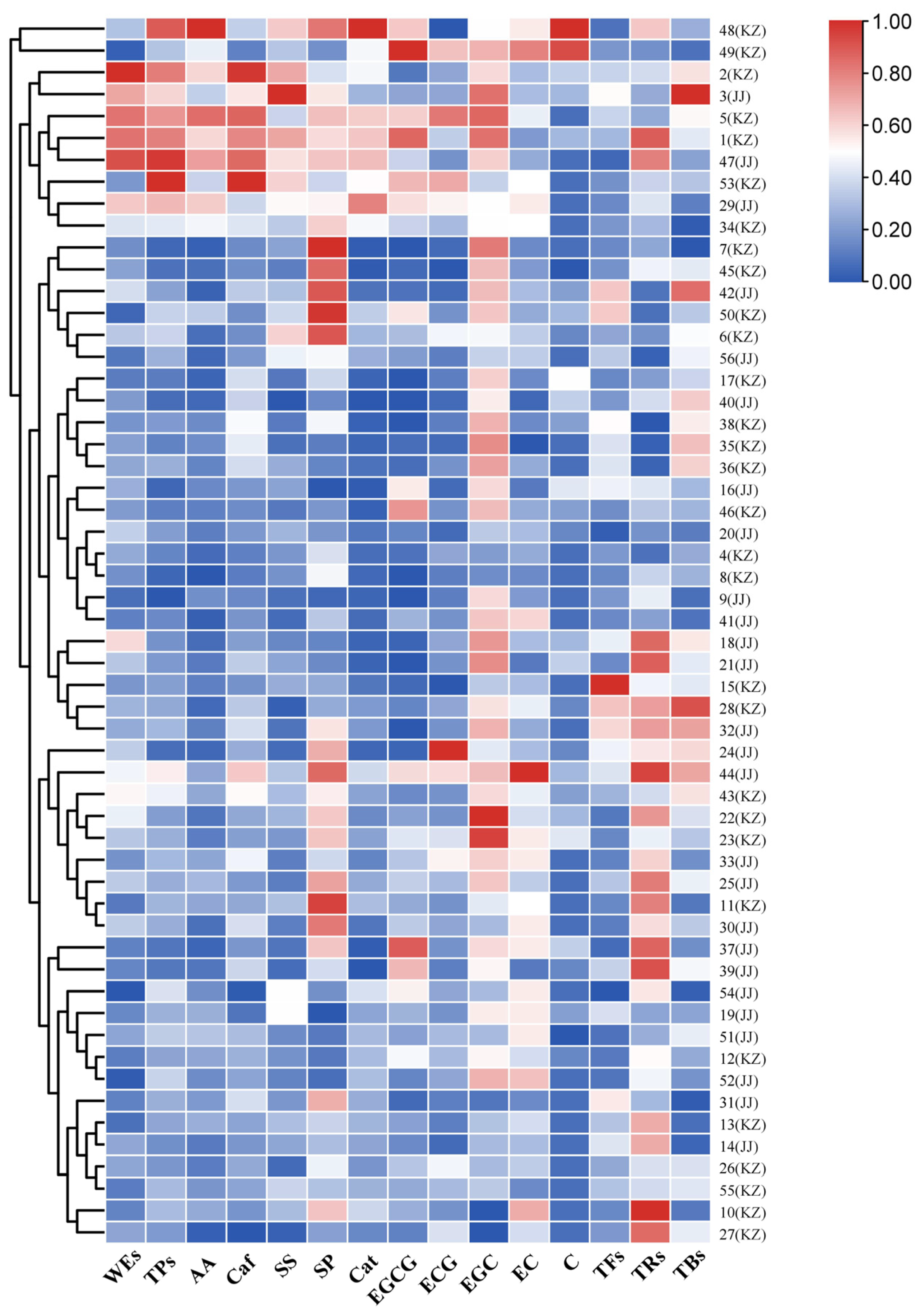
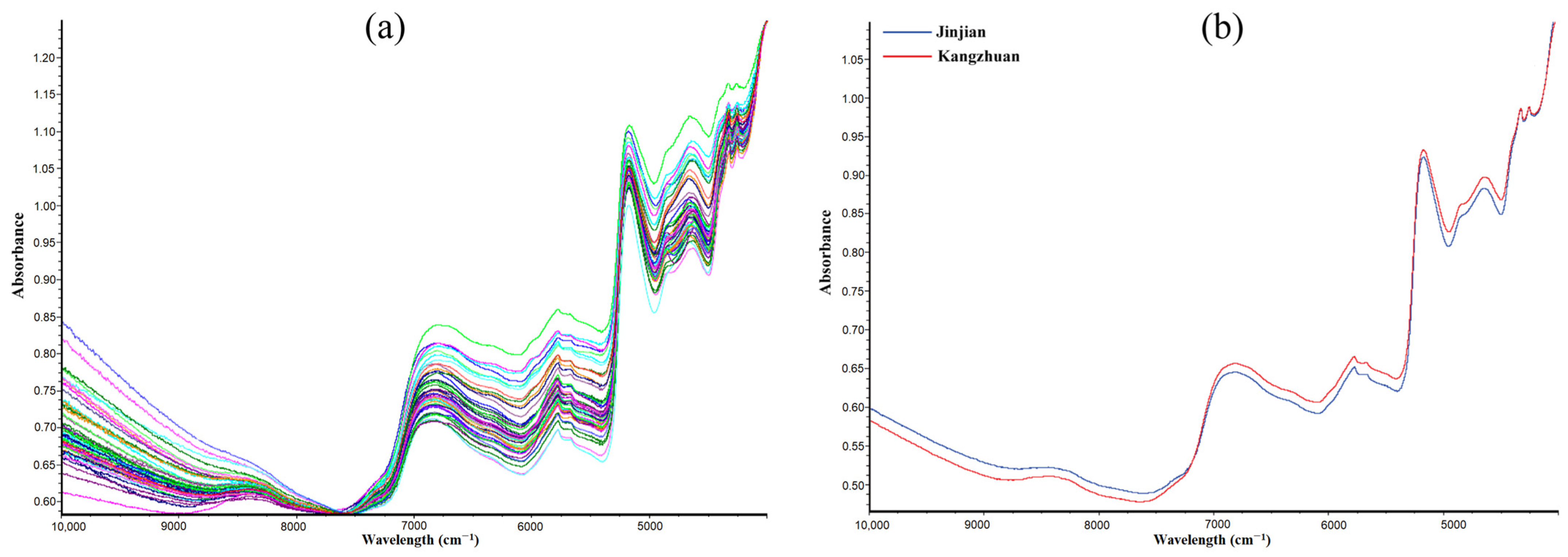
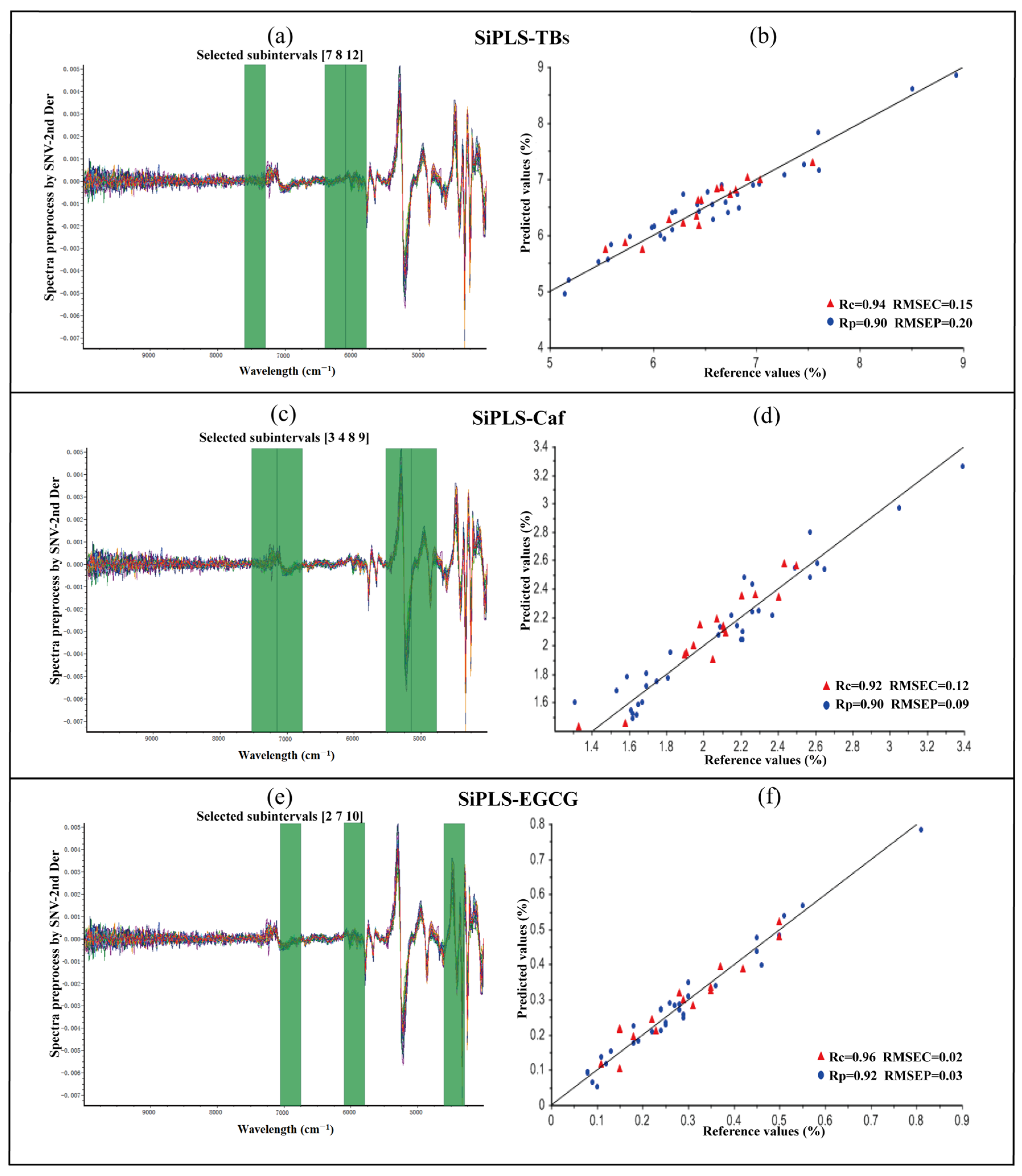

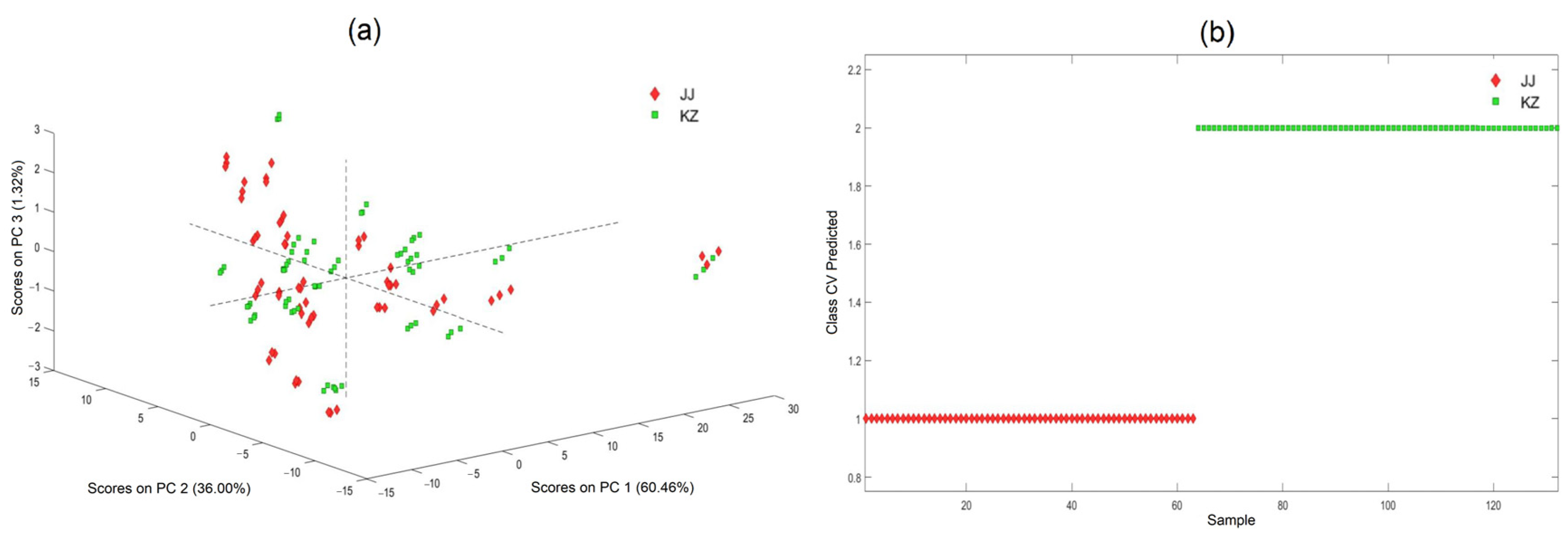
| Chemicals | Range (%) | Mean (%) | SD | CV (%) |
|---|---|---|---|---|
| WEs | 14.52–45.64 | 23.51 | 7.09 | 30.15 |
| TPs | 2.24–11.30 | 5.04 | 2.17 | 43.05 |
| AA | 0.24–2.39 | 0.71 | 0.46 | 64.78 |
| Caf | 1.21–3.71 | 2.02 | 0.56 | 27.31 |
| SS | 0.89–8.62 | 2.99 | 1.61 | 53.84 |
| SP | 0.17–1.21 | 0.64 | 0.29 | 45.31 |
| Cat | 0.67–5.76 | 1.87 | 1.10 | 58.82 |
| EGCG | 0.08–0.81 | 0.28 | 0.19 | 67.85 |
| EGC | 0.03–0.47 | 0.26 | 0.22 | 84.61 |
| ECG | 0.05–0.22 | 0.09 | 0.04 | 45.55 |
| EC | 0.03–0.23 | 0.10 | 0.04 | 36.36 |
| C | 0.02–0.16 | 0.05 | 0.03 | 60.78 |
| TFs | 0.02–0.13 | 0.05 | 0.44 | 37.73 |
| TRs | 0.64–2.21 | 1.37 | 0.56 | 31.38 |
| TBs | 5.08–9.03 | 6.51 | 0.39 | 14.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Li, X.; Han, D. Multidimensional Quality Characteristics of Sichuan South-Road Dark Tea and Its Chemical Prediction. Agronomy 2024, 14, 1582. https://doi.org/10.3390/agronomy14071582
Zou Y, Li X, Han D. Multidimensional Quality Characteristics of Sichuan South-Road Dark Tea and Its Chemical Prediction. Agronomy. 2024; 14(7):1582. https://doi.org/10.3390/agronomy14071582
Chicago/Turabian StyleZou, Yao, Xian Li, and Deyang Han. 2024. "Multidimensional Quality Characteristics of Sichuan South-Road Dark Tea and Its Chemical Prediction" Agronomy 14, no. 7: 1582. https://doi.org/10.3390/agronomy14071582




