Plant and Soil Effects of Alternative Sources of Phosphorus over Three Years of Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up and Design
2.2. Harvests, Crop and Soil Analysis
2.3. Chemical Characteristics of Mineral and Bio-Based Fertilisers
2.4. Statistics
3. Results
3.1. Nutrient and Metal Characteristics
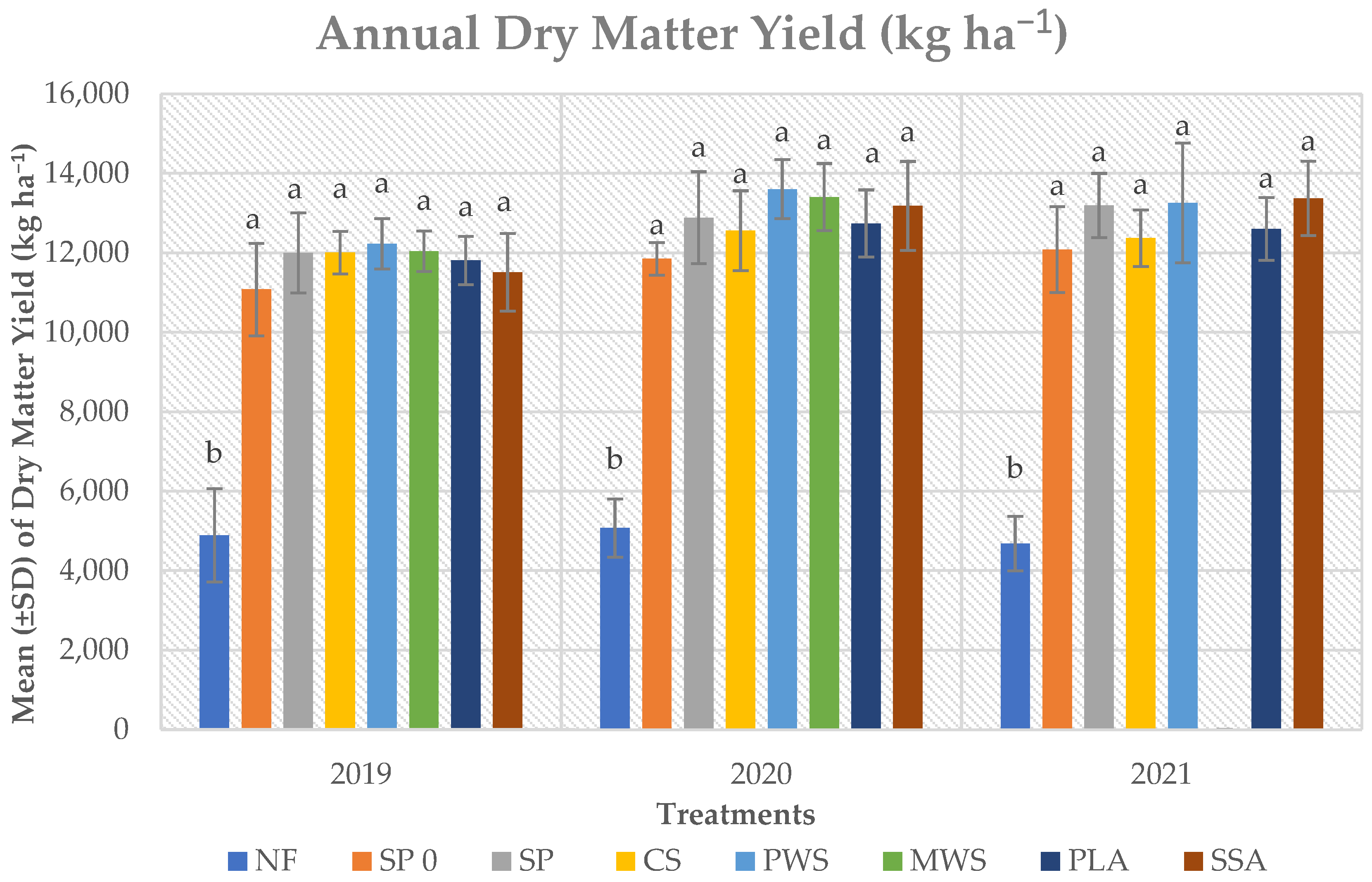
3.2. Grass Annual Yield and P Uptake by the Grass
3.2.1. Grass Yield
3.2.2. P Uptake
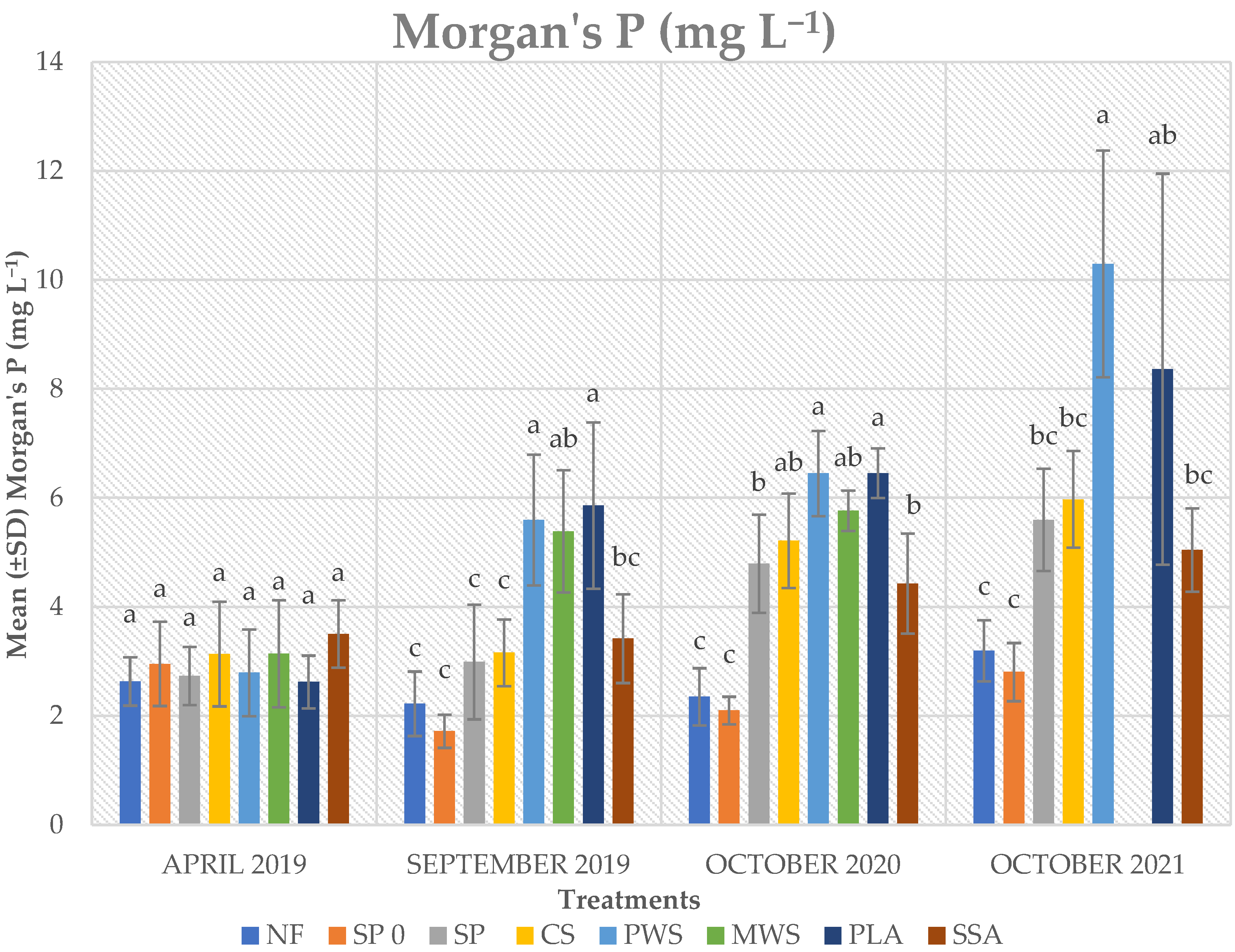
3.3. Soil P
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Skrumsager Møller, I.; White, P. Chapter 6–Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: New York, NY, USA, 2012; pp. 135–189. ISBN 9780123849052. [Google Scholar]
- Schulte, R.P.O.; Herlihy, M. Quantifying responses to phosphorus in Irish grasslands: Interactions of soil and fertiliser with yield and P concentration. Eur. J. Agron. 2007, 26, 144–153. [Google Scholar] [CrossRef]
- Jouany, C.; Morel, C.; Ziadi, N.; Bélanger, G.; Sinaj, S.; Stroia, C.; Cruz, P.; Theau, J.P.; Duru, M. Plant and soil tests to optimise phosphorus fertilisation management of grasslands. Eur. J. Agron. 2021, 125, 126–249. [Google Scholar] [CrossRef]
- Ros, M.B.H.; Koopmans, G.F.; van Groenigen, K.J.; Abolos, D.; Oenema, O.; Vos, H.M.; van Groenigen, J.W. Towards optimal use of phosphorus fertiliser. Sci. Rep. 2020, 10, 17804. [Google Scholar] [CrossRef]
- Macintosh, K.A.; Doody, D.G.; Withers, P.J.A.; McDowell, R.W.; Smith, D.R.; Johnson, L.T.; Bruulsema, T.W.; O’Flaherty, V.; McGrath, J.W. Transforming soil phosphorus fertility management strategies to support the delivery of multiple ecosystem services from agricultural systems. Sci. Total Environ. 2019, 649, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Boyce, A.; Walsh, G. Identification and Evaluation of Phosphorus Recovery Technologies in an Irish Context. Environmental Protection Agency 2016, Report No. 189, EPA Research Programme 2014–2020. Available online: https://www.epa.ie/publications/research/water/EPA-RR-189-final-web.pdf (accessed on 10 June 2024).
- Jasinski, S.M. Mineral Commodity Summaries 2019. U.S. Department of Interior and U.S. Geological Survey, Reston, Virginia. Available online: https://miningpress.com/media/briefs/usgs-mineral-commodity-summarie-2019_2963.pdf (accessed on 30 November 2023).
- Matta, S.; Stephan, K.; Stephan, J.; Lteif, R.; Goutaudier, C.; Saab, J. Phosphoric acid production by attacking phosphate rock with recycled hexafluosilicic acid. Int. J. Miner. Process. 2017, 161, 21–27. [Google Scholar] [CrossRef]
- Roberts, T.L. Cadmium and phosphorus fertilizers: The issues and the science. Procedia Eng. 2014, 83, 52–59. [Google Scholar] [CrossRef]
- Ulrich, A.E. Cadmium governance in Europe’s phosphate fertilizers: Not so fast? Sci. Total Environ. 2019, 650, 541–545. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Regulation of the European Parliament and of the Council Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No2003/2003. 2019. Available online: https://data.consilium.europa.eu/doc/document/PE-76-2018-INIT/en/pdf (accessed on 13 January 2024).
- World Health Organization (WHO). Exposure to Cadmium: A Major Public Health Concern. 2010. Available online: https://apps.who.int/iris/bitstream/handle/10665/329480/WHO-CED-PHE-EPE-19.4.3-eng.pdf (accessed on 26 February 2024).
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Lalung, J. Isolation, identification, and characterization of cadmium resistant Pseudomonas sp. M3 from industrial wastewater. Waste Manag. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Chunhabundit, R. Cadmium Exposure and Potential Health Risk from Foods in Contaminated Area, Thailand. Toxicol. Res. 2016, 32, 65–72. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Hilton, J.; Johnston, A.E.; Dawson, C.J. The Phosphate Life-Cycle: Rethinking the Options for a Finite Resource. In International Fertiliser Society; CABI Digital Library: Wallingford, UK, 2010; ISBN 978-0-85310-305-9. [Google Scholar]
- Cordell, D.; White, S. Sustainable Phosphorus Measures: Strategies and Technologies for Achieving Phosphorus Security. Agronomy 2013, 3, 86–116. [Google Scholar] [CrossRef]
- Mancho, C.; Diez-Pascual, S.; Alonso, J.; Gil-Díaz, M.; Lobo, M.C. Assessment of Recovered Struvite as a Safe and Sustainable Phosphorus Fertilizer. Environments 2023, 10, 22. [Google Scholar] [CrossRef]
- Mew, M.C.; Steiner, G.; Geissler, B. Phosphorus Supply Chain—Scientific, Technical, and Economic Foundations: A Transdisciplinary Orientation. Sustainability 2018, 10, 1087. [Google Scholar] [CrossRef]
- Mohanty, A.; Mankoti, M.; Ranjan Rout, P.; Meena, S.S.; Dewan, S.; Kalia, B.; Banu, J.R. Sustainable utilization of food waste for bioenergy production: A step towards circular bioeconomy. Int. J. Food Microbiol. 2022, 365, 109538. [Google Scholar] [CrossRef]
- Dhiman, S.; Mukherjee, G. Present scenario and future scope of food waste to biofuel production. J. Food Process Eng. 2021, 44, e13594. [Google Scholar] [CrossRef]
- Rout, P.R.; Dash, R.R.; Bhunia, P.; Lee, E.; Bae, J. Comparison between a single unit bioreactor and an integrated bioreactor for nutrient removal from domestic wastewater. Sustain. Energy Technol. Assess. 2021, 48, 101620. [Google Scholar] [CrossRef]
- O’Donovan, M.; Lewis, E.; O’Kiely, P. Requirements of future grass-based ruminant production systems in Ireland. Ir. J. Agric. Food Res. 2011, 50, 1–21. [Google Scholar]
- O’Mara, F.P. The role of grasslands in food security and climate change. Ann. Bot. 2012, 6, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, M.; Hennessy, D.; Creighton, P. Ruminant grassland production systems in Ireland. Ir. J. Agric. Food Res. 2021, 59, 225–232. [Google Scholar] [CrossRef]
- Harms, I.; Power, N.; Sultanbaeve, E.; Coopman, F.; Bouthier, A.; Trochard, R.; Denis, T.; Postma, R.; Laub, K.; De Dobbelaere, A.; et al. Exploring the Demand for Recycling-Derived Nutrients and Organic Matter in Regions of Northwest Europe 2019, WPT2, Activity 1, Deliverable 1.2. Available online: https://www.nmi-agro.nl/wp-content/uploads/2020/03/ReNu2Farm_WPT2_D1_2_NutrientDemand_2-1.pdf (accessed on 13 January 2024).
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus recovery from wastewater by struvite crystallisation: A review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Lorick, D.; Macura, B.; Ahlström, M.; Grimvall, A.; Harder, R. Effectiveness of struvite precipitation and ammonia stripping for recovery of phosphorus and nitrogen from anaerobic digestate: A systematic review. Environ. Evid. 2020, 9, 27. [Google Scholar] [CrossRef]
- Franz, M. Phosphate fertilizer from sewage sludge ash (SSA). Waste Manag. 2008, 28, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Mattenberger, H.; Fraissler, G.; Brunner, T.; Herk, P.; Hermann, L.; Obernberger, I. Sewage sludge ash to phosphorus fertiliser: Variables influencing heavy metal removal during thermochemical treatment. Waste Manag. 2008, 28, 2709–2722. [Google Scholar] [CrossRef]
- Teagasc. Major and Micronutrient Advice for Productive Agricultural Crops. Available online: https://www.teagasc.ie/media/website/publications/2020/Major--Micro-Nutrient-Advice-for-Productive-Agricultural-Crops-2020.pdf (accessed on 13 February 2024).
- Ashekuzzaman, S.M.; Forrestal, P.; Richards, K.G.; Daly, K.; Fenton, O. Grassland Phosphorus and Nitrogen Fertiliser Replacement value of Dairy Processing Dewatered Sludge. Sustain. Prod. Consum. 2021, 25, 363–373. [Google Scholar] [CrossRef]
- USEPA (undated) Memorandum. Subject: Fipronil ID#000264-00554: Updated Toxicology Hazard Assessment. In Evaluation of an Acute Neurotoxicity Study with Fipronil and Reevaluation of the Developmental Neurotoxicity Study; USEPA: Washington, DC, USA, 1996. [Google Scholar]
- Morgan, M.F. Chemical Soil Diagnosis by the Universal Soil Testing System. Connecticut Agricultural Experiment Station, Bulletin 450. 1941. Available online: https://portal.ct.gov/-/media/CAES/DOCUMENTS/Publications/Bulletins/B450pdf.pdf?la=en (accessed on 17 February 2024).
- Shi, W.; Healy, M.G.; Ashekuzzaman, S.M.; Daly, K.; Fenton, O. Mineral fertiliser equivalent value of dairy processing sludge and derived biochar using ryegrass (Lolium perenne L.) and spring wheat (Triticumaes-time). J. Environ. Manag. 2022, 321, 116012. [Google Scholar] [CrossRef] [PubMed]
- ISO 11885; Water Quality, Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). International Organization for Standardization: Geneva, Switzerland, 2007. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:11885:ed-2:v1:en (accessed on 25 February 2024).
- ISO 11261; Soil Quality, Determination of Total Nitrogen, Modified Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 1995. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:11261:ed-1:v1:en (accessed on 25 February 2024).
- IBM Corp 2023, version 28.0; IBM SPSS Statistics for Windows; IBM Corp: Armonk, NY, USA, 2023.
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Lindh, M.; Hoeber, S.; Weih, M.; Manzoni, S. Interactions of nutrient and water availability control growth and diversity effects in a Salix two-species mixture. Ecohydrology 2022, 15, e2401. [Google Scholar] [CrossRef]
- O’Donnell, C.; Barnett, D.; Harrington, J.; Power, N. The Extended Effect of Top-Dressed Recovered Struvite Fertiliser on Residual Irish Grassland Soil Phosphorus Levels Compared to Commercial Phosphorus Fertiliser. Agronomy 2022, 12, 8. [Google Scholar] [CrossRef]
- Hauck, D.; Lohr, D.; Meinken, E.; Schmidhalter, U. Phosphorus Availability from German Sewage Sludge Ashes to Plants Cultivated in Soilless Growing Media of Contrasting pH. Agronomy 2022, 12, 2610. [Google Scholar] [CrossRef]
- Ohno, T.; Erich, M.S. Effect of wood ash application on soil pH and soil test nutrient levels. Agric. Ecosyst. Environ. 1990, 32, 223–239. [Google Scholar] [CrossRef]
- Pels, J.R.; De Nie, D.S.; Kiel, J.H.A. Utilization of ashes from biomass combustion and gasification. In Proceedings of the 14th European Biomass Conference & Exhibition, Paris, France, 17–21 October 2005. ECN-RX-05-182. [Google Scholar]
- Li, X.; Rubæk, G.H.; Sørensen, P. High plant availability of phosphorus and low availability of cadmium in four biomass combustion ashes. Sci. Total Environ. 2016, 557, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Talboys, P.J.; Heppell, J.; Roose, T. Struvite: A slow-release fertiliser for sustainable phosphorus management? Plant Soil 2016, 401, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Khiari, L.; Joseph, C.A.; Gallichand, J.; Beecher, N.; Bouslama, S. Classification and Assessment Models of First Year Biosolids Phosphorus Bioavailability. Waste Biomass Valorization 2020, 11, 1443–1452. [Google Scholar] [CrossRef]
- Arenas-Montaño, V.; Fenton, O.; Moore, B.; Healy, M.G. Evaluation of the fertiliser replacement value of phosphorus-saturated filter media. J. Clean. Prod. 2021, 291, 125943. [Google Scholar] [CrossRef]
- Tumbure, A.; Schmalenberger, A. Struvites with comparable nitrogen and phosphorus composition have similar agronomic response but shape cherry tomato rhizosphere bacterial community structure differently. Appl. Soil Ecol. 2024, 195, 929–1393. [Google Scholar] [CrossRef]
- Korkusuz, A.E.; Beklioğlu, M.; Demirer, G.N. Use of blast furnace granulated slag as a substrate in vertical flow reed beds: Field application. Bioresour. Technol. 2007, 98, 2089–2101. [Google Scholar] [CrossRef] [PubMed]
- Vanden Nest, T.; Amery, F.; Fryda, L.; Boogaerts, C.; Bilbao, J.; Vandecasteele, B. Renewable P sources: P use efficiency of digestate, processed animal manure, compost, biochar and struvite. Sci. Total Environ. 2021, 750, 141699. [Google Scholar] [CrossRef] [PubMed]
- Hylander, L.D.; Simán, G. Plant availability of phosphorus sorbed to potential wastewater treatment materials. Biol. Fertil. Soils 2002, 34, 42–48. [Google Scholar] [CrossRef]
- Barrow, N.J.; Hartemink, A.E. The effects of pH on nutrient availability depend on both soils and plants. Plant Soil 2023, 487, 21–37. [Google Scholar] [CrossRef]
- Teagasc. Phosphorus Resources Maximising Nutrient Efficiency for Increased Sustainability Catchment Science into Policy. Teagasc, Johnstown Castle, Co Wexford 2015. Available online: https://www.teagasc.ie/media/website/publications/2015/Pres_Soil-Phosphorus-Trends----Dr.-David-Wall.pdf (accessed on 9 November 2023).
- Suciu, N.A.; Devivo, R.; Rizzati, N.; Capri, E. Cd content in phosphate fertilizer: Which potential risk for the environment and human health? Curr. Opin. Environ. Sci. Health 2022, 30, 100392. [Google Scholar] [CrossRef]
- Carne, G.; Leconte, S.; Sirot, V.; Breysse, N.; Badot, P.M.; Bispo, A.; Deportes, I.Z.; Dumat, C.; Rivière, G.; Crépet, A. Mass balance approach to assess the impact of cadmium decrease in mineral phosphate fertilizers on health risk: The case-study of French agricultural soils. Sci. Total Environ. 2021, 760, 143374. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Smolders, E.; Zhao, F.J.; Grant, C.; Montalvo, D. Chapter One–Managing cadmium in agricultural systems. Adv. Agron. 2021, 166, 1–129. [Google Scholar] [CrossRef]
- Ballabio, C.; Jones, A.; Panagos, P. Cadmium in topsoils of the European Union–an analysis based on LUCAS topsoil database. Sci. Total Environ. 2024, 912, 168710. [Google Scholar] [CrossRef] [PubMed]
- Codling, E.E.; Chaney, R.L.; Sherwell, J. Poultry litter ash as a potential phosphorus source for agricultural crops. J. Environ. Qual. 2002, 31, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Severin, M.; Breuer, J.; Rex, M.; Stemann, J.; Adam, C.; Van den Weghe, H.; Kücke, M. Phosphate fertilizer value of heat treated sewage sludge ash. Plant Soil Environ. 2014, 60, 555–561. [Google Scholar] [CrossRef]
- Herzel, H.; Krüger, O.; Hermann, L.; Adam, C. Sewage sludge ash— a promising secondary phosphorus source for fertilizer production. Sci. Total Environ. 2016, 542, 1136–1143. [Google Scholar] [CrossRef]
- Rech, I.; Withers, P.J.A.; Jones, D.L.; Pavinato, P.S. Solubility, diffusion and crop uptake of phosphorus in three different struvites. Sustainability 2019, 11, 134. [Google Scholar] [CrossRef]
- Hertzberger, A.J.; Cusick, R.D.; Margenot, A.J. A review and meta-analysis of the agricultural potential of struvite as a phosphorus fertilizer. Soil Sci. Soc. Am. J. 2020, 84, 653–671. [Google Scholar] [CrossRef]
- Saerens, B.; Geerts, S.; Weemaes, M. Phosphorus recovery as struvite from digested sludge–experience from the full scale. J. Environ. Manag. 2021, 280, 111743. [Google Scholar] [CrossRef]
- Karpinska, A.; Ryan, D.; Germaine, K.; Dowling, D.; Forrestal, P.; Kakouli-Duarte, T. Soil Microbial and Nematode Community Response to the Field Application of Recycled Bio-Based Fertilisers in Irish Grassland. Sustainability 2021, 13, 12342. [Google Scholar] [CrossRef]
- Ryan, D.; Karpinska, A.; Forrestal, P.J.; Ashekuzzaman, S.M.; Kakouli-Duarte, T.; Dowling, D.N.; Germaine, K.J. The Impact of Bio-Based Fertilizer Integration into Conventional Grassland Fertilization Programmes on Soil Bacterial, Fungal, and Nematode Communities. Front. Sustain. Food Syst. 2022, 6, 832841. [Google Scholar] [CrossRef]
- Deinert, L.; Ikoyi, I.; Egeter, B.; Forrestal, P.; Schmalenberger, A. Short-Term Impact of RecyclingDerived Fertilizers on Their P Supply for Perennial Ryegrass (Lolium perenne). Plants 2023, 12, 2762. [Google Scholar] [CrossRef]
- Deinert, L.; Schmalenberger, A. Reuse of Soils Fertilized with Ash as Recycling Derived Fertilizer Revealed Strong Stimulation of Microbial Communities Involved in P Mobilization in Lolium perenne Rhizospheres. Environments 2024, 11, 49. [Google Scholar] [CrossRef]
- Deinert, L.; Ashekuzzaman, S.M.; Forrestal, P.; Schmalenberger, A. The impact of struvite and ash recycling-derived fertilizers on microbial phosphorus mobilization capabilities and community structure in a Lolium perenne field trial. bioRxiv 2024. the preprint server for biology. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Sylvester-Bradley, R.; Jones, D.L.; Healey, J.R.; Talboys, P.J. Feed the crop not the soil: Rethinking phosphorus management in the food chain. Environ. Sci. Technol. 2014, 48, 6523–6530. [Google Scholar] [CrossRef]
- Stemann, J.; Peplinski, B.; Adam, C. Thermochemical treatment of sewage sludge ash with sodium salt additives for phosphorus fertilizer production–analysis of underlying chemical reactions. Waste Manag. 2015, 45, 385–390. [Google Scholar] [CrossRef]
- Sigurnjak, I.; Saju, A.; Schmalenberger, A.; Lagrange, H.; Forrestal, P.; Postma, R.; Scholl, L.; Verleden, I.; Ryan, D.; Karpinska, A.; et al. ReNu2Farm, Product Information Sheets 2022. Available online: https://www.biorefine.eu/publications/product-information-sheets/ (accessed on 9 November 2023).


| Fertiliser and Chemical Element | Super Phosphate (SP) | Cattle Slurry (CS) | Potato Waste Struvite (PWS) | Municipal Waste Struvite (MWS) | Poultry Litter Ash (PLA) | Sewage Sludge Ash (SSA) |
|---|---|---|---|---|---|---|
| TC (%DM) | 3.52 ± 0.53 | 42 ± 2.36 | 0.44 ± 0.07 | 0.36 ± 0.06 | 1.16 ± 0.17 | <0.10 |
| TN g kg−1 | 4.7 ± 0.94 | 31.4 ± 1.65 | 51.2 ± 0.25 | 50.7 ± 0.21 | 0.2 ± 0.05 | 0.3 ± 0.18 |
| P g kg−1 | 167.0 ± 33.40 | 6.1 ± 0.13 | 106.7 ± 0.82 | 100.3 ± 2.95 | 55.1 ± 5.82 | 83.9 ± 2.36 |
| K g kg−1 | 7.9 ± 1.58 | 43.0 ± 0.32 | 11.9 ± 0.07 | 0.6 ± 0.03 | 106.7 ± 3.92 | 12.6 ± 0.05 |
| S g kg−1 | 18.0 ± 3.60 | 4.4 ± 0.11 | 0.1 ± 0.06 | 0.03 ± 0.01 | 30.6 ± 1.20 | 29.7 ± 1.11 |
| Na g kg−1 | 3.0 ± 0.60 | 3.5 ± 0.01 | 0.1 ± 0.01 | 0.01 ± 0.00 | 13.5 ± 0.79 | 100.2 ± 1.34 |
| Ca g kg−1 | 212.0 ± 42.40 | 31.7 ± 0.57 | 0.4 ± 0.14 | 0.27 ± 0.01 | 155.6 ± 27.08 | 103.4 ± 0.93 |
| Mg g kg−1 | 3.8 ± 0.76 | 8.7 ± 0.01 | 99.4 ± 0.88 | 94.2 ± 2.76 | 35.3 ± 1.88 | 14.9 ± 0.23 |
| Zn mg kg−1 | 356.0 ± 71.2 | 143.0 ± 1.30 | 4.1 ± 0.54 | 4.35 ± 5.25 | 1940.3 ± 42.71 * | 1797.3 ± 33.60 * |
| Fe mg kg−1 | 914.0 ± 182.20 | 1756.0 ± 15 | 61.7 ± 9.12 | 277.5 ± 10.45 | 4632.7 ± 175.02 | 59,622.1 ± 765.57 |
| Cu mg kg−1 | 25.9 ± 5.18 | 69.8 ± 1.10 | 0.5 ± 0.06 | 0.32 ± 0.13 | 417.2 ± 3.72 * | 609.4 ± 4.01 * |
| Al mg kg−1 | 1900.0 ± 380.00 | 1321.0 ± 185 | 39.9 ± 3.06 | 34.5 ± 3.33 | 7459.5 ± 1227.75 | 52,979.9 ± 295.65 |
| Cr mg kg−1 | 94.5 ± 18.9 | 6.6 ± 0.40 | 2.8 ± 0.05 | 2.2 ± 0.10 | 20.1 ± 1.50 | 111.6 ± 3.41 |
| Mn mg kg−1 | 53.1 ± 10.62 | 218.0 ± 0.50 | 128.3 ± 1.09 | 49.2 ± 1.58 | 1915.4 ± 44.97 | 955.0 ± 4.76 |
| Ni mg kg−1 | 29.4 ± 5.88 | 3.8 ± 0.20 | <0.6 | <0.6 | 21.8 ± 0.517 | 58.7 ± 1.87 * |
| Co mg kg−1 | 0.4 ± 0.08 | 1.6 ± 0.01 | <0.3 | <0.3 | 2.5 ± 0.51 | 12.1 ± 0.55 |
| Cd mg kg−1 | 17.8 ± 3.56 | 0.2 ± 0.03 | <0.15 | <0.15 | 1.0 ± 0.09 | 0.25 ± 0.02 |
| Pb mg kg−1 | <2.0 | <2.0 | <2.0 | <2.0 | 37.2 ± 44.24 | 19.7 ± 0.99 |
| As mg kg−1 | 5.4 ± 1.08 | <1.5 | <1.5 | <1.5 | <1.5 | <1.5 |
| Mo mg kg−1 | 13.4 ± 2.68 | 3.3 ± 0.10 | <0.5 | <0.5 | 12.4 ± 2.42 | 15.5 ± 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpinska, A.; Kakouli-Duarte, T.; Ashekuzzaman, S.M.; Byrne, J.; Schmalenberger, A.; Forrestal, P.J. Plant and Soil Effects of Alternative Sources of Phosphorus over Three Years of Application. Agronomy 2024, 14, 1591. https://doi.org/10.3390/agronomy14071591
Karpinska A, Kakouli-Duarte T, Ashekuzzaman SM, Byrne J, Schmalenberger A, Forrestal PJ. Plant and Soil Effects of Alternative Sources of Phosphorus over Three Years of Application. Agronomy. 2024; 14(7):1591. https://doi.org/10.3390/agronomy14071591
Chicago/Turabian StyleKarpinska, Anna, Thomais Kakouli-Duarte, S.M. Ashekuzzaman, John Byrne, Achim Schmalenberger, and Patrick J. Forrestal. 2024. "Plant and Soil Effects of Alternative Sources of Phosphorus over Three Years of Application" Agronomy 14, no. 7: 1591. https://doi.org/10.3390/agronomy14071591






