Changes in the Bioavailability of Ionizable Herbicides in Volcanic Soils Due to Soil Acidification by Urea as Fertilizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Herbicides
2.2. Soils
2.3. Microcosm Set-Up and Persistence of Herbicides
2.4. Adsorption Experiments
2.5. Herbicide Analysis
2.6. pH Changes in Soil–Urea Microcosms
2.7. Data Analysis
3. Results and Discussion
3.1. Persistence of Herbicides
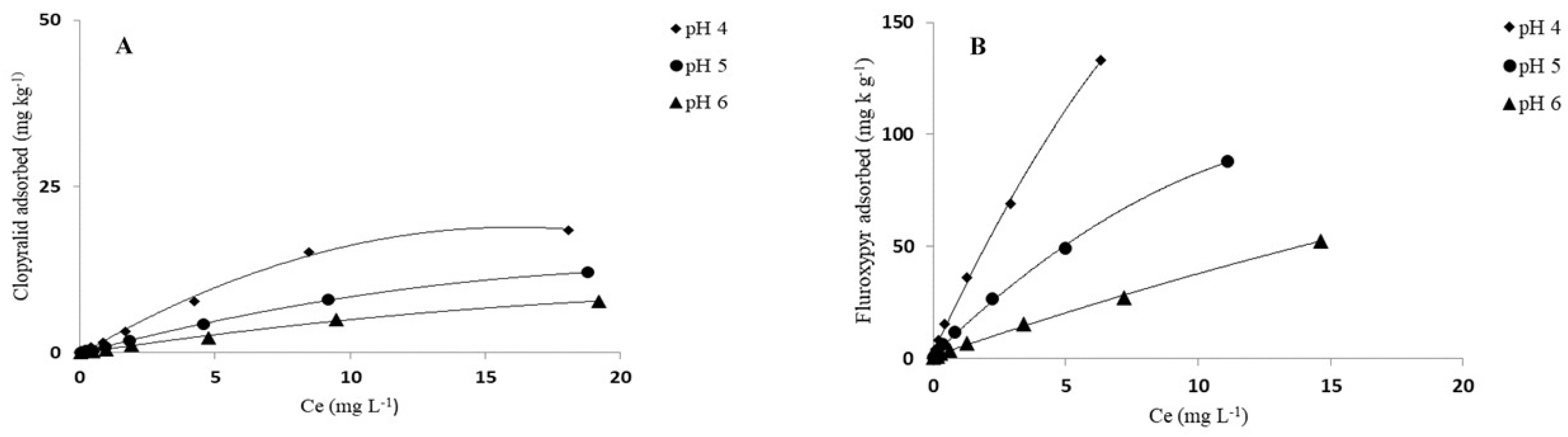
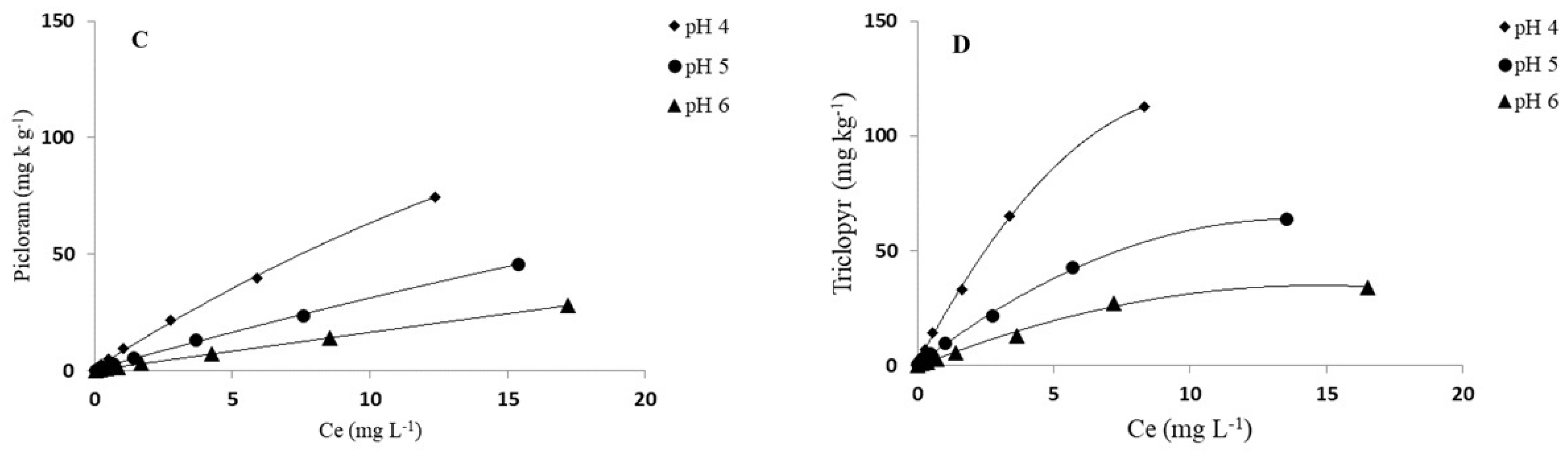
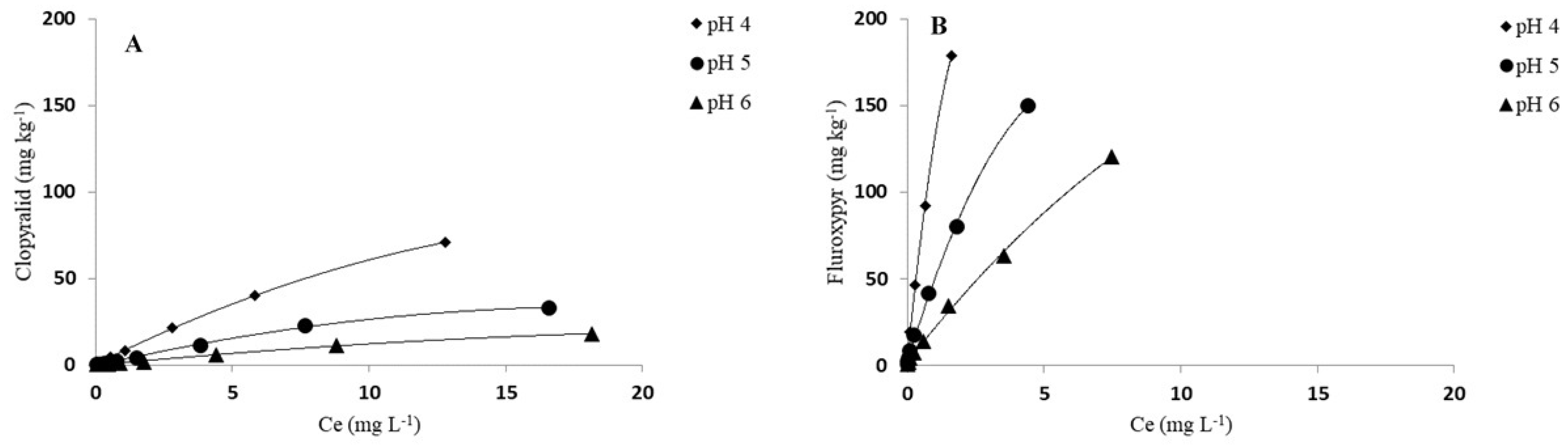
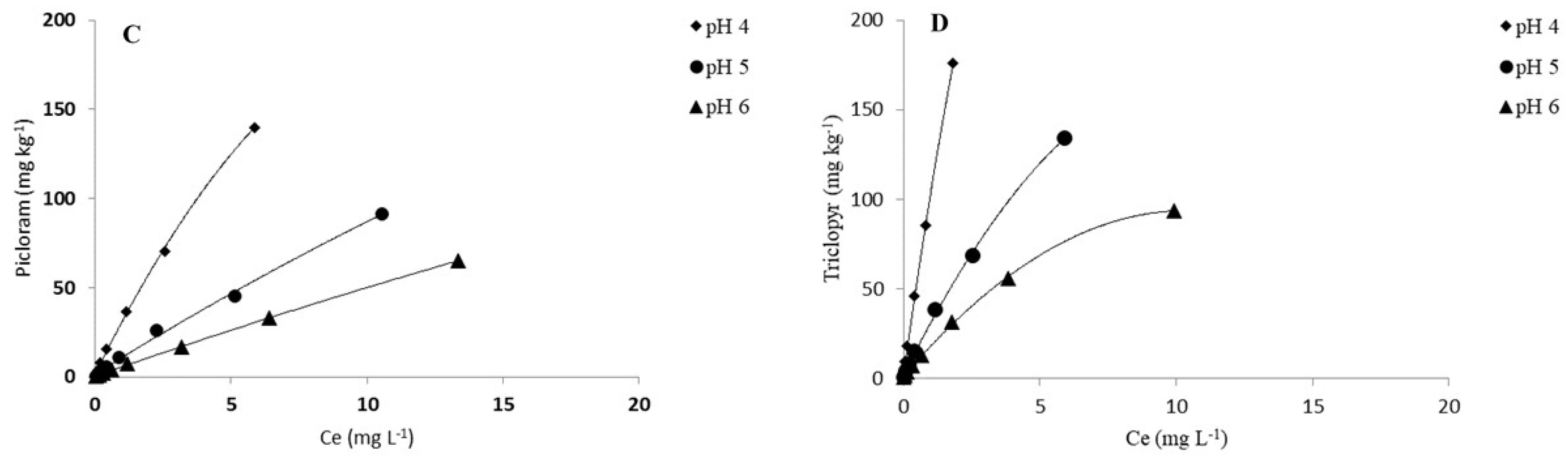
3.2. pH Effect on Adsorption of Herbicides
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Palma, G.; Spuler, M.J.; Jorquera, M.; Briceño, G. Effects of the combined application of nitrogen fertilizer and 2,4-D on nitrification ammonia oxidizers and herbicide bioavailability in a volcanic soil. A microcosm study. J. Soil Sci. Plant Nutr. 2023, 23, 4309–4317. [Google Scholar] [CrossRef]
- Dal Molin, S.J.; Ernani, P.R.; Gerber, J.M. Soil acidification and nitrogen release following application of nitrogen fertilizers. Commun. Soil Sci. Plant Anal. 2020, 51, 2551–2558. [Google Scholar] [CrossRef]
- Schroder, J.L.; Zhang, H.; Girma, K.; Raun, W.R.; Penn, C.J.; Payton, M.E. Soil Acidification from long-term use of nitrogen fertilizers on winter wheat. Soil Sci. Soc. Am. J. 2011, 75, 957–964. [Google Scholar] [CrossRef]
- Cartes, P.; Jara, A.; Demanet, R.; Mora, M.L. Urease activity and nitrogen mineralization kinetics as affected by temperature and urea input rate in southern Chilean Andisols. J. Soil Sci. Plant Nutr. 2009, 9, 69–82. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 1–9. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhou, X.; Guo, D.; Zhao, J.H.; Yan, L.; Feng, G.Z.; Gao, Q.; Yu, H.; Zhao, L.P. Soil pH is the primary factor driving the distribution and function of microorganisms in farmland soils in northeastern China. Ann. Microbiol. 2019, 69, 1461–1473. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Shen, R.F. Aluminum-nitrogen interactions in the soil-plant system. Front. Plant Sci. 2018, 9, 807. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Guan, D.; Zhou, B.; Zhao, B.; Ma, M.; Qin, J.; Jiang, X.; Chen, S.; Cao, F.; Shen, D.; et al. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms-A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Palma, G.; Jorquera, M.; Demanet, R.; Elgueta, S.; Briceño, G.; Mora, M.L. Urea fertilizer and pH influence on sorption process of flumetsulam and MCPA acidic herbicides in a volcanic soil. J. Environ. Qual. 2016, 45, 323–330. [Google Scholar] [CrossRef]
- Pinna, M.V.; Roggero, P.; Seddaiu, G.; Pusino, A. Soil sorption and leaching of active ingredients of lumax® under mineral or organic fertilization. Chemosphere 2014, 111, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Singh, N. Metribuzin mobility in soil columns as affected by urea fertilizer. Pest Manag. Sci. 2006, 62, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Singh, N. Organic manure and urea effect on metolachlor transport through packed soil columns. J. Environ. Qual. 2003, 32, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Entry, J.A. Influence of nitrogen on atrazine and 2, 4 dichlorophenoxyacetic acid mineralization in blackwater and redwater forested wetland soils. Biol. Fertil. Soils 1999, 29, 348–353. [Google Scholar] [CrossRef]
- Caracciolo, A.B.; Giuliano, G.; Grenni, P.; Cremisini, C.; Ciccoli, R.; Ubaldi, C. Effect of urea on degradation of terbuthylazine in soil. Environ. Toxicol. Chem. 2005, 24, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhou, J. Cypermethrin persistence and soil properties as affected by long-term fertilizer management. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2008, 58, 314–321. [Google Scholar] [CrossRef]
- Xie, W.; Zhou, J.; Wang, H.Y.; Chen, X.Q. Effect of nitrogen on the degradation of cypermethrin and its metabolite 3-phenoxybenzoic acid in soil. Pedosphere 2008, 18, 638–644. [Google Scholar] [CrossRef]
- Pesticide Properties DataBase (PPDB). Agriculture and Environment Research Unit. University of Hertfordshire, UK. 2013. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/index.htm (accessed on 12 March 2024).
- Pérez-Lucas, G.; Vela, N.; Abellán, M.; Fenoll, J.; Navarro, S. Use of index-based screening models to evaluate the leaching of triclopyr and fluroxypyr through a loam soil amended with vermicompost. Bull. Environ. Contam. Toxicol. 2020, 104, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Sakaliene, O.; Rice, P.; Koskinen, W.; Blažauskienė, G. Dissipation and transport of clopyralid in soil: Effect of application strategies. J. Agric. Food Chem. 2011, 59, 7891–7895. [Google Scholar] [CrossRef]
- Ulén, B.M.; Larsbo, M.; Kreuger, J.K.; Svanbäck, A. Spatial variation in herbicide leaching from a marine clay soil via subsurface drains. Pest Manag. Sci. 2014, 70, 405–414. [Google Scholar] [CrossRef]
- Palma, G.; Sánchez, A.; Olave, Y.; Encina, F.; Palma, R.; Barra, R. Pesticide levels in surface waters in an agricultural-forestry basin in Southern Chile. Chemosphere 2004, 57, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Cessna, A.J.; Grover, R.; Waite, D.T. Environmental fate of triclopyr. Rev. Environ. Contam. Toxicol. 2002, 174, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Cessna, A.; Nicholaichuk, W.; Tollefson, L. Leaching rates and preferential flow of selected herbicides through tilled and untilled soil. J. Environ. Qual. 2000, 29, 1650–1656. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Peer review of the pesticide risk assessment of the active substance clopyralid. EFSA J. 2018, 16, 5389. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Conclusion on the peer review of the pesticide risk assessment of the active substance fluroxypyr (evaluated variant fluroxypyr-methyl): Peer Review of the pesticide risk assessment of the active substance fluroxypyr. EFSA J. 2011, 9, 2091. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Conclusion on the peer review of the pesticide risk assessment of the active substance picloram: Peer review of the pesticide risk assessment of the active substance picloram. EFSA J. 2009, 7, 1390. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Conclusion regarding the peer review of the pesticide risk assessment of the active substance triclopyr. EFSA J. 2006, 4, 56r. [Google Scholar] [CrossRef]
- Kah, M.; Brown, C.D. Prediction of the adsorption of ionizable pesticides in soils. J. Agric. Food Chem. 2007, 55, 2312–2322. [Google Scholar] [CrossRef]
- Kah, M.; Brown, C.D. Adsorption of ionizable pesticides in soils. Rev. Environ. Contam. Toxicol. 2006, 188, 149–217. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Alonso, D.G.; Koskinen, W.C.; Papiernik, S.K. Comparative sorption, desorption and leaching potential of aminocyclopyrachlor and picloram. J. Environ. Sci. Health Part B-Pestic. Contam. Agric. Wastes 2013, 48, 1049–1057. [Google Scholar] [CrossRef]
- Kah, M.; Beulke, S.; Brown, C.D. Factors influencing degradation of pesticides in soil. J. Agric. Food Chem. 2007, 55, 4487–4492. [Google Scholar] [CrossRef] [PubMed]
- Tandon, S.; Singh, A. Residue behavior of clopyralid herbicide in soil and sugar beet crop under subtropical field conditions. J. Food Prot. 2022, 85, 735–739. [Google Scholar] [CrossRef]
- Passos, A.B.; Souza, M.F.; Silva, D.V.; Saraiva, D.T.; da Silva, A.A.; Zanuncio, J.C.; Gonçalves, B.F. Persistence of picloram in soil with different vegetation managements. Environ. Sci. Pollut. Res. Int. 2018, 25, 23986–23991. [Google Scholar] [CrossRef]
- Ahmad, R.; James, T.K.; Rahman, A.; Holland, P.T. Dissipation of the herbicide clopyralid in an allophanic soil: Laboratory and field studies. J. Environ. Sci. Health Part B-Pestic. Contam. Agric. Wastes 2003, 38, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, L.; Chen, L.; Pan, C. Residue dynamics of clopyralid and picloram in rape plant rapeseed and field soil. Bull. Environ. Contam. Toxicol. 2011, 86, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Douglass, C.H.; Nissen, S.J.; Meiman, P.J.; Kniss, A.R. Impacts of imazapyr and triclopyr soil residues on the growth of several restoration species. Rangel. Ecol. Manag. 2016, 69, 199–205. [Google Scholar] [CrossRef]
- Palma, G.; Demanet, R.; Jorquera, M.; Mora, M.L.; Briceño, G.; Violante, A. Effect of pH on sorption kinetic process of acidic herbicides in a volcanic soil. J. Soil Sci. Plant Nutr. 2015, 15, 549–560. [Google Scholar] [CrossRef]
- Marileo, L.G.; Jorquera, M.A.; Hernández, M.; Briceño, G.; Mora, M.L.; Demanet, R.; Palma, G. Changes in bacterial communities by post-emergent herbicides in an Andisol fertilized with urea as revealed by DGGE. Appl. Soil Ecol. 2016, 101, 141–151. [Google Scholar] [CrossRef]
- Escudey, M.; Forster, J.; Galindo, G. Relevance of organic matter in some chemical and physical characteristics of volcanic ash-derived soils. Commun. Soil Sci. Plant Anal. 2004, 35, 781–797. [Google Scholar] [CrossRef]
- Besoain, E. Mineralogía de los suelos volcánicos del centro sur de Chile. In Suelos Volcánicos de Chile; Tosso, J., Ed.; Instituto de Investigaciones Agropecuarias: Santiago, Chile, 1985; pp. 109–151. Available online: https://hdl.handle.net/20.500.14001/35623 (accessed on 12 March 2024).
- Sadzawka, A.; Carrasco, M.A.; Grez, R.; Mora, M.L.; Flores, H.; Neaman, A. Metodos de Análisis Recomendados para los Suelos de Chile. 2006. Available online: https://www.schcs.cl/wp-content/uploads/2018/11/Analisis-de-suelos.pdf (accessed on 12 March 2024).
- OECD. Test No. 307: Aerobic and Anaerobic Transformation in Soil, OECD Guidelines for the Testing of Chemicals, Section 3; OECD Publishing: Paris, France, 2002. [Google Scholar] [CrossRef]
- OECD. Test No. 106: Adsorption—Desorption Using a Batch Equilibrium Method, OECD Guidelines for the Testing of Chemicals, Section 1; OECD Publishing: Paris, France, 2000. [Google Scholar] [CrossRef]
- European Commission. SANCO/12571/2013, Guidance Document on Analytical Quality Control and Validation Procedures for Pesticide Residues Analysis in Food and Feed, European Commission, Health and Consumer Protection Directorate General. Available online: https://www.eurl-pesticides.eu/library/docs/allcrl/AqcGuidance_Sanco_2013_12571.pdf (accessed on 12 March 2024).
- European Commission. SANCO 825/00, rev. 8.1. Guidance Document on Pesticide Residue Analytical Methods, European Commission, Health and Consumer Protection Directorate General. 2010. Available online: https://www.biotecnologiebt.it/download/SANCO_825_00_rev8_1_2010.pdf (accessed on 12 March 2024).
- Tao, L.; Yang, H. Fluroxypyr biodegradation in soils by multiple factors. Environ. Monit. Assess. 2011, 175, 227–238. [Google Scholar] [CrossRef]
- Close, M.E.; Lee, R.; Sarmah, A.K.; Pang, L.; Dann, R.; Magesan, G.N.; Watt, J.P.; Vincent, K.W. Pesticide sorption and degradation characteristics in New Zealand soils-a synthesis from seven field trials. N. Z. J. Crop Hortic. Sci. 2008, 36, 9–30. [Google Scholar] [CrossRef]
- Cederlund, H.; Börjesson, E.; Jonsson, E.; Thierfelder, T. Degradation and leaching of fluroxypyr after application to railway tracks. J. Environ. Qual. 2012, 4, 1884–1892. [Google Scholar] [CrossRef]
- Prashar, P.; Shah, S. Impact of fertilizers and pesticides on soil microflora in agriculture. In Sustainable Agriculture Reviews; Springer: Cham, Switzerland, 2016; pp. 331–361. [Google Scholar] [CrossRef]
- Joner, E.J.; Eldhuset, T.D.; Lange, H.; Frostegard, A. Changes in the microbial community in a forest soil amended with aluminium in situ. Plant Soil 2005, 275, 295–304. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Gschwend, P.M.; Imboden, M. Environmental Organic Chemistry, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Caceres-Jensen, L.; Rodríguez, B.J.; Escudey, M. Impact of physical/chemical properties of volcanic ash-derived soils on mechanisms involved during sorption of ionizable and non-ionizable herbicides. In Advanced Sorption Process Applications; Edebil, D.S., Ed.; IntechOpen: London, UK, 2018; Available online: https://www.intechopen.com/chapters/63941 (accessed on 12 March 2024).
- Marco-Brown, J.L.; Gaigneaux, E.M.; Torres Sánchez, R.M.; Dos Santos, A.M. Adsorption of picloram on clays nontronite, illite and kaolinite: Equilibrium and herbicide-clays surface complexes. J. Environ. Sci. Health Part B-Pestic. Contam. Agric. Wastes 2019, 54, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, J.; Kah, M.; Brown, C.D. Adsorption and degradation of four acidic herbicides in soils from southern Spain. Pest Manag. Sci. 2008, 64, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Zabaloy, M.C.; Zanini, G.P.; Bianchinotti, V.; Gomez, M.A.; Garland, J.L. Herbicides in the Soil Environment: Linkage between Bioavailability and Microbial Ecology. In Herbicides, Theory and Applications; Larramendy, M., Ed.; IntechOpen: London, UK, 2011. Available online: https://ri.conicet.gov.ar/bitstream/handle/11336/109966/CONICET_Digital_Nro.e06921f8-1ce4-4bdd-b081-ce1d761f3b71_A.pdf?sequence=2&isAllowed=y (accessed on 12 March 2024).
- Wu, X.M.; Li, M.; Long, Y.H.; Liu, R.X.; Yu, Y.L.; Fang, H.; Li, S.N. Effects of adsorption on degradation and bioavailability of metolachlor in soil. J. Soil Sci. Plant Nutr. 2011, 11, 83–97. [Google Scholar]
- Park, J.H.; Kay, D.; Zhao, X.; Boyd, S.A.; Voice, T.C. Kinetic modeling of biovailability for sorbed-phase 2, 4-D. J. Environ. Qual. 2001, 30, 1523–1527. [Google Scholar] [CrossRef]

| Herbicide | Formula | Dose a (g a.i. ha−1) | Sw b (g L−1) | Log P c | pKa d | α e (%) | ||
|---|---|---|---|---|---|---|---|---|
| pH 4 | pH 5 | pH 6 | ||||||
| Clopyralid | 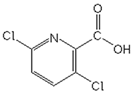 | 143 | 7.8 | −2.63 | 2.0 | 0.99 | 0.1 | 0.01 |
| Fluroxypyr | 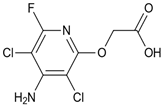 | 300 | 6.5 | 0.04 | 2.9 | 7.4 | 0.8 | 0.1 |
| Picloram | 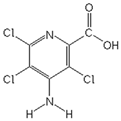 | 244 | 0.5 | −1.92 | 1.8 | 2.0 | 0.2 | 0.02 |
| Triclopyr | 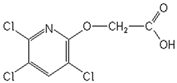 | 720 | 8.1 | 4.62 | 4.0 | 50.0 | 9.1 | 1.0 |
| Soil Order | Location a | pH | OM b (%) | CEC c (cmol(+)/kg) | Sand (%) | Silt (%) | Clay (%) | Texture |
|---|---|---|---|---|---|---|---|---|
| Andisol | FS | 5.68 | 12.0 | 8.20 | 39.7 | 42.9 | 17.3 | silty loam |
| Andisol | PNS | 5.14 | 20.0 | 2.97 | 54.8 | 41.0 | 4.2 | sandy loam |
| FS | PNS | |||||
|---|---|---|---|---|---|---|
| Treatment | k | t1/2 (d) | R2 | k | t1/2 | R2 |
| Clopyralid | ||||||
| 0N1C | 0.053 ± 0.01 | 13.1 | 0.996 | 0.045 ± 0.02 | 15.6 | 0.942 |
| 2N1C | 0.029 ± 0.01 | 23.9 | 0.981 | 0.037 ± 0.03 | 18.7 | 0.986 |
| 4N1C | 0.031± 0.02 | 22.4 | 0.995 | 0.025 ± 0.02 | 27.7 | 0.989 |
| Fluroxypyr | ||||||
| 0N1F | 0.088 ± 0.02 | 7.9 | 0.988 | 0.023 ± 0.03 | 30.1 | 0.982 |
| 2N1F | 0.074 ± 0.02 | 9.4 | 0.994 | 0.023 ± 0.03 | 30.1 | 0.993 |
| 4N1F | 0.068 ± 0.02 | 10.2 | 0.991 | 0.022 ± 0.02 | 31.5 | 0.989 |
| Picloram | ||||||
| 0N1P | 0.036 ± 0.03 | 19.3 | 0.997 | 0.037 ± 0.01 | 18.5 | 0.948 |
| 2N1P | 0.022 ± 0.02 | 31.5 | 0.998 | 0.034 ± 0.03 | 20.4 | 0.940 |
| 4N1P | 0.019 ± 0.03 | 36.5 | 0.996 | 0.025 ± 0.03 | 27.7 | 0.967 |
| Triclopyr | ||||||
| 0N1T | 0.116 ± 0.02 | 6.0 | 0.992 | 0.019 ± 0.02 | 36.5 | 0.937 |
| 2N1T | 0.121± 0.03 | 6.2 | 0.994 | 0.016 ± 0.03 | 43.3 | 0.952 |
| 4N1T | 0.109 ± 0.04 | 6.4 | 0.994 | 0.015 ± 0.03 | 46.2 | 0.948 |
| Soil | FS | PNS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Herbicide | Kf | 1/n | Kfoc | R2 | Kd | Koc | Kf | 1/n | Kfoc | R2 | Kd | Koc |
| Clopyralid | ||||||||||||
| pH 4 | 1.89 ± 0.07 | 0.87± 0.01 | 27 | 0.992 | 1.79 | 26 | 7.65 ± 0.05 | 0.92 ± 0.01 | 66 | 0.998 | 6.83 | 59 |
| pH 5 | 0.95 ± 0.05 | 0.90 ± 0.02 | 14 | 0.999 | 0.87 | 12 | 2.77 ± 0.06 | 0.97 ± 0.02 | 24 | 0.996 | 2.95 | 25 |
| pH 6 | 0.54 ± 0.02 | 0.90 ± 0.02 | 8 | 0.992 | 0.53 | 8 | 1.28 ± 0.05 | 0.88 ± 0.01 | 11 | 0.976 | 1.28 | 11 |
| Fluroxypyr | ||||||||||||
| pH 4 | 29.89 ± 0.15 | 0.80 ± 0.01 | 428 | 0.999 | 23.57 | 338 | 115.61± 0.90 | 0.73 ± 0.01 | 994 | 0.946 | 139.12 | 1196 |
| pH 5 | 13.66 ± 0.18 | 0.77 ± 0.01 | 196 | 0.998 | 9.90 | 142 | 47.04 ± 0.80 | 0.75 ± 0.01 | 405 | 0.991 | 44.25 | 380 |
| pH 6 | 5.32 ± 0.15 | 0.86 ± 0.01 | 76 | 0.999 | 3.77 | 54 | 23.18 ± 0.20 | 0.67 ± 0.01 | 199 | 0.971 | 17.90 | 154 |
| Picloram | ||||||||||||
| pH 4 | 8.65 ± 0.02 | 0.87± 0.02 | 124 | 0.998 | 6.74 | 97 | 32.23 ± 0.20 | 0.90 ± 0.02 | 277 | 0.995 | 27.75 | 239 |
| pH 5 | 3.92 ± 0.03 | 0.90 ± 0.01 | 56 | 0.999 | 3.11 | 45 | 11.80 ± 0.08 | 0.89 ± 0.01 | 101 | 0.998 | 8.82 | 76 |
| pH 6 | 1.79 ± 0.01 | 0.95 ± 0.01 | 26 | 0.999 | 1.62 | 23 | 5.83 ± 0.09 | 0.96 ± 0.02 | 50 | 0.995 | 5.12 | 44 |
| Triclopyr | ||||||||||||
| pH 4 | 21.84 ± 0.06 | 0.75 ± 0.01 | 313 | 0.991 | 19.36 | 277 | 109.40± 0.40 | 0.94 ± 0.01 | 940 | 0.992 | 103.95 | 894 |
| pH 5 | 8.73 ± 0.07 | 0.84 ± 0.02 | 125 | 0.997 | 7.48 | 107 | 31.72 ± 0.12 | 0.80 ± 0.03 | 273 | 0.997 | 26.99 | 232 |
| pH 6 | 3.83 ± 0.06 | 0.88 ± 0.02 | 55 | 0.994 | 3.75 | 54 | 17.50 ± 0.07 | 0.82 ± 0.01 | 150 | 0.997 | 14.46 | 124 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma, G.; Jorquera, M.; Ladino, A.; Benimeli, C.; Briceño, G. Changes in the Bioavailability of Ionizable Herbicides in Volcanic Soils Due to Soil Acidification by Urea as Fertilizer. Agronomy 2024, 14, 1617. https://doi.org/10.3390/agronomy14081617
Palma G, Jorquera M, Ladino A, Benimeli C, Briceño G. Changes in the Bioavailability of Ionizable Herbicides in Volcanic Soils Due to Soil Acidification by Urea as Fertilizer. Agronomy. 2024; 14(8):1617. https://doi.org/10.3390/agronomy14081617
Chicago/Turabian StylePalma, Graciela, Milko Jorquera, Aylin Ladino, Claudia Benimeli, and Gabriela Briceño. 2024. "Changes in the Bioavailability of Ionizable Herbicides in Volcanic Soils Due to Soil Acidification by Urea as Fertilizer" Agronomy 14, no. 8: 1617. https://doi.org/10.3390/agronomy14081617






