Suitable Water–Fertilizer Management and Ozone Synergy Can Enhance Substrate-Based Lettuce Yield and Water–Fertilizer Use Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Environmental Conditions
2.2. Substrate Physical Characteristics
2.3. Plant Material
2.4. Experimental Design and Treatments
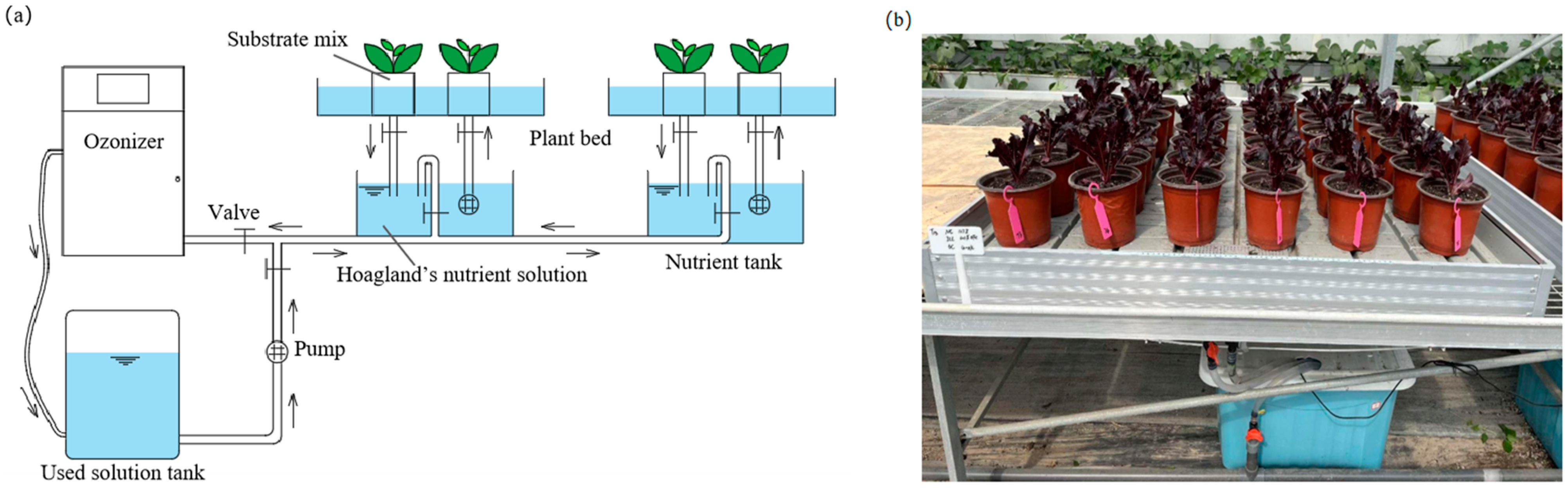
2.5. Soilless Culture Setup
2.6. Nutrient Solution Management
2.7. Analysis and Methods
- (1)
- Measurement of lettuce growth indicators:
- (2)
- Measurement of lettuce biomass and water content:
- (3)
- Measurement of lettuce nutrients:
- (4)
- Measurement of lettuce quality:
2.8. Data Processing
3. Results
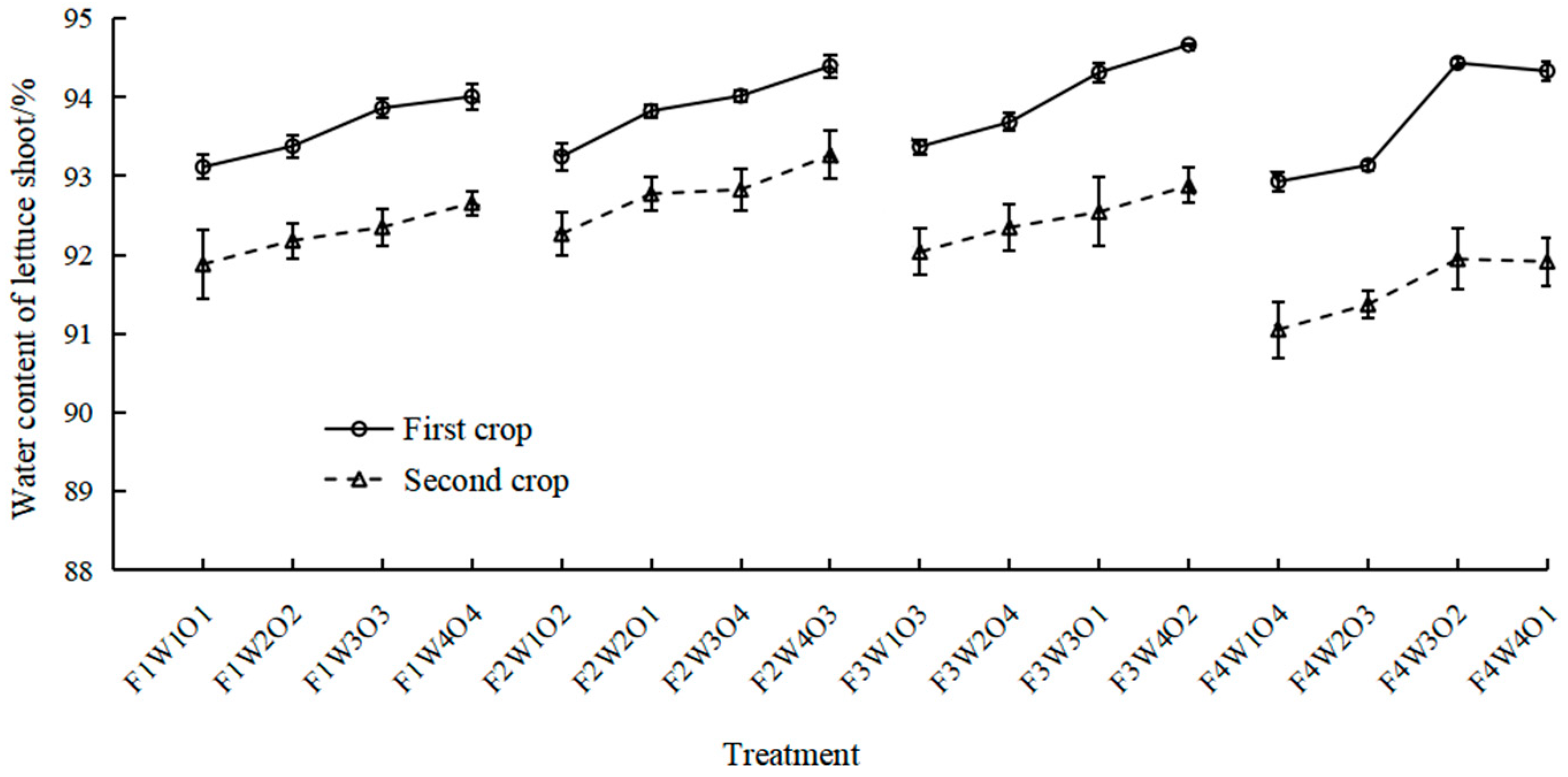
3.1. Effect of Different Treatments on Water Content of Lettuce Shoots
3.2. Effect of Different Treatments on Nutrient Concentration and Uptake in Lettuce
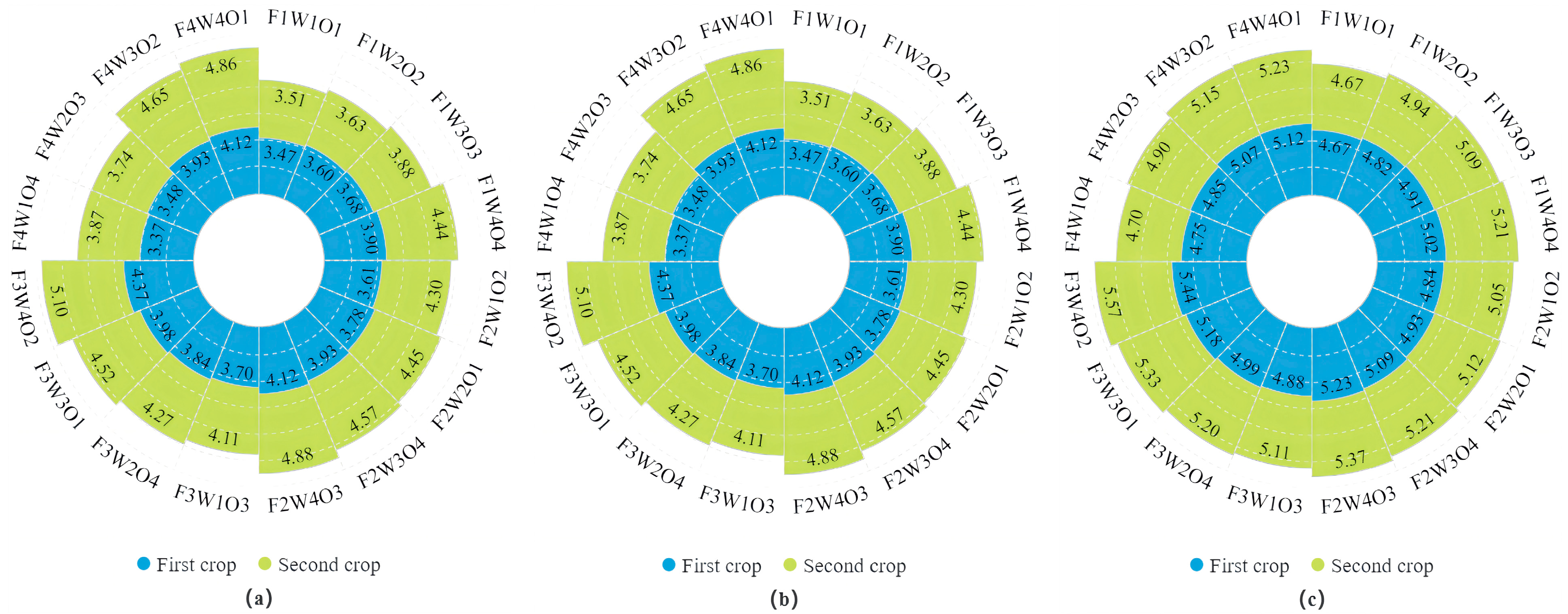
3.3. Effect of Different Treatments on Lettuce Biomass
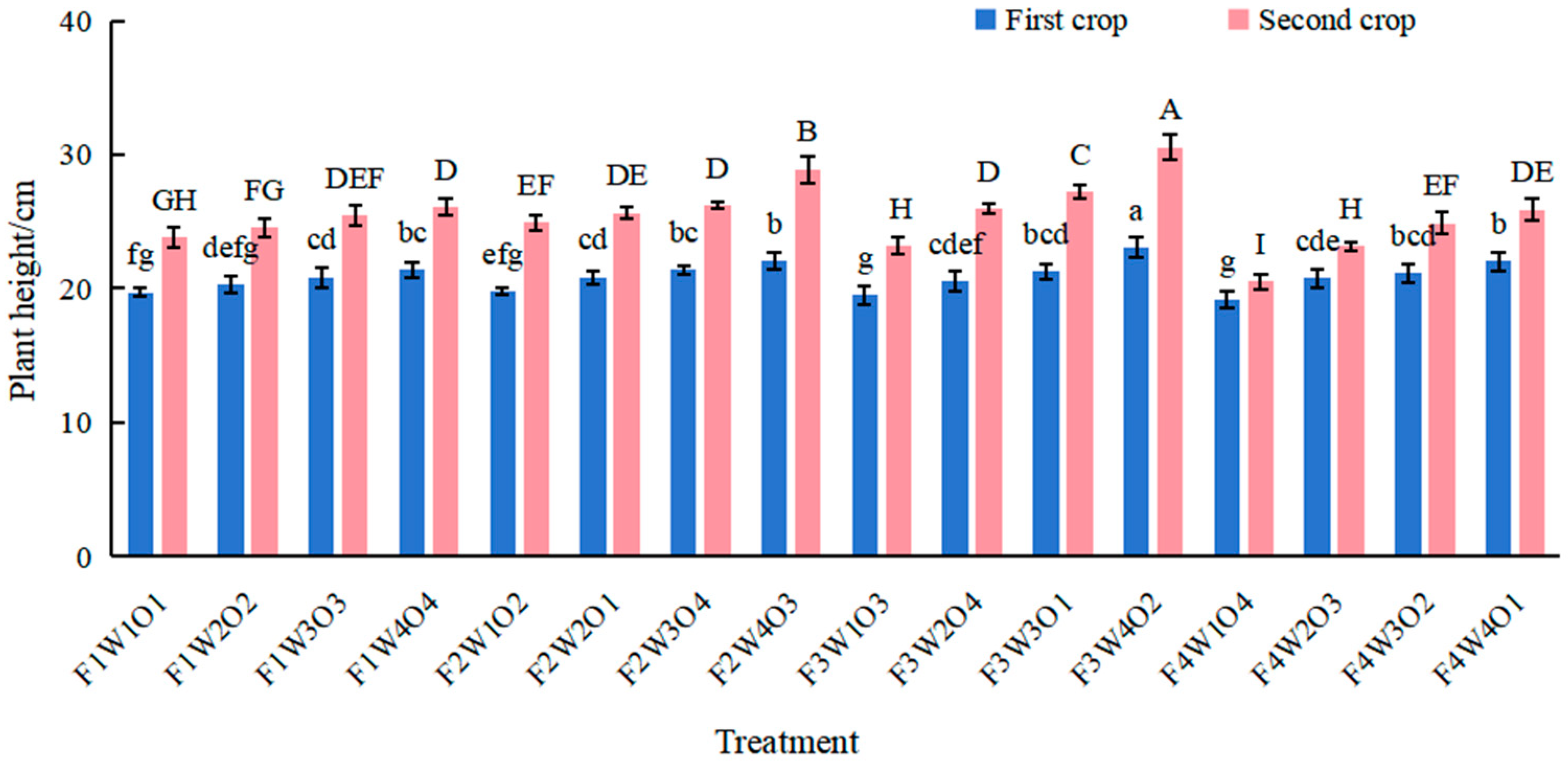
3.4. Effect of Different Treatments on Lettuce Plant Height and Crown Diameter
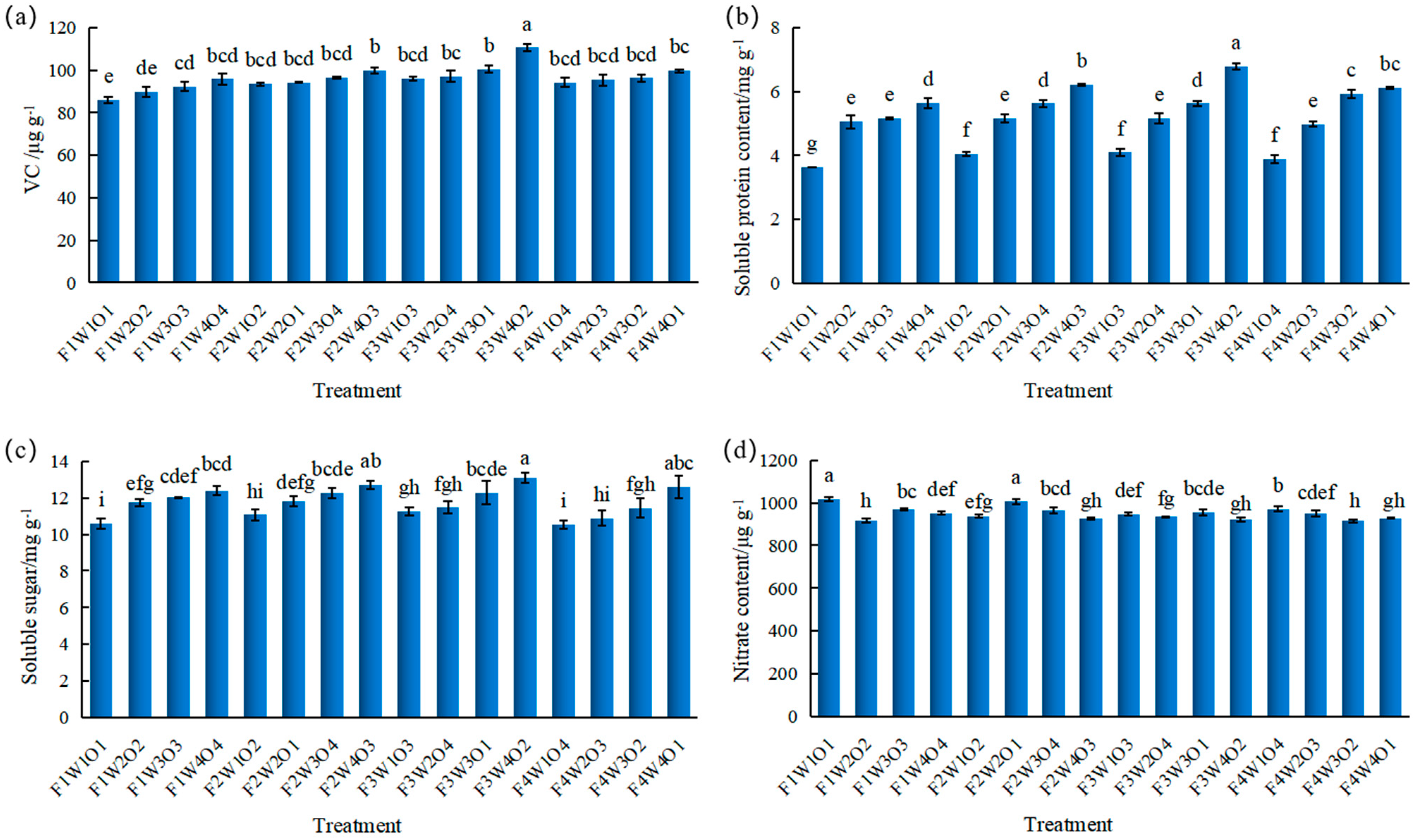
3.5. Effect of Different Treatments on Lettuce Yield and Quality
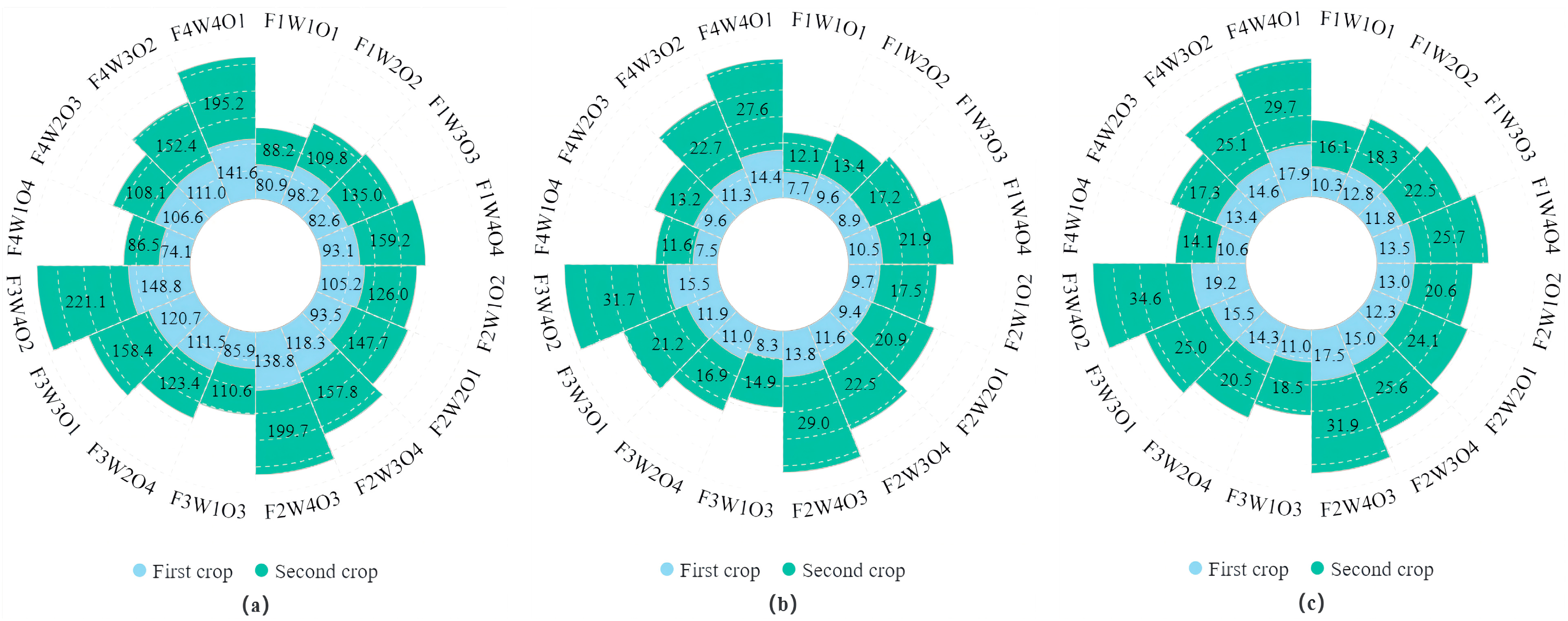
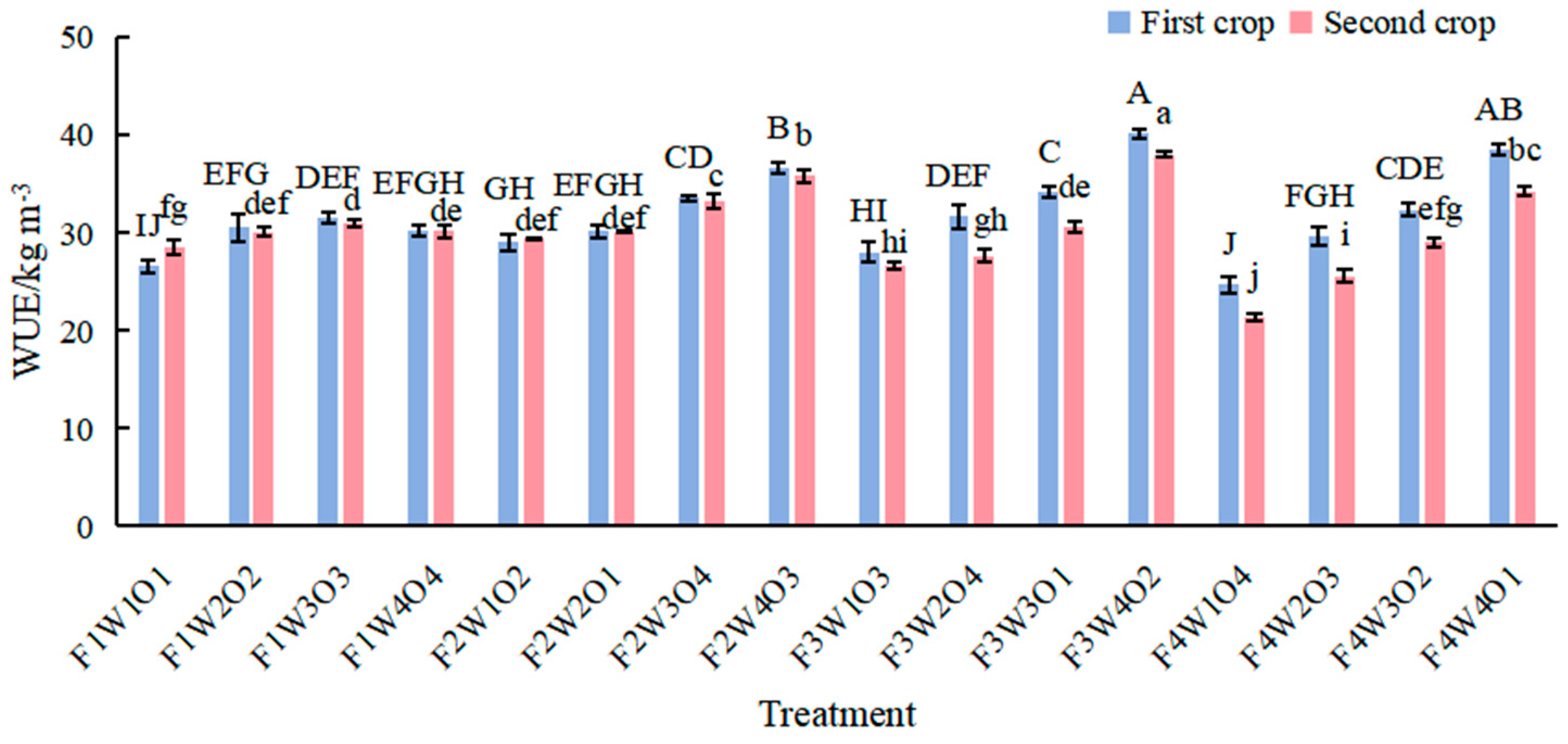
3.6. Effect of Different Treatments on Lettuce WUE
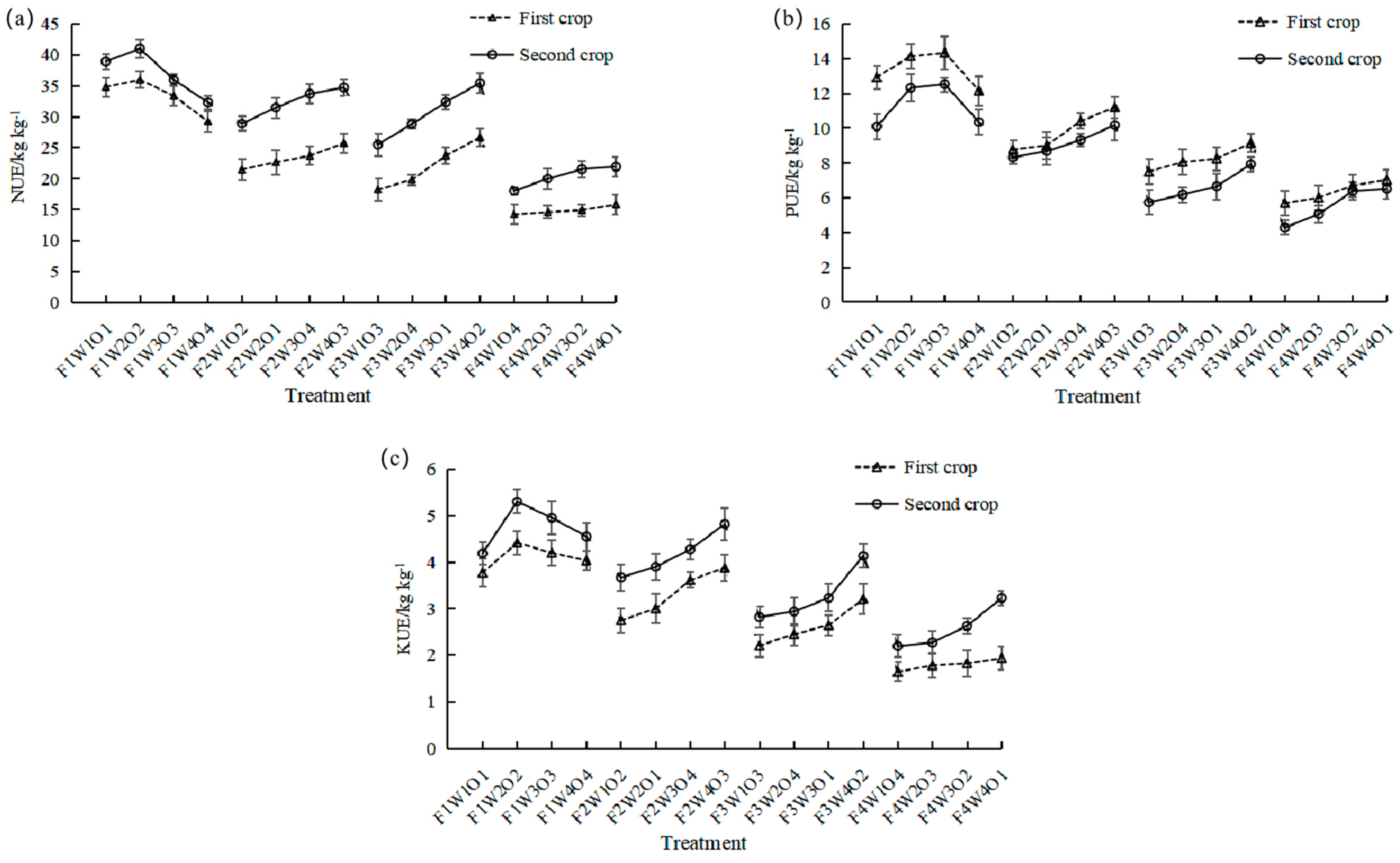
3.7. Effect of Different Treatments on Lettuce NPK Use Efficiency
3.8. Range and Variance Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Xiao, W.; Ye, Y.; He, T.; Luo, H. Rural Residential Land Expansion and Its Impacts on Cultivated Land in China between 1990 and 2020. Land Use Policy 2023, 132, 106816. [Google Scholar] [CrossRef]
- D’Amico, A.; De Boni, A.; Ottomano Palmisano, G.; Acciani, C.; Roma, R. Environmental Analysis of Soilless Tomato Production in a High-Tech Greenhouse. Clean. Environ. Syst. 2023, 11, 100137. [Google Scholar] [CrossRef]
- Voutsinos, O.; Mastoraki, M.; Ntatsi, G.; Liakopoulos, G.; Savvas, D. Comparative Assessment of Hydroponic Lettuce Production Either under Artificial Lighting, or in a Mediterranean Greenhouse during Wintertime. Agriculture 2021, 11, 503. [Google Scholar] [CrossRef]
- Goren, A.Y.; Eskisoy, D.N.; Genisoglu, S.; Okten, H.E. Microbial Desalination Cell Treated Spent Geothermal Brine as a Nutrient Medium in Hydroponic Lettuce Cultivation: Health Risk Assessment. Sci. Total Environ. 2024, 907, 167778. [Google Scholar] [CrossRef]
- Lei, C.; Engeseth, N.J. Comparison of Growth Characteristics, Functional Qualities, and Texture of Hydroponically Grown and Soil-Grown Lettuce. LWT 2021, 150, 111931. [Google Scholar] [CrossRef]
- Schmal, J.L.; Dumroese, R.K.; Davis, A.S.; Pinto, J.R.; Jacobs, D.F. Subirrigation for Production of Native Plants in Nurseries-Concepts, Current Knowledge, and Implementation. Nativ. Plants J. 2011, 12, 81–93. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Pinto, J.R.; Jacobs, D.F.; Davis, A.S.; Horiuchi, B. Subirrigation Reduces Water Use, Nitrogen Loss, and Moss Growth in a Container Nursery. Nativ. Plants J. 2006, 7, 253–261. [Google Scholar]
- Jesse, S.D.; Zhang, Y.; Margenot, A.J.; Davidson, P.C. Hydroponic Lettuce Production Using Treated Post-Hydrothermal Liquefaction Wastewater (PHW). Sustainability 2019, 11, 3605. [Google Scholar] [CrossRef]
- Cifuentes-Torres, L.; Mendoza-Espinosa, L.G.; Correa-Reyes, G.; Daesslé, L.W. Hydroponics with Wastewater: A Review of Trends and Opportunities. Water Environ. J. 2021, 35, 166–180. [Google Scholar] [CrossRef]
- Majid, M.; Khan, J.N.; Ahmad Shah, Q.M.; Masoodi, K.Z.; Afroza, B.; Parvaze, S. Evaluation of Hydroponic Systems for the Cultivation of Lettuce (Lactuca sativa L., Var. Longifolia) and Comparison with Protected Soil-Based Cultivation. Agric. Water Manag. 2021, 245, 106572. [Google Scholar] [CrossRef]
- Pei, Y.; Bie, Z. Effects of Different Irrigation Minima on the Growth and Physiological Characteristics of Lettuce under Plastic Greenhouse. Trans. Chin. Soc. Agric. Eng. 2008, 24, 207–211. [Google Scholar]
- Yazgan, S.; Ayas, S.; Demirtas, C.; Büyükangaz, H.; Candogan, B. Deficit Irrigation Effects on Lettuce (Lactuca sativa Var. Olenka) Yield in Unheated Greenhouse Condition. J. Food Agric. Environ. 2008, 6, 357–361. [Google Scholar]
- Stamford, N.P.; Felix, F.; Oliveira, W.; Silva, E.; Carolina, S.; Arnaud, T.; Freitas, A.D. Interactive Effectiveness of Microbial Fertilizer Enriched in N on Lettuce Growth and on Characteristics of an Ultisol of the Rainforest Region. Sci. Hortic. 2019, 247, 242–246. [Google Scholar] [CrossRef]
- Schmidt, D.; Santos, O.S.; Bonnecarrère, R.A.G.; Mariani, O.A.; Manfron, P.A. Desempenho de soluções nutritivas e cultivares de alface em hidroponia. Hortic. Bras. 2001, 19, 122–126. [Google Scholar] [CrossRef]
- Max, J.F.J.; Horst, W.J. Influence of Nighttime Electrical Conductivity of Substrate Solution on Fruit Cracking and Blossom-End Rot of Greenhouse Tomato in the Tropics. J. Plant Nutr. Soil Sci. 2009, 172, 829–838. [Google Scholar] [CrossRef]
- Zhang, Y.; Kiriiwa, Y.; Nukaya, A. Influence of Nutrient Concentration and Composition on the Growth, Uptake Patterns of Nutrient Elements and Fruit Coloring Disorder for Tomatoes Grown in Extremely Low-Volume Substrate. Hortic. J. 2015, 84, 37–45. [Google Scholar] [CrossRef]
- Li, Y.L.; Stanghellini, C.; Challa, H. Effect of Electrical Conductivity and Transpiration on Production of Greenhouse Tomato (Lycopersicon esculentum L.). Sci. Hortic. 2001, 88, 11–29. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Lucini, L.; Rea, E.; Colla, G. Nutrient Solution Concentration Affects Growth, Mineral Composition, Phenolic Acids, and Flavonoids in Leaves of Artichoke and Cardoon. HortScience 2012, 47, 1424–1429. [Google Scholar] [CrossRef]
- Neocleous, D.; Savvas, D. Effect of Different Macronutrient Cation Ratios on Macronutrient and Water Uptake by Melon (Cucumis melo) Grown in Recirculating Nutrient Solution. J. Plant Nutr. Soil Sci. 2015, 178, 320–332. [Google Scholar] [CrossRef]
- Gonnella, M.; Serio, F.; Conversa, G.; Santamaria, P. Production and nitrate content in lamb’s lettuce grown in floating system. Acta Hortic. 2004, 644, 61–68. [Google Scholar] [CrossRef]
- Cheng, G.; Li, Z.; Sun, L.; Li, Y.; Fu, J. Application of Microwave/Electrodeless Discharge Ultraviolet/Ozone Sterilization Technology in Water Reclamation. Process Saf. Environ. Prot. 2020, 138, 148–156. [Google Scholar] [CrossRef]
- Diksha; Samandeep; Rehal, J.; Kaur, J. Removal of Pesticide Residues in Food Using Ozone. Food Chem. Adv. 2023, 3, 100512. [Google Scholar] [CrossRef]
- Köhne, M.; Hofbauer, L.; Böttcher, D.; Tönissen, A.; Hegger, A.; Görgens, A.; Ulrich, R.; Sieme, H. Comparison of Systemic Trimethoprim-Sulfadimethoxine Treatment and Intrauterine Ozone Application as Possible Therapies for Bacterial Endometritis in Equine Practice. Front. Vet. Sci. 2023, 10, 1102149. [Google Scholar] [CrossRef]
- Khanashyam, A.C.; Shanker, M.A.; Kothakota, A.; Mahanti, N.K.; Pandiselvam, R. Ozone Applications in Milk and Meat Industry. Ozone Sci. Eng. 2022, 44, 50–65. [Google Scholar] [CrossRef]
- Krylova, L.N. Efficiency of Ozone Application for Extraction of Metals from Mineral Raw Materials. Russ. J. Non-Ferr. Met. 2022, 63, 247–255. [Google Scholar] [CrossRef]
- Gao, H.-L.; Wen, Z.-N.; Sun, B.-C.; Zou, H.-K.; Chu, G.-W. Intensification of Ozone Mass Transfer for Wastewater Treatment Using a Rotating Bar Reactor. Chem. Eng. Process.-Process Intensif. 2022, 176, 108946. [Google Scholar] [CrossRef]
- Najarian, M.; Mohammadi-Ghehsareh, A.; Fallahzade, J.; Peykanpour, E. Responses of Cucumber (Cucumissativus L.) to Ozonated Water under Varying Drought Stress Intensities. J. Plant Nutr. 2018, 41, 1–9. [Google Scholar] [CrossRef]
- Díaz-López, M.; Siles, J.A.; Ros, C.; Bastida, F.; Nicolás, E. The Effects of Ozone Treatments on the Agro-Physiological Parameters of Tomato Plants and the Soil Microbial Community. Sci. Total Environ. 2022, 812, 151429. [Google Scholar] [CrossRef]
- Yu, D.-Y.; Kang, N.; Bae, W.; Banks, M.K. Characteristics in Oxidative Degradation by Ozone for Saturated Hydrocarbons in Soil Contaminated with Diesel Fuel. Chemosphere 2007, 66, 799–807. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Osei-Owusu, J.; Chia, S.Y.; Dofuor, A.K.; Antwi-Agyakwa, A.K.; Okyere, H.; Gyan, M.; Edusei, G.; Ninsin, K.D.; Duker, R.Q.; et al. Remediation of Pesticide Residues Using Ozone: A Comprehensive Overview. Sci. Total Environ. 2023, 894, 164933. [Google Scholar] [CrossRef]
- Garrido, I.; Martínez-Escudero, C.M.; Contreras, F.; Flores, P.; Hellín, P.; Díaz-López, M.; Bastida, F.; Fenoll, J. Degradation of Four Pesticides by Ozonation under Field Conditions and Assessment of Its Influence on Soil Microbial Activity. J. Environ. Chem. Eng. 2023, 11, 110034. [Google Scholar] [CrossRef]
- Xu, J.-P.; Yu, Y.-C.; Zhang, T.; Ma, Q.; Yang, H.-B. Effects of Ozone Water Irrigation and Spraying on Physiological Characteristics and Gene Expression of Tomato Seedlings. Hortic. Res. 2021, 8, 180. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Z.; Li, Y.; Wang, Q. Effect of Different Concentrations of Ozone on In Vitro Plant Pathogens Development, Tomato Yield and Quality, Photosynthetic Activity and Enzymatic Activities. Ozone Sci. Eng. 2019, 41, 531–540. [Google Scholar] [CrossRef]
- Sharaf-Eldin, M.A.; Alshallash, K.S.; Alharbi, K.R.; Alqahtani, M.M.; Etman, A.A.; Yassin, A.M.; Azab, E.S.; El-Okkiah, S.A.F. Influence of Seed Soaking and Foliar Application Using Ozonated Water on Two Sweet Pepper Hybrids under Cold Stress. Sustainability 2022, 14, 13453. [Google Scholar] [CrossRef]
- Burkey, K.; Tisdale, R.; Zobel, R.; Ray, S.; Pursley, W. Interactive Effects of Elevated Ozone and Temperature on Growth and Yield of Soybean (Glycine max (L.) Merr.) under Field Conditions. Agronomy 2020, 10, 1803. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, C.; Song, W. Effect of Ozonated Nutrient Solution on the Growth and Root Antioxidant Capacity of Substrate and Hydroponically Cultivated Lettuce (Lactuca sativa). Ozone Sci. Eng. 2020, 42, 286–292. [Google Scholar] [CrossRef]
- Chen, H.; Dai, Z.; Jager, H.I.; Wullschleger, S.D.; Xu, J.; Schadt, C.W. Influences of Nitrogen Fertilization and Climate Regime on the Above-Ground Biomass Yields of Miscanthus and Switchgrass: A Meta-Analysis. Sustain. Energy Rev. 2019, 108, 303–311. [Google Scholar] [CrossRef]
- Cheng, Q.; Ma, J.; Ren, R.; Zheng, L.; Guo, X.; Sun, X. Effects of Fertilization Management under WSPI on Soil Nitrogen Distribution and Nitrogen Absorption in Apple Orchard in Loess Plateau. Agronomy 2020, 10, 1386. [Google Scholar] [CrossRef]
- Belay-Tedla, A.; Zhou, X.; Su, B.; Wan, S.; Luo, Y. Labile, Recalcitrant, and Microbial Carbon and Nitrogen Pools of a Tallgrass Prairie Soil in the US Great Plains Subjected to Experimental Warming and Clipping. Soil Biol. Biochem. 2009, 41, 110–116. [Google Scholar] [CrossRef]
- Yan, Y.; Lei, B.; Wang, L.; Bie, Z. Effects of Different Day and Night Temperature on the Growth and Quality of Hydroponic Lettuce. J. Chang. Veg. 2010, 24, 39–42. [Google Scholar]
- Li, R.; Zhu, Y.; Takagaki, M.; Yamori, W.; Yang, L. Effects of Root Zone Temperature on the Growth and Mineral Elements Content of Hydroponically-Grown Lettuce. Acta Agric. Shanghai 2015, 31, 48–52. (In Chinese) [Google Scholar]
- Chen, Y.; Yang, L.; He, Z. Effects of Temperature on Growth and Quality of Dormancy-Released Sedum aizoon L. IOP Conf. Ser. Earth Environ. Sci. 2019, 310, 052020. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, H.; Zhang, F.; Wang, X.; Liao, Z.; Zhang, S.; Yang, Q.; Fan, J. Effects of Irrigation and Fertilization Regimes on Tuber Yield, Water-Nutrient Uptake and Productivity of Potato under Drip Fertigation in Sandy Regions of Northern China. Agric. Water Manag. 2023, 287, 108459. [Google Scholar] [CrossRef]
- Ballester, C.; Hornbuckle, J.; Brinkhoff, J.; Quayle, W.C. Effects of Three Frequencies of Irrigation and Nitrogen Rates on Lint Yield, Nitrogen Use Efficiency and Fibre Quality of Cotton under Furrow Irrigation. Agric. Water Manag. 2021, 248, 106783. [Google Scholar] [CrossRef]
- Lefever, K.; Laubscher, C.; Ndakidemi, P. The Effects of pH and Phosphorus Concentrations on the Growth Potential of Medicinal Salvia chamelaeagnea in Hydroponic Cultivation. Acta Hortic. 2015, 1104, 29–34. [Google Scholar] [CrossRef]
- Ertek, A.; Şensoy, S.; Küçükyumuk, C.; Gedik, İ. Irrigation Frequency and Amount Affect Yield Components of Summer Squash (Cucurbita pepo L.). Agric. Water Manag. 2004, 67, 63–76. [Google Scholar] [CrossRef]
- Berners-Lee, M.; Kennelly, C.; Watson, R.; Hewitt, C.N. Current Global Food Production Is Sufficient to Meet Human Nutritional Needs in 2050 Provided There Is Radical Societal Adaptation. Elem. Sci. Anthr. 2018, 6, 52. [Google Scholar] [CrossRef]
- Miller, F.A.; Silva, C.L.M.; Brandão, T.R.S. A Review on Ozone-Based Treatments for Fruit and Vegetables Preservation. Food Eng. Rev. 2013, 5, 77–106. [Google Scholar] [CrossRef]
- Kleiber, T.; Borowiak, K.; Schroeter-Zakrzewska, A.; Budka, A.; Osiecki, S. Effect of Ozone Treatment and Light Colour on Photosynthesis and Yield of Lettuce. Sci. Hortic. 2017, 217, 130–136. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Wang, T.; Pan, J.; Zhou, B.; Muhammad, T.; Zhou, C.; Li, Y. Micro-Nano Bubble Water Oxygation: Synergistically Improving Irrigation Water Use Efficiency, Crop Yield and Quality. J. Clean. Prod. 2019, 222, 835–843. [Google Scholar] [CrossRef]
- Sey, B.K.; Manceur, A.M.; Whalen, J.K.; Gregorich, E.G.; Rochette, P. Root-Derived Respiration and Nitrous Oxide Production as Affected by Crop Phenology and Nitrogen Fertilization. Plant Soil 2010, 326, 369–379. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Xu, F.; Muhammad, T.; Li, Y. Appropriate Dissolved Oxygen Concentration and Application Stage of Micro-Nano Bubble Water Oxygation in Greenhouse Crop Plantation. Agric. Water Manag. 2019, 223, 105713. [Google Scholar] [CrossRef]
- Cengiz, M.; Uslu, M.O.; Balcioglu, I. Treatment of E. coli HB101 and the TetM Gene by Fenton’s Reagent and Ozone in Cow Manure. J. Environ. Manage. 2010, 91, 2590–2593. [Google Scholar] [CrossRef] [PubMed]
- Karaca, H.; Velioglu, Y.S. Effects of Ozone Treatments on Microbial Quality and Some Chemical Properties of Lettuce, Spinach, and Parsley. Postharvest Biol. Technol. 2014, 88, 46–53. [Google Scholar] [CrossRef]
- Rai, R.; Agrawal, M.; Kumar Choudhary, K.; Agrawal, S.B.; Emberson, L.; Büker, P. Application of Ethylene Diurea (EDU) in Assessing the Response of a Tropical Soybean Cultivar to Ambient O3: Nitrogen Metabolism, Antioxidants, Reproductive Development and Yield. Ecotoxicol. Environ. Saf. 2015, 112, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Kwak, M.J.; Jeong, S.G.; Woo, S.Y. Individual and Interactive Effects of Elevated Ozone and Temperature on Plant Responses. Horticulturae 2022, 8, 211. [Google Scholar] [CrossRef]
- Wilkinson, S.; Mills, G.; Illidge, R.; Davies, W.J. How Is Ozone Pollution Reducing Our Food Supply? J. Exp. Bot. 2012, 63, 527–536. [Google Scholar] [CrossRef]
- Guo, G.; Li, X.; Kuai, J.; Zhang, X.; Peng, X.; Xu, Y.; Zeng, G.; Liu, J.; Zhang, C.; Lin, J. Estimation of Annual Soil CO2 Efflux under the Erosion and Deposition Conditions by Measuring and Modeling Its Respiration Rate in Southern China. J. Environ. Manage. 2024, 351, 119686. [Google Scholar] [CrossRef] [PubMed]
- Dejie, K.; Liu, N.; Li, H.; Wang, W.; Li, N.; Ren, G.; Yang, G. Effect of Nitrogen Fertilizer on Soil CO2 Emission Depends on Crop Rotation Strategy. Sustainability 2020, 12, 5271. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, L.; Dai, Y.; Di, H.-J.; He, J.-Z. pH-Dependent Distribution of Soil Ammonia Oxidizers across a Large Geographical Scale as Revealed by High-Throughput Pyrosequencing. J. Soils Sediments 2013, 13, 1439–1449. [Google Scholar] [CrossRef]
- Sharma, A.; Sepehya, S.; Sharma, R.; Kumar, A. Impact of Various Cropping Systems on the Status and Distribution of Selected Micronutrients in Subtropical Region of Himachal Pradesh, India. Int. J. Plant Soil Sci. 2023, 35, 454–463. [Google Scholar] [CrossRef]
- Pan, Z.; He, P.; Fan, D.; Jiang, R.; Song, D.; Song, L.; Zhou, W.; He, W. Global Impact of Enhanced-Efficiency Fertilizers on Vegetable Productivity and Reactive Nitrogen Losses. Sci. Total Environ. 2024, 926, 172016. [Google Scholar] [CrossRef]



| Bulk Density (g·cm−3) | Total Pore Porosity (%) | Ventilation Pore Porosity (%) | Water-Holding Porosity (%) |
|---|---|---|---|
| 0.22 | 67.52 | 20.84 | 46.68 |
| pH | EC (μS·cm−1) | N (g·kg−1) | P (g·kg−1) | K (g·kg−1) | SOM (g·kg−1) |
|---|---|---|---|---|---|
| 6.7 | 380 | 20.35 | 0.21 | 5.64 | 164 |
| Formulation Elements | Dissolved Substance | Concentration (mg·L−1) |
|---|---|---|
| Major elements | Ca(NO3)2·4H2O | 945 |
| MgSO4 | 493 | |
| (NH4)3PO4 | 115 | |
| KNO3 | 607 | |
| Ferrous salt | DPTA-Fe-11 | 30 |
| Minor elements | HBO3 | 2.86 |
| CuSO4·5H2O | 0.08 | |
| ZnSO4·7H2O | 0.22 | |
| MnSO4·4H2O | 2.13 | |
| (NH4)6Mo7O24·4H2O | 0.02 |
| Treatment | Nutrient (F) Solution Concentration (%) | Lower Irrigation Threshold (W) (%FC) | Ozone (O) Concentration (mg·L−1) | EC (mS·cm−1) | pH |
|---|---|---|---|---|---|
| F1W1O1 | 50 (F1) | 40 (W1) | 0 (O1) | 1.3 | 6.52 |
| F1W2O2 | 50 | 55 (W2) | 1 (O2) | 1.3 | 6.53 |
| F1W3O3 | 50 | 70 (W3) | 2 (O3) | 1.3 | 6.52 |
| F1W4O4 | 50 | 85 (W4) | 4 (O4) | 1.3 | 6.51 |
| F2W1O2 | 75 (F2) | 40 | 1 | 1.8 | 6.51 |
| F2W2O1 | 75 | 55 | 0 | 1.8 | 6.53 |
| F2W3O4 | 75 | 70 | 4 | 1.8 | 6.52 |
| F2W4O3 | 75 | 85 | 2 | 1.8 | 6.51 |
| F3W1O3 | 100 (F3) | 40 | 2 | 2.2 | 6.50 |
| F3W2O4 | 100 | 55 | 4 | 2.2 | 6.52 |
| F3W3O1 | 100 | 70 | 0 | 2.2 | 6.51 |
| F3W4O2 | 100 | 85 | 1 | 2.2 | 6.51 |
| F4W1O4 | 125 (F4) | 40 | 1 | 2.6 | 6.50 |
| F4W2O3 | 125 | 55 | 4 | 2.6 | 6.49 |
| F4W3O2 | 125 | 70 | 2 | 2.6 | 6.48 |
| F4W4O1 | 125 | 85 | 0 | 2.6 | 6.50 |
| Crops | Sampling Time | ||||
|---|---|---|---|---|---|
| First | Second | Third | Fourth | Fifth | |
| First crop | 27-March | 2-April | 8-April | 14-April | 20-April |
| Second crop | 9-May | 15-May | 21-May | 27-May | 2-June |
| Treatment | Shoot Fresh Weight (g) | Shoot Dry Weight (g) | Root Fresh Weight (g) | Root Dry Weight (g) | Root to Shoot Ratio | Shoot Dry Matter Rate (%) |
|---|---|---|---|---|---|---|
| F1W1O1 | 34.93 ± 1.20 i | 2.20 ± 0.11 g | 5.92 ± 0.14 i | 0.43 ± 0.06 h | 0.169 ± 0.003 d | 6.27 ± 0.51 d |
| F1W2O2 | 41.27 ± 2.19 gh | 2.65 ± 0.12 de | 7.46 ± 0.10 de | 0.49 ± 0.03 cdefg | 0.181 ± 0.009 c | 6.41 ± 0.25 cd |
| F1W3O3 | 44.44 ± 1.14 efg | 2.40 ± 0.11 fg | 7.00 ± 0.18 fg | 0.46 ± 0.05 fgh | 0.158 ± 0.002 f | 5.39 ± 0.36 i |
| F1W4O4 | 45.16 ± 1.38 ef | 2.68 ± 0.14 cde | 7.37 ± 0.17 e | 0.50 ± 0.03 bcdef | 0.163 ± 0.002 ef | 5.93 ± 0.39 ef |
| F2W1O2 | 40.87 ± 1.61 gh | 2.68 ± 0.17 cde | 7.87 ± 0.12 c | 0.52 ± 0.01 bcde | 0.193 ± 0.005 b | 6.55 ± 0.45 c |
| F2W2O1 | 43.16 ± 1.27 fgh | 2.50 ± 0.04 ef | 7.70 ± 0.29 cd | 0.49 ± 0.01 cdefgh | 0.178 ± 0.003 c | 5.78 ± 0.08 fg |
| F2W3O4 | 50.19 ± 0.95 cd | 2.95 ± 0.09 b | 7.43 ± 0.13 e | 0.47 ± 0.02 efgh | 0.148 ± 0.000 j | 5.87 ± 0.06 ef |
| F2W4O3 | 59.48 ± 1.44 b | 3.34 ± 0.16 a | 8.32 ± 0.18 b | 0.54 ± 0.02 abc | 0.140 ± 0.001 h | 5.61 ± 0.14 gh |
| F3W1O3 | 39.89 ± 1.82 h | 2.24 ± 0.11 g | 7.22 ± 0.19 ef | 0.46 ± 0.03 fgh | 0.181 ± 0.004 c | 5.61 ± 0.57 gh |
| F3W2O4 | 47.53 ± 2.16 de | 2.86 ± 0.07 bc | 7.90 ± 0.16 c | 0.49 ± 0.02 cdefgh | 0.166 ± 0.006 de | 6.01 ± 0.34 e |
| F3W3O1 | 52.49 ± 1.29 c | 2.92 ± 0.02 b | 7.79 ± 0.14 c | 0.52 ± 0.01 abcd | 0.148 ± 0.001 j | 5.56 ± 0.27 gh |
| F3W4O2 | 66.19 ± 1.37 a | 3.53 ± 0.07 a | 8.68 ± 0.27 a | 0.55 ± 0.04 ab | 0.131 ± 0.002 i | 5.33 ± 0.19 i |
| F4W1O4 | 31.57 ± 1.20 i | 2.23 ± 0.12 g | 6.52 ± 0.07 h | 0.47 ± 0.04 efgh | 0.207 ± 0.006 a | 7.07 ± 0.13 a |
| F4W2O3 | 40.31 ± 1.42 h | 2.77 ± 0.10 bcd | 6.88 ± 0.10 g | 0.44 ± 0.03 gh | 0.171 ± 0.004 d | 6.86 ± 0.06 b |
| F4W3O2 | 51.58 ± 1.27 c | 2.87 ± 0.07 bc | 7.76 ± 0.20 c | 0.48 ± 0.04 defgh | 0.150 ± 0.002 j | 5.57 ± 0.05 h |
| F4W4O1 | 61.46 ± 1.09 b | 3.48 ± 0.13 a | 8.57 ± 0.16 a | 0.57 ± 0.05 a | 0.139 ± 0.004 h | 5.67 ± 0.12 gh |
| Treatment | Shoot Fresh Weight (g) | Shoot Dry Weight (g) | Root Fresh Weight (g) | Root Dry Weight (g) | Root to Shoot Ratio | Shoot Dry Matter Rate (%) |
|---|---|---|---|---|---|---|
| F1W1O1 | 45.08 ± 1.66 i | 3.43 ± 0.12 i | 6.48 ± 0.13 j | 0.50 ± 0.04 f | 0.144 ± 0.003 d | 7.60 ± 0.17 ghi |
| F1W2O2 | 51.95 ± 1.05 gh | 3.70 ± 0.09 h | 7.25 ± 0.08 i | 0.53 ± 0.07 ef | 0.140 ± 0.001 e | 7.12 ± 0.03 j |
| F1W3O3 | 59.24 ± 1.34 ef | 4.41 ± 0.18 f | 8.26 ± 0.11 g | 0.63 ± 0.04 bcde | 0.139 ± 0.002 e | 7.45 ± 0.17 i |
| F1W4O4 | 62.47 ± 1.89 de | 4.93 ± 0.08 d | 9.67 ± 0.09 c | 0.74 ± 0.01 b | 0.155 ± 0.003 c | 7.86 ± 0.22 ef |
| F2W1O2 | 52.69 ± 0.66 g | 4.07 ± 0.10 g | 8.24 ± 0.17 g | 0.64 ± 0.07 bcde | 0.156 ± 0.002 c | 7.73 ± 0.56 fgh |
| F2W2O1 | 57.08 ± 0.43 f | 4.69 ± 0.09 e | 9.40 ± 0.15 d | 0.74 ± 0.06 b | 0.165 ± 0.002 b | 8.22 ± 0.77 cd |
| F2W3O4 | 63.19 ± 2.02 d | 4.91 ± 0.07 d | 8.86 ± 0.14 e | 0.67 ± 0.11 bcd | 0.140 ± 0.002 e | 7.76 ± 0.13 fg |
| F2W4O3 | 76.49 ± 1.65 b | 5.94 ± 0.13 b | 10.82 ± 0.14 b | 0.92 ± 0.08 a | 0.141 ± 0.002 de | 7.76 ± 0.39 fg |
| F3W1O3 | 43.35 ± 1.09 i | 3.62 ± 0.10 h | 7.11 ± 0.18 i | 0.57 ± 0.03 def | 0.164 ± 0.001 b | 8.35 ± 0.34 bc |
| F3W2O4 | 49.06 ± 1.52 h | 3.95 ± 0.05 g | 7.64 ± 0.06 h | 0.61 ± 0.07 cdef | 0.156 ± 0.004 c | 8.05 ± 0.17 de |
| F3W3O1 | 62.10 ± 1.58 de | 4.69 ± 0.16 e | 9.00 ± 0.20 e | 0.71 ± 0.12 bc | 0.145 ± 0.001 d | 7.55 ± 0.11 hi |
| F3W4O2 | 81.46 ± 0.90 a | 6.21 ± 0.15 a | 11.53 ± 0.06 a | 1.02 ± 0.10 a | 0.142 ± 0.001 de | 7.62 ± 0.19 ghi |
| F4W1O4 | 33.54 ± 0.76 j | 3.00 ± 0.06 j | 6.06 ± 0.06 k | 0.49 ± 0.02 f | 0.181 ± 0.002 a | 8.94 ± 0.13 a |
| F4W2O3 | 43.69 ± 1.55 i | 3.53 ± 0.10 hi | 6.23 ± 0.07 k | 0.50 ± 0.08 f | 0.143 ± 0.003 de | 8.08 ± 0.05 de |
| F4W3O2 | 57.87 ± 1.16 f | 4.87 ± 0.13 d | 7.46 ± 0.23 h | 0.62 ± 0.08 cde | 0.129 ± 0.003 f | 8.42 ± 0.39 b |
| F4W4O1 | 70.46 ± 1.61 c | 5.67 ± 0.10 c | 8.53 ± 0.18 f | 0.69 ± 0.06 bc | 0.121 ± 0.001 j | 8.04 ± 0.48 de |
| Treatment | Yield (t·ha−1) | |
|---|---|---|
| First Crop | Second Crop | |
| F1W1O1 | 10.14 ± 0.46 i | 11.46 ± 0.42 i |
| F1W2O2 | 10.49 ± 0.56 gh | 13.20 ± 0.27 gh |
| F1W3O3 | 11.30 ± 0.29 efg | 15.06 ± 0.34 ef |
| F1W4O4 | 11.48 ± 0.35 ef | 17.08 ± 0.35 de |
| F2W1O2 | 10.39 ± 0.41 gh | 13.39 ± 0.17 g |
| F2W2O1 | 10.97 ± 0.32 fgh | 14.51 ± 0.11 f |
| F2W3O4 | 12.76 ± 0.24 cd | 16.06 ± 0.51 d |
| F2W4O3 | 15.62 ± 0.28 b | 20.59 ± 0.46 b |
| F3W1O3 | 8.88 ± 0.30 h | 11.02 ± 0.28 i |
| F3W2O4 | 12.08 ± 0.55 de | 12.47 ± 0.39 h |
| F3W3O1 | 13.34 ± 0.33 c | 15.78 ± 0.40 de |
| F3W4O2 | 16.82 ± 0.35 a | 20.70 ± 0.23 a |
| F4W1O4 | 8.02 ± 0.30 i | 8.52 ± 0.19 j |
| F4W2O3 | 10.25 ± 0.36 h | 11.1 ± 0.39 i |
| F4W3O2 | 13.11 ± 0.32 c | 14.71 ± 0.30 f |
| F4W4O1 | 15.12 ± 0.37 b | 17.91 ± 0.41 c |
| Factor | F | W | O | F × O | F × W | W × O | ||
|---|---|---|---|---|---|---|---|---|
| Yield | First crop | 11.34 ** | 37.87 ** | 7.36 ** | 42.82 ** | 13.30 ** | 17.28 ** | |
| Second crop | 0.74 ** | 2.44 ** | 0.47 ** | 2.83 ** | 0.85 ** | 1.12 ** | ||
| Quality | VC | First crop | 811.51 ** | 751.33 ** | 49.29 * | 859.55 ** | 157.51 ** | 919.73 ** |
| Soluble protein | First crop | 2.50 ** | 44.72 ** | 1.45 ** | 45.20 ** | 1.93 ** | 2.98 ** | |
| Soluble sugar | First crop | 4.19 ** | 29.54 ** | 0.34 ns | 31.39 ** | 2.19 * | 6.04 ** | |
| Nitrate | First crop | 7052.55 ** | 10,592.41 ** | 23,398.08 ** | 22,392.49 ** | 3519.16 ** | 18,853.63 ** | |
| WUE | First crop | 123.45 ** | 735.37 ** | 79.88 ** | 825.00 ** | 169.51 ** | 213.08 ** | |
| Second crop | 175.03 ** | 586.75 ** | 113.55 ** | 678.85 ** | 205.65 ** | 267.14 ** | ||
| FUE | NUE | First crop | 2774.92 ** | 42.34 * | 86.72 ** | 234.19 ** | 278.57 ** | 2966.77 ** |
| Second crop | 2365.07 ** | 109.62 ** | 135.09 ** | 378.69 ** | 404.16 ** | 2634.14 ** | ||
| PUE | First crop | 427.57 ** | 15.36 ** | 5.03 * | 33.86 ** | 23.53 ** | 446.07 ** | |
| Second crop | 321.16 ** | 28.20 ** | 12.92 ** | 39.25 ** | 23.96 ** | 332.20 ** | ||
| KUE | First crop | 46.38 ** | 3.90 ** | 0.43 ns | 6.16 ** | 2.69 ** | 48.64 ** | |
| Second crop | 44.04 ** | 7.76 ** | 1.64 ** | 11.16 ** | 5.05 ** | 47.44 ** | ||
| Factor | Index | Yield (t·ha−1) | VC (μg·g−1) | Protein (mg·g−1) | Soluble Sugar (mg·g−1) | Nitrate (μg·g−1) | WUE (kg·m−3) | NUE (%) | PUE (%) | KUE (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Crop | Second Crop | First Crop | Second Crop | First Crop | Second Crop | First Crop | Second Crop | First Crop | Second Crop | ||||||
| F | K1 | 43.40 | 56.80 | 364.41 | 19.48 | 46.75 | 3856 | 118.88 | 119.77 | 133.39 | 148.26 | 53.59 | 45.32 | 16.42 | 19.00 |
| K2 | 49.73 | 64.55 | 384.11 | 21.05 | 47.83 | 3836 | 129.31 | 128.51 | 93.55 | 128.81 | 39.42 | 36.51 | 13.24 | 16.66 | |
| K3 | 51.12 | 59.98 | 404.32 | 21.64 | 48.08 | 3760 | 134.03 | 122.92 | 88.39 | 122.12 | 32.98 | 26.53 | 10.51 | 13.12 | |
| K4 | 46.50 | 52.25 | 385.76 | 20.89 | 45.49 | 3765 | 125.27 | 110.27 | 59.47 | 81.47 | 25.45 | 22.25 | 7.18 | 10.33 | |
| k1 | 10.58 | 14.20 | 91.10 | 4.87 | 11.69 | 964 | 29.72 | 29.94 | 33.35 | 37.06 | 13.40 | 11.33 | 4.10 | 4.75 | |
| k2 | 12.43 | 16.14 | 96.03 | 5.26 | 11.96 | 959 | 32.33 | 32.13 | 23.39 | 32.20 | 9.85 | 9.13 | 3.31 | 4.17 | |
| k3 | 12.78 | 14.99 | 101.08 | 5.41 | 12.02 | 940 | 33.51 | 30.73 | 22.10 | 30.53 | 8.25 | 6.63 | 2.63 | 3.28 | |
| k4 | 11.62 | 13.06 | 96.44 | 5.22 | 11.37 | 941 | 31.32 | 27.57 | 14.87 | 20.37 | 6.36 | 5.56 | 1.80 | 2.58 | |
| Range | 1.93 | 3.07 | 9.98 | 0.54 | 0.65 | 24 | 3.79 | 4.56 | 18.48 | 16.70 | 7.04 | 5.77 | 2.31 | 2.17 | |
| W | K1 | 37.43 | 43.39 | 369.68 | 15.64 | 43.42 | 3874 | 108.36 | 106.02 | 88.73 | 111.32 | 34.92 | 28.46 | 10.37 | 12.88 |
| K2 | 43.79 | 51.28 | 376.81 | 20.36 | 45.90 | 3811 | 121.99 | 113.39 | 93.04 | 121.30 | 37.25 | 32.27 | 11.65 | 14.41 | |
| K3 | 50.51 | 61.61 | 385.90 | 22.33 | 48.02 | 3803 | 131.67 | 123.80 | 95.67 | 123.61 | 39.73 | 34.90 | 12.28 | 15.09 | |
| K4 | 59.04 | 76.28 | 406.22 | 24.75 | 50.81 | 3729 | 145.47 | 138.26 | 97.37 | 124.43 | 39.54 | 34.99 | 13.05 | 16.73 | |
| k1 | 9.36 | 11.10 | 92.42 | 3.91 | 10.86 | 969 | 27.09 | 26.50 | 22.18 | 27.83 | 8.73 | 7.12 | 2.59 | 3.22 | |
| k2 | 10.95 | 12.82 | 94.20 | 5.09 | 11.48 | 953 | 30.50 | 28.35 | 23.26 | 30.33 | 9.31 | 8.07 | 2.91 | 3.60 | |
| k3 | 12.63 | 15.40 | 96.48 | 5.58 | 12.00 | 951 | 32.92 | 30.95 | 23.92 | 30.90 | 9.93 | 8.73 | 3.07 | 3.77 | |
| k4 | 14.76 | 19.07 | 101.55 | 6.19 | 12.70 | 932 | 36.37 | 34.57 | 24.34 | 31.11 | 9.89 | 8.75 | 3.26 | 4.18 | |
| Range | 5.40 | 7.97 | 9.14 | 2.28 | 1.85 | 36 | 9.28 | 8.06 | 2.16 | 3.28 | 1.20 | 1.63 | 0.67 | 0.96 | |
| O | K1 | 49.57 | 59.66 | 380.62 | 20.55 | 47.29 | 3907 | 129.43 | 123.47 | 96.96 | 124.73 | 37.24 | 31.97 | 11.35 | 14.55 |
| K2 | 50.81 | 62.01 | 390.24 | 21.80 | 47.35 | 3693 | 132.10 | 126.62 | 98.96 | 126.88 | 38.78 | 34.98 | 12.20 | 15.74 | |
| K3 | 46.04 | 57.77 | 384.11 | 20.45 | 46.85 | 3794 | 125.77 | 118.93 | 91.91 | 116.19 | 39.07 | 33.52 | 12.06 | 14.87 | |
| K4 | 44.34 | 54.14 | 383.63 | 20.28 | 46.66 | 3823 | 120.18 | 112.44 | 86.97 | 112.86 | 36.35 | 30.15 | 11.74 | 13.96 | |
| k1 | 12.39 | 14.91 | 95.15 | 5.14 | 11.82 | 977 | 32.36 | 30.87 | 24.24 | 31.18 | 9.31 | 7.99 | 2.84 | 3.64 | |
| k2 | 12.70 | 15.50 | 97.56 | 5.45 | 11.84 | 923 | 33.03 | 31.66 | 24.74 | 31.72 | 9.70 | 8.74 | 3.05 | 3.93 | |
| k3 | 11.51 | 14.44 | 96.03 | 5.11 | 11.71 | 949 | 31.44 | 29.73 | 22.98 | 29.05 | 9.77 | 8.38 | 3.02 | 3.72 | |
| k4 | 11.08 | 13.53 | 95.91 | 5.07 | 11.66 | 956 | 30.05 | 28.11 | 21.74 | 28.22 | 9.09 | 7.54 | 2.93 | 3.49 | |
| Range | 1.62 | 1.97 | 2.41 | 0.38 | 0.17 | 53 | 2.98 | 3.54 | 3.00 | 3.51 | 0.68 | 1.21 | 0.21 | 0.44 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Guo, S.; Feng, J.; Li, D.; Yang, S.; Zhou, X. Suitable Water–Fertilizer Management and Ozone Synergy Can Enhance Substrate-Based Lettuce Yield and Water–Fertilizer Use Efficiency. Agronomy 2024, 14, 1619. https://doi.org/10.3390/agronomy14081619
Zhao Q, Guo S, Feng J, Li D, Yang S, Zhou X. Suitable Water–Fertilizer Management and Ozone Synergy Can Enhance Substrate-Based Lettuce Yield and Water–Fertilizer Use Efficiency. Agronomy. 2024; 14(8):1619. https://doi.org/10.3390/agronomy14081619
Chicago/Turabian StyleZhao, Qingqing, Shulong Guo, Junjie Feng, Dongwei Li, Shenjiao Yang, and Xinguo Zhou. 2024. "Suitable Water–Fertilizer Management and Ozone Synergy Can Enhance Substrate-Based Lettuce Yield and Water–Fertilizer Use Efficiency" Agronomy 14, no. 8: 1619. https://doi.org/10.3390/agronomy14081619
APA StyleZhao, Q., Guo, S., Feng, J., Li, D., Yang, S., & Zhou, X. (2024). Suitable Water–Fertilizer Management and Ozone Synergy Can Enhance Substrate-Based Lettuce Yield and Water–Fertilizer Use Efficiency. Agronomy, 14(8), 1619. https://doi.org/10.3390/agronomy14081619








