Physiological Factors Associated with Interspecific Variations in Drought Tolerance in Centipedegrass
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Environment
2.2. Experimental Design
2.3. Measurements of Growth and Physiological Indices
2.4. Determination of Osmoregulants
2.5. Statistical Analysis
3. Results
3.1. Soil Moisture Status after Drought and Rehydration
3.2. Effects of Drought and Rehydration on Turf Quality
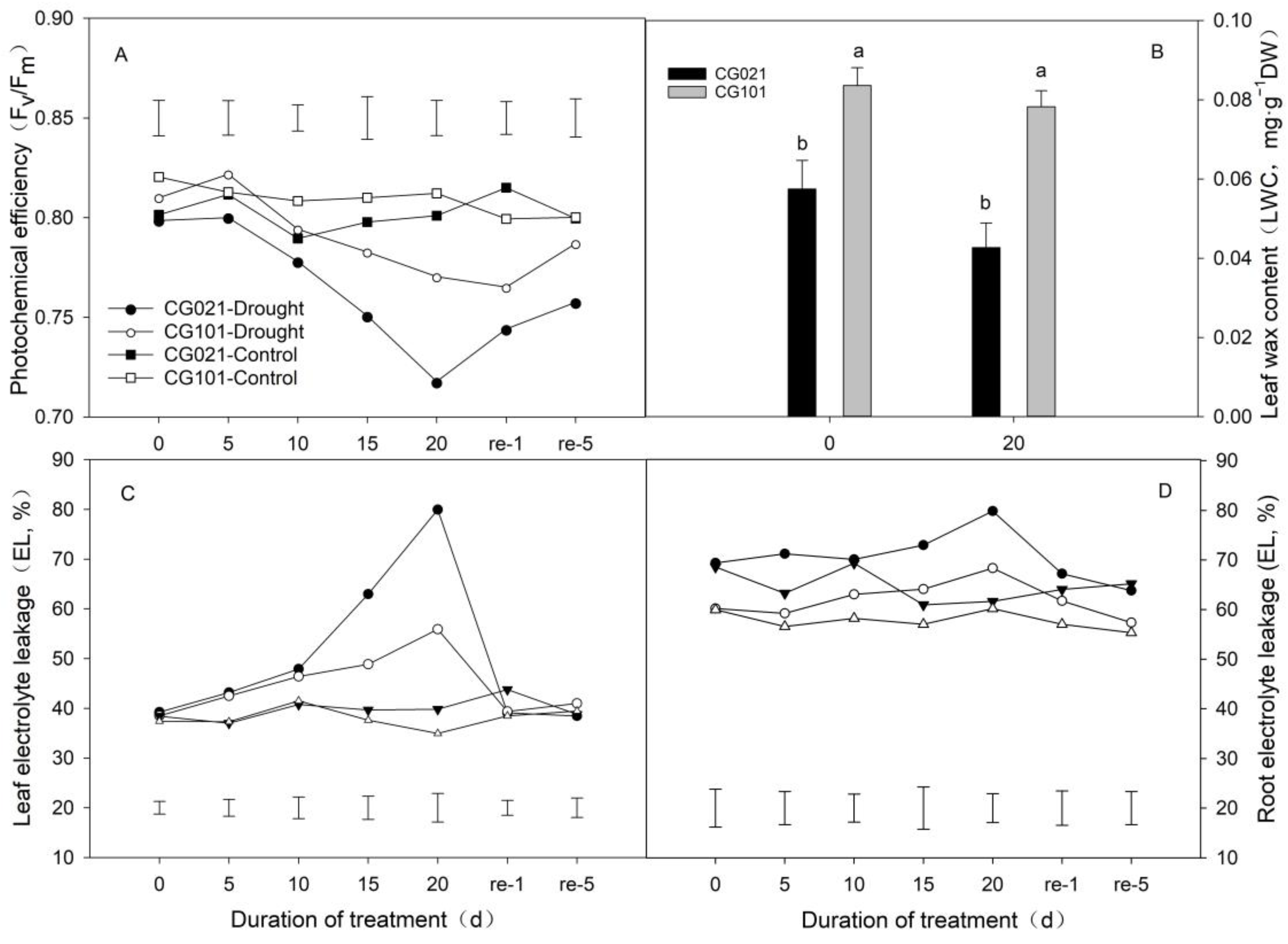
3.3. Impacts of Drought and Rehydration on Photochemical Efficiency, Electrolyte Leakage, and Leaf Wax Content
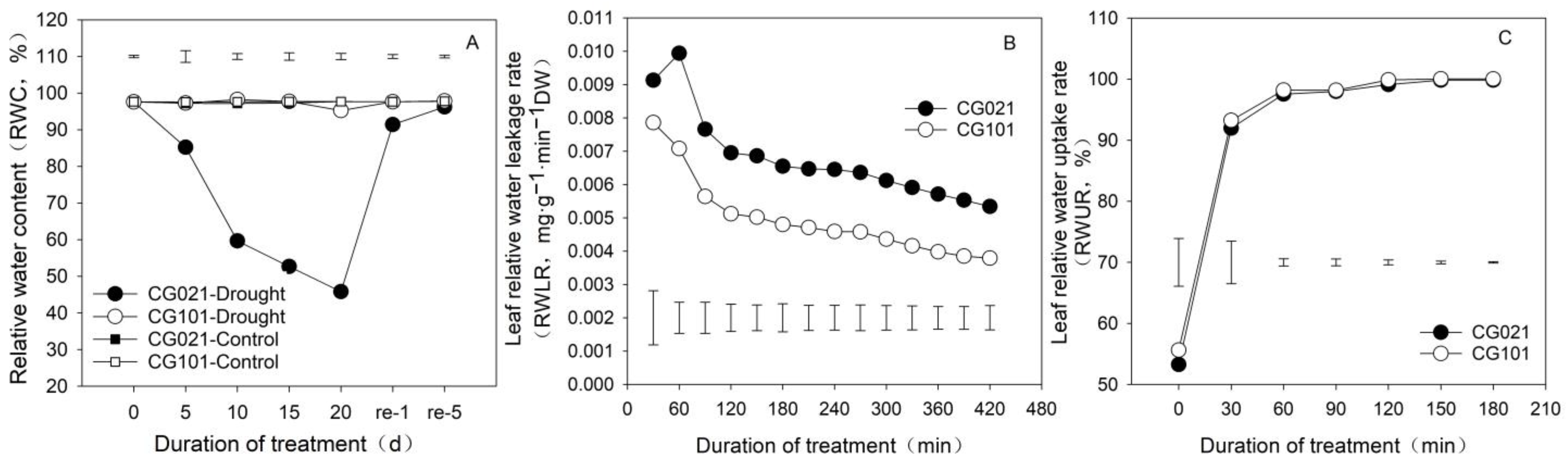
3.4. Influence of Drought and Rehydration on Leaf Water Status
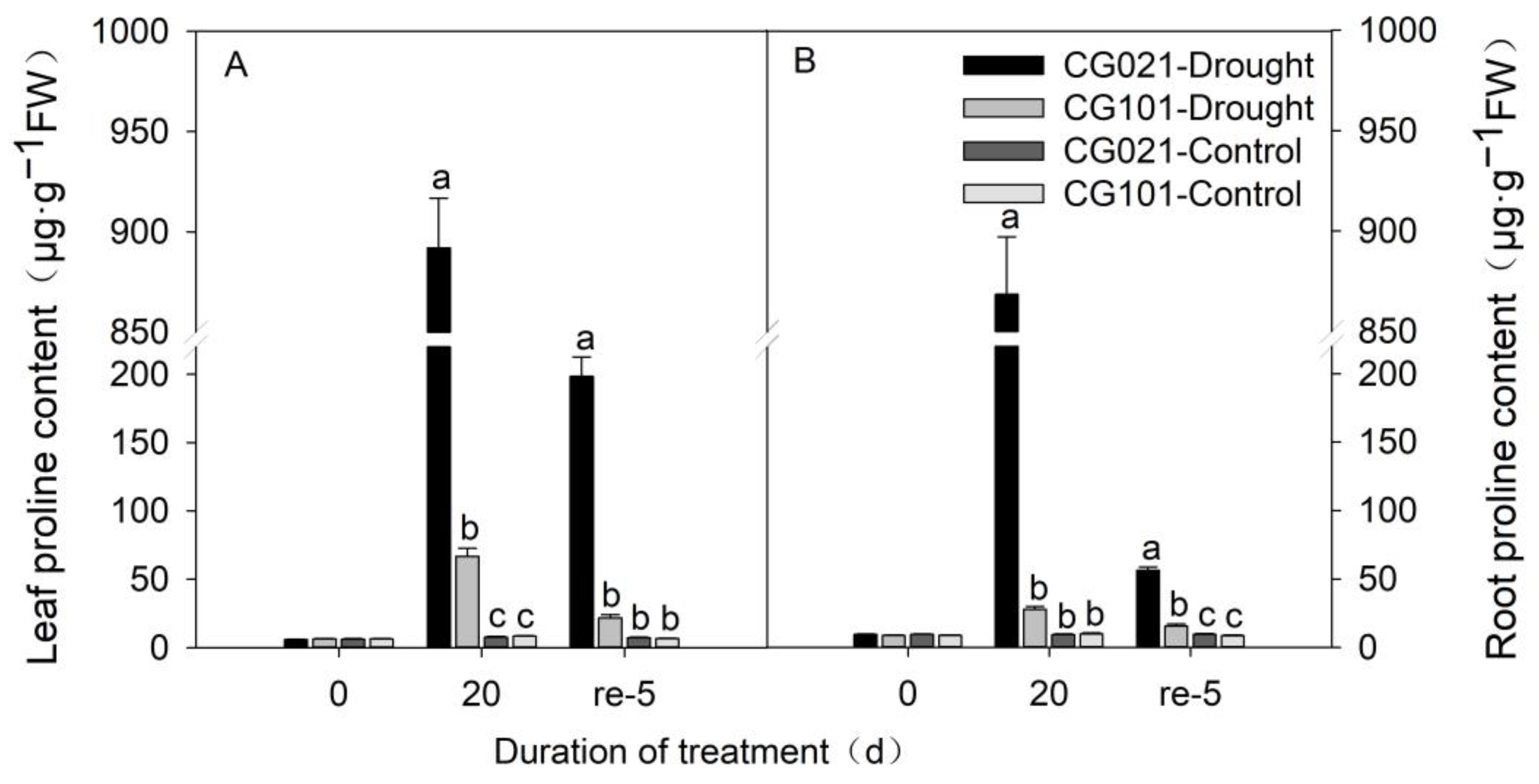
3.5. Impact of Drought and Rehydration on Proline Content in Leaves and Roots
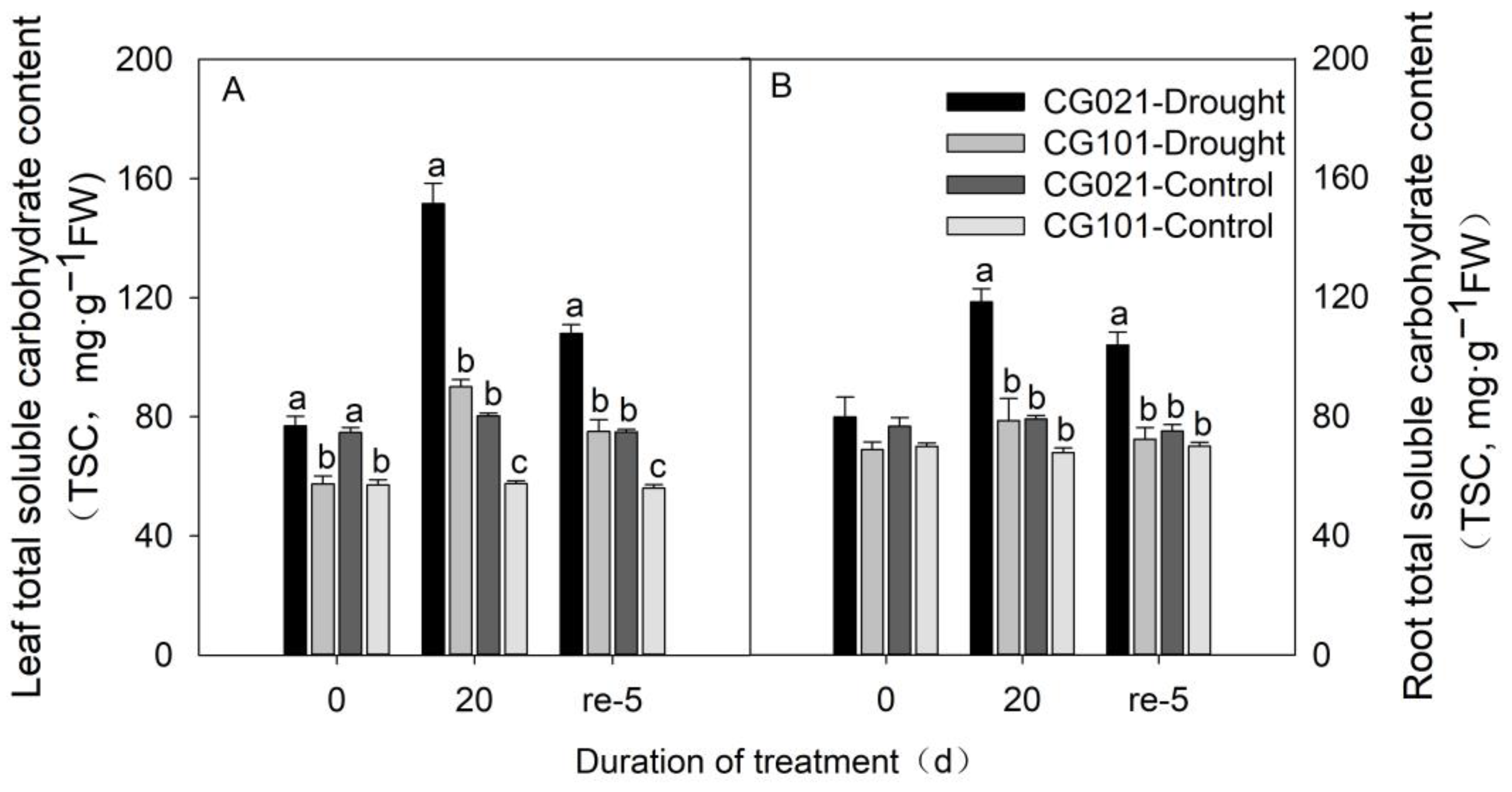
3.6. Influence of Drought and Rehydration on Total Soluble Carbohydrate Content in Leaves and Roots
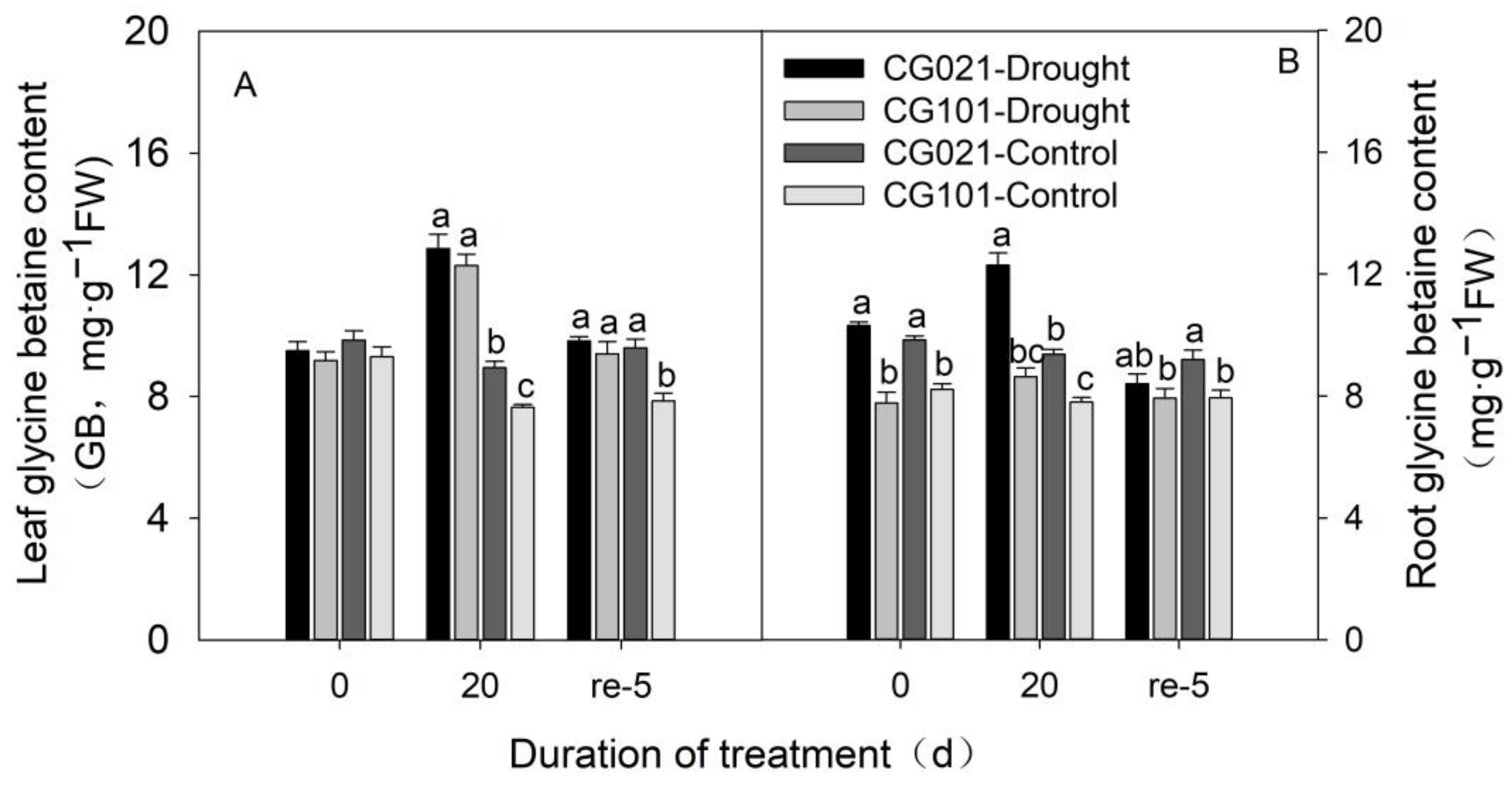
3.7. Impact of Drought and Rehydration on Glycine Betaine Content in Leaves and Roots
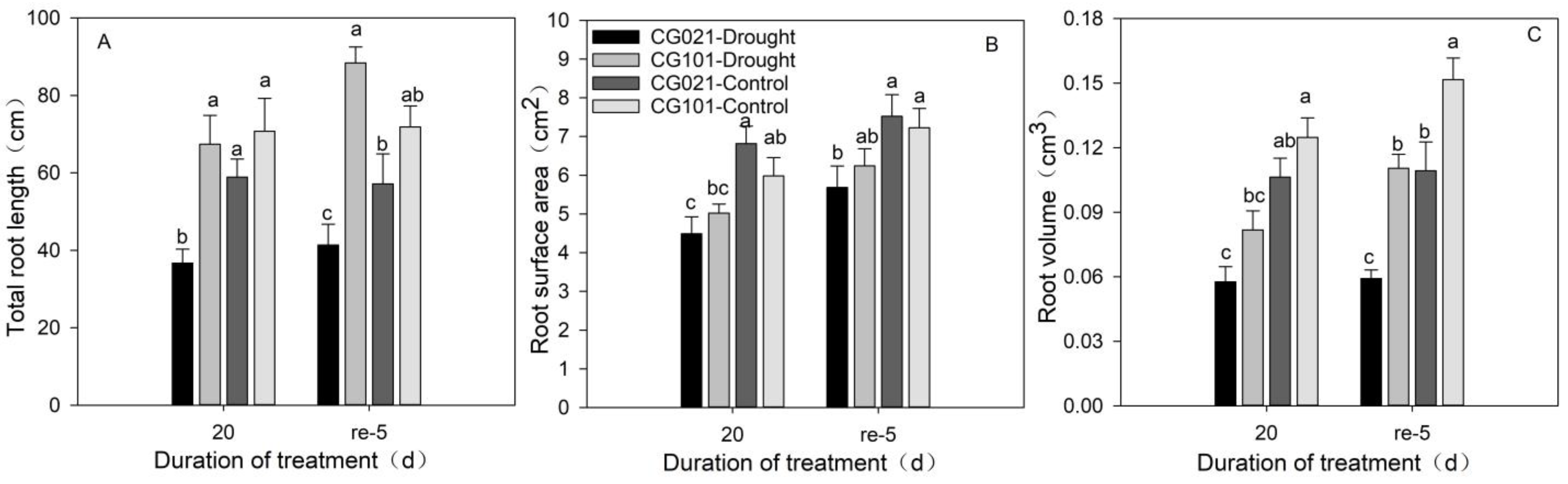
3.8. Effects of Drought and Rehydration on Root Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hirata, M. Development and establishment of centipede grass (Eremochloa ophiuroides) in southwestern Japan. Trop. Grassl. 2007, 41, 100–112. [Google Scholar]
- Islam, M.A.; Hirata, M. Centipedegrass (Eremochloa ophiuroides (Munro) Hack.): Growth behavior and multipurpose usages. Grassl. Sci. 2010, 51, 183–190. [Google Scholar] [CrossRef]
- Wang, J.; Zi, H.; Wang, R.; Liu, J.; Wang, H.; Chen, R.; Li, L.; Guo, H.; Chen, J.; Li, J. A high-quality chromosome-scale assembly of the centipedegrass [Eremochloa ophiuroides (Munro) Hack.] genome provides insights into chromosomal structural evolution and prostrate growth habit. Hortic. Res. 2021, 8, 201. [Google Scholar] [CrossRef]
- Schwartz, B.M.; Harris-Shultz, K.R.; Contreras, R.N.; Hans, C.S.; Milla-Lewis, S.R. Creation of artificial triploid and tetraploid centipedegrass using colchicine and breeding. Int. Turfgrass Res. J. 2013, 12, 327–333. [Google Scholar]
- Milla-Lewis, S.R.; Kimball, J.A.; Zuleta, M.C.; Harris-Shultz, K.R.; Schwartz, B.M.; Hanna, W.W. Use of sequence-related amplified polymorphism (SRAP) markers for comparing levels of genetic diversity in centipedegrass (Eremochloa ophiuroides (Munro) Hack.) germplasm. Genet. Resour. Crop Evol. 2012, 59, 1517–1526. [Google Scholar] [CrossRef]
- Guo, A.; Liu, J.; Guo, H. The preliminary identification of several drought resistance warm-season turf grasses. Pratacultural Sci. 2002, 19, 61–63. [Google Scholar]
- Huang, B.; Duncan, R.R.; Carrow, R.N. Drought-resistance mechanisms of seven warm-season turfgrasses under surface soil drying: I. Shoot response. Crop Sci. 1997, 37, 1858–1863. [Google Scholar] [CrossRef]
- Huang, B.; Duncan, R.R.; Carrow, R.N. Drought-resistance mechanisms of seven warm-season turfgrasses under surface soil drying: II. root aspects. Crop Sci. 1997, 37, 1863–1869. [Google Scholar] [CrossRef]
- Yu, J.; Liu, M.X.; Yang, Z.M.; Huang, B. Growth and physiological factors involved in interspecific variations in drought tolerance and postdrought recovery in warm- and cool-season turfgrass species. J. Am. Soc. Hortic. Sci. 2015, 140, 459–465. [Google Scholar] [CrossRef]
- Yan, J.; Chen, J.; Zhang, T.; Liu, J.; Liu, H. Evaluation of aluminum tolerance and nutrient uptake of 50 centipedegrass accessions and cultivars. J. Am. Soc. Hortic. Sci. 2009, 44, 857–861. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, F.; Chen, Z.; Huang, C.; Li, X.; Luo, X.; Liu, P.; Bai, C.; Liu, G. Evaluation of drought tolerance of 15 centipedegrass ecotypes. Trop. Agric. Eng. 2017, 41, 7–15. [Google Scholar]
- Zheng, Y.; Cai, Q.; Liu, J. Response of centipedegrass (Eremochloa ophiuroides (Munro.) Hack.) accessions to water stress. Acta Agrestia Sin. 2005, 5, 102–105. [Google Scholar]
- Yang, Z.M.; Yu, J.J.; Merewitz, E.; Huang, B.R. Differential effects of abscisic acid and glycine betaine on physiological responses to drought and salinity stress for two perennial grass species. J. Am. Soc. Hortic. Sci. 2012, 137, 96–106. [Google Scholar] [CrossRef]
- Yang, Z.M.; Xu, L.X.; Yu, J.J.; DaCosta, M.; Huang, B.R. Changes in carbohydrate metabolism in two kentucky bluegrass cultivars during drought stress and recovery. J. Am. Soc. Hortic. Sci. 2013, 138, 24–30. [Google Scholar] [CrossRef]
- Burgess, P.; Huang, B. Effects of sequential application of plant growth regulators and osmoregulants on drought tolerance of creeping bentgrass (Agrostis stolonifera). Crop Sci. 2014, 54, 837–844. [Google Scholar] [CrossRef]
- Yu, J.; Fan, N.; Hao, T.; Bian, Y.; Zhuang, L.; Li, Q.; Yang, Z. Ethionine-mitigation of drought stress associated with changes in root viability, antioxidant defense, osmotic adjustment, and endogenous hormones in tall fescue. Plant Growth Regul. 2023, 100, 119–132. [Google Scholar] [CrossRef]
- Dong, S.; Dong, Y.; Yang, L.; Qin, X.; Wang, W.; Cui, G.; Liu, L. Effects of drought stress and re-watering on osmotic adjustment ability and yield of soybean. J. Northeast Agric. Univ. 2020, 27, 8. [Google Scholar]
- Tamirat, W.; Mengs, B. Role of biochemical traits on coffee for drought: A review article. Eur. J. Biotech. Biosci. 2020, 8, 22–27. [Google Scholar]
- Shaw, B.; Thomas, T.H.; Cooke, D.T. Responses of sugar beet (Beta vulgaris L.) to drought and nutrient deficiency stress. Plant Growth Regul. 2002, 37, 77–83. [Google Scholar] [CrossRef]
- Iqbal, S.; Bano, A. Effect of drought and abscisic acid application on the osmotic adjustment of four wheat cultivars. J. Chem. Soc. Pak. 2010, 32, 13–19. [Google Scholar]
- Nayyar, H. Variation in osmoregulation in differentially drought-sensitive wheat genotypes involves calcium. Biol. Plant. 2003, 47, 541–547. [Google Scholar] [CrossRef]
- Liu, E.; Zong, H.; Guo, Z.; Li, Y. Effects of drought, salt and chilling stresses on proline accumulation in shoot of rice seedlings. J. Trop. Subtrop. Bot. 2000, 8, 235–238. [Google Scholar]
- Turgeon, A. Turfgrass Management, 8th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity techniques for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Hu, L.X.; Wang, Z.L.; Du, H.M.; Huang, B.R. Differential accumulation of dehydrins in response to water stress for hybrid and common bermudagrass genotypes differing in drought tolerance. J. Plant Physiol. 2010, 167, 103–109. [Google Scholar] [CrossRef]
- Merewitz, E.B.; Gianfagna, T.; Huang, B.R. Effects of SAG12-ipt and HSP18.2-ipt expression on cytokinin production, root growth, and leaf senescence in creeping bentgrass exposed to drought stress. J. Am. Soc. Hortic. Sci. 2010, 135, 230–239. [Google Scholar] [CrossRef]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Liu, N.; Shen, Y.; Huang, B. Osmoregulants involved in osmotic adjustment for differential drought tolerance in different bentgrass genotypes. J. Am. Soc. Hortic. Sci. 2015, 140, 605–613. [Google Scholar] [CrossRef]
- Dong, H.; Yu, H.; Bao, Z.; Guo, X.; Peng, J.; Yao, Z.; Chen, G.; Qu, S.; Dong, H. The ABI2-dependent abscisic acid signalling controls HrpN-induced drought tolerance in Arabidopsis. Planta 2005, 221, 313–327. [Google Scholar] [CrossRef]
- Wang, G.P.; Zhang, X.Y.; Li, F.; Luo, Y.; Wang, W. Overaccumulation of glycine betaine enhances tolerance to drought and heat stress in wheat leaves in the protection of photosynthesis. Photosynthetica 2010, 48, 117–126. [Google Scholar] [CrossRef]
- Kou, X.; Han, W.; Kang, J. Responses of root system architecture to water stress at multiple levels: A meta-analysis of trials under controlled conditions. Front. Plant Sci. 2022, 13, 1085409. [Google Scholar] [CrossRef]
- Xu, L.; Han, L.; Huang, B. Antioxidant enzyme activities and gene expression patterns in leaves of kentucky bluegrass in response to drought and post-drought recovery. J. Am. Soc. Hortic. Sci. 2011, 136, 247–255. [Google Scholar] [CrossRef]
- Qian, Y.; Fry, J. Water relations and drought tolerance of four turfgrasses. J. Am. Soc. Hort. Sci. 1997, 122, 129–133. [Google Scholar] [CrossRef]
- Jiang, Y.; Carrow, R.N. Broadband spectral reflectance models of turfgrass species and cultivars to drought stress. Crop Sci. 2007, 47, 1611–1618. [Google Scholar] [CrossRef]
- Du, H.; Wang, Z.; Huang, B. Differential responses of tall fescue and zoysiagrass to heat and drought stress. Acta Hortic. 2008, 783, 207–213. [Google Scholar]
- Yu, J.; Li, R.; Liu, M.X.; Yang, Z.M. Ecophysiological mechanisms associated with drought tolerance and post-drought recovery in warm- and cool-season turfgrasses. Acta Prataculturae Sin. 2016, 25, 86–93. [Google Scholar]
- Li, Q.; Li, R.; He, F.; Yang, Z.; Yu, J. Growth and physiological effects of chitosan on heat tolerance in creeping bentgrass (Agrostis stolonifera). Grass Res. 2022, 2, 6. [Google Scholar] [CrossRef]
- Seo, P.J.; Lee, S.B.; Suh, M.C.; Park, M.J.; Go, Y.S.; Park, C.M. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell 2011, 23, 1138–1152. [Google Scholar] [CrossRef]
- Aharoni, A.; Dixit, S.; Jetter, R.; Thoenes, E.; Pereira, A.A. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 2004, 16, 2463–2480. [Google Scholar] [CrossRef]
- Zhang, J.; Broeckling, C.D.; Blancaflor, E.; Sledge, M.K.; Sumner, L.; Wang, Z. Overexpression of WXP1, a putative Medicago truncatula AP2 domain-containing transcription factor gene, increases cuticular wax accumulation and enhances drought tolerance in transgenic alfalfa (Medicago sativa). Plant J. 2005, 42, 689–707. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Broeckling, C.D.; Sumner, L.W.; Wang, Z.Y. Heterologous expression of two Medicago truncatula putative ERF transcription factor genes, WXP1 and WXP2, in Arabidopsis led to increased leaf wax accumulation and improved drought tolerance, but differential response in freezing tolerance. Plant Mol. Biol. 2007, 64, 265–278. [Google Scholar] [CrossRef]
- Li, A.; Lv, D.; Zhang, Y.; Zhagn, D.; Zong, Y.; Shi, X.; Li, P. Elevated CO2 concentration enhances drought resistance of soybean by regulating cell structure, cuticular wax synthesis, photosynthesis, and oxidative stress response. Plant Physiol. Biochem. 2024, 206, 108266. [Google Scholar] [CrossRef]
- Premachandra, G.S.; Hirofumi, S.; Kounosuke, F.; Shoitsu, O. Leaf water relations, osmotic adjustment, cell membrane stability, epicuticular wax load and growth as affected by increasing water deficits in sorghum. J. Exp. Bot. 1992, 43, 1569–1576. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Xie, Y.; Wang, Y.; Duan, L.; Zhang, M.; Li, Z. Ethephon improved drought tolerance in maize seedlings by modulating cuticular wax biosynthesis and membrane stability. Agric. Econ. Blackwell 2017, 214, 123–133. [Google Scholar] [CrossRef]
- Ran, J.; Ding, Q.; Wang, G.; Shen, Y.; Gao, Z.; Gao, Y.; Ma, X.; Hou, X. The developmental mechanism of the root system of cultivated terrestrial watercress. Plants 2023, 12, 3523. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Wang, X.; Dai, M.; Peng, Y. Crop root system architecture in drought response. J. Genet. Genom. 2024, in press. [Google Scholar] [CrossRef]
- Sun, J.; Meyer, W.; Cross, J.; Huang, B. Growth and physiological traits of canopy and root systems associated with drought resistance in tall fescue. Crop Sci. 2013, 53, 575–584. [Google Scholar] [CrossRef]
- Kaur, K.; Gupta, A.K.; Kaur, N. Effect of water deficit on carbohydrate status and enzymes of carbohydrate metabolism in seedlings of wheat cultivars. Indian J. Biochem. Biophys. 2007, 44, 223–230. [Google Scholar]
- Wang, Y.G.; Abulaiti, A.; Qi, M. Differing response to drought stress of two Cynodon dactylon varieties. Acta Prataculturae Sin. 2006, 15, 58–64. [Google Scholar]
- Hanson, A.D.; Nelsen, C.E.; Pedersen, A.R.; Everson, E.H. Capacity for proline accumulation during water stress in barley and its implications for breeding for drought resistance. Crop Sci. 1979, 19, 489–493. [Google Scholar] [CrossRef]
- Richards, R.A.; Thurling, N. Genetic analysis of drought stress response in rapeseed (Brassica campestris and B. napus). III. physiological characters. Euphytica 1979, 28, 755–759. [Google Scholar] [CrossRef]
- Moftah, A.E.; Michel, B.E. The effect of sodium chloride on solute potential and proline accumulation in soybean leaves. Plant Physiol. 1987, 83, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, J.K. Proline Accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of arabidopsis. Plant Physiol. 1997, 114, 591. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Yu, J.; Xu, M.; Wang, S.; He, J.; Ai, L. Physiological Factors Associated with Interspecific Variations in Drought Tolerance in Centipedegrass. Agronomy 2024, 14, 1624. https://doi.org/10.3390/agronomy14081624
Song Y, Yu J, Xu M, Wang S, He J, Ai L. Physiological Factors Associated with Interspecific Variations in Drought Tolerance in Centipedegrass. Agronomy. 2024; 14(8):1624. https://doi.org/10.3390/agronomy14081624
Chicago/Turabian StyleSong, Yali, Jingjin Yu, Mao Xu, Sheng Wang, Jin He, and Lijiao Ai. 2024. "Physiological Factors Associated with Interspecific Variations in Drought Tolerance in Centipedegrass" Agronomy 14, no. 8: 1624. https://doi.org/10.3390/agronomy14081624
APA StyleSong, Y., Yu, J., Xu, M., Wang, S., He, J., & Ai, L. (2024). Physiological Factors Associated with Interspecific Variations in Drought Tolerance in Centipedegrass. Agronomy, 14(8), 1624. https://doi.org/10.3390/agronomy14081624






