Relationship between Chilling Accumulation and Heat Requirement for Flowering in Peach Varieties of Different Chilling Requirements
Abstract
1. Introduction
2. Materials and Methods
2.1. Temperature Data Collection
2.2. Materials
2.3. Peach Twig CA Treatment and Data Calculation
2.4. Scanning Electron Microscopy of Peach Buds
3. Results
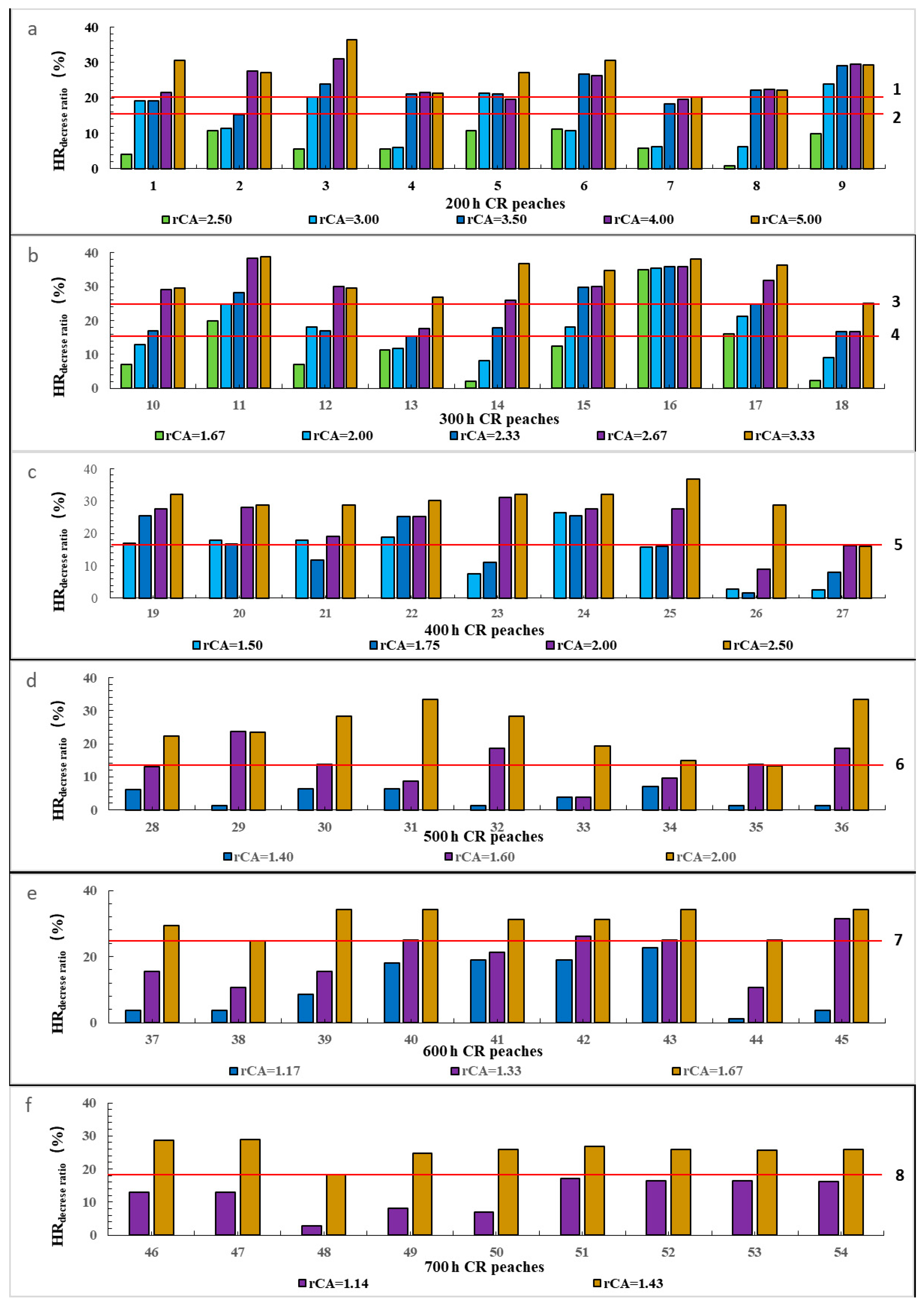
3.1. Effect of Increasing CA on HR for Flowering in Peach Varieties of Different CRs
3.1.1. Effect of Increasing CA on FFD in Peach Varieties of Different CRs
3.1.2. Impact of Increasing CA on HR in Peaches of Different CRs
3.1.3. Relationship between rCA and drHR of Peaches with Different CRs
3.2. SEM Analysis of the Effect of CA and GDHN on the Developmental Phenology of Flower Buds
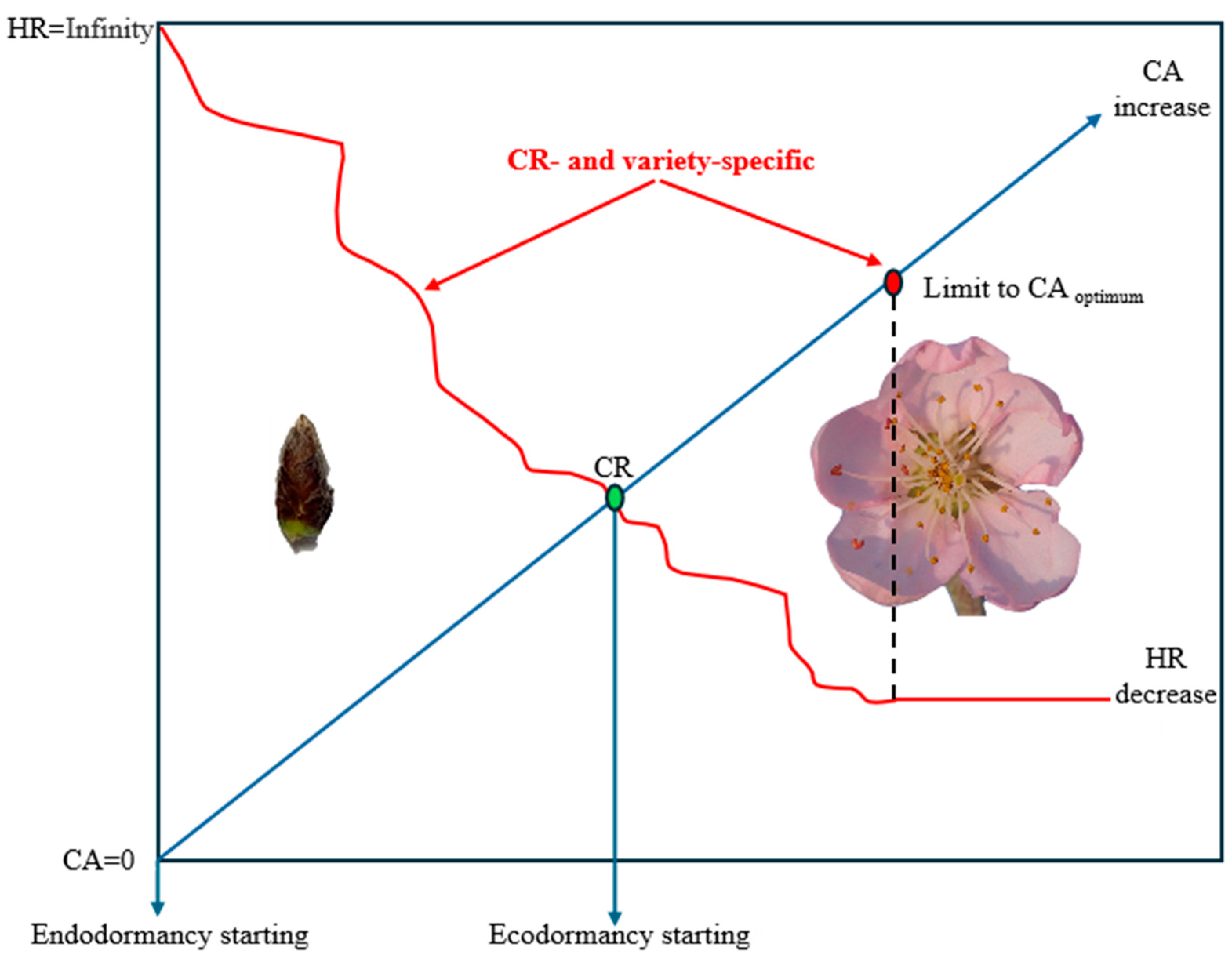
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Zhang, M.; Cai, Z.; Shen, Z.; Ma, R.; Xu, Z.; Su, Z.; Yu, M. Effects of chilling and heat requirement differences between peach floral bud and leaf bud on their phenological process. J. Plant Genet. Resour. 2021, 22, 1281–1292. [Google Scholar]
- Yan, J.; Zhao, B.; Sun, M.; Song, H.; Cai, Z.; Li, J.; Su, Z.; Zhang, M.; Shen, Z.; Xu, J.; et al. Adaptability of peach under air temperature change based on chilling requirement. Acta Hortic. Sin. 2023, 50, 724–736. [Google Scholar]
- Douglas, G.B.; Gasic, K. Peach [Prunus persica (L.) Batsch] cultivars differ in apparent base temperature and growing degree hour requirement for floral bud break. Front. Plant Sci. 2022, 13, 801606. [Google Scholar]
- Pawasut, A.; Fujishige, N.; Yamane, K.; Yamaki, Y.; Honjo, H. Relationships between chilling and heat requirement for flowering in ornamental peaches. Engei Gakkai Zasshi 2004, 73, 519–523. [Google Scholar] [CrossRef][Green Version]
- Razavi, F.; Hajilou, J.; Tabatabaei, S.J.; Dadpour, M.R. Comparison of chilling and heat requirement in some peach and apricot cultivars. Res. Plant Biol. 2011, 1, 40–47. [Google Scholar]
- Zhao, X.; Han, X.; Wang, Q.; Wang, X.; Chen, X.; Li, L.; Fu, X.; Gao, D. Early bud break 1 triggers bud break in peach trees by regulating hormone metabolism, the cell cycle, and cell wall modifications. J. Exp. Bot. 2020, 71, 3512–3523. [Google Scholar] [CrossRef] [PubMed]
- Atagul, O.; Calle, A.; Demirel, G.; Lawton, J.M.; Bridges, W.C.; Gasic, K. Estimating heat requirement for flowering in peach germplasm. Agronomy 2022, 12, 1002. [Google Scholar] [CrossRef]
- Rom, R.C.; Harrington, E.H. The effect of varying temperature regimes on degree-days to bloom in the Elberta peach. Proc. Am. Soc. Hortic. Sci. 1966, 88, 239–244. [Google Scholar]
- Couvillon, G.A.; Hendershott, C.H. A characterization of the “After-Rest” period of flower buds of two peach cultivars of different chilling requirements. J. Am. Soc. Hortic. Sci. 1974, 99, 23–26. [Google Scholar] [CrossRef]
- Couvillon, G.A.; Erez, A. Influence of prolonged exposure to chilling temperatures on bud break and heat requirement for bloom of several fruit species. J. Am. Soc. Hortic. Sci. 1985, 110, 47–50. [Google Scholar] [CrossRef]
- Scalabrelli, G.; Couvillon, G.A. The effect of temperature and bud type on rest completion and the GDHC requirement for budbreak in ‘Redhaven’ peach. J. Am. Soc. Hortic. Sci. 1986, 111, 537–540. [Google Scholar] [CrossRef]
- Gariglio, N.; González Rossia, D.E.; Mendow, M.; Reig, C.; Agusti, M. Effect of artificial chilling on the depth of endodormancy and vegetative and flower budbreak of peach and nectarine cultivars using excised shoots. Sci. Hortic. 2006, 108, 371–377. [Google Scholar] [CrossRef]
- Harrington, C.A.; Gould, P.J.; St. Clair, J.B. Modeling the effects of winter environment on dormancy release of Douglas-fir. For. Ecol. Manag. 2010, 259, 798–808. [Google Scholar] [CrossRef]
- Okie, W.R.; Blackburn, B. Increasing Chilling Reduces Heat Requirement for Floral Budbreak in Peach. HortScience 2011, 46, 245–252. [Google Scholar] [CrossRef]
- Tan, Y.; Li, L.; Li, D.; Chen, D.; Leng, C.; Gao, D. Relationship between Chilling and Heat in Budburst Regulation of Peaches for Protected Cultivation. Chin. J. Appl. Environ. Biol. 2012, 18, 728–733. [Google Scholar] [CrossRef]
- Lin, S.; Wang, H.; Ge, Q.; Hu, Z. Effects of chilling on heat requirement of spring phenology vary between years. Agric. For. Meteorol. 2022, 312, 108718. [Google Scholar] [CrossRef]
- Polgar, C.A.; Primack, R.B. Leaf-out phenology of temperate woody plants: From trees to ecosystems. New Phytol. 2011, 191, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Flynn, D.F.B.; Wolkovich, E.M. Temperature and photoperiod drive spring phenology across all species in a temperate forest community. New Phytol. 2018, 219, 1353–1362. [Google Scholar] [CrossRef]
- Du, Y.J.; Pan, Y.Q.; Ma, K.P. Moderate chilling requirement controls budburst for subtropical species in China. Agric. For. Meteorol. 2019, 278, 107693. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Ge, Q.; Dai, J. The interactive effects of chilling, photoperiod, and forcing temperature on flowering phenology of temperate woody plants. Front. Plant Sci. 2020, 11, 443. [Google Scholar] [CrossRef]
- Morin, X.; Lechowicz, M.J.; Augspurger, C.; O’keefe, J.; Viner, D.; Chuine, I. Leaf phenology in 22 North American tree species during the 21st century. Glob. Chang. Biol. 2009, 15, 961–975. [Google Scholar] [CrossRef]
- Polgar, C.A.; Gallinat, A.; Primack, R.B. Drivers of leaf-out phenology and their implications for species invasions: Insights from Thoreau’s Concord. New Phytol. 2014, 202, 106–115. [Google Scholar] [CrossRef]
- Bennie, J.; Kubin, E.; Wiltshire, A.; Huntley, B.; Baxter, R. Predicting spatial and temporal patterns of bud-burst and spring frost risk in north-west Europe: The implications of local adaptation to climate. Glob. Change Biol. 2010, 16, 1503–1514. [Google Scholar] [CrossRef]
- Clark, J.S.; Melillo, J.; Mohan, J.; Salk, C. The seasonal timing of warming that controls onset of the growing season. Glob. Chang. Biol. 2014, 20, 1136–1145. [Google Scholar] [CrossRef]
- Demirel, G.; Calle, A.; Lawton, J.M.; Atagul, O.; Fu, W.; Gasic, K. Ppe.CR.1 DNA test for predicting chilling requirement in peach. Sci. Rep. 2023, 13, 987. [Google Scholar] [CrossRef]
- Parker, L.E.; Abatzoglou, J.T. Warming winters reduce chill accumulation for peach production in the Southeastern United States. Climate 2019, 7, 94. [Google Scholar] [CrossRef]
- Li, Y.; Cao, K.; Li, N.; Zhu, G.; Fang, W.; Chen, C.; Wang, X.; Guo, J.; Wang, Q.; Ding, T.; et al. Genomic analyses provide insights into peach local adaptation and responses to climate change. Genome Res. 2021, 31, 592–606. [Google Scholar] [CrossRef]
- Citadin, I.; Raseira, M.C.B.; Herter, F.G.; Silva, J.D. Heat requirement for blooming and leafing in peach. HortScience 2001, 36, 305–307. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, M.H.; Peng, B.; Su, Z.; Xu, Z.; Cai, Z.; Yang, J.; Ma, R.; Yu, M.; Shen, Z. Predicting chilling requirement of peach floral buds using electronic nose. Sci. Hortic. 2021, 290, 110517. [Google Scholar] [CrossRef]
- Luedeling, E.; Guo, L.; Dai, J.; Leslie, C.; Blanke, M.M. Differential responses of trees to temperature variation during the chilling and forcing phases. Agric. For. Meteorol. 2013, 181, 33–42. [Google Scholar] [CrossRef]
- Harrington, C.A.; Gould, P.J. Tradeoffs between chilling and forcing in satisfying dormancy requirements for pacific northwest tree species. Front. Plant Sci. 2015, 6, 120. [Google Scholar] [CrossRef]
- Campoy, J.A.; Ruiz, D.; Cook, N.; Allderman, L.; Egea, J. High temperatures and time to budbreak in low chill apricot ‘Palsteyn’. towards a better understanding of chill and heat requirements fulfillment. Sci. Hortic. 2011, 129, 649–655. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J.; Salazar, J.A.; Campoy, J.A. Chilling and heat requirements of Japanese plum cultivars for flowering. Sci. Hortic. 2018, 242, 164–169. [Google Scholar] [CrossRef]
- Montazeran, A.; Khadivi, A.; Khaleghi, A. The first report: Chilling and heat requirements of seedless barberry (Berberis vulgaris L. var. asperma). Sci. Hortic. 2018, 231, 188–193. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Diaz-Vivancos, P.; Acosta-Motos, J.R.; Alburquerque, N.; Martínez, D.; Carrera, E.; García-Bruntón, J.; Barba-Espín, G. Interplay among antioxidant system, hormone profile and carbohydrate metabolism during bud dormancy breaking in a high-chill peach variety. Antioxidants 2021, 10, 560. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Diaz-Vivancos, P.; Martinez-Sanchez, G.; Alburquerque, N.; Martínez, D.; Barba-Espín, G.; Acosta-Motos, J.R.; Carrera, E.; García-Bruntón, J. Physiological and biochemical characterization of bud dormancy: Evolution of carbohydrate and antioxidant metabolisms and hormonal profile in a low chill peach variety. Sci. Hortic. 2021, 281, 109957. [Google Scholar] [CrossRef]



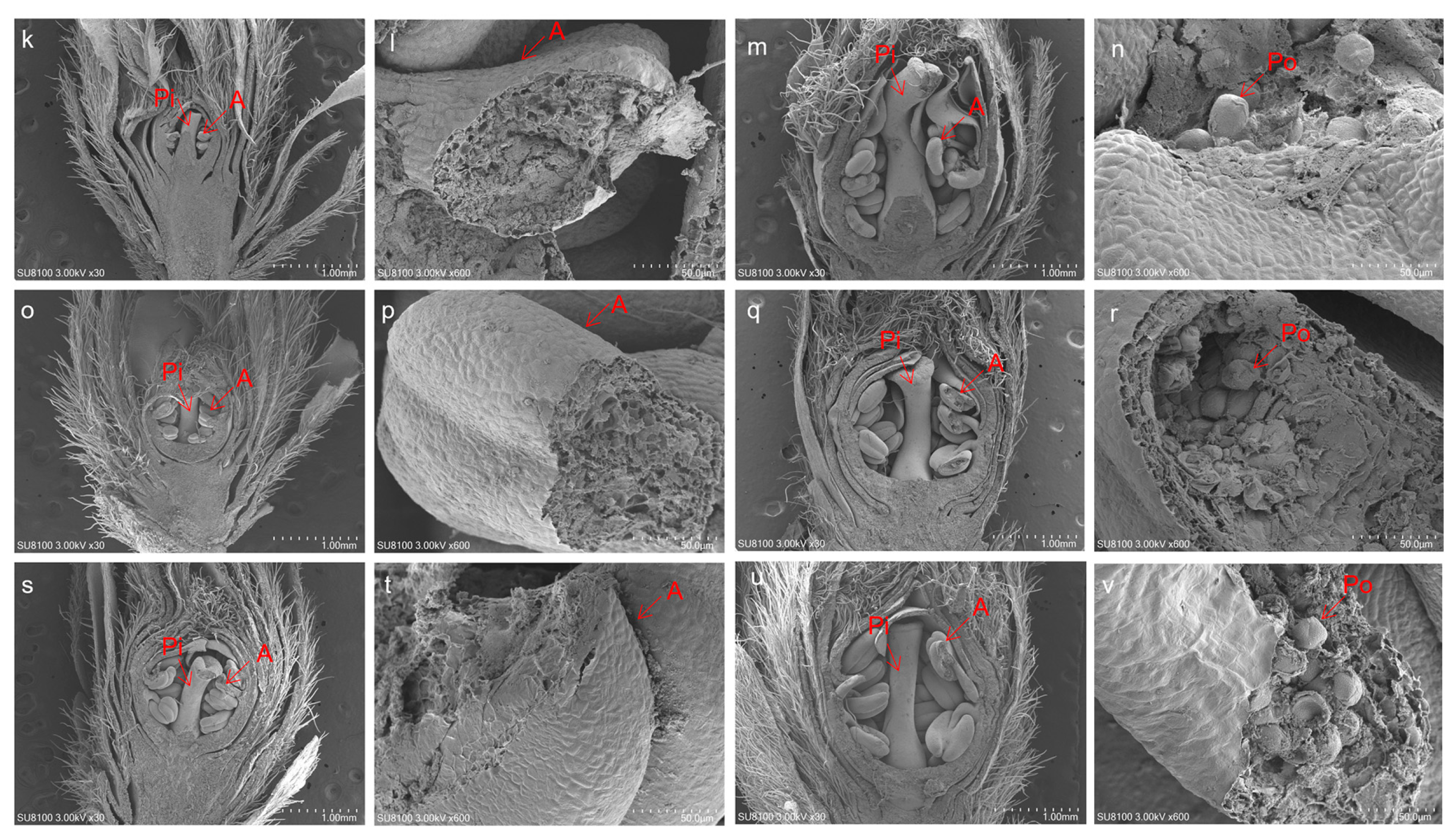
| CA (h)/GDHN (GDH °C) | Flower Bud Collection Date | Days of Bud Cultivation | Bud Diameter (mm) | Phenotypic State Description |
|---|---|---|---|---|
| 0/0 | 12 November 2023 | 0 | 2.2 ± 0.2 | -- |
| 19 November 2023 | 7 | 2.2 ± 0.3 | No significant change | |
| 26 November 2023 | 14 | 2.4 ± 0.1 | No significant change | |
| 3 December 2023 | 21 | 2.8 ± 0.3 | Slight enlargement of flower buds was noted relative to the preceding state | |
| 200/0 | 5 December 2023 | 0 | 2.1 ± 0.2 | No significant difference relative to day 0 of CA = 0 h |
| 12 December 2023 | 7 | 2.3 ± 0.2 | No significant changes were observed relative to the preceding state | |
| 19 December 2023 | 14 | 2.7 ± 0.1 | Slight enlargement of flower buds relative to the preceding state | |
| 26 December 2023 | 21 | 4.0 ± 0.4 | Significant enlargement of flower buds and conspicuous development of the reproductive organs, with >25% flower buds showing green scales | |
| 400/0 | 25 December 2023 | 0 | 2.3 ± 0.2 | No significant changes were observed relative to day 0 of CA = 0 h and 200 h |
| 2 January 2024 | 7 | 2.6 ± 0.4 | Slight enlargement of flower buds relative to the preceding state | |
| 7 January 2024 | 12 | 3.7 ± 0.4 | Significant enlargement of the flower buds and conspicuous development of the reproductive organs, with >25% flower buds showing green scales | |
| 12 January 2024 | 17 | 4.2 ± 0.3 | >25% flower buds showing red scales | |
| 15 January 2024 | 21 | 5.3 ± 0.6 | The first flower bloomed | |
| 600/603 | 8 January 2024 | 0 | 2.7 ± 0.3 | Significant enlargement of the flower buds and conspicuous development of the reproductive organs were observed compared to day 0 of CA = 0 h, 200 h and 400 h |
| 15 January 2024 | 7 | 4.0 ± 0.5 | Significant enlargement of the flower buds and conspicuous development of the reproductive organs relative to the preceding state | |
| 19 January 2024 | 11 | 4.3 ± 0.5 | >50% flower buds showing green scales | |
| 24 January 2024 | 16 | 5.7 ± 0.9 | The first flower bloomed | |
| 800/1343 | 24 January 2024 | 0 | 3.1 ± 0.3 | Significant enlargement of the flower buds and conspicuous development of the reproductive organs relative to day 0 of CA = 600 h |
| 29 January 2024 | 5 | 3.8 ± 0.2 | Significant enlargement of the flower buds and conspicuous development of the reproductive organs, with >25% flower buds showing red scales | |
| 5 February 2024 | 12 | 5.6 ± 0.7 | The first flower bloomed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Cai, Z.; Chen, Z.; Zhang, B.; Li, J.; Xu, J.; Ma, R.; Yu, M.; Shen, Z. Relationship between Chilling Accumulation and Heat Requirement for Flowering in Peach Varieties of Different Chilling Requirements. Agronomy 2024, 14, 1637. https://doi.org/10.3390/agronomy14081637
Yan J, Cai Z, Chen Z, Zhang B, Li J, Xu J, Ma R, Yu M, Shen Z. Relationship between Chilling Accumulation and Heat Requirement for Flowering in Peach Varieties of Different Chilling Requirements. Agronomy. 2024; 14(8):1637. https://doi.org/10.3390/agronomy14081637
Chicago/Turabian StyleYan, Juan, Zhixiang Cai, Zheng Chen, Binbin Zhang, Jiyao Li, Jianlan Xu, Ruijuan Ma, Mingliang Yu, and Zhijun Shen. 2024. "Relationship between Chilling Accumulation and Heat Requirement for Flowering in Peach Varieties of Different Chilling Requirements" Agronomy 14, no. 8: 1637. https://doi.org/10.3390/agronomy14081637
APA StyleYan, J., Cai, Z., Chen, Z., Zhang, B., Li, J., Xu, J., Ma, R., Yu, M., & Shen, Z. (2024). Relationship between Chilling Accumulation and Heat Requirement for Flowering in Peach Varieties of Different Chilling Requirements. Agronomy, 14(8), 1637. https://doi.org/10.3390/agronomy14081637








