Accelerated Iron Evolution in Quaternary Red Soils through Anthropogenic Land Use Activities
Abstract
:1. Introduction
2. Materials and Methods
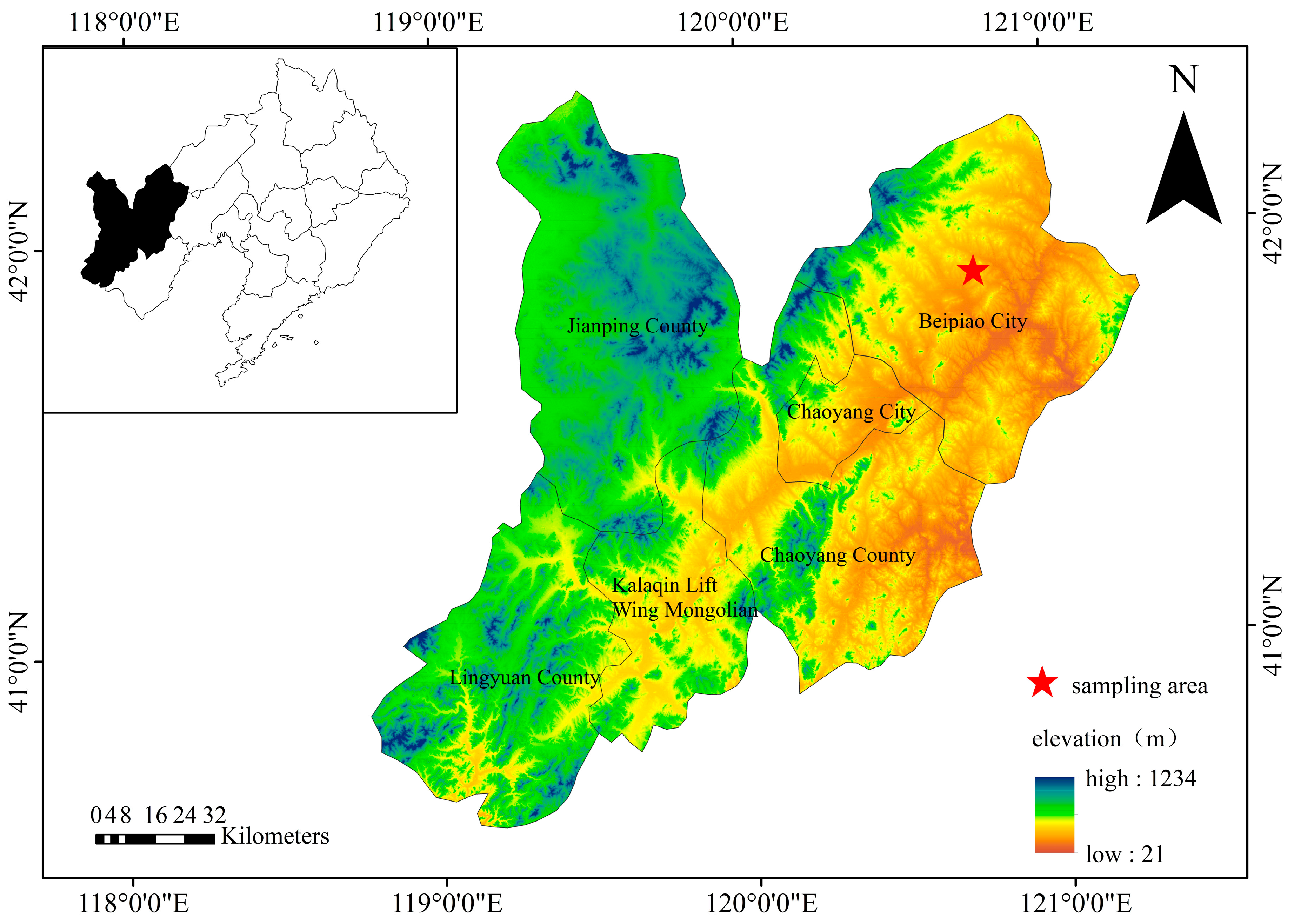
2.1. Study Area
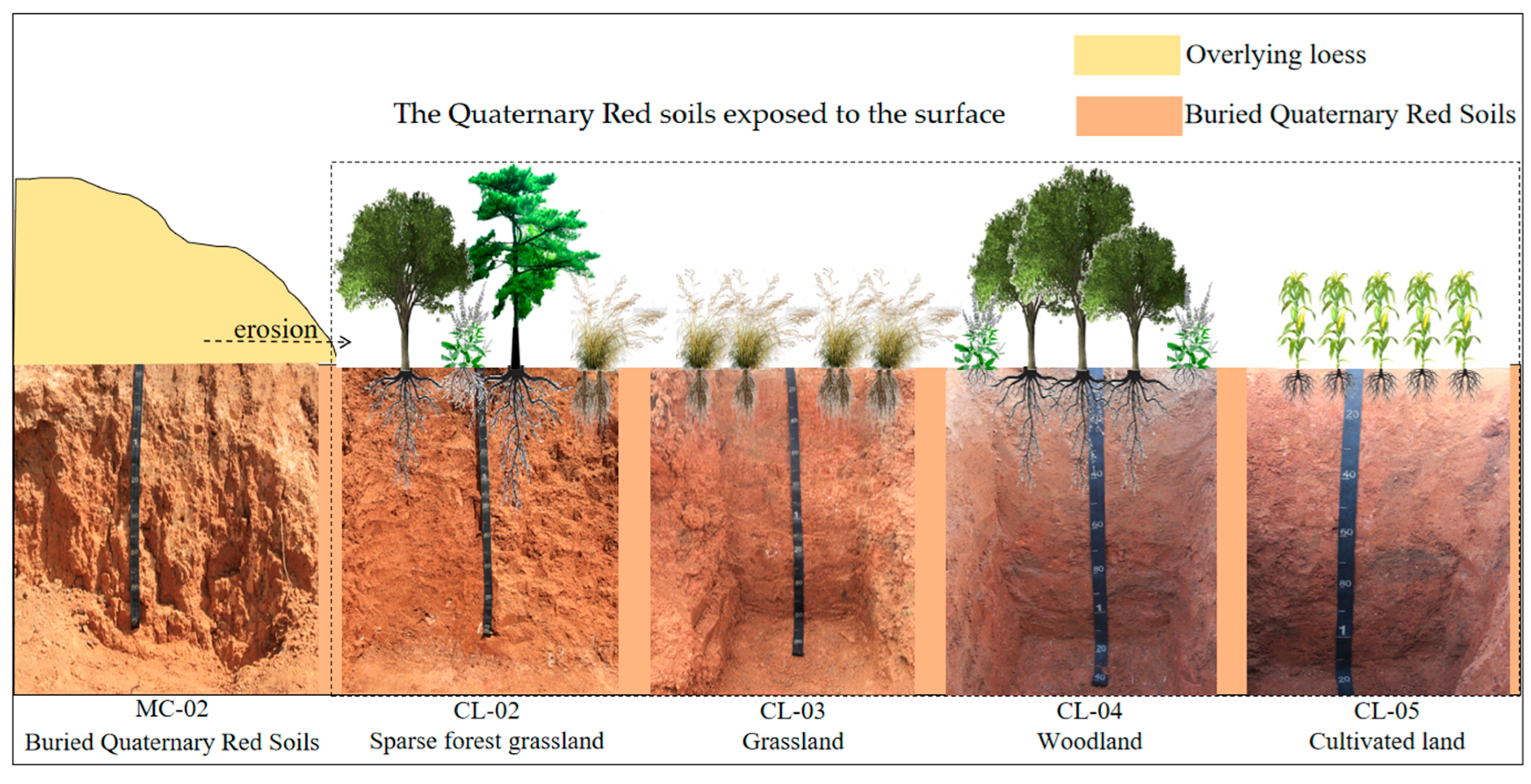
2.2. Soil Samplings and Preprocessing
2.3. Measurement Methods
2.3.1. Measurement Methods
2.3.2. Determinations of Iron in Different Forms
2.4. Quantitative Calculation of Iron Index and Soil Pedogenic Process
2.5. Data Processing
3. Results
3.1. Basic Physical and Chemical Properties
3.2. Iron Composition Characteristics of Quaternary Red Soils under Different Land Use Patterns
3.2.1. Variation Characteristics of Iron Content in Different Forms with Soil Depth
- (1)
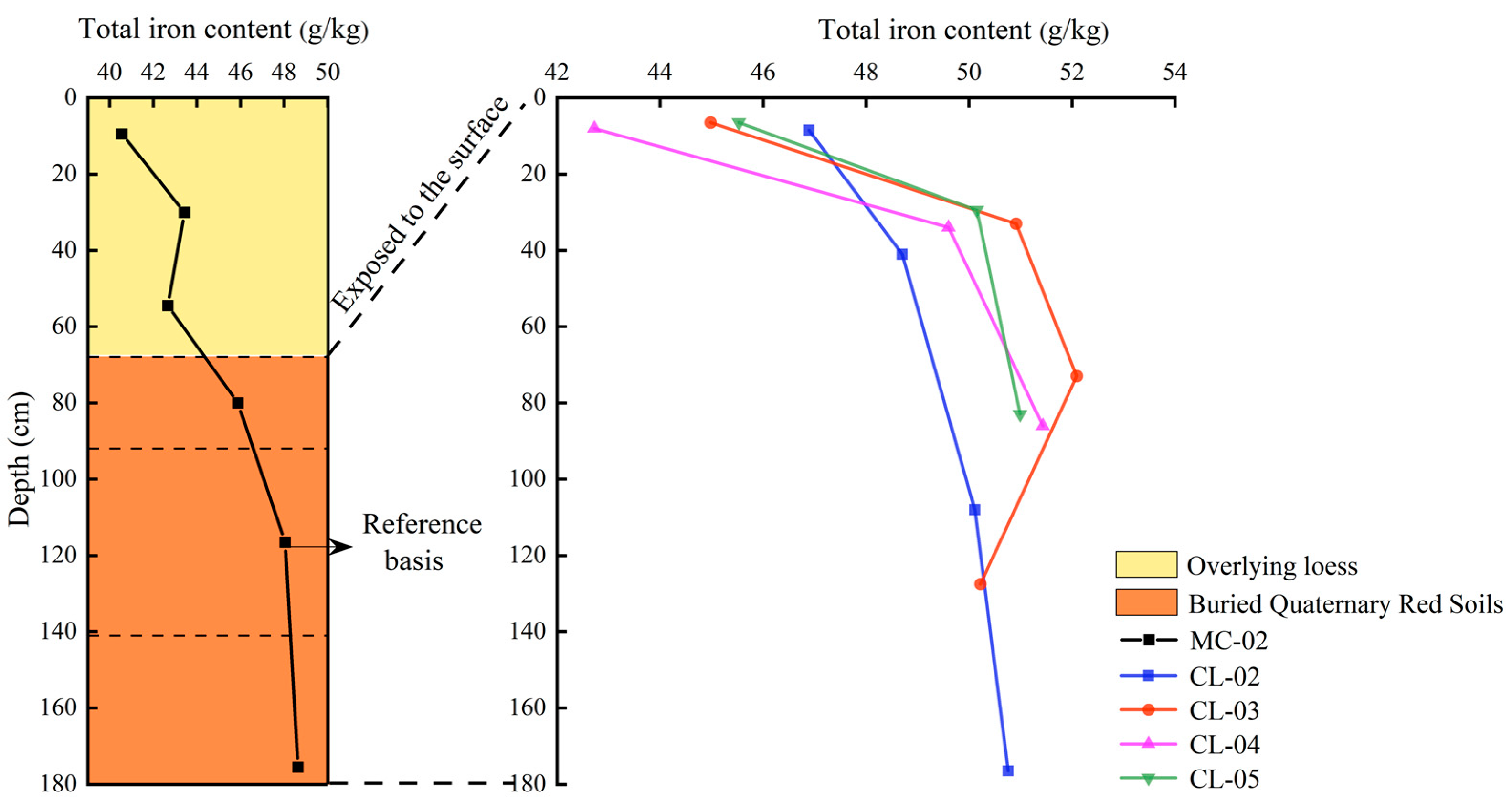
- Variation characteristics of total iron content with profile depth
- (2)
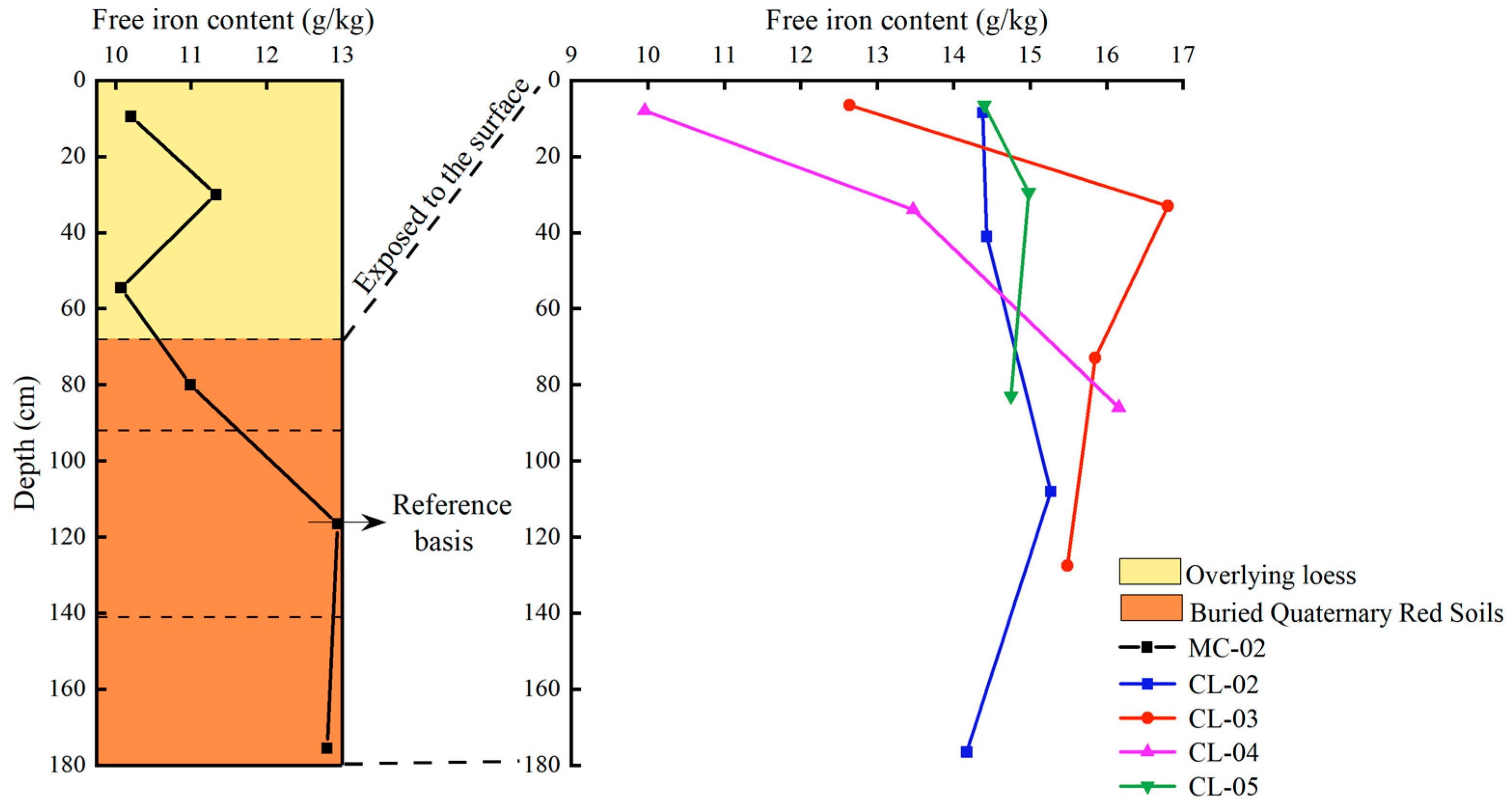
- Variation of free iron content with profile depth
- (3)
- Variation of silicate-bound iron pool content with profile depth
- (4)
- Variation characteristics of poorly crystalline iron oxides content with profile depth
- (5)
- Variation characteristics of crystalline oxides content with profile depth
3.2.2. Variation Characteristics of Iron Content Ratio between Different Forms in Quaternary Red Soils with Profile Depth
- (1)
- Variation characteristics of iron freeness with profile depth
- (2)
- Variation characteristics of iron active index with profile depth
- (3)
- Variation characteristics of iron crystallinity with profile depth
3.3. Changes of Iron in Different Forms of Quaternary Red Soils
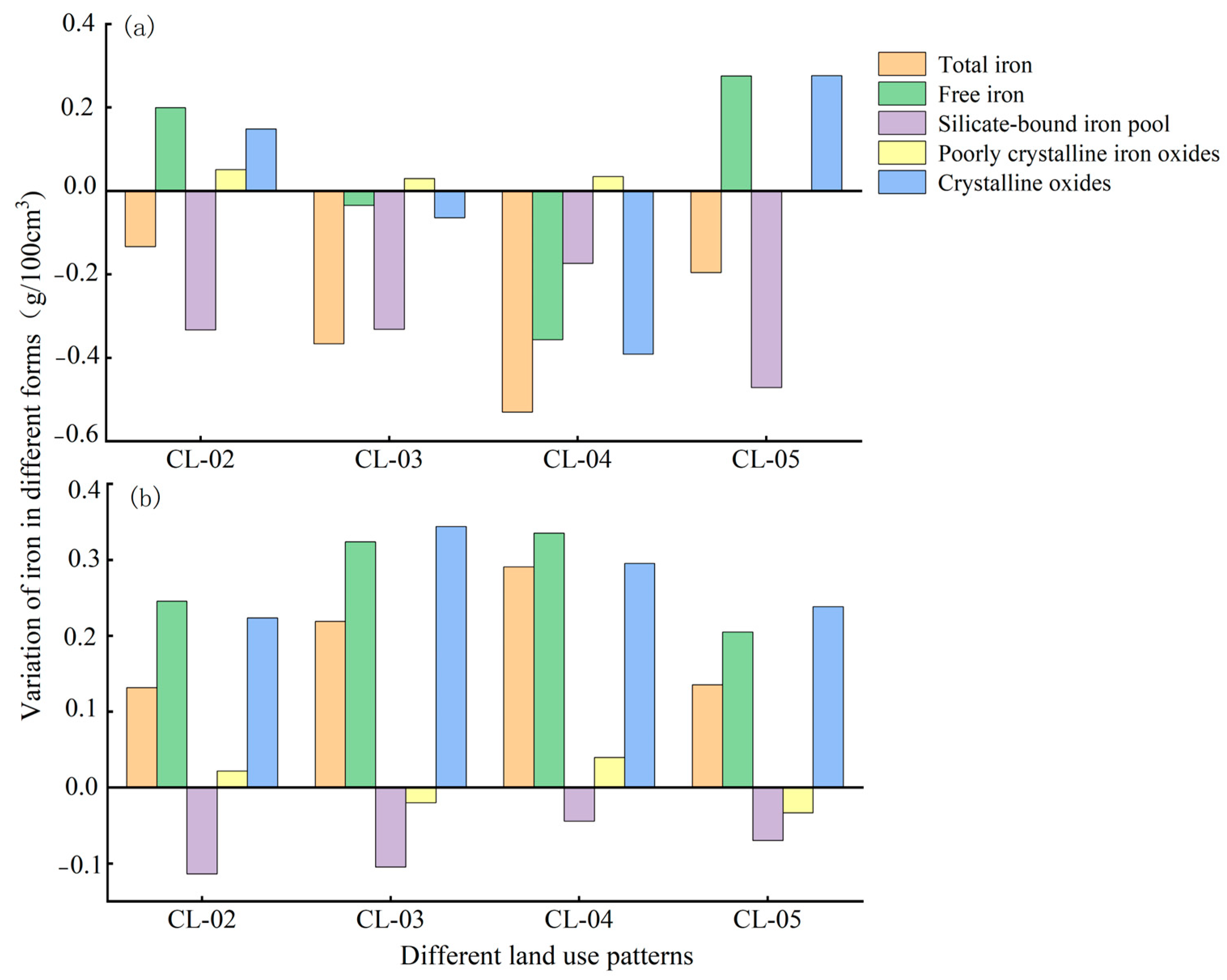
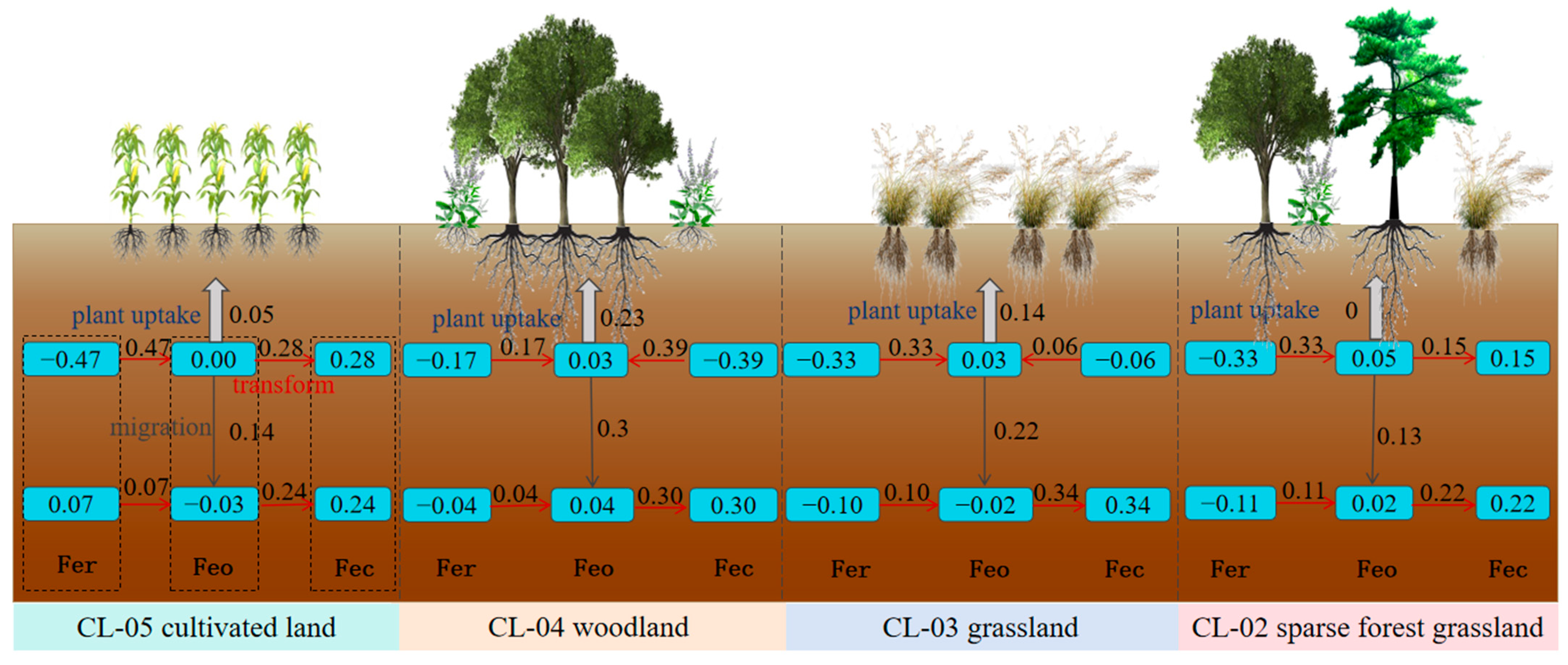
3.3.1. Loss/Gain Characteristics of Iron in Different Forms
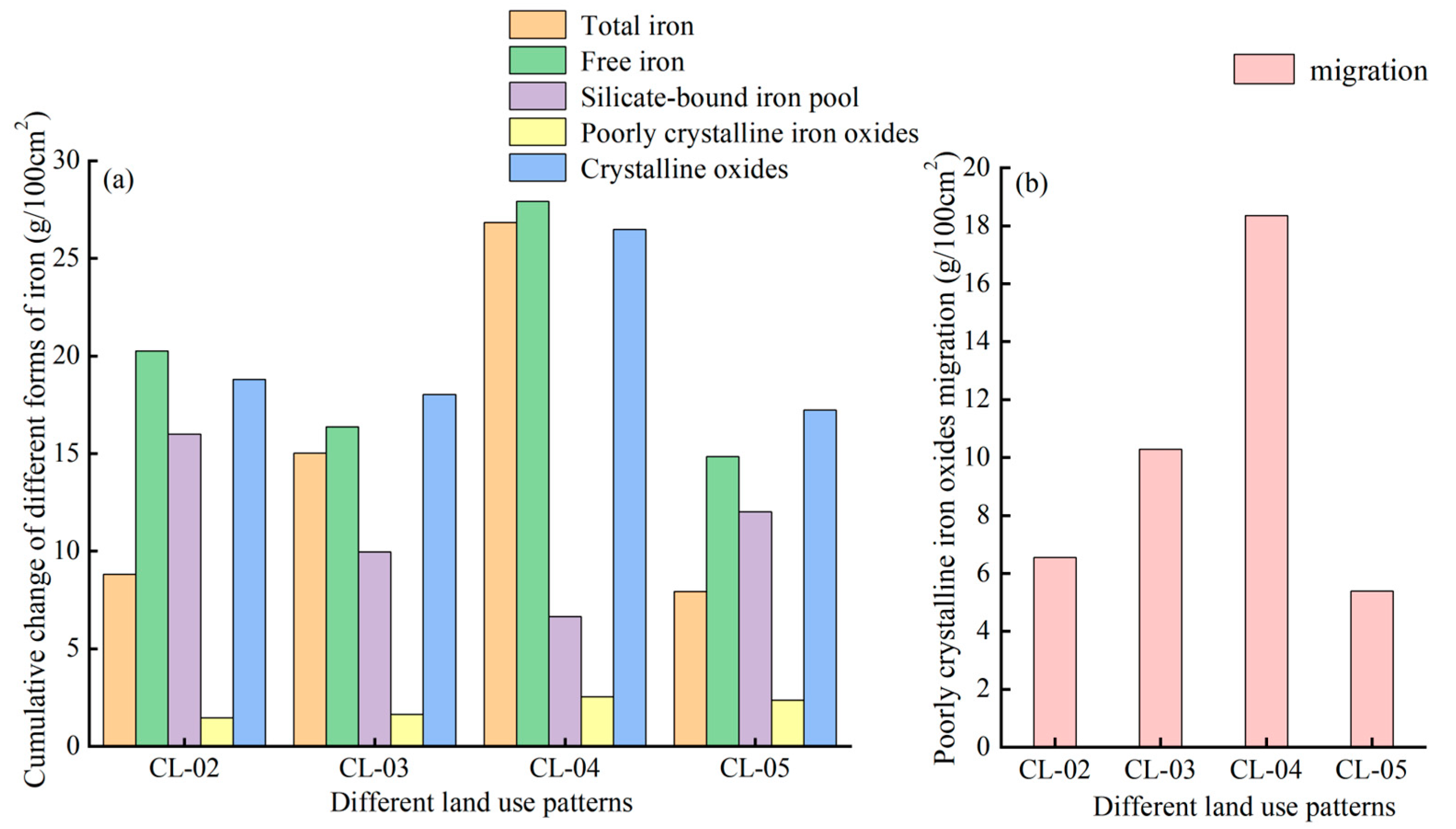
3.3.2. Cumulative Changes and Migrations of Iron in Different Forms
3.4. The Transformation Rate of Iron between Different Forms
4. Discussion
4.1. Iron Composition Characteristics in Different Forms of Quaternary Red Soils
4.2. Migration and Transformation of Iron in Different Forms in Quaternary Red Soils
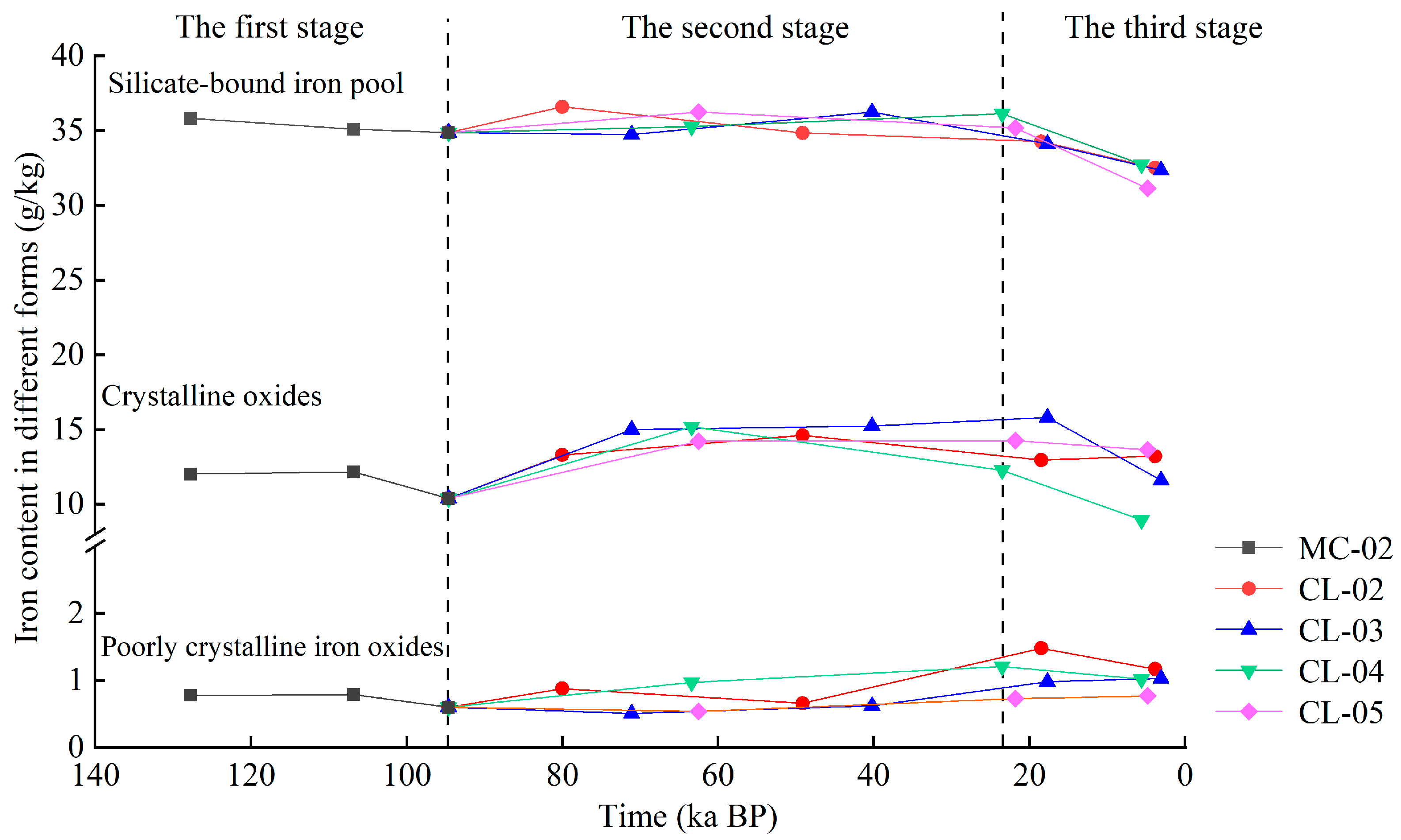
4.3. Evolution Characteristics of Iron in Different Forms in Quaternary Red Soils over Time
5. Conclusions
- (1)
- Anthropogenic land use activities in the middle temperate subhumid region have altered the iron composition of Quaternary red soils. The total iron content has increased due to anthropogenic influences. Some of the silicate-bound iron in the topsoil and subsoil has undergone weathering to form free iron, with cultivated land showing the highest transformation intensity.
- (2)
- In this region, the conversion of iron forms within the soil profile surpasses migration and is dominated by migration. Land use patterns affect the conversion of poorly crystalline to crystalline iron oxides in both topsoil and subsoil, with grassland exhibiting the highest transformation intensity in the subsoil.
- (3)
- Over time, anthropogenic activities have accelerated the evolution of iron morphology in these soils. Three distinct stages (140–94 ka BP; 94–24 ka BP; 24 ka BP to the present) of iron content change were observed, correlating with periods of human land use. Since 24 ka BP, the conversion rate between iron oxide forms has increased significantly due to intensified human activities.
- (4)
- After considering various factors such as suitability, stability, and sustainability, it is recommended that rational planning and utilization of Quaternary red soil resources should be conducted. Scientific planning or adjustments to land use should be carried out in the distribution areas, with reference to different patterns of land coverage. For essential cultivated land, protective farming practices such as no-till, reduced tillage, and straw return, along with the scientific application of organic fertilizers, should be adopted to promote the conversion of crystalline iron into poorly crystalline iron and improve the availability of soil iron. In low mountainous and hilly areas with severe soil erosion, some of the cultivated land or grassland located on higher ground with steep slopes can be moderately converted into woodland through returning farmland to forest or afforestation, with the aim of achieving functions such as windbreaks, sand fixation, and water conservation. This approach will enhance soil fertility, ensure food security, and mitigate issues such as soil erosion and water evaporation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giannetta, B.; Plaza, C.; Thompson, A.; Plante, A.F.; Zaccone, C. Iron speciation in soil size fractions under different land uses. Geoderma 2022, 418, 115842. [Google Scholar] [CrossRef]
- Gao, M.; Li, H.; Xie, Z.; Li, Z.; Luo, Z.; Yu, R.; Lü, C.; He, J. The fate of Arsenic associated with the transformation of iron oxides in soils: The mineralogical evidence. Sci. Total Environ. 2024, 914, 169795. [Google Scholar] [CrossRef] [PubMed]
- He, N.Z. Iron nutrition of plants. Prog. Soil Sci. 1986, 1, 19–23. [Google Scholar]
- Herndon, E.; AlBashaireh, A.; Singer, D.; Chowdhury, T.R.; Gu, B.; Graham, D. Influence of iron redox cycling on organo-mineral associations in Arctic tundra soil. Geochim. Cosmochim. Acta 2017, 207, 210–231. [Google Scholar] [CrossRef]
- Li, M.Y.; Zhang, X.T.; Liu, H.Y. Effects of Water Management on the Transformation of Iron Oxide Forms in Paddy Soils and Its Coupling with Changes in Cadmium Activity. Environ. Sci. 2022, 43, 4301–4312. [Google Scholar]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Cappellen, P.V.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K. Biogeochemical redox processes and their impact on contaminant dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kirsten, M.; Mikutta, R.; Vogel, C.; Thompson, A.; Mueller, C.W.; Kimaro, D.N.; Bergsma, H.L.; Feger, K.-H.; Kalbitz, K. Iron oxides and aluminous clays selectively control soil carbon storage and stability in the humid tropics. Sci. Rep. 2021, 11, 5076. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Lu, X.H.; Luo, D. Characteristics of Iron Oxides and their Relationship with Soil Types in the Karst Mountainous Areas of Guizhou. Chin. J. Soil Sci. 2021, 52, 505–514. [Google Scholar]
- Zhang, C.C.; Sun, Z.X.; Wang, Q.B.; Jiang, Y.Y.; Duan, S.Y.; Sun, F.J. Characteristics of Bacterial and Fungal Communities in Quaternary Red Soils under Different Land-use Patterns. Chin. J. Soil Sci. 2023, 54, 897–912. [Google Scholar]
- Liu, L.W.; Gong, Z.T. Development and evolution of red paleosols. Mar. Geol. Quat. Geol. 2000, 20, 37–42. [Google Scholar]
- Duan, S.Y.; Sun, Z.X.; Wang, Q.B.; Jiang, Y.Y.; Sun, F.J. Comparison of Aggregate Composition in Quaternary Red Soils under Different Land Use Patterns. Chin. J. Soil Sci. 2020, 51, 587–596. [Google Scholar]
- Owliaie, H.; Adhami, E.; Ghiri, M.N. Changes in soil magnetic properties and iron oxides following land use change (case study: Mokhtar Plain, Kohgilouye Province). J. Water Soil 2022, 36, 267–282. [Google Scholar]
- Korkanç, S.Y.; Korkanç, M.; Amiri, A.F. Effects of land use/cover change on heavy metal distribution of soils in wetlands and ecological risk assessment. Sci. Total Environ. 2024, 923, 171603. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, X.; Xue, Z.; Huo, L.; Jiang, M.; Lu, X.; Zou, Y. Distribution characteristics of iron, carbon, nitrogen and phosphorus in the surface soils of different land use types near Xingkai Lake. J. Soil Sediment. 2019, 19, 275–285. [Google Scholar] [CrossRef]
- Li, J.; Richter, D.D. Effects of two-century land use changes on soil iron crystallinity and accumulation in Southeastern Piedmont region, USA. Geoderma 2012, 173, 184–191. [Google Scholar] [CrossRef]
- Yang, D.W.; Zhang, M.K.; Zhang, P.Q.; Liu, Q.Q.; Dong, J.; Li, X.; Huang, X. Changes of Soil Clay Minerals and Iron Oxides after Paddy Field Converted into Forest Land in Plain Areas. Soils 2020, 52, 567–574. [Google Scholar]
- Wu, B.; Meng, X.; Yao, H.; Amelung, W. Iron dynamics and isotope fractionation in soil and rice during 2000 years of rice cultivation. Plant Soil 2024, 495, 615–629. [Google Scholar] [CrossRef]
- Sun, D.D.; Liu, X.; Yang, J.S.; Yu, J.B.; Wang, Z.K.; Zhou, D.; Li, Y.Z.; Yu, Y.; Ning, K. Effects of returning years from farmland to wetland on the content and distribution of soil iron oxides in the Yellow River Delta. Chin. J. Ecol. 2023, 42, 2359–2367. [Google Scholar]
- Huang, L.M.; Shao, M.A.; Chen, L.M.; Han, G.Z.; Zhang, G.L. Evolution Characteristics of Iron Oxides and Magnetic Susceptibility in Stagnic Anthrosols along Chronosequences. Acta Pedol. Sin. 2017, 54, 1–13. [Google Scholar]
- Guo, S.; Cai, Y.; Ren, J.; Guan, Y.; Xin, D.; Long, X. Formation and migration of magnetic particles associated with iron oxide transformation at a hillslope scale. Catena 2021, 197, 104944. [Google Scholar] [CrossRef]
- Wang, Q.B.; Han, C.L.; Sun, F.J.; Sun, Z.X. Chinese Soil History Liaoning Volume; Science Press: Beijing, China, 2010. [Google Scholar]
- Jia, W.J. Liaoning Soil; Liaoning Science and Technology Press: Shenyang, China, 1992. [Google Scholar]
- Chinese Soil Taxonomy Research Group; Institute of Soil Science Chinese Academy of Sciences; Cooperative Research Group on Chinese Soil Taxonomy. Keys to Chinese Soil Taxonomy, 3rd ed.; University of Science and Technology of China Press: Hefei, China, 2001. (In Chinese) [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA; NRCS Publications: Washington, DC, USA, 2014. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Zhang, G.L.; Gong, Z.T. Methods for Laboratory Analysis of Soil Investigation; Science Press: Beijing, China, 2012. [Google Scholar]
- Wagner, D.; Gattinger, A.; Embacher, A.; Pfeiffer, E.M.; Schloter, M.; Lipski, A. Methanogenic activity and biomass in Holocene permafrost deposits of the Lena Delta, Siberian Arctic and its implication for the global methane budget. Glob. Change Biol. 2007, 13, 1089–1099. [Google Scholar] [CrossRef]
- Zheng, R.C.; Shen, R.S.; Qian, Q.W. Rapid analysis of soil-lithium carbonate, boric acid, graphite powder crucible melting method. Soils 1985, 17, 34–35. [Google Scholar]
- Yu, Z.Y. About the arrangement and application of soil analysis data in soil general survey. Chin. J. Soil Sci. 1984, 5, 224–227. [Google Scholar]
- Lu, Y.C.; Yin, G.M.; Chen, J.; Liu, A.G. Photoluminescence dating of Quaternary sediments. Earth Sci. 1995, 6, 668. [Google Scholar]
- Blume, H.; Schwertmann, U. Genetic evaluation of profile distribution of aluminum, iron, and manganese oxides. Soil Sci. Soc. Am. J. 1969, 33, 438–444. [Google Scholar] [CrossRef]
- Schwertmann, U. The effect of pedogenic environments on iron oxide minerals. Adv. Soil Sci. 1985, 1, 171–200. [Google Scholar]
- Schwertmann, U. Occurrence and formation of iron oxides in various pedo-environments. In Iron in Soils and Clay Minerals; Springer: Dordrecht, The Netherlands, 1988; pp. 267–308. [Google Scholar]
- Brewer, R. Fabric and mineral analysis of soils. Soil Sci 1965, 100, 73. [Google Scholar] [CrossRef]
- Stolt, M.; Baker, J. Quantitative comparison of soil and saprolite genesis: Examples from the Virginia Blue Ridge and Piedmont. Southeast. Geol. 2000, 39, 3–4. [Google Scholar]
- Schwertmann, U.; Taylor, R.M. Iron oxides. Miner. Soil Environ. 1989, 1, 379–438. [Google Scholar]
- Postma, D.; Boesen, C.; Kristiansen, H.; Larsen, F. Nitrate reduction in an unconfined sandy aquifer: Water chemistry, reduction processes, and geochemical modeling. Water Resour. Res. 1991, 27, 2027–2045. [Google Scholar] [CrossRef]
- Schjønning, P.; Thomsen, I.K.; Petersen, S.O.; Kristensen, K.; Christensen, B.T. Relating soil microbial activity to water content and tillage-induced differences in soil structure. Geoderma 2011, 163, 256–264. [Google Scholar] [CrossRef]
- Regelink, I.C.; Stoof, C.R.; Rousseva, S.; Weng, L.; Lair, G.J.; Kram, P.; Nikolaidis, N.P.; Kercheva, M.; Banwart, S.; Comans, R.N. Linkages between aggregate formation, porosity and soil chemical properties. Geoderma 2015, 247, 24–37. [Google Scholar] [CrossRef]
- Xia, X.; Han, X.; Zhai, Y. Activation of iron oxide minerals in an aquifer by humic acid to promote adsorption of organic molecules. J. Environ. Manag. 2024, 356, 120543. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gélinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef] [PubMed]
- ThomasArrigo, L.K.; Byrne, J.M.; Kappler, A.; Kretzschmar, R. Impact of organic matter on iron (II)-catalyzed mineral transformations in ferrihydrite–organic matter coprecipitates. Environ. Sci. Technol. 2018, 52, 12316–12326. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.F. Influence of iron oxides and organic matter on magnetic susceptibility in the loess-paleosol sequence. Acta Pedol. Sin. 2004, 41, 7–12. [Google Scholar]
- Wang, J.L.; Liu, Z.Y. Iron transformation in rice rhizosphere. Acta Pedol. Sin. 1992, 29, 364–385. [Google Scholar]
- Anderson, W.; Parkpian, P. Plant availability of an iron waste product utilized as an agricultural fertilizer on calcareous soil. J. Plant Nutr. 1984, 7, 223–233. [Google Scholar] [CrossRef]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol. Biochem. 2002, 34, 139–162. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, N.; Li, J.; Wang, Y.; Liu, Y.; Cao, M.; Yan, Q. Characterization of humic acids from original coal and its oxidization production. Sci. Rep. 2021, 11, 15381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Yuan, L.; Lin, Z.A.; Li, Y.T.; Hu, S.W.; Zhao, B.Q. Advances in humic acid for promoting plant growth and its mechanism. J. Plant Nutr. Fert. 2017, 23, 1065–1076. [Google Scholar]
- DiDonato, N.; Chen, H.; Waggoner, D.; Hatcher, P. Potential origin and formation for molecular components of humic acids in soils. Geochim. Cosmochim. Ac. 2016, 178, 210–222. [Google Scholar] [CrossRef]
- Duan, S.Y.; Sun, Z.X.; Wang, Q.B.; Jiang, Y.Y.; Han, C.L.; Zhang, Y.W.; Lv, M.F.; Sun, F.J.; Chen, L.M. Characteristics of Soil Organic Carbon Distribution of Quaternary Red Soils under Different Land Use Patterns. Chin. J. Soil Sci. 2021, 52, 1078–1084. [Google Scholar]
- Li, T.J.; Zhao, Y.; Zhang, K.L.; Zhang, Y.S.; Wang, Y. Soil Geography, 3rd ed.; Higher Education Press: Beijing, China, 2004. [Google Scholar]
- Sun, W.G.; Gan, Z.T.; Sun, Z.G.; Li, L.L.; Sun, J.K.; Sun, W.L.; Mou, X.J.; Wang, L.L. Spatial Distribution Characteristics of Fe and Mn Contents in the New-born Coastal Marshes in the Yellow River Estuary. Environ. Sci 2013, 34, 4411–4419. [Google Scholar]
- Li, Y.F.; Yang, Y.F.; Wang, Y.Y.; Liu, W.J.; Zhang, P.J. Characteristics of soil iron forms in wetlands with various restoration ages around the Caizi Lake, Anhui Province. Acta Sci. Circumstantiae 2015, 35, 3234–3241. [Google Scholar]
- Drever, J.I.; Vance, G.F. Role of soil organic acids in mineral weathering processes. Org. Acids Geol. Process. 1994, 138–161. [Google Scholar]
- Tan, K.H. Degradation of soil minerals by organic acids. Interact. Soil Miner. Nat. Org. Microbes 1986, 17, 1–27. [Google Scholar]
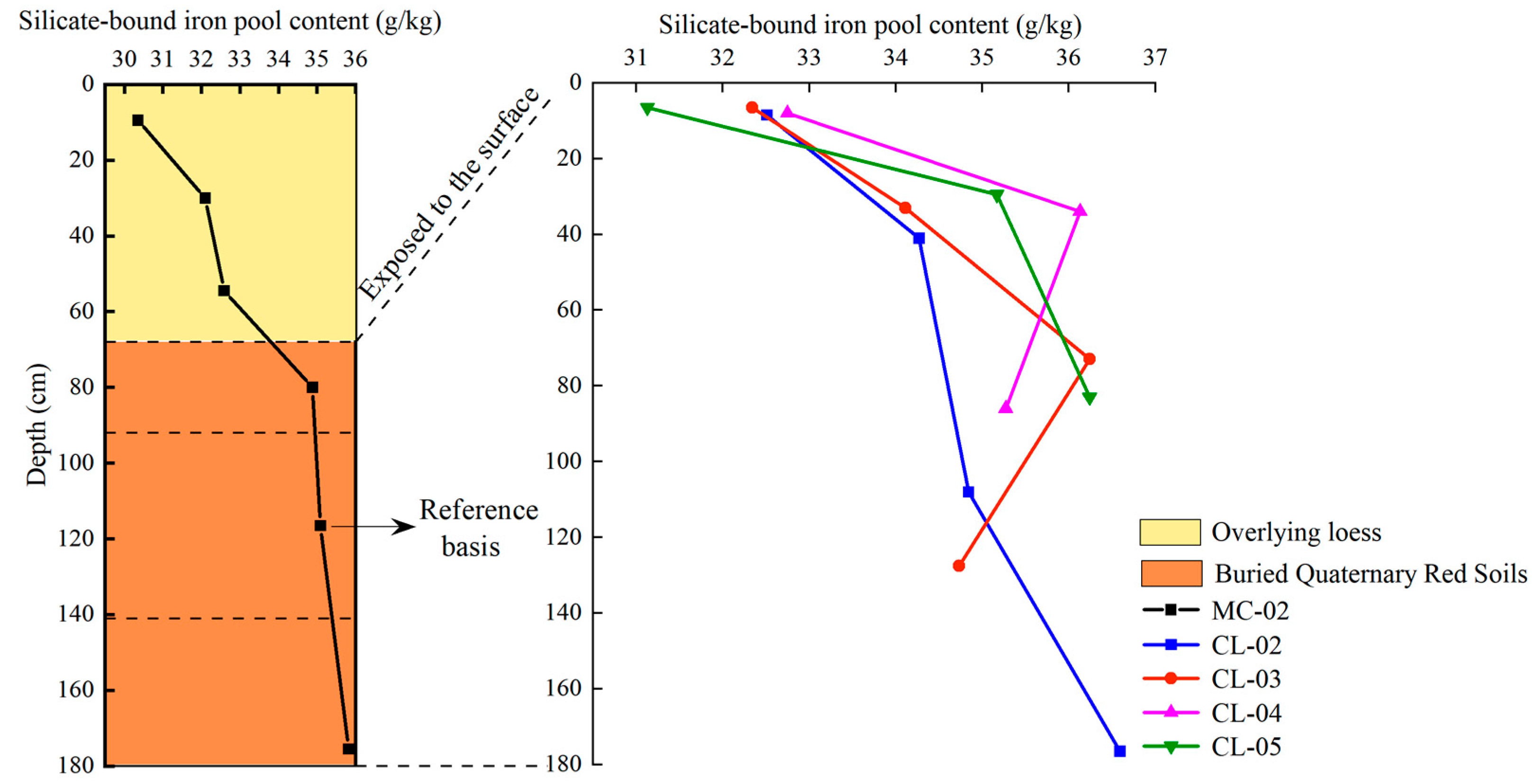
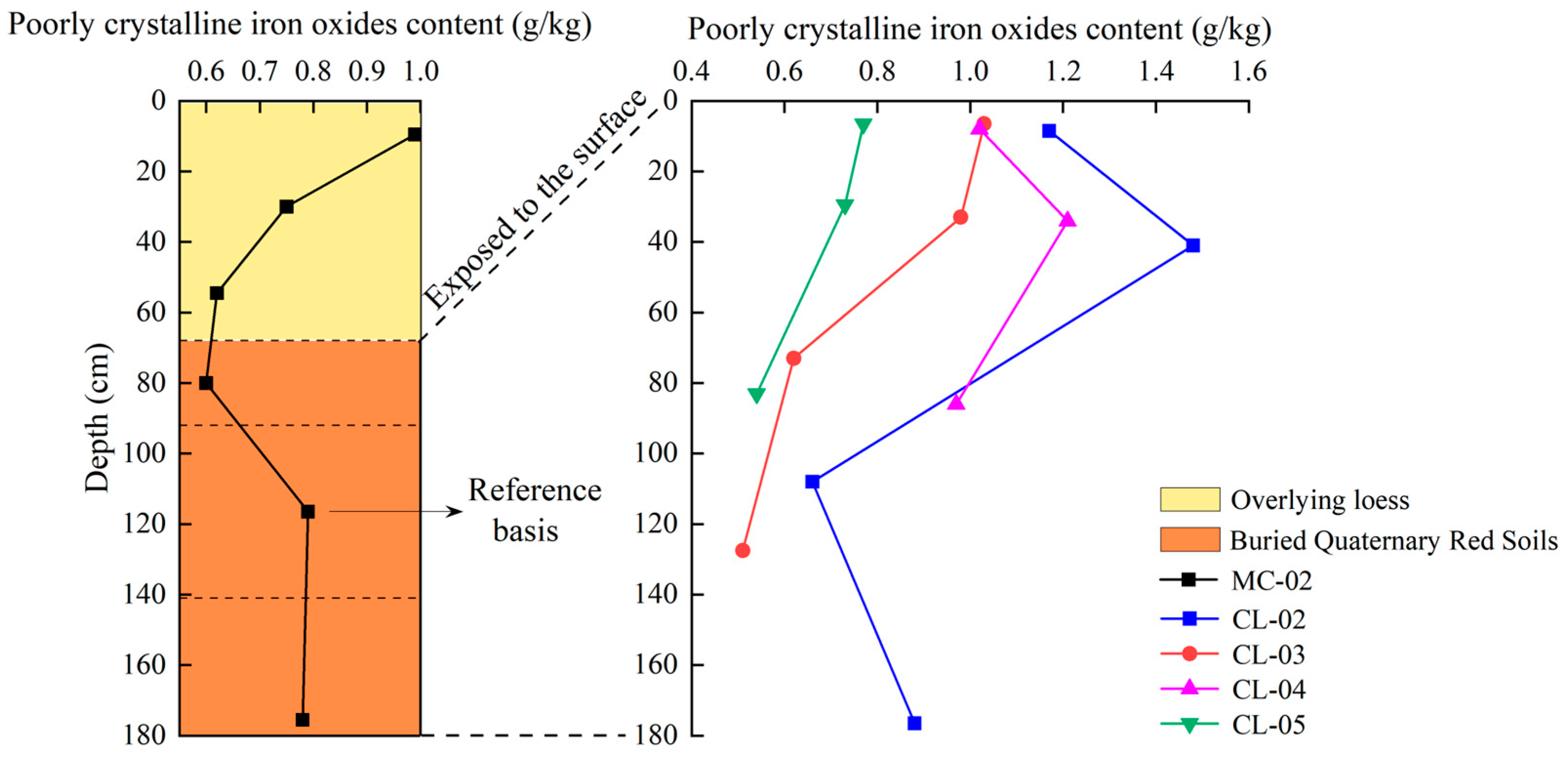

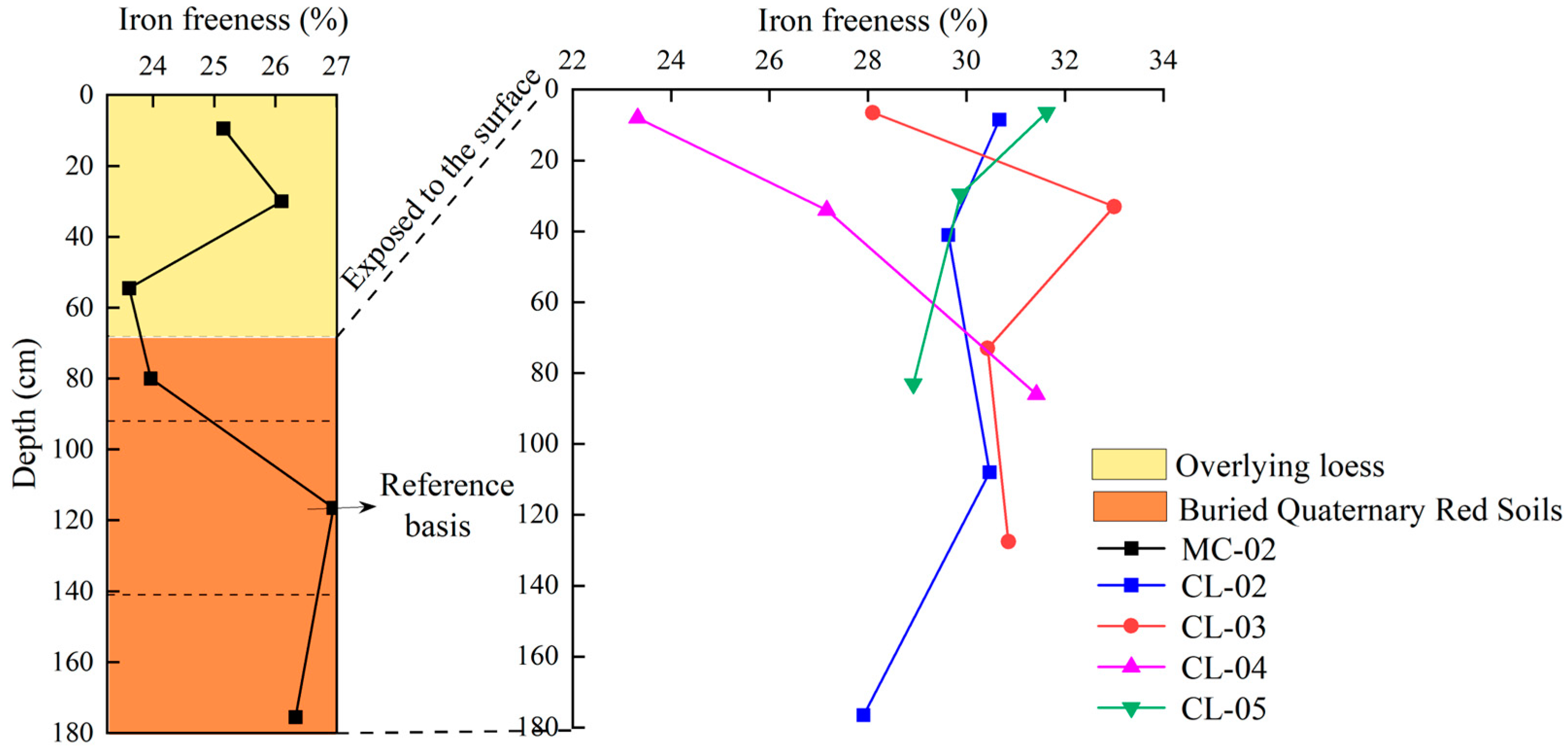
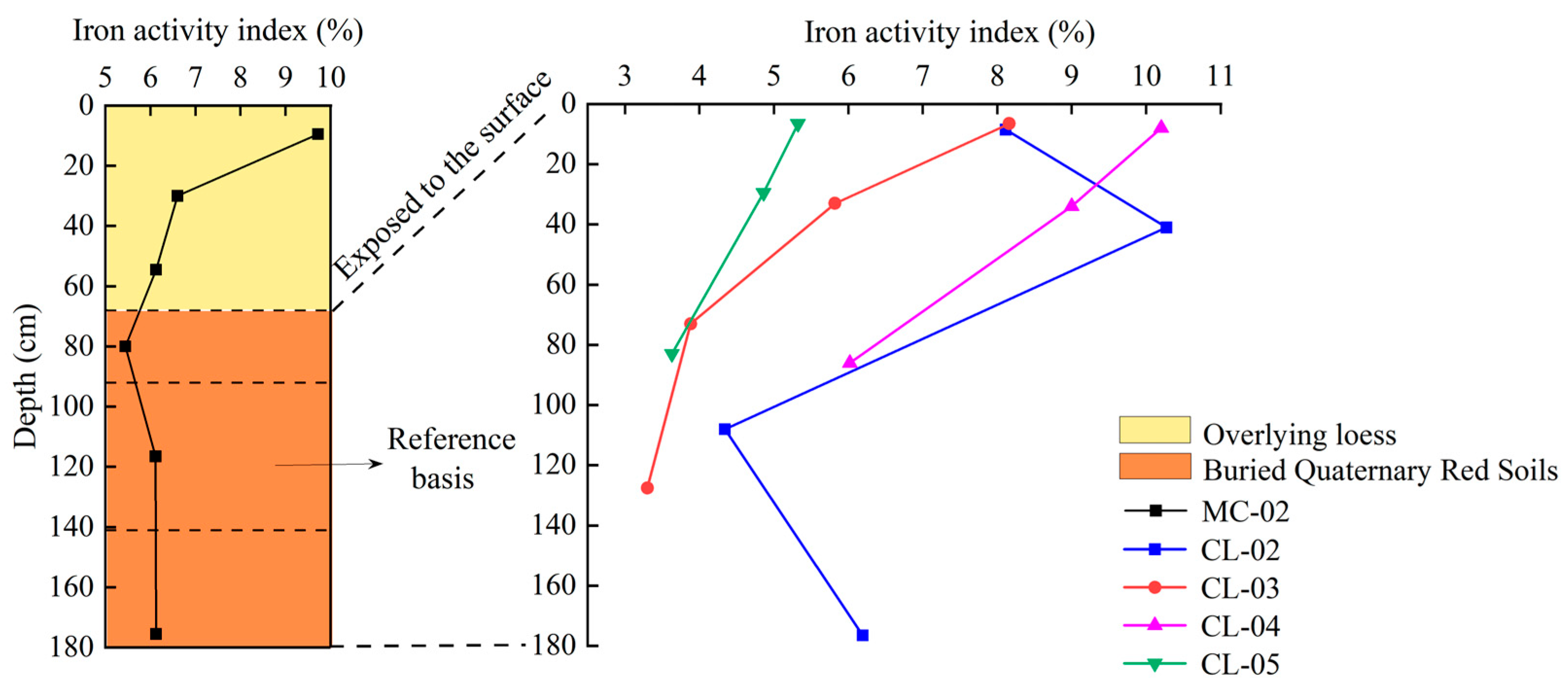
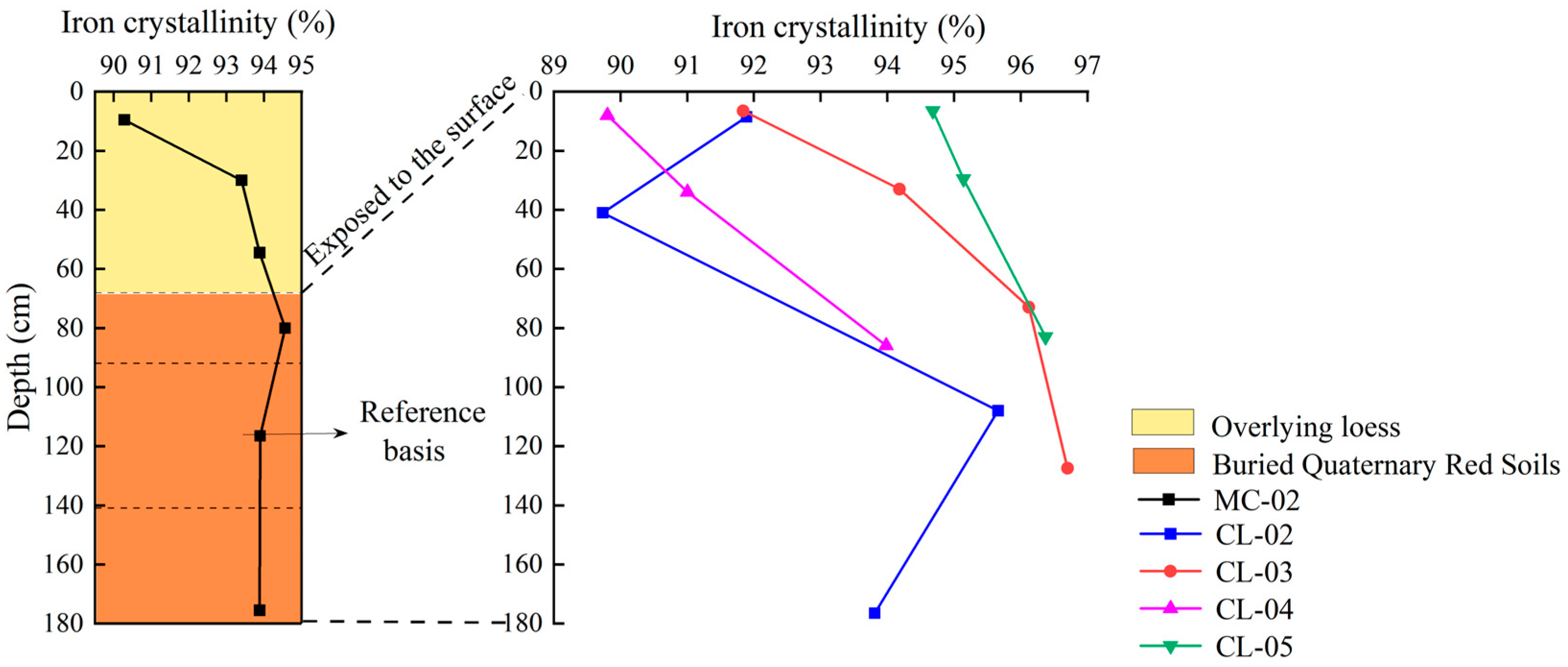

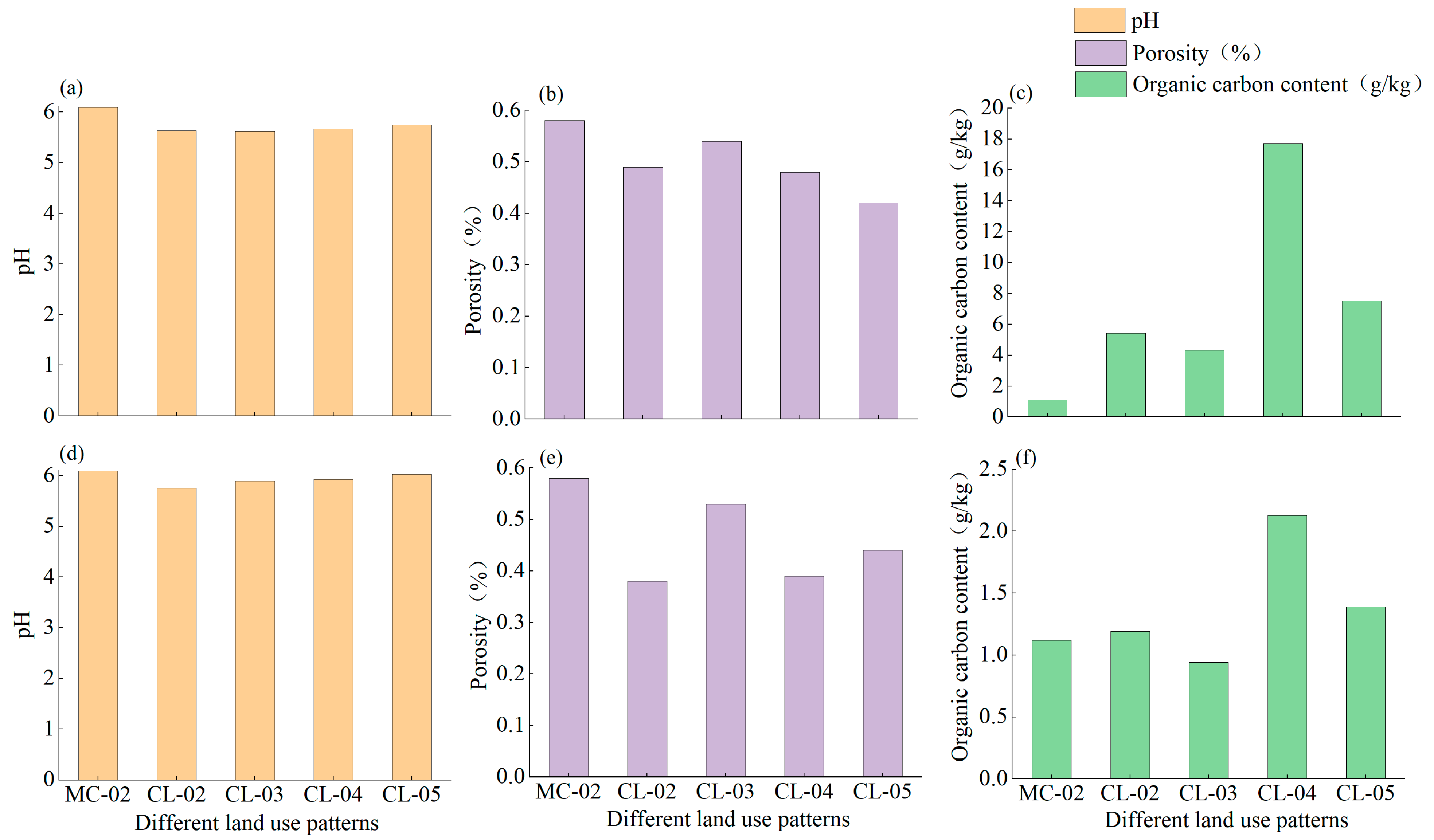

| Moisture Content (%) | pH | Bulk Density (g cm−3) | Sand Content (%) | Silt Content (%) | Clay Content (%) | Organic Carbon (g kg−1) | Total Nitrogen (g kg−1) | Total Manganese (%) | |
|---|---|---|---|---|---|---|---|---|---|
| MC-02 | 5.85 | 6.09 | 1.12 | 20.58 | 56.24 | 23.18 | 1.12 | 0.19 | 0.09 |
| CL-02 | 17.79 | 5.74 | 1.61 | 18.72 | 55.54 | 25.74 | 1.55 | 0.24 | 0.08 |
| CL-03 | 17.46 | 5.87 | 1.23 | 19.87 | 52.71 | 27.42 | 1.22 | 0.24 | 0.07 |
| CL-04 | 20.57 | 5.90 | 1.59 | 20.38 | 53.30 | 26.32 | 4.21 | 0.44 | 0.09 |
| CL-05 | 19.58 | 6.00 | 1.49 | 18.62 | 56.44 | 24.91 | 3.54 | 0.47 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.-C.; Sun, Z.-X.; Jiang, Y.-Y.; Duan, S.-Y. Accelerated Iron Evolution in Quaternary Red Soils through Anthropogenic Land Use Activities. Agronomy 2024, 14, 1669. https://doi.org/10.3390/agronomy14081669
Zhang C-C, Sun Z-X, Jiang Y-Y, Duan S-Y. Accelerated Iron Evolution in Quaternary Red Soils through Anthropogenic Land Use Activities. Agronomy. 2024; 14(8):1669. https://doi.org/10.3390/agronomy14081669
Chicago/Turabian StyleZhang, Cheng-Cheng, Zhong-Xiu Sun, Ying-Ying Jiang, and Si-Yi Duan. 2024. "Accelerated Iron Evolution in Quaternary Red Soils through Anthropogenic Land Use Activities" Agronomy 14, no. 8: 1669. https://doi.org/10.3390/agronomy14081669
APA StyleZhang, C.-C., Sun, Z.-X., Jiang, Y.-Y., & Duan, S.-Y. (2024). Accelerated Iron Evolution in Quaternary Red Soils through Anthropogenic Land Use Activities. Agronomy, 14(8), 1669. https://doi.org/10.3390/agronomy14081669






