Species-Dependent Response of Brassica chinensis L. to Elevated CO2 Gradients Influences Uptake and Utilization of Soil Nitrogen, Phosphorus and Potassium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Experimental Materials
2.3. Sampling Harvest
2.4. Determination of N, P and K in Plants and Soils
2.5. Determination of Soil Enzymatic Activity
2.6. Statistical Analysis
3. Results
3.1. Plant Biomass Production
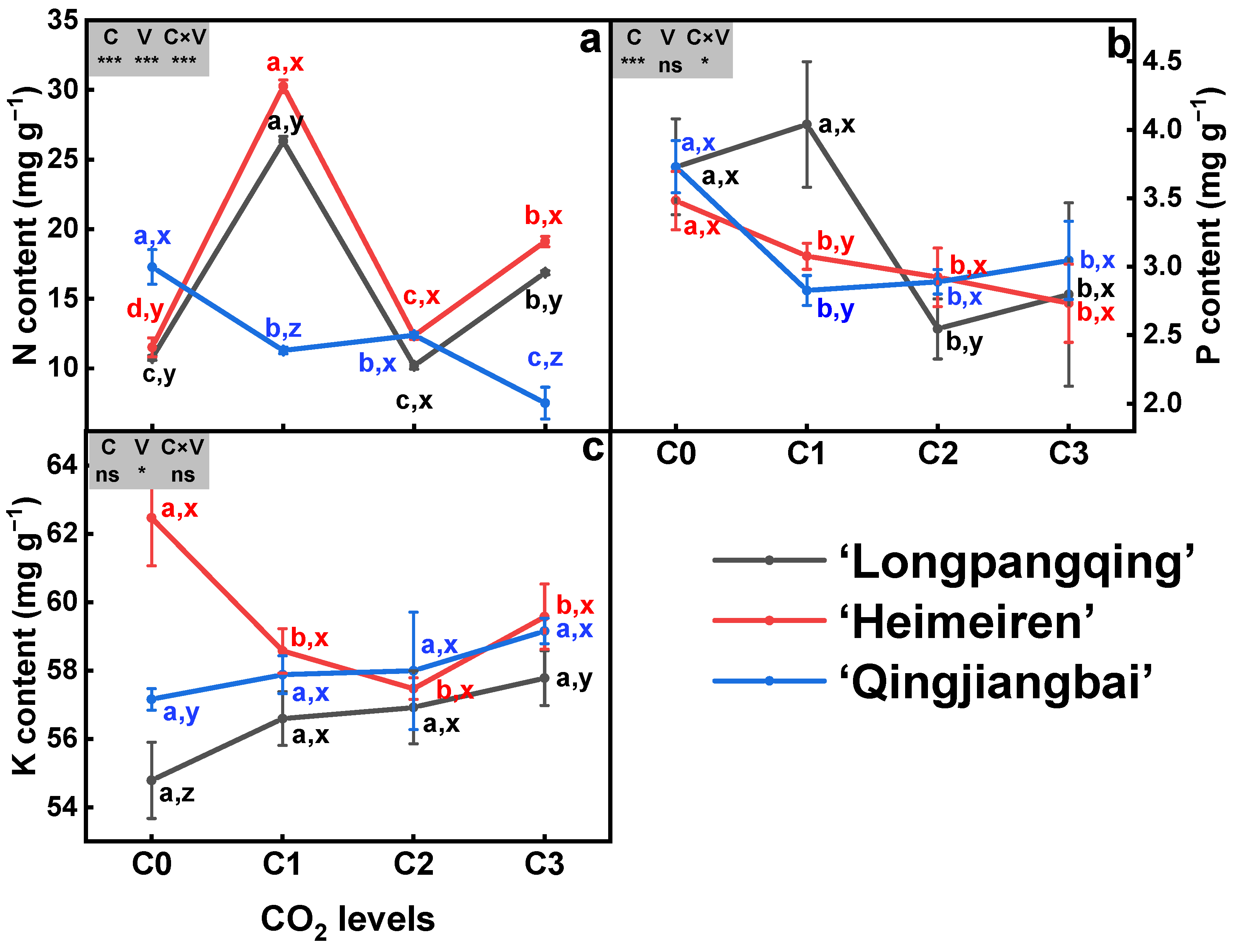
3.2. Plant N, P and K Content
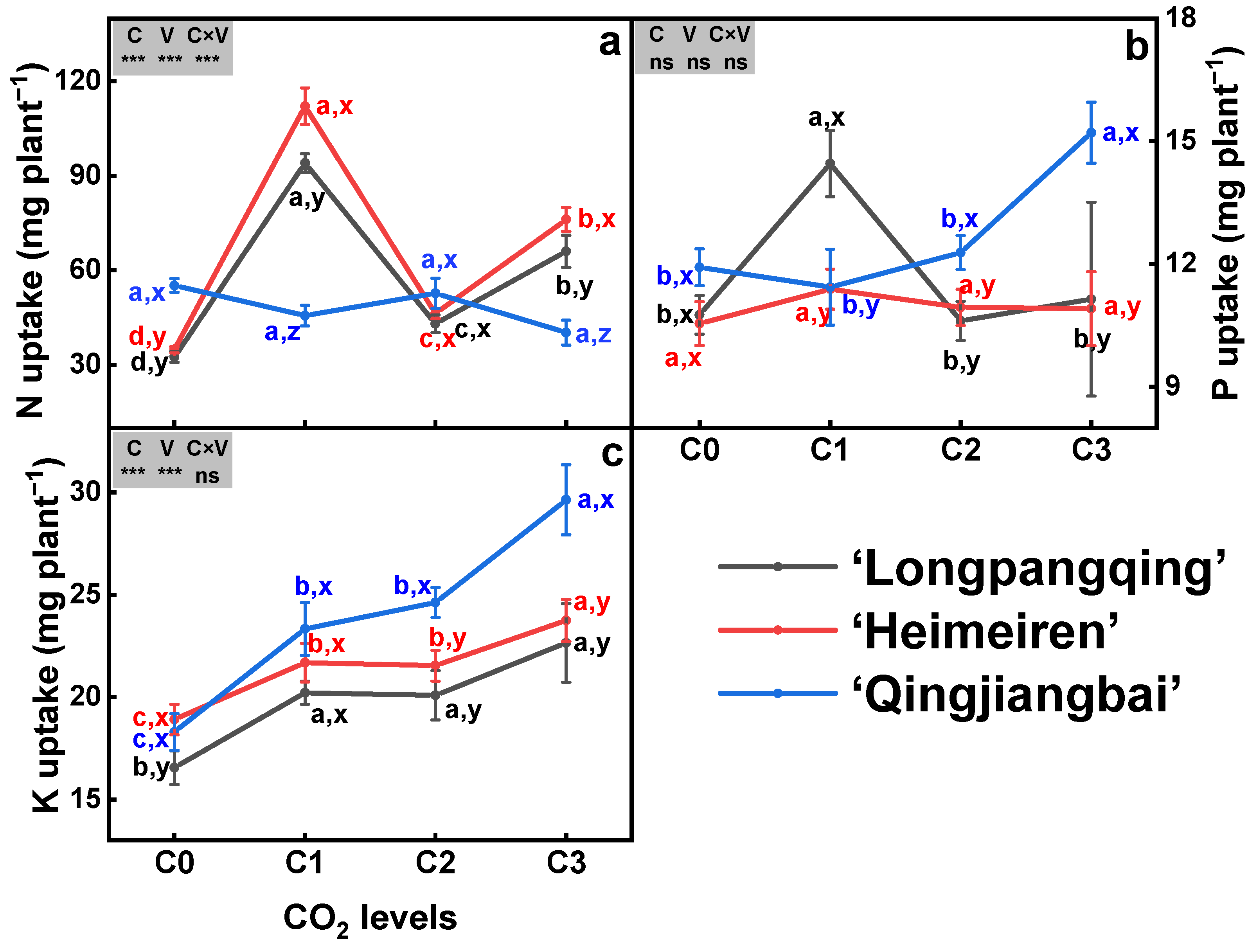
3.3. Plant N, P and K Uptake
3.4. Plant N, P and K Use Efficiency
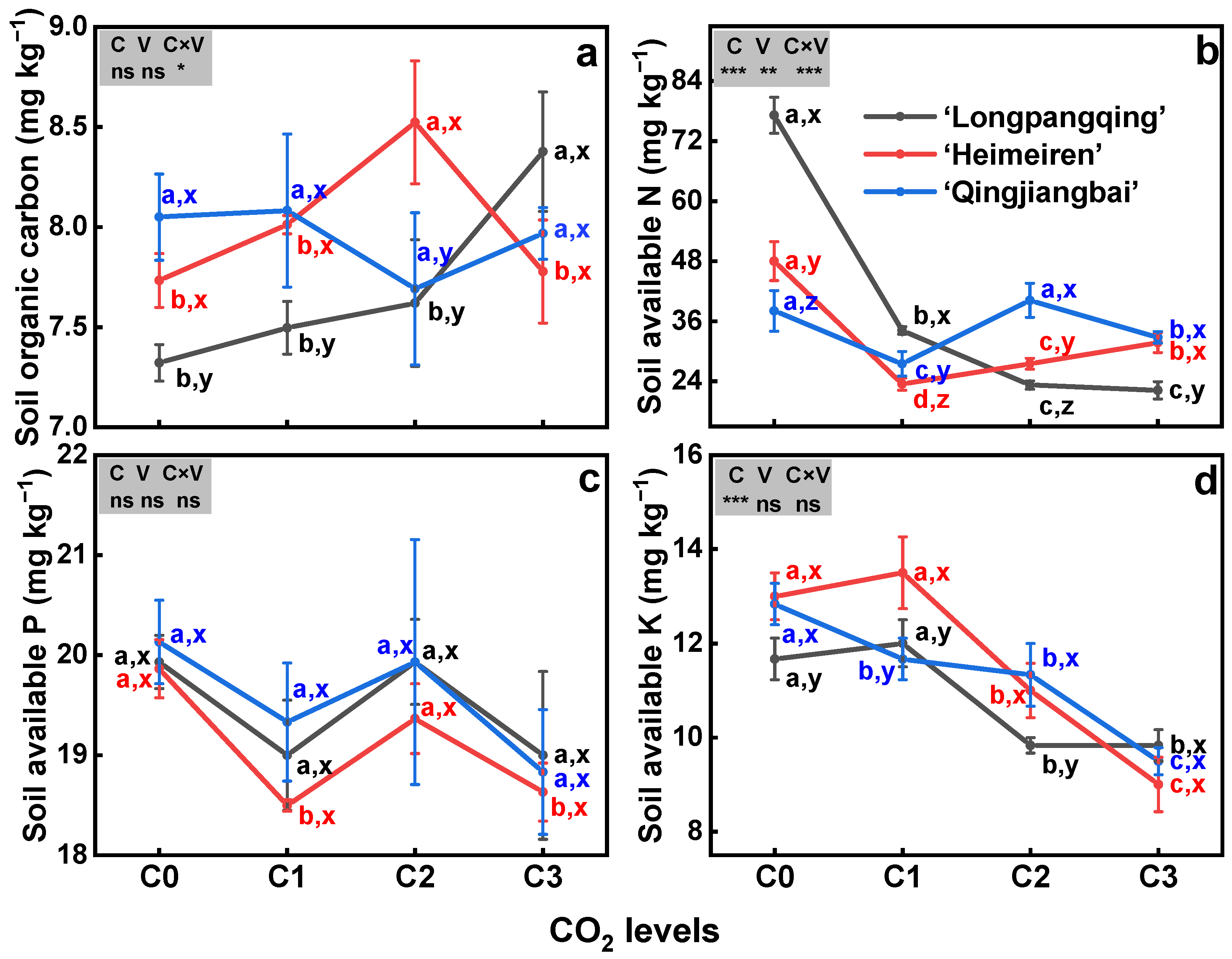
3.5. Soil Available Nutrients
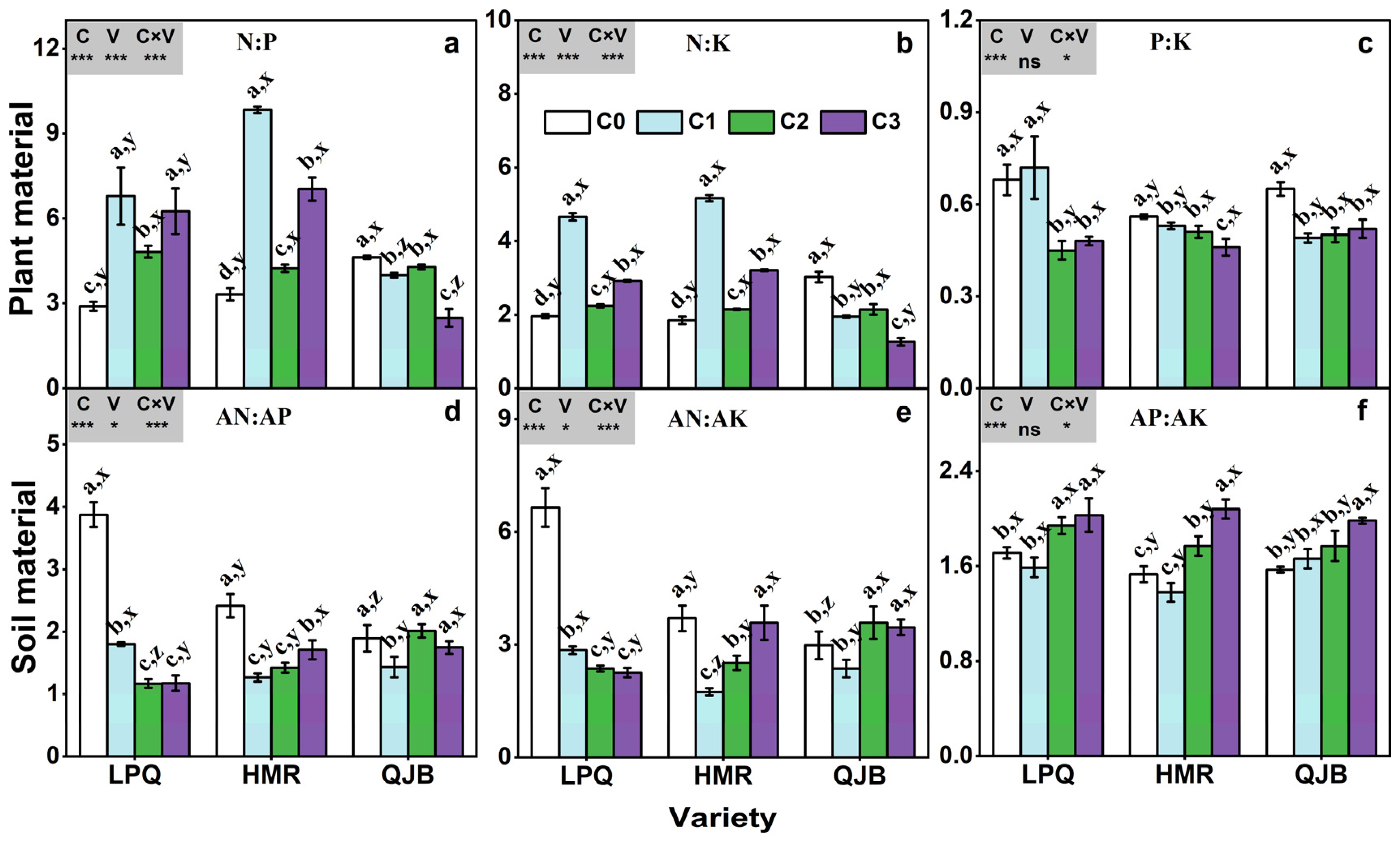
3.6. N:P:K Ratios
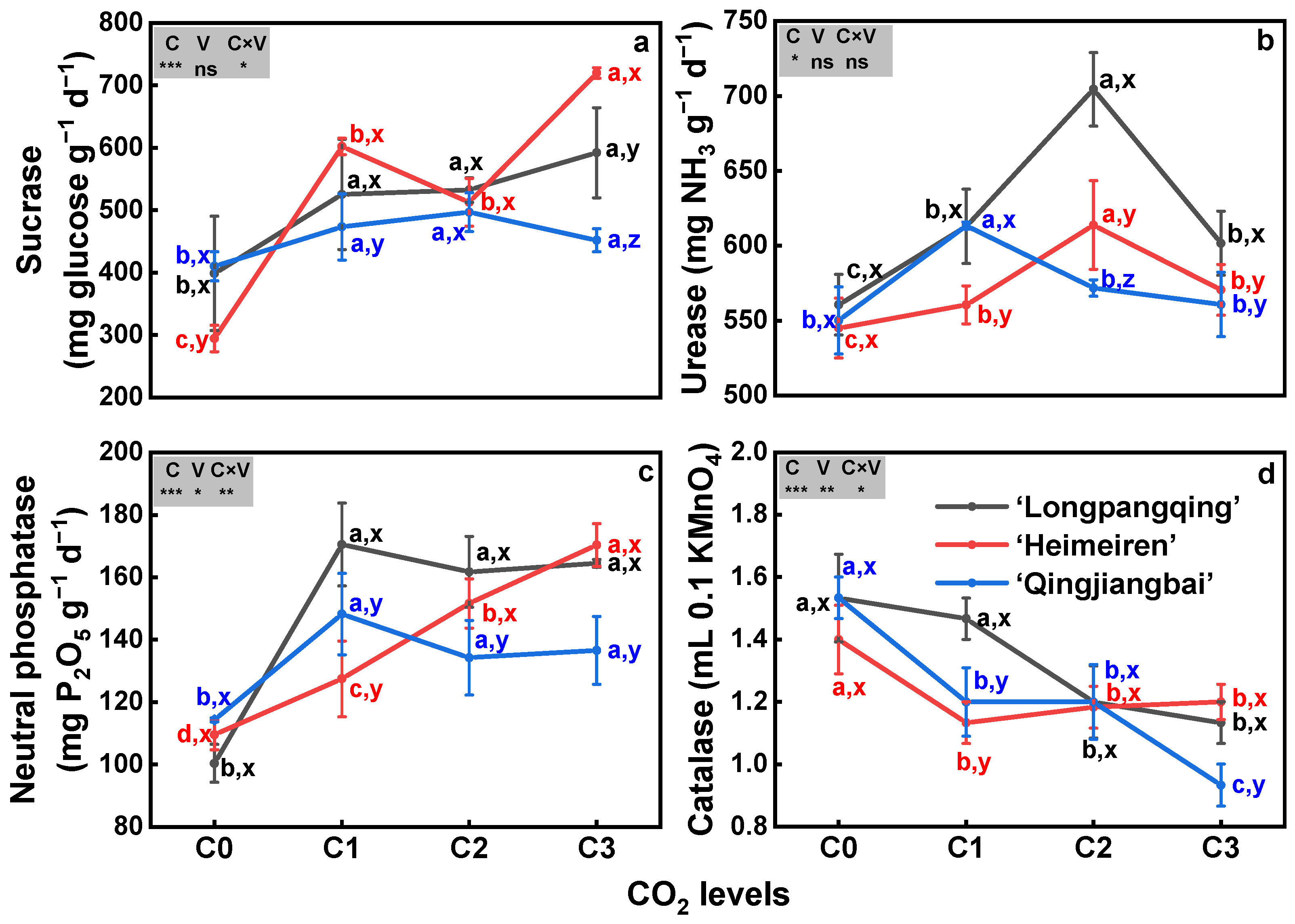
3.7. Soil Enzymatic Activities
3.8. Correlations
4. Discussion
4.1. eCO2 Linearly Increases Biomass Production of the Three Tested B. chinensis Varieties
4.2. Effect of eCO2 on N, P and K Uptake Depends on the Variety of B. chinensis
4.3. eCO2 Improves N, P and K Use Efficiency of B. chinensis
4.4. eCO2 Stimulates Soil Enzyme Activities to Reduce Soil Nutrient Limitation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| aCO2 | atmospheric carbon dioxide |
| eCO2 | elevated carbon dioxide |
| B. chinensis | Brassica chinensis |
| C | carbon |
| N | nitrogen |
| P | phosphorus |
| K | potassium |
| FACE | free-air CO2 enrichment |
| ANOVA | analysis of variance |
| SOC | soil organic carbon |
| AN | available nitrogen |
| AP | available phosphorus |
| AK | available potassium |
| NUE | nitrogen use efficiency |
| PUE | phosphorus use efficiency |
| KUE | potassium use efficiency |
References
- Kläring, H.P.; Hauschild, C.; Heissner, A.; Bar-Yosef, B. Model-based control of CO2 concentration in greenhouses at ambient levels increases cucumber yield. Agric. For. Meteorol. 2007, 143, 208–216. [Google Scholar] [CrossRef]
- Dong, J.L.; Gruda, N.; Li, X.; Tang, Y.; Zhang, P.J.; Duan, Z.Q. Sustainable vegetable production under changing climate: The impact of elevated CO2 on yield of vegetables and the interactions with environments—A review. J. Clean. Prod. 2020, 253, 119920. [Google Scholar] [CrossRef]
- Willits, D.H.; Peet, M.M. Predicting yield responses to different greenhouse CO2 enrichment schemes-cucumbers and tomatoes. Agric. For. Meteorol. 1989, 44, 275–293. [Google Scholar] [CrossRef]
- Holley, J.; Mattson, N.; Ashenafi, E.; Nyman, M. The impact of CO2 enrichment on biomass, carotenoids, xanthophyll, and mineral content of Lettuce (Lactuca sativa L.). Horticulturae 2022, 8, 820. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wu, Z.M. CO2 enhances low-nitrogen adaption by promoting amino acid metabolism in Brassica napus. Plant Physiol. Biochem. 2023, 201, 107864. [Google Scholar] [CrossRef] [PubMed]
- Jo, N.Y.; Lee, J.; Byeon, J.E.; Park, H.J.; Ryoo, J.W.; Hwang, S.G. Elevated CO2 concentration induces changes in plant growth, transcriptome, and antioxidant activity in fennel. Front. Plant Sci. 2022, 13, 1067713. [Google Scholar] [CrossRef]
- Li, X.; Dong, J.L.; Gruda, N.S.; Chu, W.Y.; Duan, Z.Q. Interactive effects of the CO2 enrichment and nitrogen supply on the biomass accumulation, gas exchange properties, and mineral elements concentrations in cucumber plants at different growth stages. Agronomy 2020, 10, 139. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Lugassi, N.; Egbaria, A.; Granot, D.; Yermiyahu, U. The role of nitrogen in photosynthetic acclimation to elevated [CO2] in tomatoes. Plant Soil 2019, 434, 397–411. [Google Scholar] [CrossRef]
- Deng, Q.; Hui, D.F.; Luo, Y.Q.; Elser, J.; Wang, Y.P.; Loladze, I.; Zhang, Q.F.; Dennis, S. Down-regulation of tissue N:P ratios in terrestrial plants by elevated CO2. Ecology 2015, 96, 3354–3362. [Google Scholar] [CrossRef]
- Lenka, N.K.; Lenka, S.; Mahapatra, P.; Sharma, N.; Kumar, S.; Aher, S.B.; Yashona, D.S. The fate of 15N labeled urea in a soybean-wheat cropping sequence under elevated CO2 and/or temperature. Agric. Ecosyst. Environ. 2019, 282, 23–29. [Google Scholar] [CrossRef]
- Du, C.J.; Wang, X.D.; Zhang, M.Y.; Jing, J.; Gao, Y.H. Effects of elevated CO2 on plant C-N-P stoichiometry in terrestrial ecosystems: A meta-analysis. Sci. Total Environ. 2019, 650, 697–708. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Varghese, E.; Dash, P.K.; Padhy, S.R.; Das, A.; Santra, P.; Mohapatra, T. Anticipated atmospheric CO2 elevation differentially influenced the soil microbial diversities in crop, grassland, and forest: A meta-analysis. Rhizosphere 2023, 25, 100630. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.B.; Gruda, N.S.; Wang, Z.Y.; Duan, Z.Q.; Müller, C.; Li, X. The regulation of gross nitrogen transformation rates in greenhouse soil cultivated with cucumber plants under elevated atmospheric [CO2] and increased soil temperature. Geoderma 2023, 439, 116680. [Google Scholar] [CrossRef]
- Tan, M.D.; Zong, R.; Lin, H.X.; Dhital, Y.P.; Ayantobo, O.O.; Chen, P.P.; Li, H.Q.; Chen, R.; Wang, Z.H. Responses of soil nutrient and enzyme activities to long-term mulched drip irrigation (MDI) after the conversion of wasteland to cropland. Appl. Soil Ecol. 2023, 190, 104976. [Google Scholar] [CrossRef]
- Jin, J.; Armstrong, R.; Tang, C.X. Impact of elevated CO2 on grain nutrient concentration varies with crops and soils—A long-term FACE study. Sci. Total Environ. 2019, 651, 2641–2647. [Google Scholar] [CrossRef]
- Loladze, I. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 2014, 3, e02245. [Google Scholar] [CrossRef]
- Zhu, X.C.; Song, F.B.; Liu, S.Q.; Liu, F.L. Arbuscular mycorrhiza improves growth, nitrogen uptake, and nitrogen use efficiency in wheat grown under elevated CO2. Mycorrhiza 2016, 26, 133–140. [Google Scholar] [CrossRef]
- Piñero, M.C.; Otálora, G.; Porras, M.E.; Sánchez-Guerrero, M.C.; Lorenzo, P.; Medrano, E.; del Amor, F.M. The form in which nitrogen is supplied affects the polyamines, amino acids, and mineral composition of sweet pepper fruit under an elevated CO2 concentration. J. Agric. Food Chem. 2017, 65, 711–717. [Google Scholar] [CrossRef]
- Pérez-López, U.; Sgherri, C.; Miranda-Apodaca, J.; Micaelli, F.; Lacuesta, M.; Mena-Petite, A.; Quartacci, M.F.; Muñoz-Rueda, A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Biochem. 2018, 123, 233–241. [Google Scholar] [CrossRef]
- Lupitu, A.; Moisa, C.; Gavrilas, S.; Dochia, M.; Chambre, D.; Ciutina, V.; Copolovici, D.M.; Copolovici, L. The influence of elevated CO2 on volatile emissions, photosynthetic characteristics, and pigment content in Brassicaceae plants species and varieties. Plants 2022, 11, 973. [Google Scholar] [CrossRef]
- Lupitu, A.; Moisa, C.; Bortes, F.; Peteleu, D.; Dochia, M.; Chambre, D.; Ciutina, V.; Copolovici, D.M.; Copolovici, L. The impact of increased CO2 and drought stress on the secondary metabolites of cauliflower (Brassica oleracea var. botrytis) and cabbage (Brassica oleracea var. capitata). Plants 2023, 12, 3098. [Google Scholar] [CrossRef] [PubMed]
- Rangaswamy, T.C.; Sridhara, S.; Manoj, K.N.; Gopakkali, P.; Ramesh, N.; Shokralla, S.; El-Abedin, T.Z.K.; Almutairi, K.F.; Elansary, H.O. Impact of elevated CO2 and temperature on growth, development and nutrient uptake of tomato. Horticulturae 2021, 7, 509. [Google Scholar] [CrossRef]
- Shi, S.M.; Xu, X.; Dong, X.S.; Xu, C.Y.; Qiu, Y.L.; He, X.H. Photosynthetic acclimation and growth responses to elevated CO2 associate with leaf nitrogen and phosphorus concentrations in mulberry (Morus multicaulis Perr.). Forests 2021, 12, 660. [Google Scholar] [CrossRef]
- Li, Y.S.; Yu, Z.H.; Liu, X.B.; Mathesius, U.; Wang, G.H.; Tang, C.X.; Wu, J.J.; Liu, J.D.; Zhang, S.Q.; Jin, J. Elevated CO2 increases nitrogen fixation at the reproductive phase contributing to various yield responses of soybean varieties. Front. Plant Sci. 2017, 8, 1546. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Armstrong, B.; Rajashekar, C.B. Rajashekar. Elevated carbon dioxide level suppresses nutritional quality of lettuce and spinach. Am. J. Plant Sci. 2016, 7, 246–258. [Google Scholar] [CrossRef]
- Singh, A.K.; Rai, A.; Kushwaha, M.; Chauhan, P.S.; Pandey, V.; Singh, N. Tree growth rate regulate the influence of elevated CO2 on soil biochemical responses under tropical condition. J. Environ. Manag. 2019, 231, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Liu, B.; Gilna, B.; Zhang, Y.L.; Zhu, C.W.; Ma, H.L.; Pang, J.; Chen, G.P.; Zhu, J.G. Elevated CO2 effects on nutrient competition between a C3 crop (Oryzasativa L.) and a C4 weed (Echinochloa crusgalli L.). Nutr. Cycl. Agroecosys. 2011, 89, 93–104. [Google Scholar] [CrossRef]
- Chakwizira, E.; Dunbar, H.J.; Andrews, M.; Moot, D.J.; Teixeira, E. Elevated carbon-dioxide effects on wheat grain quality differed under contrasting nitrogen and phosphorus fertiliser supply. Ann. Appl. Biol. 2024, 184, 152–162. [Google Scholar] [CrossRef]
- Saleh, A.M.; Abdel-Mawgoud, M.; Hassan, A.R.; Habeeb, T.H.; Yehia, R.S.; AbdElgawad, H. Global metabolic changes induced by Arbuscular mycorrhizal fungi in oregano plants grown under ambient and elevated levels of atmospheric CO2. Plant Physiol. Biochem. 2020, 151, 255–263. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, H. Alteration in biochemical constituents and nutrients partitioning of Asparagus racemosus in response to elevated atmospheric CO2 concentration. Environ. Sci. Pollut. Res. 2022, 29, 6812–6821. [Google Scholar] [CrossRef]
- Saha, S.; Chakraborty, D.; Sehgal, V.K.; Pal, M. Potential impact of rising atmospheric CO2 on quality of grains in chickpea (Cicer arietinum L.). Food Chem. 2015, 187, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Usue, P.L.; Jon, M.A.; Maite, L.; Amaia, M.P.; Alberto, M.R. Growth and nutritional quality improvement in two differently pigmented lettuce varieties grown under elevated CO2 and/or salinity. Sci. Hortic. 2015, 195, 56–66. [Google Scholar] [CrossRef]
- Duan, B.; Zhang, Y.; Xu, G.; Chen, J.; Paquette, A.; Peng, S. Long-term responses of plant growth, soil microbial communities and soil enzyme activities to elevated CO2 and neighbouring plants. Agric. For. Meteorol. 2015, 213, 91–101. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Roy, K.S.; Neogi, S.; Manna, M.C.; Adhya, T.K.; Rao, K.S.; Nayak, A.K. Influence of elevated carbon dioxide and temperature on belowground carbon allocation and enzyme activities in tropical flooded soil planted with rice. Environ. Monit. Assess. 2013, 185, 8659–8671. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.J.; Bai, E.D.; Piao, S.L.; Chen, N.; Zhao, G.; Zheng, Z.T.; Zhu, Y.X. The stimulatory effect of elevated CO2 on soil respiration is unaffected by N addition. Sci. Total Environ. 2022, 813, 151907. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.Z.; Schjoerring, J.K. Effects of elevated atmospheric CO2 on physiology and yield of wheat (Triticum aestivum L.): A meta-analytic test of current hypotheses. Agric. Ecosyst. Environ. 2013, 178, 57–63. [Google Scholar] [CrossRef]
- Kelley, A.M.; Fay, P.A.; Polley, H.W.; Gill, R.A.; Jackson, R.B. Atmospheric CO2 and soil extracellular enzyme activity: A meta-analysis and CO2 gradient experiment. Ecosphere 2011, 2, 96. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Adak, T.; Munda, S.; Kumar, U.; Berliner, J.; Pokhare, S.S.; Jambhulkar, N.N.; Jena, M. Effect of elevated CO2 on chlorpyriphos degradation and soil microbial activities in tropical rice soil. Environ. Monit. Assess. 2016, 188, 105. [Google Scholar] [CrossRef]
- Larson, J.L.; Zak, D.R.; Sinsabaugh, R.L. Extracellular enzyme activity beneath temperate trees growing under elevated carbon dioxide and ozone. Soil Sci. Soc. Am. J. 2002, 66, 1848–1856. [Google Scholar] [CrossRef]
- Piñeiro, J.; Pathare, V.; Ochoa-Hueso, R.; Carrillo, Y.; Power, S.A. No CO2 fertilization effect on plant growth despite enhanced rhizosphere enzyme activity in a low phosphorus soil. Plant Soil 2022, 471, 359–374. [Google Scholar] [CrossRef]
- Piñeiro, J.; Ochoa-Hueso, R.; Serrano-Grijalva, L.; Power, S.A. Phosphorus and water supply independently control productivity and soil enzyme activity responses to elevated CO2 in an understorey community from a Eucalyptus woodland. Plant Soil 2023, 483, 643–657. [Google Scholar] [CrossRef]
- Shi, S.; Luo, X.; Wen, M.; Dong, X.; Sharifi, S.; Xie, D.; He, X. Funneliformis mosseae improves growth and nutrient accumulation in wheat by facilitating soil nutrient uptake under elevated CO2 at daytime, not nighttime. J. Fungi 2021, 7, 458. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, C.; Dai, H. Soil Agrochemical Analysis and Environmental Monitoring Techniques(M); Chinese Dadi Press: Beijing, China, 2008; pp. 18–64. [Google Scholar]
- Carvalho, J.M.; Barreto, R.F.; Prado, R.D.; Habermann, E.; Martinez, C.A.; Branco, R.B.F. Elevated [CO2] and warming increase the macronutrient use efficiency and biomass of Stylosanthes capitata Vogel under field conditions. J. Agron. Crop Sci. 2020, 206, 597–606. [Google Scholar] [CrossRef]
- Guan, S.Y.; Zhang, D.; Zhang, Z. Soil Enzyme and Its Research Methods (M); Agricultural Press: Beijing, China, 1986; pp. 274–340. [Google Scholar]
- Suboktagin, S.; Khurshid, G.; Bilal, M.; Abbassi, A.Z.; Kwon, S.Y.; Ahmad, R. Improvement of photosynthesis in changing environment: Approaches, achievements and prospects. Plant Biotechnol. Rep. 2024, 18, 21–32. [Google Scholar] [CrossRef]
- Srivastava, A.C.; Pal, M.; Das, M.; Sengupta, U.K. Growth, CO2 exchange rate and dry matter partitioning in mungbean (Vigna radiata L.) grown under elevated CO2. Indian J. Exp. Biol. 2001, 39, 572–577. [Google Scholar]
- Gamez, A.L.; Han, X.; Aranjuelo, I. Differential effect of free-air CO2 enrichment (FACE) in different organs and growth stages of two cultivars of durum wheat. Plants 2023, 12, 686. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–371. [Google Scholar] [CrossRef]
- Hu, S.W.; Chen, W.; Tong, K.C.; Wang, Y.X.; Jing, L.Q.; Wang, Y.L.; Yang, L.X. Response of rice growth and leaf physiology to elevated CO2 concentrations: A meta-analysis of 20-year FACE studies. Sci. Total Environ. 2022, 807, 151017. [Google Scholar] [CrossRef]
- Jablonski, L.M.; Wang, X.Z.; Curtis, P.S. Plant reproduction under elevated CO2 conditions: A meta-analysis of reports on 79 crop and wild species. New Phytol. 2002, 156, 9–26. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Davey, P.A.; Bernacchi, C.J.; Dermody, O.C.; Heaton, E.A.; Moore, D.J.; Morgan, P.B.; Naidu, S.L.; Ra, H.S.Y.; Zhu, X.G. A meta-analysis of elevated [CO2] effects on soybean (Glycine max) physiology, growth and yield. Glob. Chang. Biol. 2002, 8, 695–709. [Google Scholar] [CrossRef]
- Becker, C.; Kläring, H.P. CO2 enrichment can produce high red leaf lettuce yield while increasing most flavonoid glycoside and some caffeic acid derivative concentrations. Food Chem. 2016, 199, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Samuel, E.B.; Krishna, N.; Gioia, D.M.; Raymond, M.W.; Robert, C.M.; Cary, A.M. Growth and photosynthetic responses of Chinese cabbage (Brassica rapa L. cv. Tokyo Bekana) to continuously elevated carbon dioxide in a simulated space station “Veggie” crop-production environment. Life Sci. Space Res. 2020, 27, 83–88. [Google Scholar] [CrossRef]
- Long, S.P.; Ainsworth, E.A.; Leakey, A.D.; Nosberger, J.; Ort, D.R. Food for thought: Lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 2006, 312, 1918–1921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Lv, H.J.; Wang, D.S.; Deng, J.C.; Song, W.J.; Makeen, K.; Shen, Q.R.; Xu, G.H. Partial nitrate nutrition amends photosynthetic characteristics in rice (Oryza sativa L. var. japonica) differing in nitrogen use efficiency. Plant Growth Regul. 2011, 63, 235–242. [Google Scholar] [CrossRef]
- Anjana; Umar, S.; Abrol, Y.P.; Iqbal, M. Modulation of nitrogen-utilization efficiency in wheat genotypes differing in nitrate reductase activity. J. Plant Nutr. 2011, 34, 920–933. [Google Scholar] [CrossRef]
- Drake, B.G.; Gonzalez-Meler, M.A.; Long, S.P. More efficient plants: A consequence of rising atmospheric CO2? Annu. Rev. Plant Physiol. 1997, 48, 609–639. [Google Scholar] [CrossRef]
- Becklin, K.M.; Mullinix, G.W.R.; Ward, J.K. Host plant physiology and mycorrhizal functioning shift across a glacial through future CO2 gradient. Plant Physiol. 2016, 172, 789–801. [Google Scholar] [CrossRef]
- Denis, F.; Michael, D.; Yin, X.; Anne, C.; Sandrine, R.; Armelle, S.; Delphine, L. Genotypic variation in source and sink traits affects the response of photosynthesis and growth to elevated atmospheric CO2. Plant Cell Environ. 2020, 43, 579–593. [Google Scholar] [CrossRef]
- Yang, L.X.; Huang, H.Y.; Yang, H.J.; Dong, G.C.; Liu, H.J.; Liu, G.; Zhu, J.G.; Wang, Y.L. Seasonal changes in the effects of free-air CO2 enrichment (FACE) ion nitrogen (N) uptake and utilization of rice at three levels of N fertilization. Field Crop Res. 2007, 100, 189–199. [Google Scholar] [CrossRef]
- Yang, L.X.; Wang, Y.L.; Huang, J.Y.; Zhu, J.G.; Yang, H.J.; Liu, G.; Liu, H.J.; Dong, G.C.; Hu, J. Seasonal changes in the effects of free-air CO2 enrichment (FACE) on phosphorus uptake and utilization of rice at three levels of nitrogen fertilization. Field Crop Res. 2007, 102, 141–150. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, J.S.; Xu, X.; Liu, G.; Zhu, J.G. Effects of free-air CO2enrichment (FACE) and nitrogen (N) supply on N uptake and utilization of indica and japonica cultivars (Oryza sativa L.). Ecol. Process. 2020, 9, 35. [Google Scholar] [CrossRef]
- Wang, B.; Guo, C.; Wan, Y.F.; Li, J.L.; Ju, X.T.; Cai, W.W.; You, S.C.; Qin, X.B.; Wilkes, A.; Li, Y.E. Air warming and CO2 enrichment increase N use efficiency and decrease N surplus in a Chinese double rice cropping system. Sci. Total Environ. 2020, 706, 136063. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.H.; Li, Y.C.; Guo, Z.W.; Li, Y.Q.; Pan, W.T.; Chen, S.L. Elevated CO2 and O3 levels influence the uptake and leaf concentration of mineral N, P, K in Phyllostachys edulis (Carrière) J.Houz. and Oligostachyum lubricum (wen) King f. Forests 2018, 9, 195. [Google Scholar] [CrossRef]
- Pang, J.Y.; Ryan, M.H.; Lambers, H.; Siddique, K.H.M. Phosphorus acquisition and utilisation in crop legumes under global change. Curr. Opin. Plant Biol. 2018, 45, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.H.; Du, T.S.; Li, X.N.; Fang, L.; Liu, F.L. Interactive effects of CO2 concentration elevation and nitrogen fertilization on water and nitrogen use efficiency of tomato grown under reduced irrigation regimes. Agric. Water Manag. 2018, 202, 174–182. [Google Scholar] [CrossRef]
- An, Y.A.; Wan, S.Q.; Zhou, X.H.; Subedar, A.A.; Wallace, L.L.; Luo, Y.Q. Plant nitrogen concentration, use efficiency, and contents in a tallgrass prairie ecosystem under experimental warming. Glob. Chang. Biol. 2005, 11, 1733–1744. [Google Scholar] [CrossRef]
- Xue, S.; Yang, X.M.; Liu, G.B.; Gai, L.T.; Zhang, C.S.; Ritsema, C.J.; Geissen, V. Effects of elevated CO2 and drought on the microbial biomass and enzymatic activities in the rhizospheres of two grass species in Chinese loess soil. Geoderma 2017, 286, 25–34. [Google Scholar] [CrossRef]
- Sulman, B.N.; Phillips, R.P.; Oishi, A.C.; Shevliakova, E.; Pacala, S.W. Microbe-driven turnover offsets mineral-mediated storage of soil carbon under elevated CO2. Nat. Clim. Chang. 2014, 4, 1099–1102. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Bhatia, A.; Chakrabarti, B.; Saha, N.D.; Pramanik, P.; Ghosh, A.; Das, S.; Singh, G.; Singh, S.D. Elevated CO2 alters aggregate carbon and microbial community but does not affect total soil organic C in the semi-arid tropics. Appl. Soil Ecol. 2023, 187, 104843. [Google Scholar] [CrossRef]
- Xu, Q.; Lu, J.; Dijkstra, F.A.; Yin, L.; Wang, P.; Cheng, W. Elevated CO2 and nitrogen interactively affect the rhizosphere priming effect of Cunninghamia lanceolata. Soil Biol. Biochem. 2023, 187, 109219. [Google Scholar] [CrossRef]


| CO2 Level | Variety | Shoot Biomass (g plant−1) | Root Biomass (g plant−1) | Total Biomass (g plant−1) |
|---|---|---|---|---|
| C0 | ‘Longpangqing’ | 2.62 ± 0.11 b,x | 0.27 ± 0.01 c,x | 2.89 ± 0.10 b,x |
| ‘Heimeiren’ | 2.77 ± 0.13 b,x | 0.26 ± 0.01 c,x | 3.03 ± 0.13 c,x | |
| ‘Qingjiangbai’ | 2.96 ± 0.08 c,x | 0.24 ± 0.02 c,x | 3.20 ± 0.09 c,x | |
| C1 | ‘Longpangqing’ | 3.21 ± 0.09 a,y | 0.33 ± 0.01 b,x | 3.57 ± 0.09 a,x |
| ‘Heimeiren’ | 3.35 ± 0.13 a,y | 0.35 ± 0.01 b,x | 3.71 ± 0.22 b,x | |
| ‘Qingjiangbai’ | 3.71 ± 0.18 b,x | 0.32 ± 0.01 b,x | 4.04 ± 0.17 b,x | |
| C2 | ‘Longpangqing’ | 3.16 ± 0.08 a,y | 0.36 ± 0.01 b,y | 3.53 ± 0.08 a,y |
| ‘Heimeiren’ | 3.29 ± 0.14 a,y | 0.36 ± 0.01 a,y | 3.65 ± 0.13 b,y | |
| ‘Qingjiangbai’ | 3.82 ± 0.17 b,x | 0.43 ± 0.00 a,x | 4.25 ± 0.17 b,x | |
| C3 | ‘Longpangqing’ | 3.47 ± 0.10 a,y | 0.44 ± 0.01 a,y | 3.91 ± 0.09 a,y |
| ‘Heimeiren’ | 3.55 ± 0.13 a,y | 0.45 ± 0.02 a,y | 3.99 ± 0.15 a,y | |
| ‘Qingjiangbai’ | 4.45 ± 0.15 a,x | 0.49 ± 0.01 a,x | 4.94 ± 0.15 a,x | |
| ANOVA | ||||
| CO2 | *** | *** | *** | |
| Variety | *** | * | *** | |
| CO2 × Variety | ns | * | ns |
| CO2 Level | Variety | Nitrogen Use Efficiency (g plant−1) | Phosphorus Use Efficiency (g plant−1) | Potassium Use Efficiency (g plant−1) |
|---|---|---|---|---|
| C0 | ‘Longpangqing’ | 268 ± 11 a,x | 780 ± 71 c,x | 528 ± 29 b,x |
| ‘Heimeiren’ | 263 ± 13 b,x | 870 ± 26 c,x | 485 ± 30 b,x | |
| ‘Qingjiangbai’ | 186 ± 14 c,y | 860 ± 56 c,x | 560 ± 22 c,x | |
| C1 | ‘Longpangqing’ | 135 ± 4 c,y | 884 ± 18 c,z | 632 ± 21 a,y |
| ‘Heimeiren’ | 122 ± 7 d,y | 1206 ± 79 b,y | 633 ± 38 a,y | |
| ‘Qingjiangbai’ | 357 ± 19 b,x | 1425 ± 60 b,x | 698 ± 27 b,x | |
| C2 | ‘Longpangqing’ | 288 ± 7 a,y | 1173 ± 70 b,y | 619 ± 20 a,y |
| ‘Heimeiren’ | 303 ± 9 a,y | 1287 ± 76 b,y | 652 ± 18 a,y | |
| ‘Qingjiangbai’ | 343 ± 10 b,x | 1470 ± 36 b,x | 734 ± 32 b,x | |
| C3 | ‘Longpangqing’ | 231 ± 16 b,y | 1427 ± 119 a,x | 676 ± 43 a,y |
| ‘Heimeiren’ | 209 ± 14 c,y | 1471 ± 101 a,x | 672 ± 51 a,y | |
| ‘Qingjiangbai’ | 626 ± 24 a,x | 1658 ± 157 a,x | 846 ± 38 a,x | |
| ANOVA | ||||
| CO2 | *** | *** | *** | |
| Variety | *** | ** | *** | |
| CO2 × Variety | *** | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.; Wang, X.; Li, H.; Song, J.; He, X.; Yang, Z. Species-Dependent Response of Brassica chinensis L. to Elevated CO2 Gradients Influences Uptake and Utilization of Soil Nitrogen, Phosphorus and Potassium. Agronomy 2024, 14, 1684. https://doi.org/10.3390/agronomy14081684
Shi S, Wang X, Li H, Song J, He X, Yang Z. Species-Dependent Response of Brassica chinensis L. to Elevated CO2 Gradients Influences Uptake and Utilization of Soil Nitrogen, Phosphorus and Potassium. Agronomy. 2024; 14(8):1684. https://doi.org/10.3390/agronomy14081684
Chicago/Turabian StyleShi, Songmei, Xinju Wang, Huakang Li, Jiajun Song, Xinhua He, and Zhengan Yang. 2024. "Species-Dependent Response of Brassica chinensis L. to Elevated CO2 Gradients Influences Uptake and Utilization of Soil Nitrogen, Phosphorus and Potassium" Agronomy 14, no. 8: 1684. https://doi.org/10.3390/agronomy14081684





