The Role of Organic Matter in Phosphorus Retention in Eutrophic and Dystrophic Terrestrial Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Material, Location, and Samples Preparation
2.2. Basic Soil Characteristics
2.3. Content of Phosphorus Forms
2.4. Statistics
3. Results and Discussion
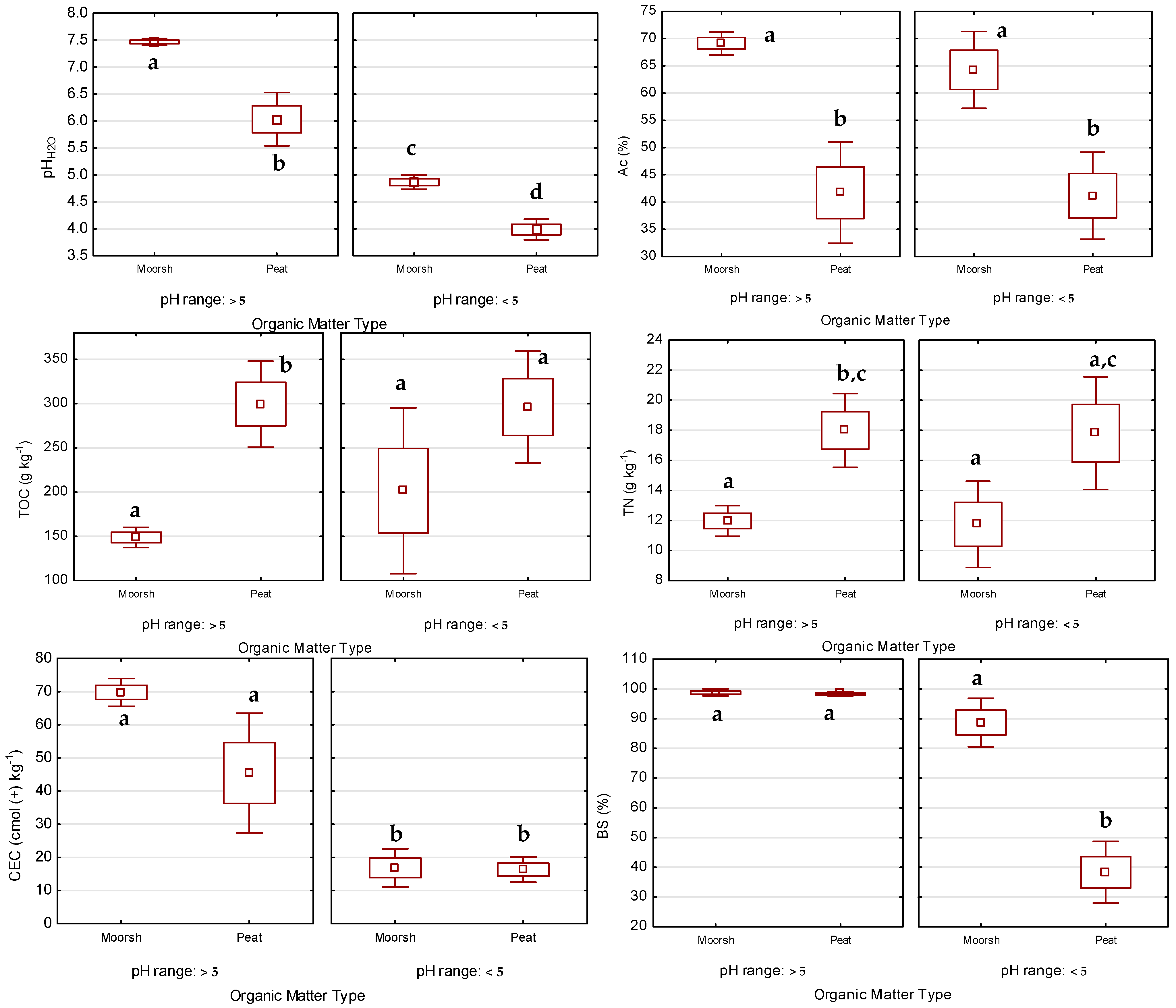
3.1. Soil Basic Characteristics against the Background of Organic Matter Transformations
3.1.1. Physical Properties
3.1.2. Chemical and Physicochemical Properties
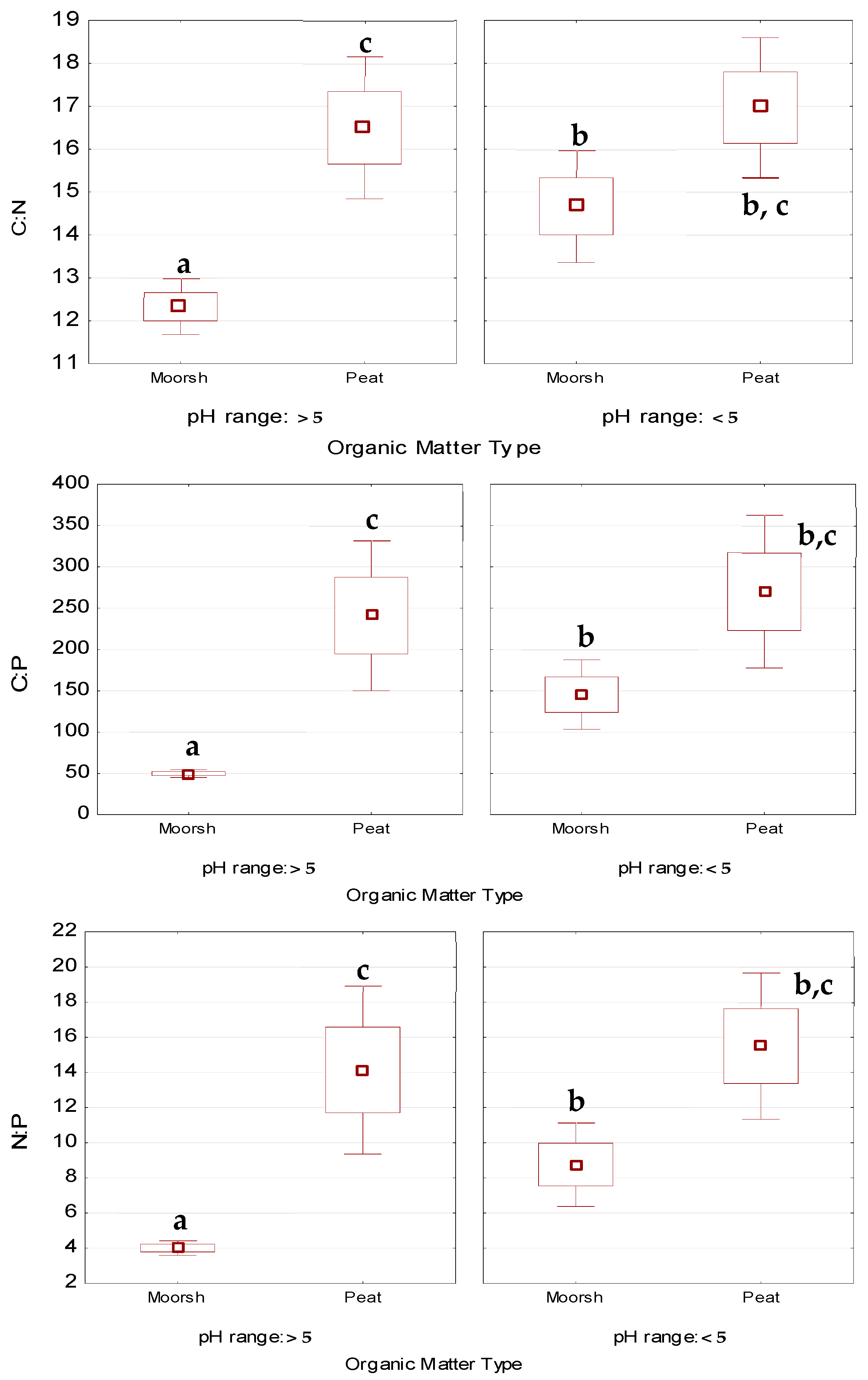
3.1.3. Macronutrient Conversion—C:N, N:P, and C:P Ratios
3.2. Phosphorus Behavior in Histosols
3.2.1. Phosphorus Forms
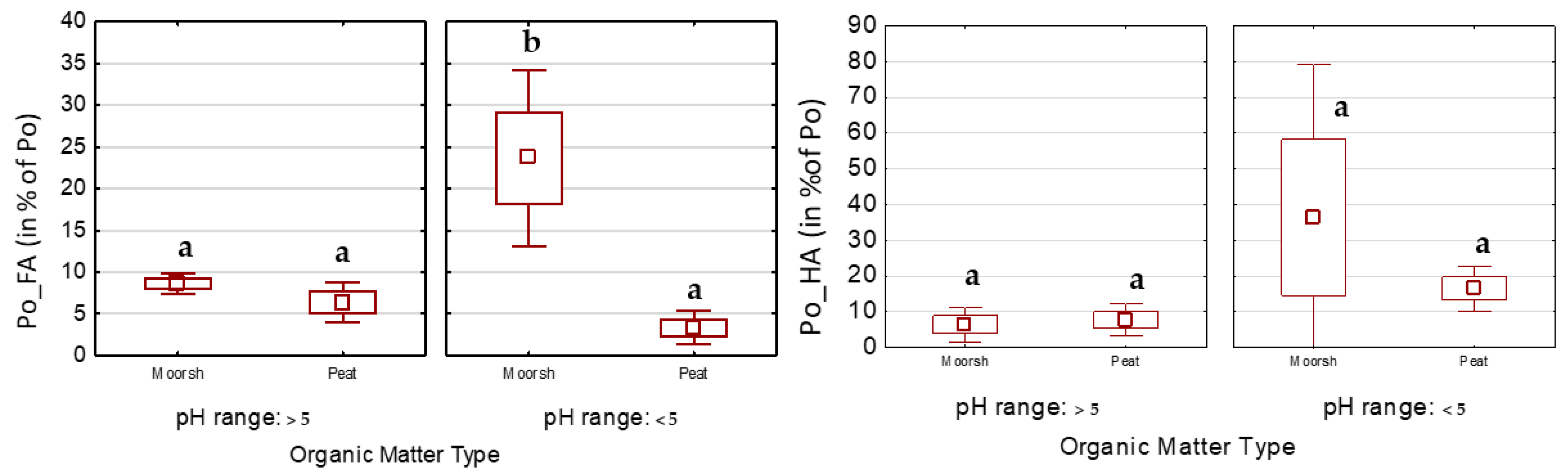
3.2.2. Retention of P in Humic and Fulvic Acids
3.3. Relationships between Organic P in Fulvic and Humic Acids and Selected Properties of the Histosols Studied
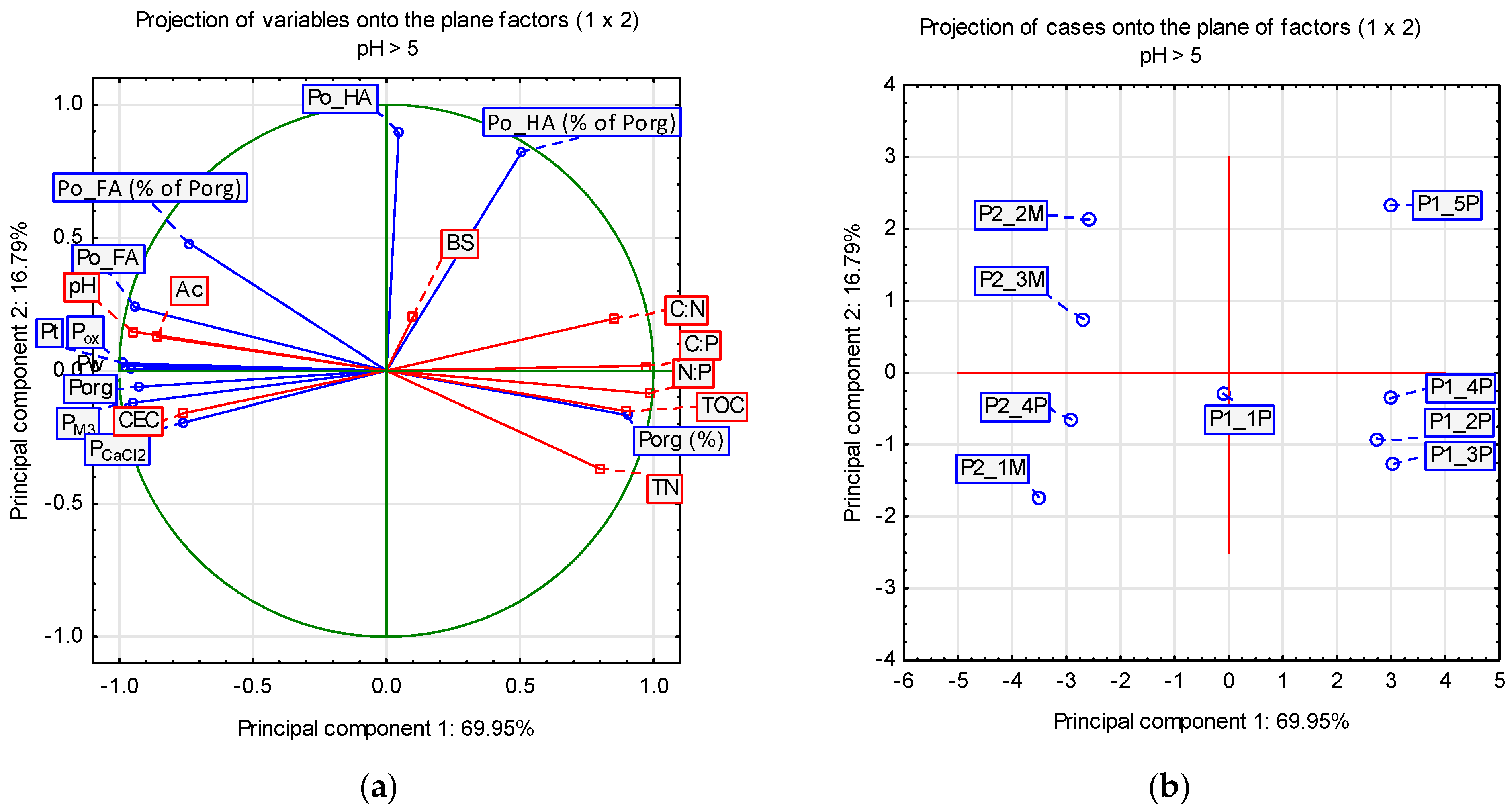
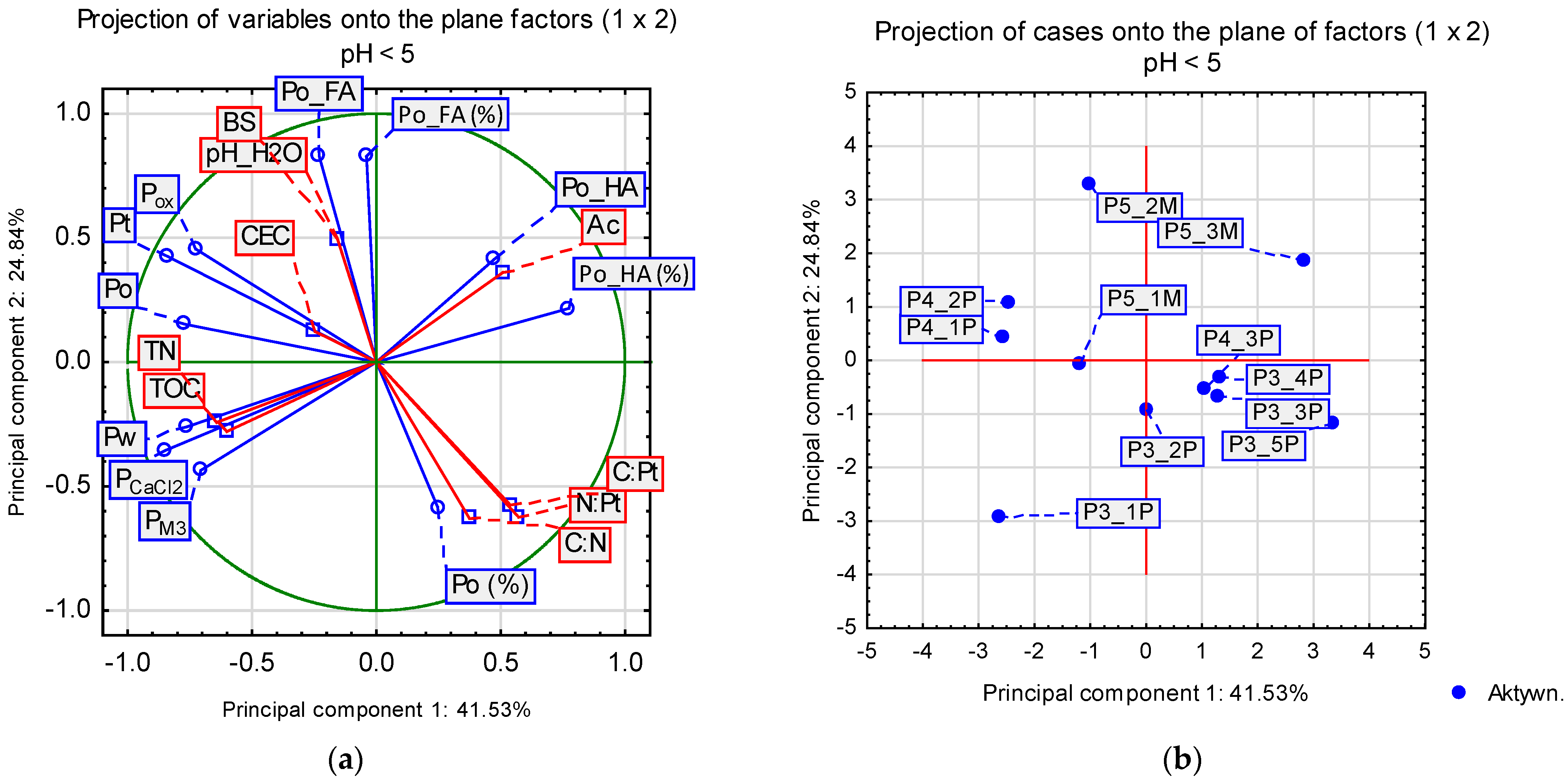
3.4. Principal Component Analysis on the Main Factors Governing the Connections between Organic Matter Transformations and Phosphorus Retention in Eutrophic (pH > 5) and Dystrophic (pH < 5) Histosols
4. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, Y.; Weng, L.; Hiemstra, T. Interaction of fulvic acid with soil organo-mineral nano-aggregates and corresponding phosphate release. Geoderma 2024, 441, 116737. [Google Scholar] [CrossRef]
- Murphy, B. Key soil functional properties affected by soil organic matter-Evidence from published literature. IOP Conf. Ser. Earth Environ. Sci. 2015, 25, 012008. [Google Scholar] [CrossRef]
- Whitbread, A.M. Soil Organic Matter: Its Fractionation and Role in Soil Structure. In Proceedings of the Soil Organic Matter Management for Sustainable Agriculture, Ubon, Thailand, 24–26 August 1994. [Google Scholar]
- Chenu, C.; Angers, D.A.; Barré, P.; Derrien, D.; Arrouays, D.; Balesdent, J. Increasing organic stocks in agricultural soils: Knowledge gaps and potential innovations. Soil Tillage Res. 2019, 188, 41–52. [Google Scholar] [CrossRef]
- Weber, J.; Chen, Y.; Jamroz, E.; Miano, T. Preface: Humic substances in the environment. J. Soils Sediments 2018, 18, 2665–2667. [Google Scholar] [CrossRef]
- Jamroz, E.; Jerzykiewicz, M. Humic fractions as indicators of soil organic matter responses to clear-cutting in mountain and lowland conditions of southwestern Poland. Land Degrad. Dev. 2022, 33, 368–378. [Google Scholar] [CrossRef]
- Obalum, S.E.; Chibuike, G.U.; Peth, S.; Ouyang, Y. Soil organic matter as sole indicator of soil degradation. Environ. Monit. Assess. 2017, 189, 176. [Google Scholar] [CrossRef]
- Martin, M.; Celi, L.; Bonifacio, E.; Nardi, S.; Barberis, E. Characteristics of soil organic matter in a limnic histosol of the alpine morainic system. Soil Sci. 2006, 171, 527–540. [Google Scholar] [CrossRef]
- Pu, Y.; Luo, Y.; Zhang, S.; Li, T.; Yuan, D.; Xu, X.; Qian, H.; Wang, G.; Jia, Y.; Li, Y. Transformation of phosphorus forms and its regulation on phosphorus availability across differently degraded marsh soils. Land Degrad. Dev. 2024, 35, 264–279. [Google Scholar] [CrossRef]
- Żukowski, E. Transformation of Organic Matter in Soils of Drained Peat Bogs in the Valley of Upper Reach of the Muchawka River. Ph.D. Thesis, Uniwersytet Przyrodniczo-Humanistyczny w Siedlcach, Siedlce, Poland, 2023. (In Polish). [Google Scholar]
- Glina, B.; Piernik, A.; Mocek-Płóciniak, A.; Maier, A.; Glatzel, S. Drivers controlling spatial and temporal variation of microbial properties and dissolved organic forms (DOC and DON) in fen soils with persistently low water tables. Glob. Ecol. Conserv. 2021, 27, e01605. [Google Scholar] [CrossRef]
- Bogacz, A.; Glina, B.; Januszewska, K.; Midor, A.; Tomaszewska, K.; Podlaska, M.; Telega, P.; Dradrach, A. The effect of long-term peatlands drainage on the properties of soils in Microrelief in the Długie Mokradło Bog (Central sudetes–SW Poland). Polish J. Soil Sci. 2017, 50, 237–247. [Google Scholar] [CrossRef]
- Łachacz, A.; Kalisz, B.; Sowiński, P.; Smreczak, B.; Niedźwiecki, J. Transformation of Organic Soils Due to Artificial Drainage and Agricultural Use in Poland. Agriculture 2023, 13, 634. [Google Scholar] [CrossRef]
- Becher, M.; Pakuła, K.; Jaremko, D. Phosphorus accumulation in the dehydrated peat soils of the Liwiec river valley. J. Ecol. Eng. 2020, 21, 213–220. [Google Scholar] [CrossRef]
- Negassa, W.; Acksel, A.; Eckhardt, K.U.; Regier, T.; Leinweber, P. Soil organic matter characteristics in drained and rewetted peatlands of northern Germany: Chemical and spectroscopic analyses. Geoderma 2019, 353, 468–481. [Google Scholar] [CrossRef]
- Negassa, W.; Eckhardt, K.U.; Regier, T.; Leinweber, P. Dissolved organic matter concentration, molecular composition, and functional groups in contrasting management practices of peatlands. J. Environ. Qual. 2021, 50, 1364–1380. [Google Scholar] [CrossRef]
- Hunt, J.F.; Ohno, T.; He, Z.; Honeycutt, C.W.; Dail, D.B. Influence of Decomposition on Chemical Properties of Plant- and Manure-Derived Dissolved Organic Matter and Sorption to Goethite. J. Environ. Qual. 2007, 36, 135–143. [Google Scholar] [CrossRef]
- Oliveira, J.F.; de Oliveira, J.C.S.; Ruiz, D.B.; de Cesare Barbosa, G.M.; Filho, J.T. Changes in carbon and phosphorus storages and humic substances in a Ferralsol, after tillage and animal manures applications. Soil Tillage Res. 2022, 220, 105358. [Google Scholar] [CrossRef]
- Vanden Nest, T.; Amery, F.; Fryda, L.; Boogaerts, C.; Bilbao, J.; Vandecasteele, B. Renewable P sources: P use efficiency of digestate, processed animal manure, compost, biochar and struvite. Sci. Total Environ. 2021, 750, 141699. [Google Scholar] [CrossRef]
- Desmidt, E.; Ghyselbrecht, K.; Zhang, Y.; Pinoy, L.; Van Der Bruggen, B.; Verstraete, W.; Rabaey, K.; Meesschaert, B. Global phosphorus scarcity and full-scale P-recovery techniques: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 336–384. [Google Scholar] [CrossRef]
- Amarh, F.; Voegborlo, R.B.; Essuman, E.K.; Agorku, E.S.; Tettey, C.O.; Kortei, N.K. Effects of soil depth and characteristics on phosphorus adsorption isotherms of different land utilization types: Phosphorus adsorption isotherms of soil. Soil Tillage Res. 2021, 213, 105139. [Google Scholar] [CrossRef]
- Pöthig, R.; Behrendt, H.; Opitz, D.; Furrer, G. A universal method to assess the potential of phosphorus loss from soil to aquatic ecosystems. Environ. Sci. Pollut. Res. 2010, 17, 497–504. [Google Scholar] [CrossRef]
- Nair, V.D. Soil phosphorus saturation ratio for risk assessment in land use systems. Front. Environ. Sci. 2014, 2, 6. [Google Scholar] [CrossRef]
- Messiga, A.J.; Lam, C.; Li, Y. Phosphorus saturation index and water-extractable phosphorus in high-legacy phosphorus soils in southern British Columbia, Canada. Can. J. Soil Sci. 2021, 101, 365–377. [Google Scholar] [CrossRef]
- McDowell, R.W.; Condron, L.M.; Stewart, I. Variation in environmentally- and agronomically-significant soil phosphorus concentrations with time since stopping the application of phosphorus fertilisers. Geoderma 2016, 280, 67–72. [Google Scholar] [CrossRef]
- Regelink, I.C.; Egene, C.E.; Tack, F.M.G.; Meers, E. Speciation of P in solid organic fertilisers from digestate and biowaste. Agronomy 2021, 11, 2233. [Google Scholar] [CrossRef]
- Jindo, K.; Audette, Y.; Olivares, F.L.; Canellas, L.P.; Smith, D.S.; Voroney, R.P. Biotic and abiotic effects of soil organic matter on the phytoavailable phosphorus in soils: A review. Chem. Biol. Technol. Agric. 2023, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Guppy, C.N.; Menzies, N.W.; Moody, P.W.; Blamey, F.P.C. Competitive sorption reactions between phosphorus and organic matter in soil: A review. Aust. J. Soil Res. 2005, 43, 189–202. [Google Scholar] [CrossRef]
- Debicka, M.; Kocowicz, A.; Weber, J.; Jamroz, E. Organic matter effects on phosphorus sorption in sandy soils. Arch. Agron. Soil Sci. 2015, 62, 840–855. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Yang, X. Effect of organic matter on phosphorus adsorption and desorption in a black soil from Northeast China. Soil Tillage Res. 2019, 187, 85–91. [Google Scholar] [CrossRef]
- Hawkins, J.M.B.; Vermeiren, C.; Blackwell, M.S.A.; Darch, T.; Granger, S.J.; Dunham, S.J.; Hernandez-Allica, J.; Smolders, E.; McGrath, S. The effect of soil organic matter on long-term availability of phosphorus in soil: Evaluation in a biological P mining experiment. Geoderma 2022, 423, 115965. [Google Scholar] [CrossRef]
- Horta, C. Fertilisation with Compost: Effects on Soil Phosphorus Sorption and on Phosphorus Availability in Acid Soils. Open J. Soil Sci. 2019, 9, 255–268. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, B.; He, N.; Yu, G.; Wang, T.; Chen, W.; Tian, J. Phosphorus and carbon competitive sorption-desorption and associated non-point loss respond to natural rainfall events. J. Hydrol. 2014, 517, 447–457. [Google Scholar] [CrossRef]
- Audette, Y.; Smith, D.S.; Parsons, C.T.; Chen, W.; Rezanezhad, F.; Van Cappellen, P. Phosphorus binding to soil organic matter via ternary complexes with calcium. Chemosphere 2020, 260, 127624. [Google Scholar] [CrossRef]
- Strawn, D.G. Sorption mechanisms of chemicals in soils. Soil Syst. 2021, 5, 13. [Google Scholar] [CrossRef]
- Chase, A.J.; Erich, M.S.; Ohno, T. Bioavailability of Phosphorus on Iron (Oxy)hydroxide Not Affected by Soil Amendment–Derived Organic Matter. Agric. Environ. Lett. 2018, 3, 170042. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Lin, L.; Tian, B.; Pei, Y. Effect of low molecular weight organic acids on phosphorus adsorption by ferric-alum water treatment residuals. J. Hazard. Mater. 2012, 203–204, 145–150. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, J.; Fu, Q.; Hong, C.; Hu, H.; Violante, A. Phosphate adsorption on uncoated and humic acid-coated iron oxides. J. Soils Sediments 2016, 16, 1911–1920. [Google Scholar] [CrossRef]
- Debicka, M.; Morshedizad, M.; Leinweber, P. The Effects of Dissolved Organic Matter Derived from Agricultural Waste Materials on Phosphorus Sorption in Sandy Soils. Agriculture 2023, 13, 2164. [Google Scholar] [CrossRef]
- Hunt, J.F.; He, Z. Characteristics of plant-derived water-extractable organic matter and its effects on phosphorus sorption behavior. In Labile Organic Matter—Chemical Compositions, Function, and Significance in Soil and the Environment; Wiley: Hoboken, NJ, USA, 2015; Volume 70124, pp. 99–118. [Google Scholar] [CrossRef]
- Hiradate, S.; Uchida, N. Effects of soil organic matter on ph-dependent phosphate sorption by soils. Soil Sci. Plant Nutr. 2004, 50, 665–675. [Google Scholar] [CrossRef]
- Du, Z.Y.; Wang, Q.H.; Liu, F.C.; Ma, H.L.; Ma, B.Y.; MalhI, S.S. Movement of Phosphorus in a Calcareous Soil as Affected by Humic Acid. Pedosphere 2013, 23, 229–235. [Google Scholar] [CrossRef]
- Sokołowska, Z.; Szajdak, L.; Boguta, P. Effect of phosphates on dissolved organic matter release from peat-muck soils. Int. Agrophysics 2011, 25, 173–180. [Google Scholar]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the Role of Humic Acids on Crop Performance and Soil Health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Cheng, W.P.; Chi, F.H.; Yu, R.F. Effect of phosphate on removal of humic substances by aluminum sulfate coagulant. J. Colloid Interface Sci. 2004, 272, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Debska, B.; Banach-szott, M. Properties of humic acids of soil under different management regimes. Polish J. Soil Sci. 2023, 42, 131–138. [Google Scholar]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical structure and biological activity of humic substances define their role as plant growth promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef] [PubMed]
- Makarov, M.I.; Malysheva, T.I. Phosphorus in humus acids. Eurasian Soil Sci. 2006, 39, 1208–1216. [Google Scholar] [CrossRef]
- Gorham, E. Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecol. Appl. 1991, 1, 182–195. [Google Scholar] [CrossRef]
- Lasota, J.; Błońska, E. C:N:P stoichiometry as an indicator of Histosol drainage in lowland and mountain forest ecosystems. For. Ecosyst. 2021, 8, 39. [Google Scholar] [CrossRef]
- Charman, D. Peatlands and Environmental Change; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002. [Google Scholar]
- Joosten, H.; Tanneberger, F.; Moen, A. Mires and Peatlands of Europe: Status Distribution and Conservation; Schweizerbart Science Publishers: Stuttgart, Germany, 2017; ISBN 9783510653836. [Google Scholar]
- Błońska, E.; Lasota, J. Soil organic matter accumulation and carbon fractions along a moisture gradient of forest soils. Forests 2017, 8, 448. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022; Volume 4, ISBN 9798986245119. [Google Scholar]
- Debicka, M.; Bogacz, A.; Kowalczyk, K. Phosphorus behaviour and its basic indices under organic matter transformation in variable moisture conditions: A case study of fen organic soils in the odra river valley, Poland. Agronomy 2021, 11, 1997. [Google Scholar] [CrossRef]
- Okruszko, H. Soil properties in drained peatlands (in polish with English summary). Zesz. Probl. Post. Nauk Rol. 1967, 72, 13–27. [Google Scholar]
- Lynn, W.C.; McKinzie, W.E.; Grossman, R.B. Field laboratory tests for characterization of histosols. In Histosols: Their Characteristics, Classification, and Use; Wiley: Hoboken, NJ, USA, 1974; pp. 11–20. ISBN 9780891189015. [Google Scholar]
- Okruszko, H. Determination on specific gravity of hydrogenic soils on the basis of their minerl particles content (in Polish; summary in English). Wiadomości Inst. Melior. Użytków Zelonych Użytków Zelonych 1971, 10, 47–54. [Google Scholar] [CrossRef]
- Saunders, W.M.H.; Williams, E.G. Observations on the determination of total organic phosphorus in soils. J. Soil Sci. 1955, 6, 254–267. [Google Scholar] [CrossRef]
- Walker, T.W.; Adams, A.F.R. Studies on soil organic matter. J. Soil Sci. 1958, 85, 307–318. [Google Scholar] [CrossRef]
- Shah, R.; Syers, J.K.; Williams, J.D.H.; Walker, T.W. The forms of inorganic phosphorus extracted from soils by n sulphuric acid. New Zeal. J. Agric. Res. 1968, 11, 184–192. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; van Vark, W. Soil analysis procedures using 0.01 M calcium chloride as extraction reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Kovar, J.L.; Pierzynski, G.M. Methods of Phosphorus Analysis for Soils, Sediments, Residuals and Waters. South. Coop. Ser. Bull. 2009, 408, 131. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica (Data Analysis Software System). 2017, Version 13. Available online: http://statistica.com (accessed on 29 September 2021).
- Okruszko, H. Transformation on fen-peat soil decomposition under the impact of draining. Zesz. Probl. Post. Nauk Rol. 1993, 406, 3–73. [Google Scholar]
- Gawlik, J. Division of differently silted formation into classes according to their state of secondary transformation. Acta Agrophysica 2000, 26, 17–24. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy. A Basic System of Soil Classification for Making and Interpreting Soil Surveys; USDA Natural Resources Conservation Service: Washington, DC, USA, 1975. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 13th ed.; USDA Natural Resources Conservation Service: Washington, DC, USA, 2022; Volume 12, ISBN 0926487221. [Google Scholar]
- Stevenson, F.J.; Cole, M.A. Cycles of Soil, 2nd ed.; John Wiley & Sons, INC.: New York, NY, USA, 1999; Volume 144. [Google Scholar]
- Parent, L.E.; Viau, A.A.; Anctil, F. Nitrogen and phosphorus fractions as indicators of organic soil quality. Suo 2000, 51, 71–81. [Google Scholar]
- Leifeld, J.; Klein, K.; Wüst-Galley, C. Soil organic matter stoichiometry as indicator for peatland degradation. Sci. Rep. 2020, 10, 7634. [Google Scholar] [CrossRef]
- Watmough, S.; Gilbert-Parkes, S.; Basiliko, N.; Lamit, L.J.; Lilleskov, E.A.; Andersen, R.; del Aguila-Pasquel, J.; Artz, R.E.; Benscoter, B.W.; Borken, W.; et al. Variation in carbon and nitrogen concentrations among peatland categories at the global scale. PLoS ONE 2022, 17, e0275149. [Google Scholar] [CrossRef]
- Becher, M.; Tołoczko, W.; Godlewska, A.; Pakuła, K.; Żukowski, E. Fractional Composition of Organic Matter and Properties of Humic Acids in the Soils of Drained Bogs of the Siedlce Heights in Eastern Poland. J. Ecol. Eng. 2022, 23, 208–222. [Google Scholar] [CrossRef]
- Jamroz, E.; Kocowicz, A.; Bekier, J.; Weber, J. Properties of soil organic matter in Podzols under mountain dwarf pine (Pinus mugo Turra.) and norway spruce (Picea abies (L.) Karst.) in various stages of dieback in the east sudety mountains, poland. For. Ecol. Manag. 2014, 330, 261–270. [Google Scholar] [CrossRef]
- Biester, H.; Knorr, K.H.; Schellekens, J.; Basler, A.; Hermanns, Y.M. Comparison of different methods to determine the degree of peat decomposition in peat bogs. Biogeosciences 2014, 11, 2691–2707. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef]
- Bai, J.; Ye, X.; Jia, J.; Zhang, G.; Zhao, Q.; Cui, B.; Liu, X. Phosphorus sorption-desorption and effects of temperature, pH and salinity on phosphorus sorption in marsh soils from coastal wetlands with different flooding conditions. Chemosphere 2017, 188, 677–688. [Google Scholar] [CrossRef]
- Cui, H.; Ou, Y.; Wang, L.; Yan, B.; Han, L.; Li, Y. Change in the distribution of phosphorus fractions in aggregates under different land uses: A case in sanjiang plain, Northeast China. Int. J. Environ. Res. Public Health 2019, 16, 212. [Google Scholar] [CrossRef]
- Li, J.; Ding, Y.; Shi, Z. Binding Properties of Fulvic Acid before and after Fractionation on Ferrihydrite: Effects of Phosphate. ACS Earth Space Chem. 2021, 5, 1535–1543. [Google Scholar] [CrossRef]
- Hua, Q.-X.; Li, J.-Y.; Zhou, J.-M.; Wang, H.-Y.; Du, C.-W.; Chen, X.-Q. Enhancement of Phosphorus Solubility by Humic Substances in Ferrosols. Pedosphere 2008, 18, 533–538. [Google Scholar] [CrossRef]
- Tan, K.H. Humic Matter in Soil and the Environment, Principles and Controversies, 2nd ed.; CRC-Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2014; ISBN 978-1-4822-3446-6. [Google Scholar]
- Senesi, N.; Loffredo, E. Soil Humic Substances. In Biopolymers Online; Wiley: Hoboken, NJ, USA, 2005; ISBN 9783527600038. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; Wiley: Hoboken, NJ, USA, 1994; ISBN 9780471594741. [Google Scholar]
- Kononova, M.M. Soil Organic Matter, Its Nature, Properties and Methods of Study; Publishing House of the Academia of Science of the U.S.S.R.: Moscow, Russia, 1963. [Google Scholar]
- Rashad, M.; Hafez, M.; Popov, A.I. Humic substances composition and properties as an environmentally sustainable system: A review and way forward to soil conservation. J. Plant Nutr. 2022, 45, 1072–1122. [Google Scholar] [CrossRef]
- Gerke, J. Humic (organic matter)-Al(Fe)-phosphate complexes: An underestimated phosphate form in soils and source of plant-available phosphate. Soil Sci. 2010, 175, 417–425. [Google Scholar] [CrossRef]
- Fahmi, A.; Radjagukguk, B.; Purwanto, B.H.; Hanudin, E. The Role of Peat Layers on Iron Dynamics in Peatlands. J. TANAH Trop. (J. Trop. Soils) 2010, 15, 195–201. [Google Scholar] [CrossRef]
- Borie, F.; Zunino, H.; Martínez, L. Macromolecule-P associations and inositol phosphates in some chilean volcanic soils of temperate regions. Commun. Soil Sci. Plant Anal. 1989, 20, 1881–1894. [Google Scholar] [CrossRef]
- Dormaar, J.F. Extraction of organic phosphorus in Chernozemic soils. Plant Soil 1968, 28, 268–279. [Google Scholar] [CrossRef]
- Ivanoff, D.B.; Reddy, K.R.; Robinson, S. Chemical fractionation of organic phosphorus in selected Histosols. Soil Sci. 1998, 163, 36–45. [Google Scholar] [CrossRef]
- Hong, J.K.; Yamane, I. Inositol phosphate and inositol in humic acid and fulvic acid fractions extracted by three methods. Soil Sci. Plant Nutr. 1980, 26, 491–496. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.G.; Pigna, M. Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef]
- Jones, D.L.; Oburger, E. Solubilization of phosphorus by soil microorganisms. In Phosphorus in Action; Bünemann, E., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26, pp. 169–198. ISBN 978-3-642-15270-2. [Google Scholar]
- Richardson, A.E.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; Smith, S.E.; Harvey, P.R.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; et al. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 2011, 349, 121–156. [Google Scholar] [CrossRef]
- Reddy, K.R.; DeLaune, R.D.; Inglett, P.W. Biogeochemistry of Wetlands; CRC Press: Boca Raton, FL, USA, 2022; ISBN 9780429155833. [Google Scholar]
| Profile No. | Soil Horizon | Horizon Depth [cm] | ρw | ρo | Pc | W1 | Fibre Volume (%) | A/B | PI | Von Post Scale | OM Type | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g cm−3 | % | A | B | |||||||||
| P1 | Oe | 0–14 | 1.80 | 0.22 | 87.8 | 0.27 | 44 | 9 | 4.7 | 4 | H4 | hemic |
| Oa1 | 14–34 | 1.96 | 0.27 | 86.2 | 0.45 | 29 | 6 | 5.0 | 3 | H5 | sapric | |
| Oa2 | 34–62 | 1.81 | 0.22 | 87.8 | 0.44 | 34 | 9 | 3.7 | 3 | H6 | sapric | |
| Oa3 | 62–70 | 1.89 | 0.25 | 86.8 | 0.28 | 44 | 8 | 5.7 | 3 | H5 | sapric | |
| Oa4 | 70–83 | 1.86 | 0.24 | 87.1 | 0.27 | 47 | 5 | 9.0 | 3 | H6 | sapric | |
| Lc | 83–106 | 2.37 | 0.42 | 82.2 | n.d. | 47 | 13 | 3.5 | 3 | H5 | sapric | |
| P2 | M1 | 0–10 | 2.19 | 0.36 | 83.6 | 0.76 | 50 | 8 | 6.4 | 2 | n.d. | sapric |
| M2 | 10–25 | 2.21 | 0.37 | 83.2 | 0.60 | 73 | 7 | 11.0 | 3 | n.d. | sapric | |
| M3 | 25–42 | 2.23 | 0.38 | 82.9 | 0.50 | 86 | 9 | 9.6 | 3 | n.d. | sapric | |
| Oa | 42–55 | 2.14 | 0.34 | 84.1 | 0.45 | 58 | 4 | 14.0 | 3 | H6 | sapric | |
| Lcca | 55–70 | 2.29 | 0.39 | 83.0 | 0.31 | not determined | ||||||
| P3 | Oa1 | 0–15 | 1.80 | 0.22 | 87.7 | 0.16 | 31 | 9 | 3.4 | 3 | H5 | sapric |
| Oa2 | 15–27 | 2.05 | 0.31 | 84.8 | 0.24 | 56 | 8 | 7.0 | 1 | H5 | sapric | |
| Oa3 | 27–37 | 1.83 | 0.22 | 87.9 | 0.30 | 49 | 10 | 4.9 | 2 | H6 | sapric | |
| Oa4 | 37–49 | 1.87 | 0.22 | 87.1 | 0.23 | 48 | 3 | 16.0 | 3 | H7 | sapric | |
| Ogy | 49–79 | 2.11 | 0.33 | 84.3 | 0.32 | 27 | 5 | 5.4 | 4 | H6 | sapric | |
| P4 | Oe | 0–5 | 1.75 | 0.20 | 88.6 | n.d. | n.d. | n.d. | n.d. | 4 | H4 | hemic |
| Oa1 | 5–25 | 1.85 | 0.23 | 87.5 | 0.38 | 51 | 6 | 8.5 | 1 | H6 | sapric | |
| Oa2 | 25–33 | 1.94 | 0.27 | 86.1 | 0.33 | 43 | 3 | 14.3 | 1 | H6 | sapric | |
| Lc | 33–38 | 2.30 | 0.40 | 82.6 | 0.31 | 24 | 4 | 6.0 | 1 | H6 | sapric | |
| P5 | M1 | 0–10 | 2.16 | 0.35 | 83.8 | 0.63 | n.d. | n.d. | n.d. | 1 | n.d. | sapric |
| M2 | 10–23 | 2.08 | 0.32 | 84.6 | 0.76 | n.d. | n.d. | n.d. | 2 | n.d. | sapric | |
| M3 | 23–39 | 2.21 | 0.36 | 83.7 | 0.46 | n.d. | n.d. | n.d. | 1 | n.d. | sapric | |
| Soil Profile | Soil Horizon | Depth (in cm) | Pt | Po | POX | PM3 | PCaCl2 | PW |
|---|---|---|---|---|---|---|---|---|
| mg kg−1 | ||||||||
| P1 | Oe | 0–14 | 2099.3 | 1048.0 | 1308.8 | 3.7 | 1.7 | 2.9 |
| Oa1 | 14–34 | 1216.3 | 990.3 | 551.0 | 1.5 | 0.9 | 1.1 | |
| Oa2 | 34–62 | 1009.3 | 758.3 | 430.8 | 1.2 | 0.7 | 1.0 | |
| Oa3 | 62–70 | 1096.3 | 682.2 | 539.0 | 1.1 | 0.3 | 0.6 | |
| Oa4 | 70–83 | 899.5 | 583.9 | 450.4 | 1.4 | 0.3 | 0.5 | |
| P2 | M1 | 0–10 | 2922.8 | 1323.5 | 1825.5 | 9.1 | 5.7 | 8.1 |
| M2 | 10–25 | 3027.5 | 1376.5 | 1838.8 | 5.8 | 2.9 | 6.9 | |
| M3 | 25–42 | 3011.8 | 1317.5 | 1960.5 | 5.6 | 2.5 | 6.5 | |
| Oa | 42–55 | 3141.3 | 1211.8 | 2315.5 | 5.5 | 1.0 | 4.7 | |
| P3 | Oa1 | 0–15 | 1312.2 | 1032.0 | 571.4 | 14.4 | 5.1 | 9.9 |
| Oa2 | 15–27 | 1374.0 | 1044.6 | 826.9 | 4.0 | 2.2 | 3.4 | |
| Oa3 | 27–37 | 1151.0 | 876.1 | 665.4 | 2.4 | 1.6 | 2.2 | |
| Oa4 | 37–49 | 1027.2 | 744.8 | 539.9 | 2.4 | 1.7 | 2.3 | |
| Ogy | 49–79 | 230.4 | 137.7 | 72.0 | 2.2 | 0.3 | 0.0 | |
| P4 | Oe | 0–5 | 2296.2 | 839.3 | 1712.9 | 8.2 | 3.5 | 5.2 |
| Oa1 | 5–25 | 2359.5 | 1305.0 | 1815.9 | 5.1 | 2.8 | 3.9 | |
| Oa2 | 25–33 | 1159.0 | 739.7 | 734.4 | 2.0 | 1.2 | 2.0 | |
| P5 | M1 | 0–10 | 1327.7 | 716.0 | 710.4 | 10.8 | 2.6 | 8.3 |
| M2 | 10–23 | 1881.0 | 1035.8 | 1102.9 | 2.6 | 1.6 | 3.6 | |
| M3 | 23–39 | 974.2 | 554.8 | 616.9 | 2.7 | 1.2 | 2.7 | |
| Soil Profile | Soil Horizon | Depth (in cm) | Po_FA | Po_HA | ΣPo | Po_FA | Po_HA | ΣPo | Po_FA | Po_HA | ΣPo |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg kg−1 | % of TP | % of Po | |||||||||
| P1 | Oe | 0–14 | 73.01 | 61.14 | 134.15 | 3.48 | 2.91 | 6.39 | 6.97 | 5.83 | 12.80 |
| Oa1 | 14–34 | 34.37 | 65.24 | 99.61 | 2.83 | 5.36 | 8.19 | 3.47 | 6.59 | 10.06 | |
| Oa2 | 34–62 | 29.36 | 43.64 | 73.00 | 2.91 | 4.32 | 7.23 | 3.87 | 5.76 | 9.63 | |
| Oa3 | 62–70 | 28.89 | 60.34 | 89.24 | 2.64 | 5.50 | 8.14 | 4.24 | 8.85 | 13.08 | |
| Oa4 | 70–83 | 50.70 | 106.00 | 156.71 | 5.64 | 11.78 | 17.42 | 8.68 | 18.15 | 26.84 | |
| P2 | M1 | 0–10 | 97.19 | 27.25 | 124.44 | 3.33 | 0.93 | 4.26 | 7.34 | 2.06 | 9.40 |
| M2 | 10–25 | 123.91 | 145.01 | 268.92 | 4.09 | 4.79 | 8.88 | 9.00 | 10.53 | 19.54 | |
| M3 | 25–42 | 123.44 | 88.09 | 211.53 | 4.10 | 2.92 | 7.02 | 9.37 | 6.69 | 16.06 | |
| Oa | 42–55 | 134.15 | 19.07 | 153.22 | 4.27 | 0.61 | 4.88 | 11.07 | 1.57 | 12.64 | |
| P3 | Oa1 | 0–15 | 13.83 | 57.17 | 71.00 | 1.05 | 4.36 | 5.41 | 1.34 | 5.54 | 6.88 |
| Oa2 | 15–27 | 11.39 | 142.31 | 153.70 | 0.83 | 10.36 | 11.19 | 1.09 | 13.62 | 14.71 | |
| Oa3 | 27–37 | 7.60 | 216.28 | 223.88 | 0.66 | 18.79 | 19.45 | 0.87 | 24.69 | 25.55 | |
| Oa4 | 37–49 | 51.06 | 161.66 | 212.72 | 4.97 | 15.74 | 20.71 | 6.85 | 21.70 | 28.56 | |
| Ogy | 49–79 | 2.74 | 44.10 | 46.84 | 1.19 | 19.14 | 20.33 | 1.99 | 32.02 | 34.01 | |
| P4 | Oe | 0–5 | 42.63 | 91.52 | 134.15 | 1.86 | 3.99 | 5.84 | 5.08 | 10.90 | 15.98 |
| Oa1 | 5–25 | 105.53 | 104.39 | 209.92 | 4.47 | 4.42 | 8.90 | 8.09 | 8.00 | 16.09 | |
| Oa2 | 25–33 | 9.48 | 117.01 | 126.48 | 0.82 | 10.10 | 10.91 | 1.28 | 15.82 | 17.10 | |
| P5 | M1 | 0–10 | 122.36 | 126.57 | 248.92 | 9.22 | 9.53 | 18.75 | 17.09 | 17.68 | 34.77 |
| M2 | 10–23 | 355.82 | 120.39 | 476.20 | 18.92 | 6.40 | 25.32 | 34.35 | 11.62 | 45.98 | |
| M3 | 23–39 | 107.85 | 443.40 | 551.25 | 11.07 | 45.51 | 56.58 | 19.44 | 79.93 | 99.37 | |
| Variable | Po_FA | Po_HA | ||
|---|---|---|---|---|
| pH < 5 | pH > 5 | pH < 5 | pH > 5 | |
| Pt | - | 0.88 | - | - |
| Po | - | 0.78 | - | - |
| PM3 | - | 0.83 | - | - |
| POX | - | 0.9 | - | - |
| PCaCl2 | - | 0.71 | - | - |
| PW | - | 0.75 | - | - |
| Po (%) | - | −0.83 | - | - |
| TOC | - | - | - | - |
| TN | - | −0.72 | - | - |
| Ac | - | - | - | - |
| CEC | - | 0.78 | - | - |
| BS | - | - | - | - |
| pH | 0.68 | 0.76 | - | - |
| C:N | −0.70 | −0.73 | - | - |
| C:P | −0.75 | −0.78 | - | - |
| N:P | −0.75 | −0.85 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debicka, M. The Role of Organic Matter in Phosphorus Retention in Eutrophic and Dystrophic Terrestrial Ecosystems. Agronomy 2024, 14, 1688. https://doi.org/10.3390/agronomy14081688
Debicka M. The Role of Organic Matter in Phosphorus Retention in Eutrophic and Dystrophic Terrestrial Ecosystems. Agronomy. 2024; 14(8):1688. https://doi.org/10.3390/agronomy14081688
Chicago/Turabian StyleDebicka, Magdalena. 2024. "The Role of Organic Matter in Phosphorus Retention in Eutrophic and Dystrophic Terrestrial Ecosystems" Agronomy 14, no. 8: 1688. https://doi.org/10.3390/agronomy14081688





