Photosynthetic and Physiological Responses of Different Maize Varieties to Mesotrione
Abstract
:1. Introduction
2. Materials and Methods
2.1. Determination of Net Photosynthetic Rate and Photosynthetic Parameters
2.2. Measurement of Photosynthetic Pigment Contents
2.3. Measurement of Chlorophyll Fluorescence Parameters
2.4. MDA Content and Conductivity Determination
2.5. Antioxidant Enzyme Activity Assays
2.6. HPPD Activity Assay
2.7. Data Processing and Statistical Analysis
3. Results
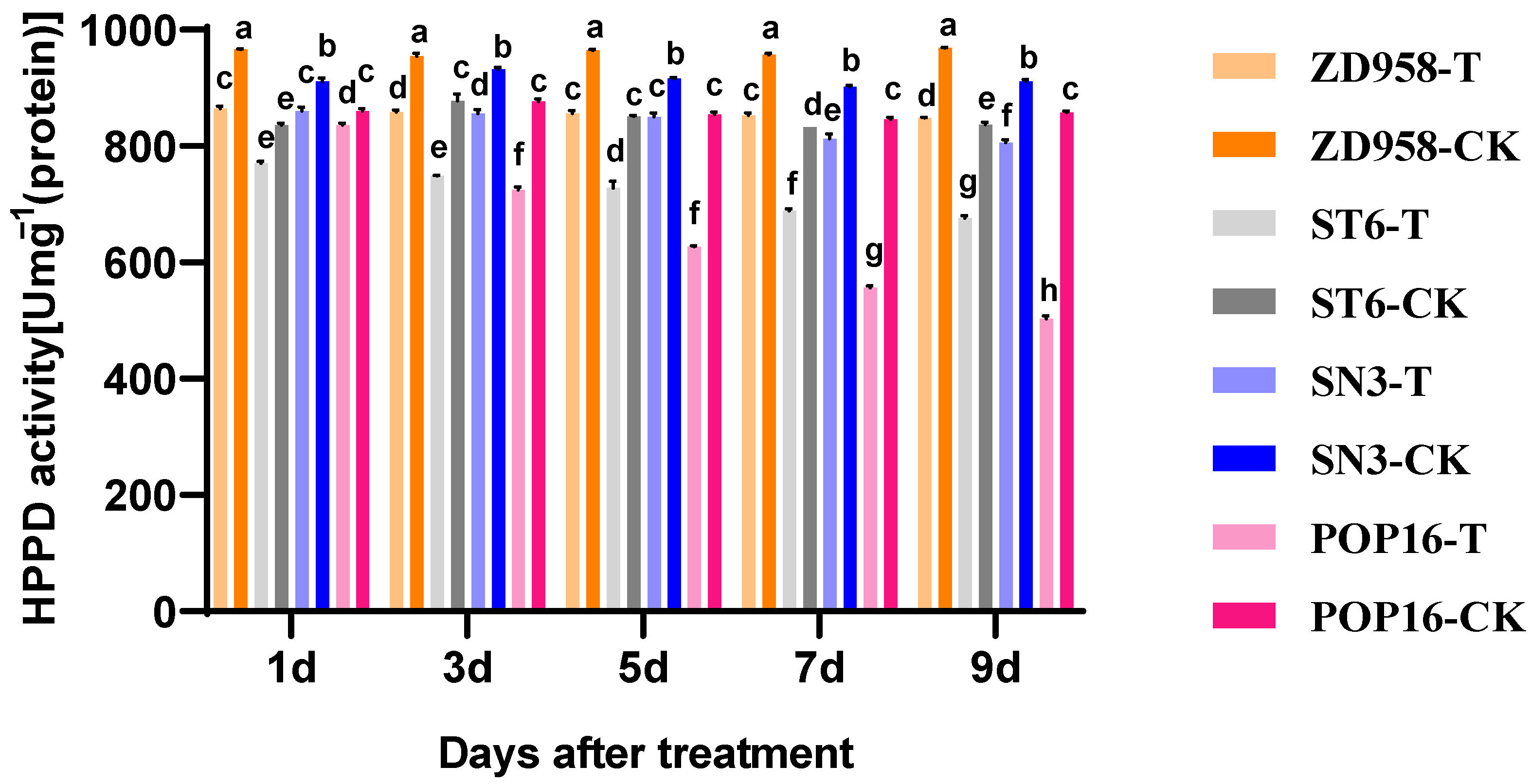
3.1. Effect of MET Treatment on HPPD Activity of Leaves
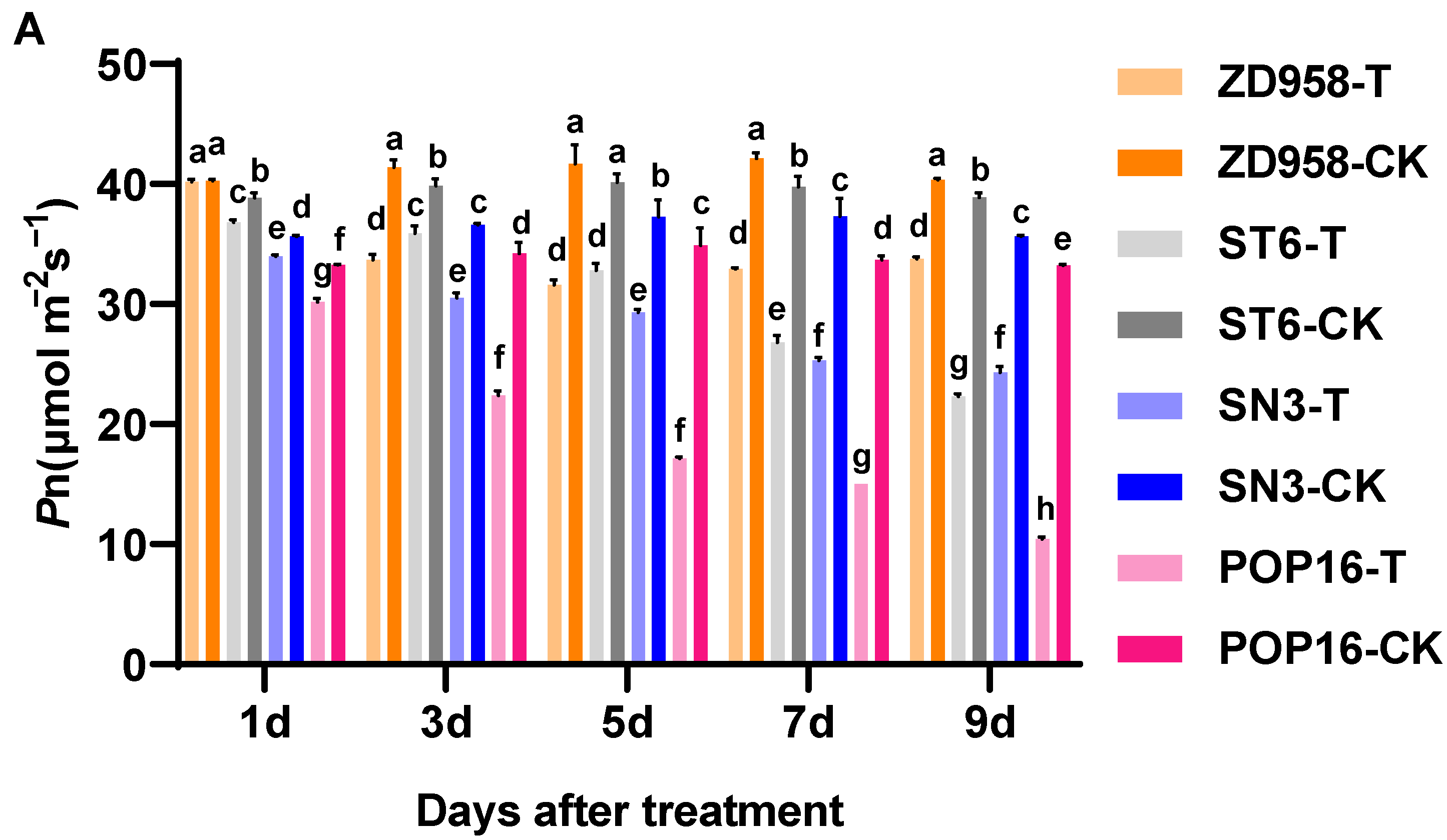
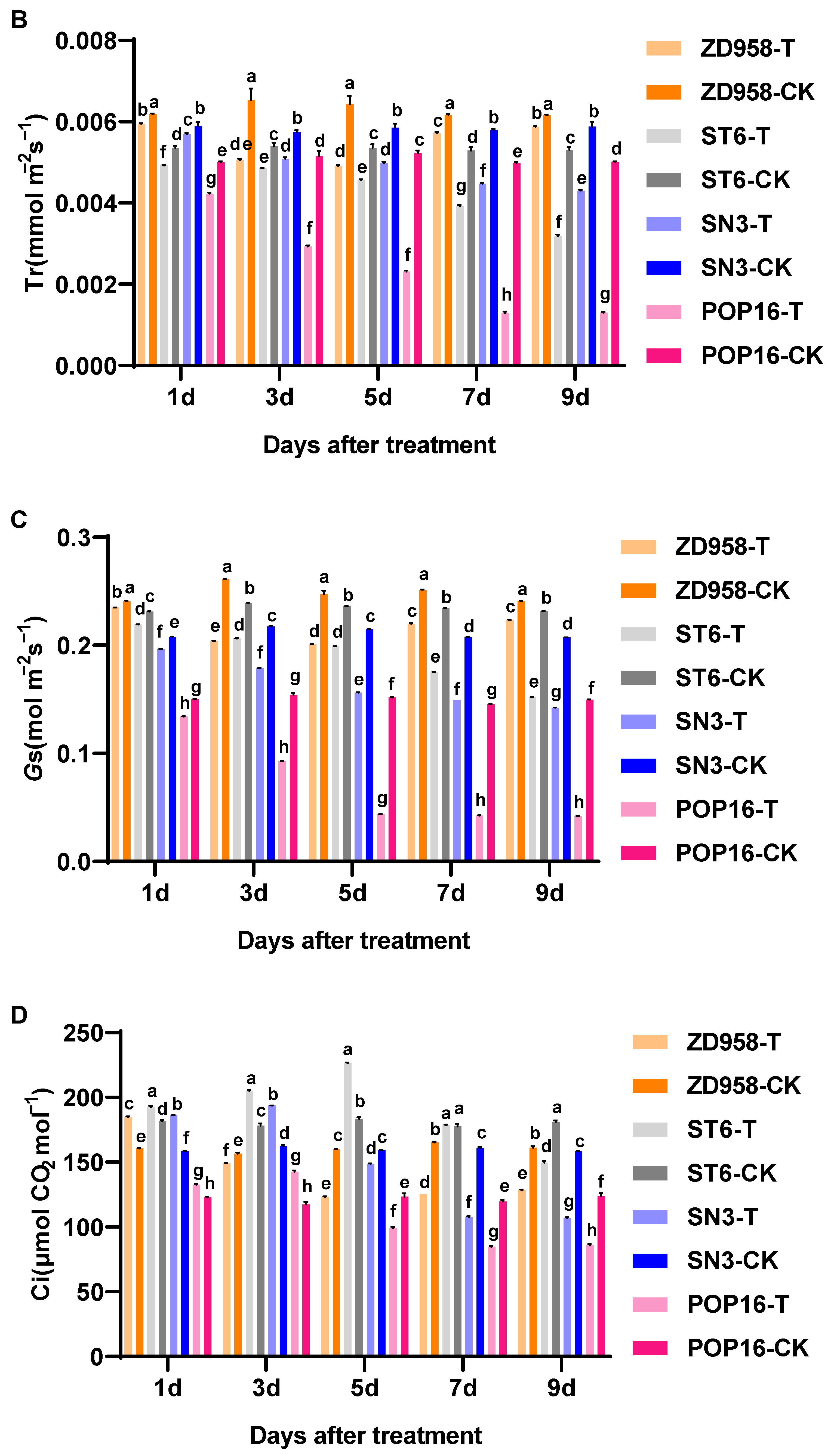
3.2. Effect of MET Treatment on Net Photosynthetic Rate and Photosynthetic Parameters of Leaves
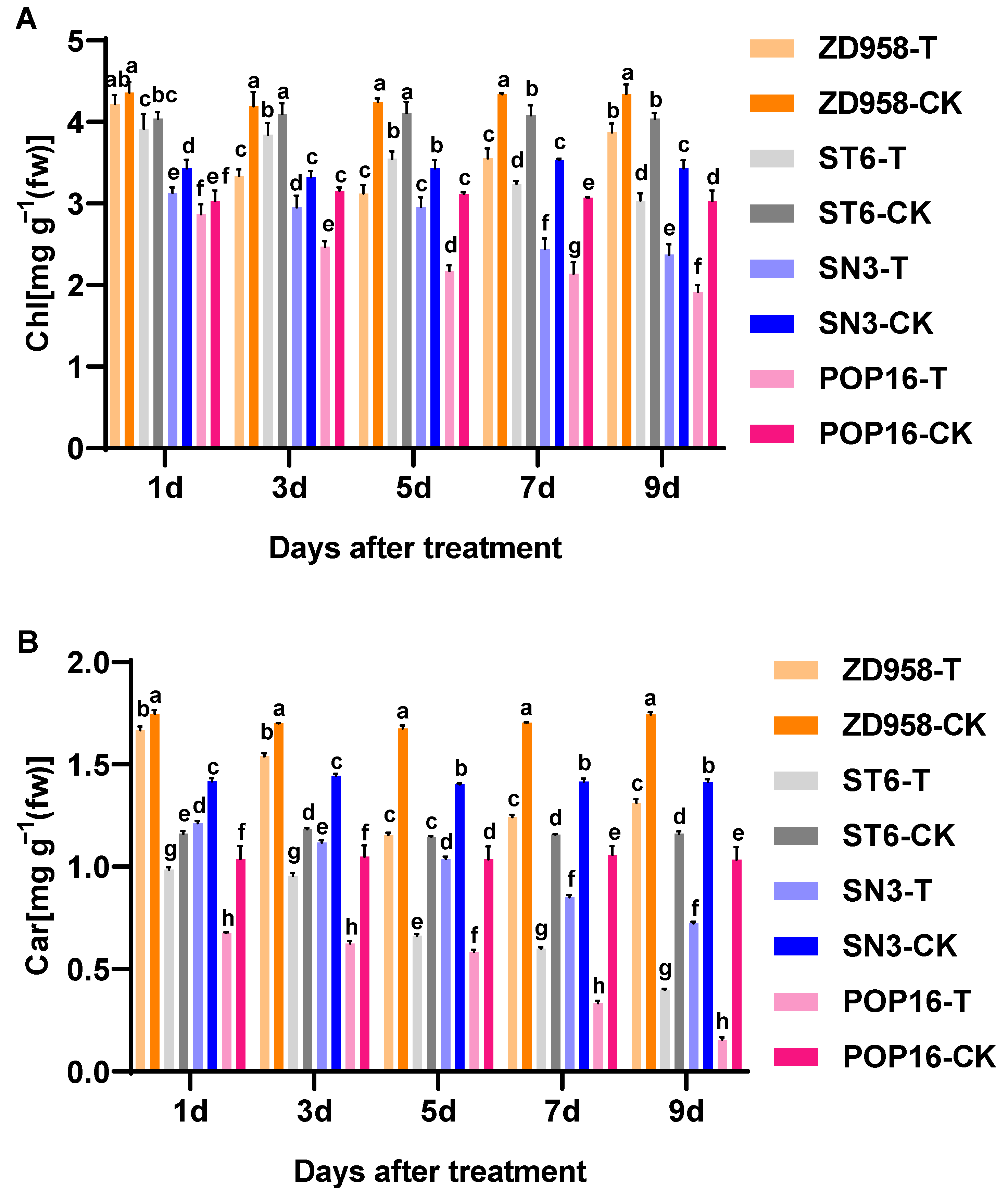
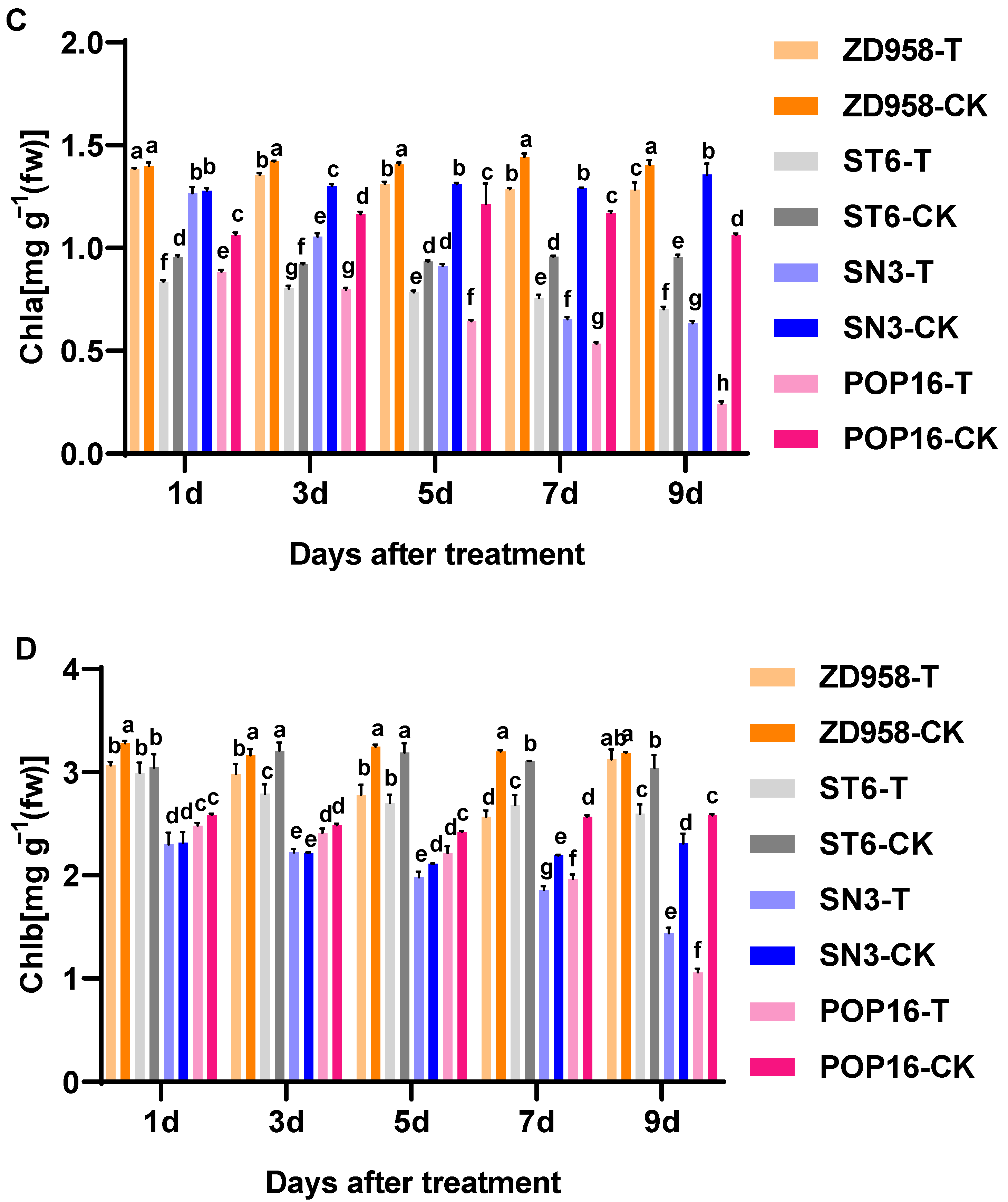
3.3. Effects of MET Treatment on Chlorophyll Contents of Leaves
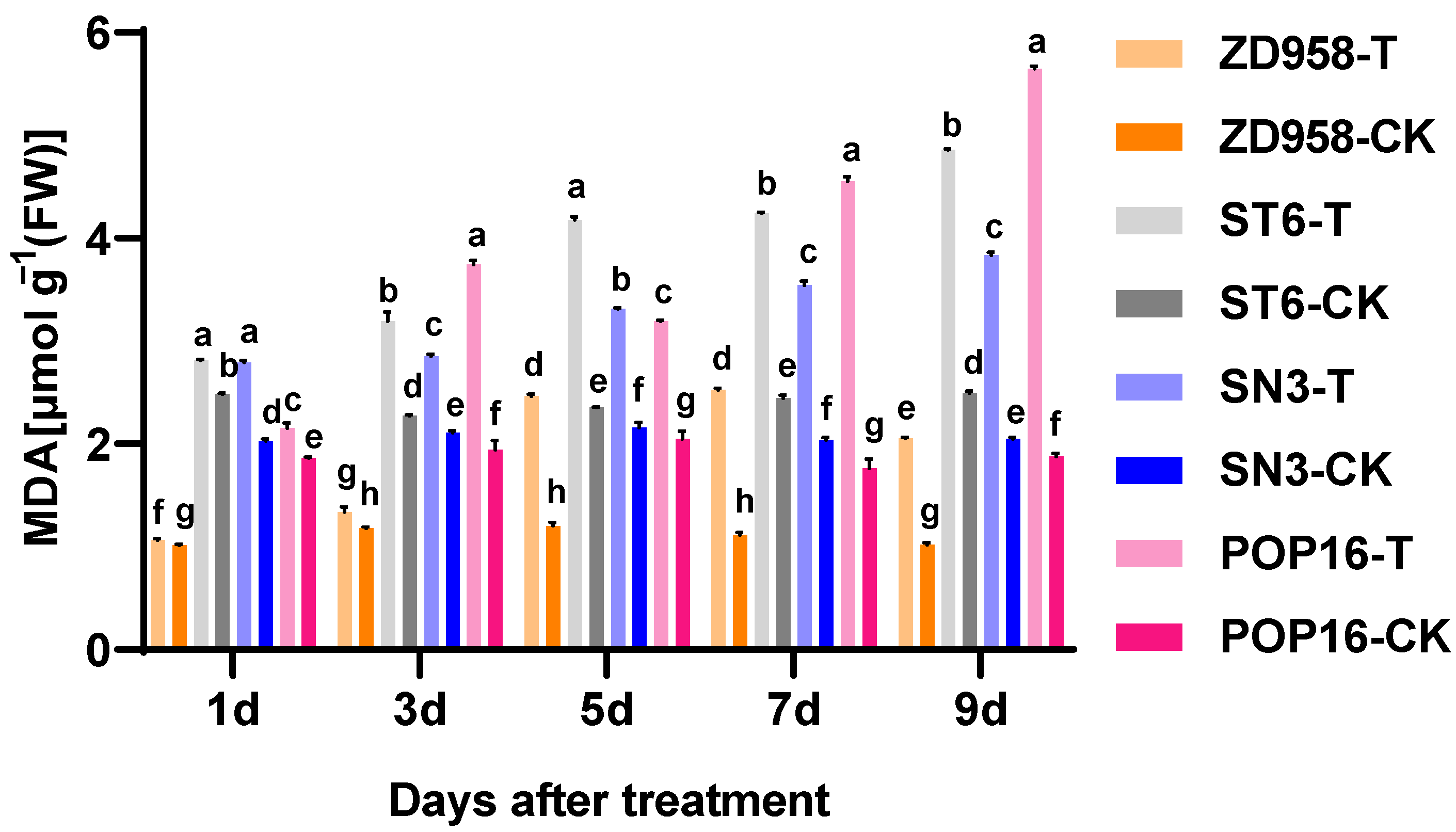
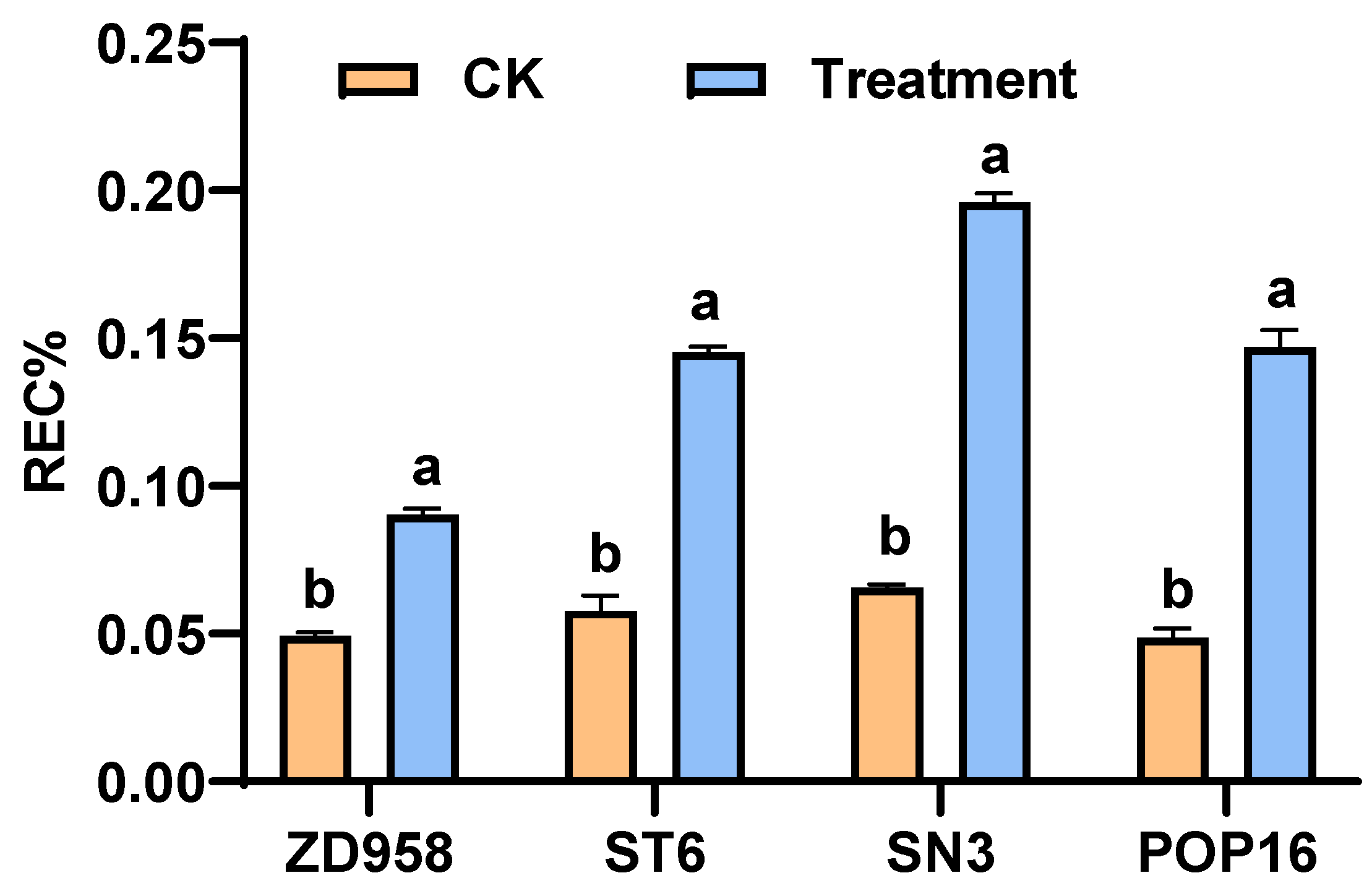
3.4. Effects of MET Treatment on Lipid Peroxidation and Conductivity in Leaves
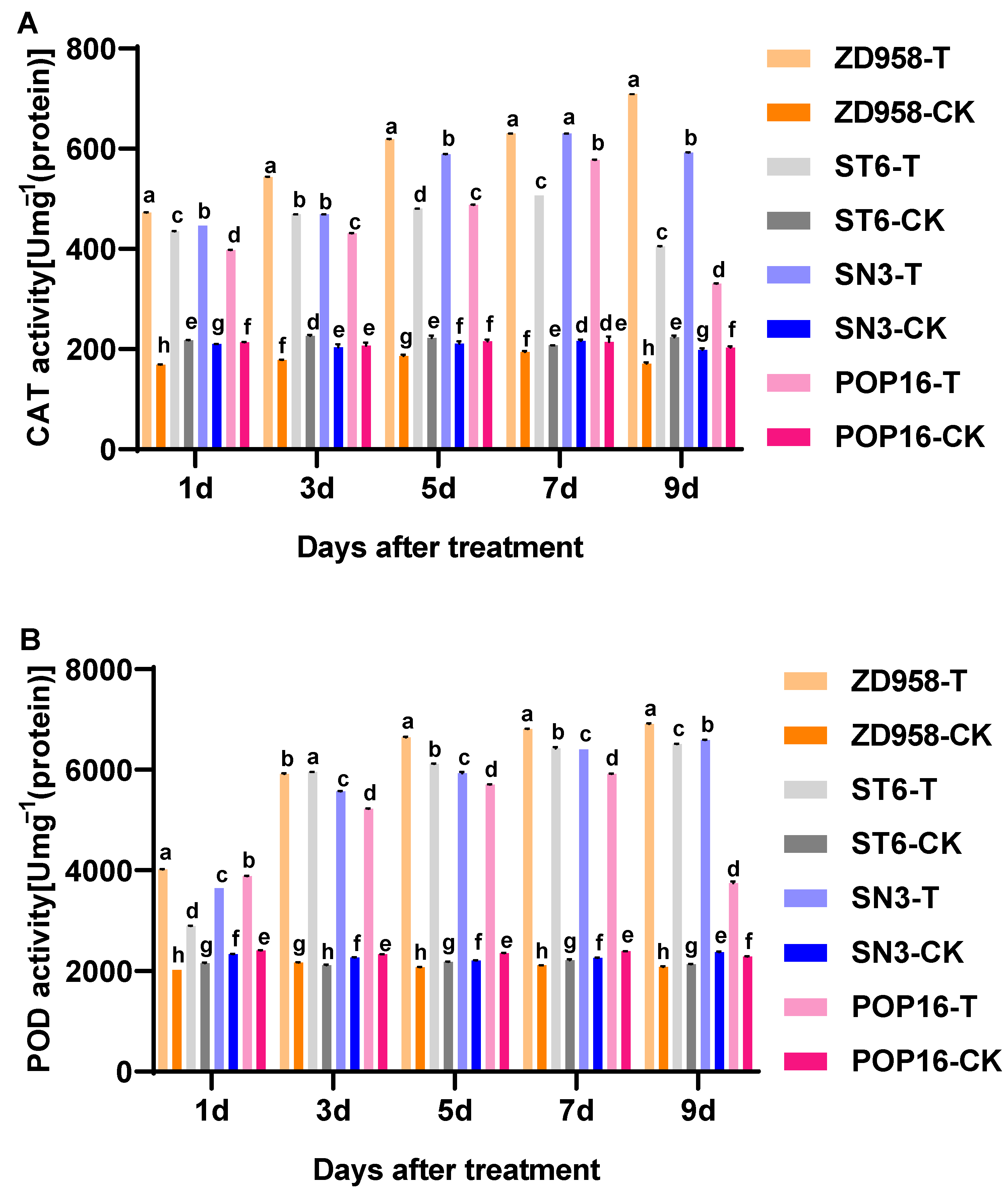
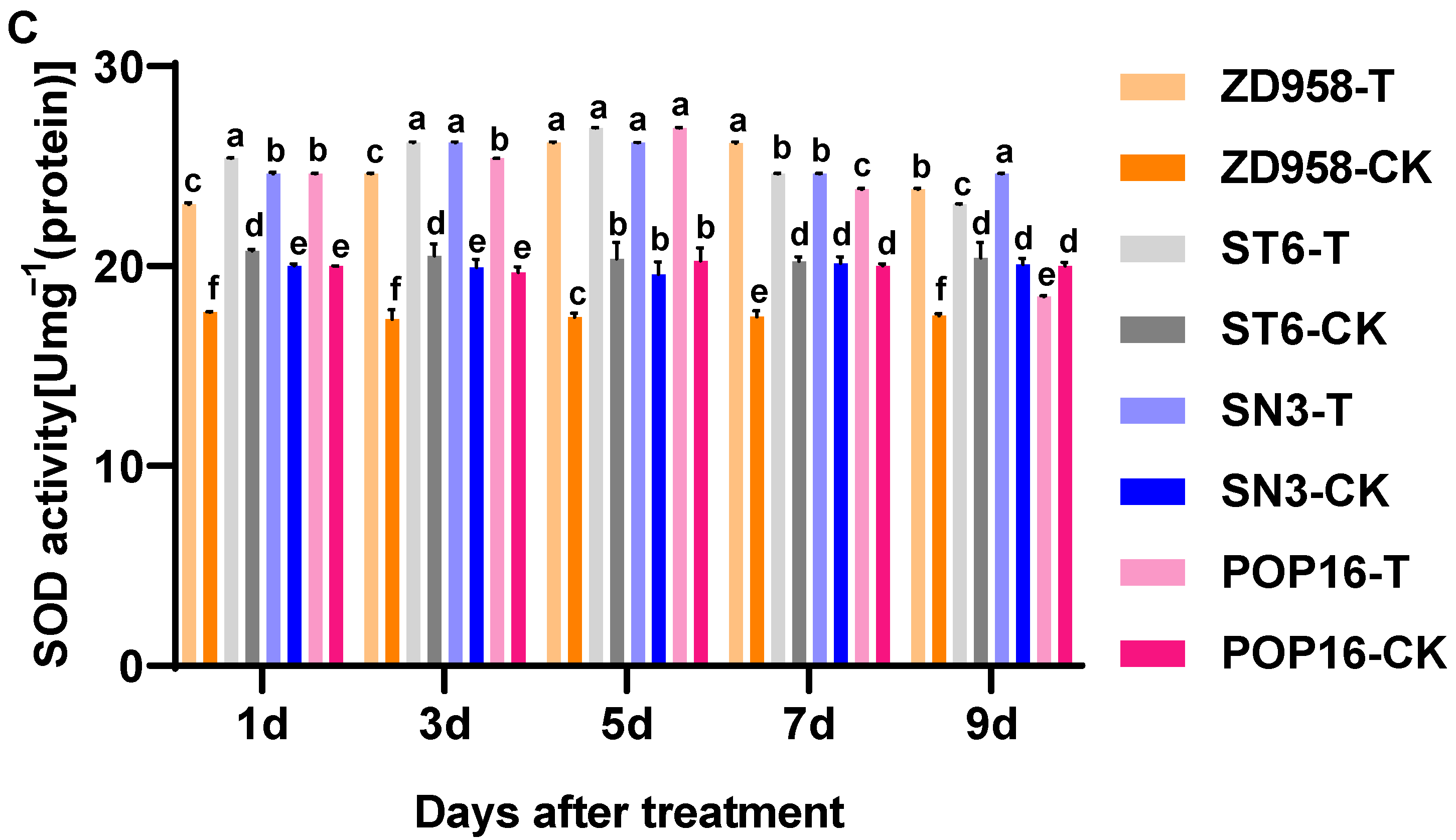
3.5. Effects of MET Treatment on Antioxidant Enzyme Activities in Leaves
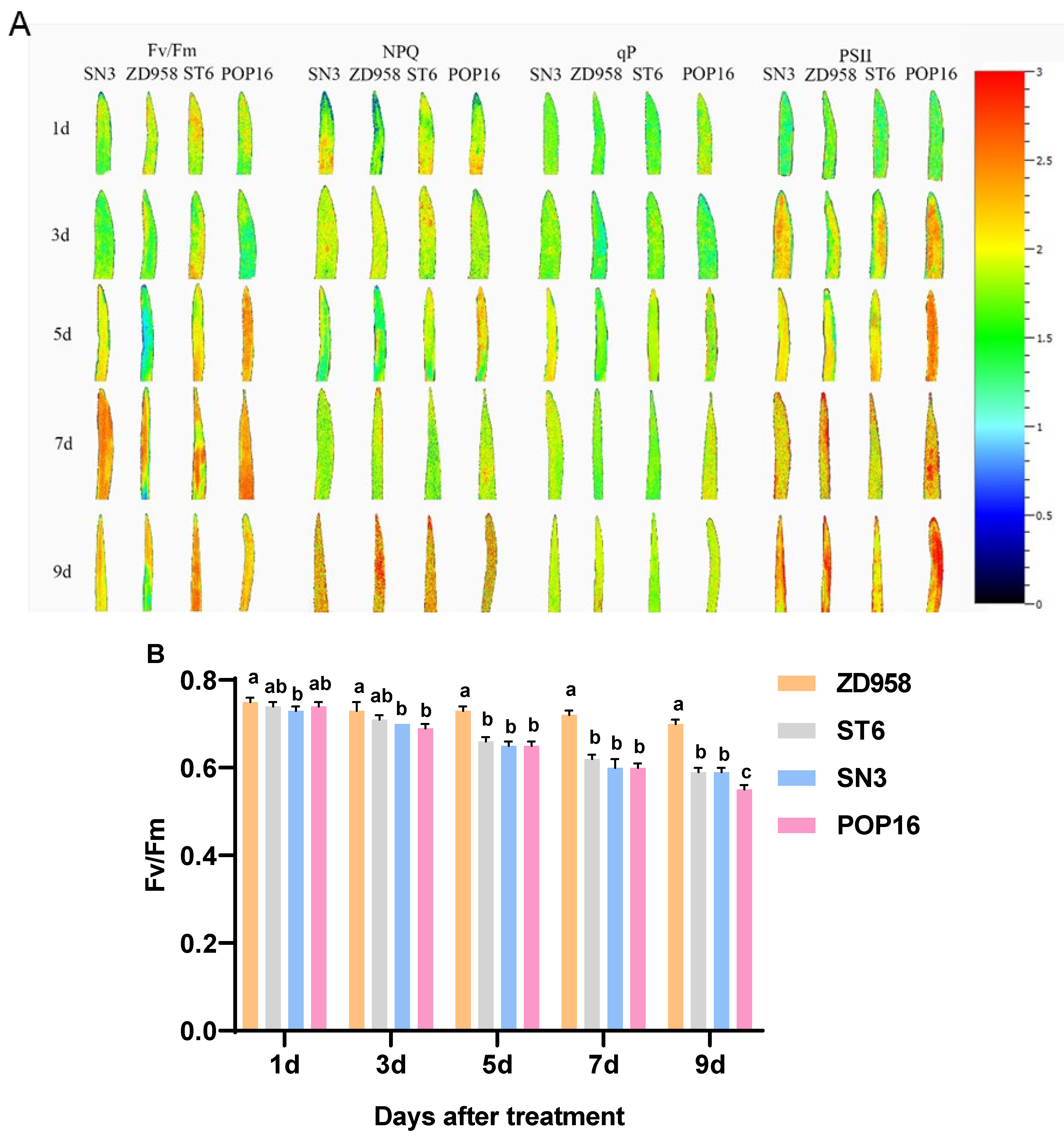
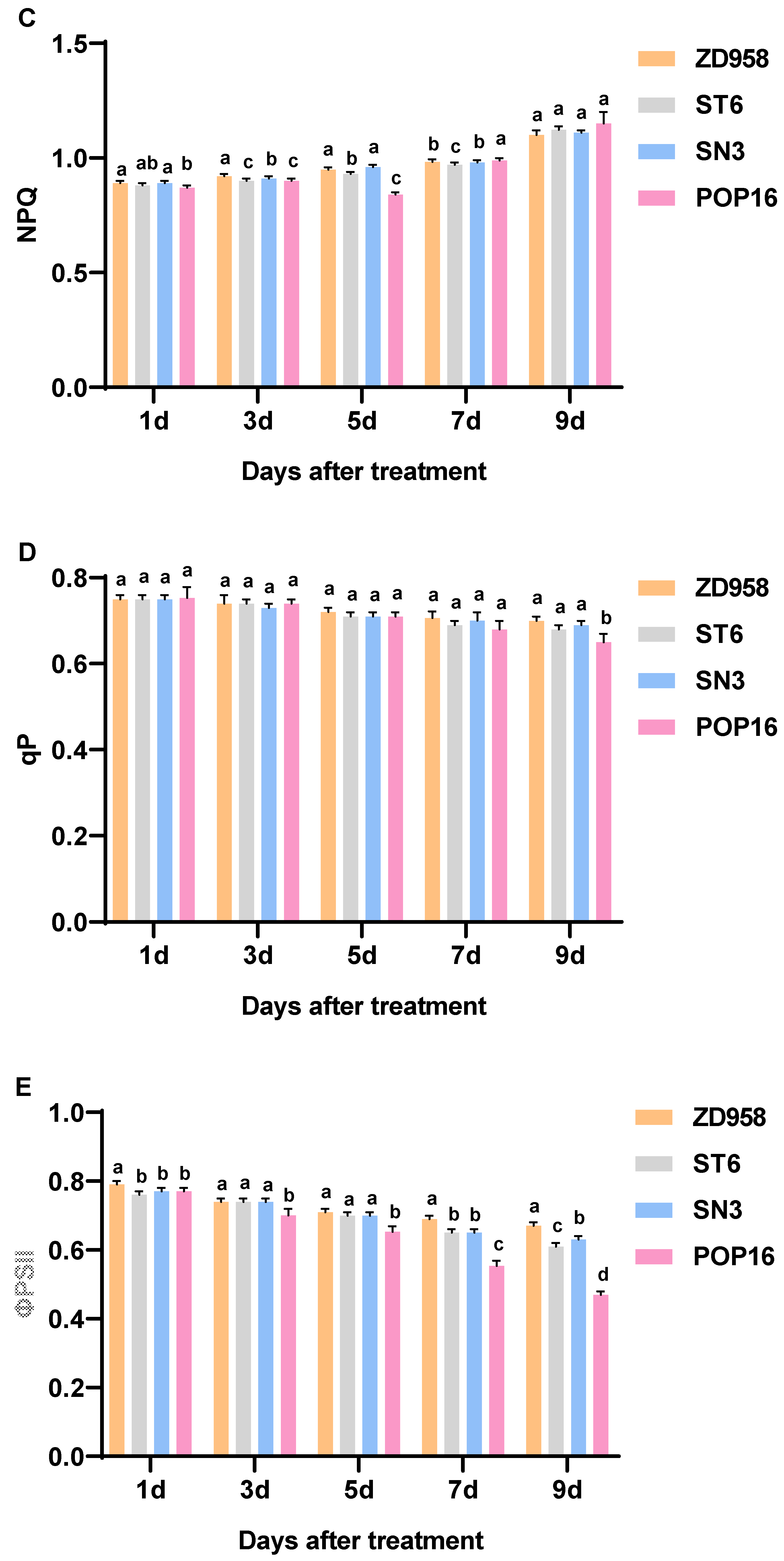
3.6. Effects of MET Treatment on Leaf Chlorophyll Fluorescence Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wang, Y.Q.; Gao, F.L.; Gao, G.Y.; Zhao, J.Y.; Wang, X.G.; Zhang, R. Production and cultivated area variation in cereal, rice, wheat and maize in China (1998–2016). Agronomy 2019, 9, 222. [Google Scholar] [CrossRef]
- Kumar, R.; Bohra, J.S. Effect of NPKS and Zn application on growth, yield, economics and quality of baby corn. Arch. Agron. Soil Sci. 2014, 60, 1193–1206. [Google Scholar] [CrossRef]
- Dai, S.H.; Georgelis, N.; Bedair, M.; Hong, Y.J.; Qi, Q.G.; Larue, C.T.; Sitoula, B.; Huang, W.; Krebel, B.; Shepard, M.; et al. Ectopic expression of a rice triketone dioxygenase gene confers mesotrione tolerance in soybean. Pest Manag. 2022, 78, 2816–2827. [Google Scholar] [CrossRef] [PubMed]
- Andert, S. The Method and Timing of Weed Control Affect the Productivity of Intercropped Maize (Zea mays L.) and Bean (Phaseolus vulgaris L.). Agriculture 2021, 11, 380. [Google Scholar] [CrossRef]
- Mitchell, G.; Bartlett, D.W.; Fraser, T.E.; Hawkes, T.R.; Holt, D.C.; Townson, J.K.; A Wichert, R. Mesotrione: A new selective herbicide for use in maize. Pest Manag. Sci. 2001, 57, 120–128. [Google Scholar] [CrossRef]
- Fu, Y.X.; Zhang, Z.Y.; Guo, W.Y.Z.; Dai, Y.J.; Wang, Z.Y.; Yang, W.; Yang, G. In vivo fluorescent screening for HPPD-targeted herbicide discovery. Pest Manag. Sci. 2022, 78, 4947–4955. [Google Scholar] [CrossRef]
- Ahrens, H.; Lange, G.; Müller, T.; Rosinger, C.; Willms, L.; van Almsick, A. 4-Hydroxyphenylpyruvate dioxygenase inhibitors in combination with safeners: Solutions for modern and sustainable agriculture. Angew. Chem. Int. Ed. Engl. 2013, 52, 9388–9398. [Google Scholar] [CrossRef] [PubMed]
- Sutton, P.; Richards, C.; Buren, L.; Glasgow, L. Activity of mesotrione on resistant weeds in maize. Pest Manag. Sci. 2002, 58, 981–984. [Google Scholar] [CrossRef]
- Ndikuryayo, F.; Moosavi, B.; Yang, W.C.; Yang, G.F. 4-Hydroxyphenylpyruvate dioxygenase inhibitors: From chemical biology to agrochemicals. J. Agric. Food Chem. 2017, 65, 8523–8537. [Google Scholar] [CrossRef]
- Jbir-Koubaa, R.; Charfeddine, S.; Ellouz, W.; Saidi, M.N.; Drira, N.; Gargouri-Bouzid, R.; Nouri-Ellouz, O. Investigation of the response to salinity and to oxidative stress of interspecific potato somatic hybrids grown in a greenhouse. Plant Cell Tissue Organ Cult. 2015, 120, 933–947. [Google Scholar] [CrossRef]
- Song, Z.W.; Gao, H.J.; Zhu, P.; Peng, C.; Deng, A.X.; Zheng, C.; Mannaf, A.; Islam, N.; Zhang, W. Organic amendments increase corn yield by enhancing soil resilience to climate change. Crop J. 2015, 3, 110–117. [Google Scholar] [CrossRef]
- Quintero, C.; Valderrama, M.; Becerra, A.; Daniliuc, C.G.; Rojas, R.S. Synthesis of 4(3H) quinazolinimines by reaction of (E)-N-(aryl)-acetimidoyl or -benzimidoyl chloride with amines. Org. Biomol. Chem. 2015, 13, 6183–6193. [Google Scholar] [CrossRef]
- Yang, J.G.; Sun, N.X.; Xiong, Q.C.; Yang, R. Effect of Moxonidine on the Uveoscleral Outflow: Role of 2-Adrenoceptors or I1 Imidazoline Receptors. Curr. Eye Res. 2009, 34, 287–296. [Google Scholar] [CrossRef]
- Zhang, M.P.; Zhang, C.J.; Yu, G.H.; Jiang, Y.Z.; Strasser, R.J.; Yuan, Z.-Y.; Yang, X.-S.; Chen, G.-X. Changes in chloroplast ultrastructure, fatty acid components of thylakoid membrane chlorophyll a fluorescence transient in flag leaves of a super-high-yield hybrid rice and its parents during the reproductive stage. J. Plant Physiol. 2010, 167, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.; Qiu, C.W.; Zhang, C.; Cao, F.; Shuijin, Z.; Wu, F. Comparative physiological analysis in the tolerance to salinity and drought individual combination in two cotton genotypes with contrasting salt tolerance. Physiol. Plant 2019, 165, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Steckel, L.E.; Thompson, M.A.; Hayes, R.M. Herbicide options for controlling glyphosate-tolerant corn in a corn replant situation. Weed Technol. 2009, 23, 243–246. [Google Scholar] [CrossRef]
- Crow, W.D.; Steckel, L.E.; Mueller, T.C.; Hayes, R.M. Management of large, glyphosate-resistant palmer amaranth (Amaranthus palmeri S.) in Corn. Weed Technol. 2016, 30, 611–616. [Google Scholar] [CrossRef]
- Chahal, P.S.; Varanasi, V.K.; Jugulam, M.; Jhala, A.J. Glyphosate-resistant palmer amaranth (Amaranthus palmeri S.) in nebraska: Confirmation, EPSPS gene amplification, and response to post corn and soybean herbicides. Weed Technol. 2017, 31, 80–93. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Schreiber, U. Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: An overview. Chlorophyll A Fluoresc. 2004, 19, 279–319. [Google Scholar] [CrossRef]
- Hodges, D.M. Chilling Effects on Antioxidant Systems of Maize (Zea mays L.); University of Ottawa: Ottawa, ON, Canada, 1995. [Google Scholar] [CrossRef]
- Li, A.X.; Han, Y.Y.; Wang, X.; Chen, Y.H.; Zhao, M.R.; Zhou, S.-M.; Wang, W. Root-specific expression of wheat expansin gene TaEXPB23 enhances root growth and water stress tolerance in tobacco. Environ. Exp. Bot. 2015, 110, 73–84. [Google Scholar] [CrossRef]
- Abedi, T.; Pakniyat, H. Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J. Genet. Plant Breed. 2010, 46, 27–34. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-b- and ozone-induced biochemical changes in antioxidant enzymes of arabidopsis thaliana. Plant Physiol. Biochem. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Meth. Enzym. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Frankart, C.; Eullaffroy, P.; Vernet, G. Comparative effects of four herbicides on non-photochemical fluorescence quenching in Lemna minor. Environ. Exp. Bot. 2003, 49, 159–168. [Google Scholar] [CrossRef]
- Ramel, F.; Sulmon, C.; Bogard, M.; Couée, I.; Gouesbet, G. Differential patterns of reactive oxygen species and antioxidative mechanisms during atrazine injury and sucrose-induced tolerance in Arabidopsis thaliana plantlets. BMC Plant Biol. 2009, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Chen, W.; Sheng, G.D.; Xu, X.; Liu, W.; Fu, Z.; Fu, Z. Effects of glufosinate on antioxidant enzymes, subcellular structure, and gene expression in the unicellular green alga Chlorella vulgaris. Aquat. Toxicol. 2008, 88, 301–307. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, H. Prometryne-induced oxidative stress and impact on antioxidant enzymes in wheat. Ecotoxicol. Environ. Saf. 2009, 72, 1687–1693. [Google Scholar] [CrossRef]
- Platiša, J.; Veljović-Jovanović, S.; Kukavica, B.; Vinterhalter, B.; Smigocki, A.; Ninković, S. Induction of peroxidases and superoxide dismutases in transformed embryogenic calli of alfalfa (Medicago sativa L.). J. Plant Physiol. 2008, 165, 895–900. [Google Scholar] [CrossRef]
- Geoffroy, L.; Frankart, C.; Eullaffroy, P. Comparison of different physiological parameter responses in Lemna minor and Scenedesmus obliquus exposed to herbicide flumioxazin. Environ. Pollut. 2004, 131, 233–241. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Shin, J.S.; Shin, D.Y.; Hyun, K.H.; Burgos, N.R.; Lee, S.; Kuk, Y.I. Tolerance to paraquat-mediated oxidative and environmental stresses in squash (Cucurbita spp.) leaves of various ages. Pestic. Biochem. Physiol. 2011, 99, 65–76. [Google Scholar] [CrossRef]
- Tarchoune, I.; Sgherri, C.; Izzo, R.; Lachaal, M.; Ouerghi, Z.; Navari-Izzo, F. Antioxidative responses of ocimum basilicum to sodium chloride or sodium sulphate salinization. Plant Physiol. Biochem. 2010, 48, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.M.; Nemat Alla, M.M. Oxidative stress in herbicide-treated broad bean and maize plants. Acta Physiol. Plant. 2005, 27, 429–438. [Google Scholar] [CrossRef]
- Wu, G.L.; Cui, J.; Tao, L.; Yang, H. Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa L.). Ecotoxicology 2010, 19, 124–132. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, S.; Wang, L.; Wang, S.; Yu, N.; Zhong, X. Photosynthetic and Physiological Responses of Different Maize Varieties to Mesotrione. Agronomy 2024, 14, 1701. https://doi.org/10.3390/agronomy14081701
Sun S, Wang L, Wang S, Yu N, Zhong X. Photosynthetic and Physiological Responses of Different Maize Varieties to Mesotrione. Agronomy. 2024; 14(8):1701. https://doi.org/10.3390/agronomy14081701
Chicago/Turabian StyleSun, Shufeng, Liru Wang, Shuang Wang, Na Yu, and Xuemei Zhong. 2024. "Photosynthetic and Physiological Responses of Different Maize Varieties to Mesotrione" Agronomy 14, no. 8: 1701. https://doi.org/10.3390/agronomy14081701
APA StyleSun, S., Wang, L., Wang, S., Yu, N., & Zhong, X. (2024). Photosynthetic and Physiological Responses of Different Maize Varieties to Mesotrione. Agronomy, 14(8), 1701. https://doi.org/10.3390/agronomy14081701



