Evaluation of the Improvement Effect of Chemical Fertilizer Combined with Different Additives on Newly Built Paddy Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Experimental Design
2.3. Determination Items and Methods
2.4. Evaluation of Soil Quality
2.4.1. Construction of TDS and MDS
2.4.2. Calculate the Index Score
2.4.3. Determine the Index Weight
2.4.4. Calculation of SQI
2.5. Statistical Analysis
3. Results
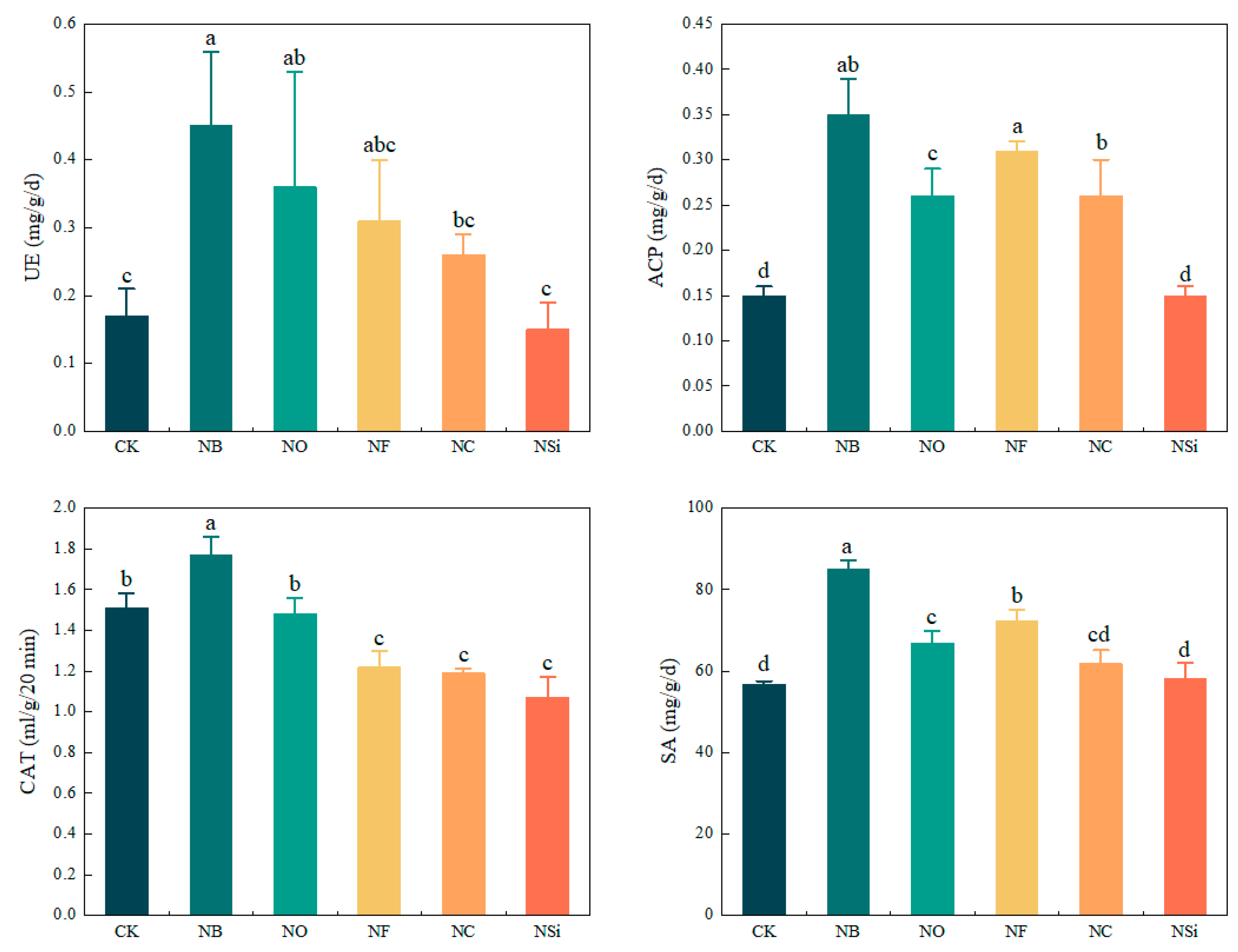
3.1. Effects of Different Treatments on Physical and Chemical Properties of Topsoil and Soil Enzyme Activity
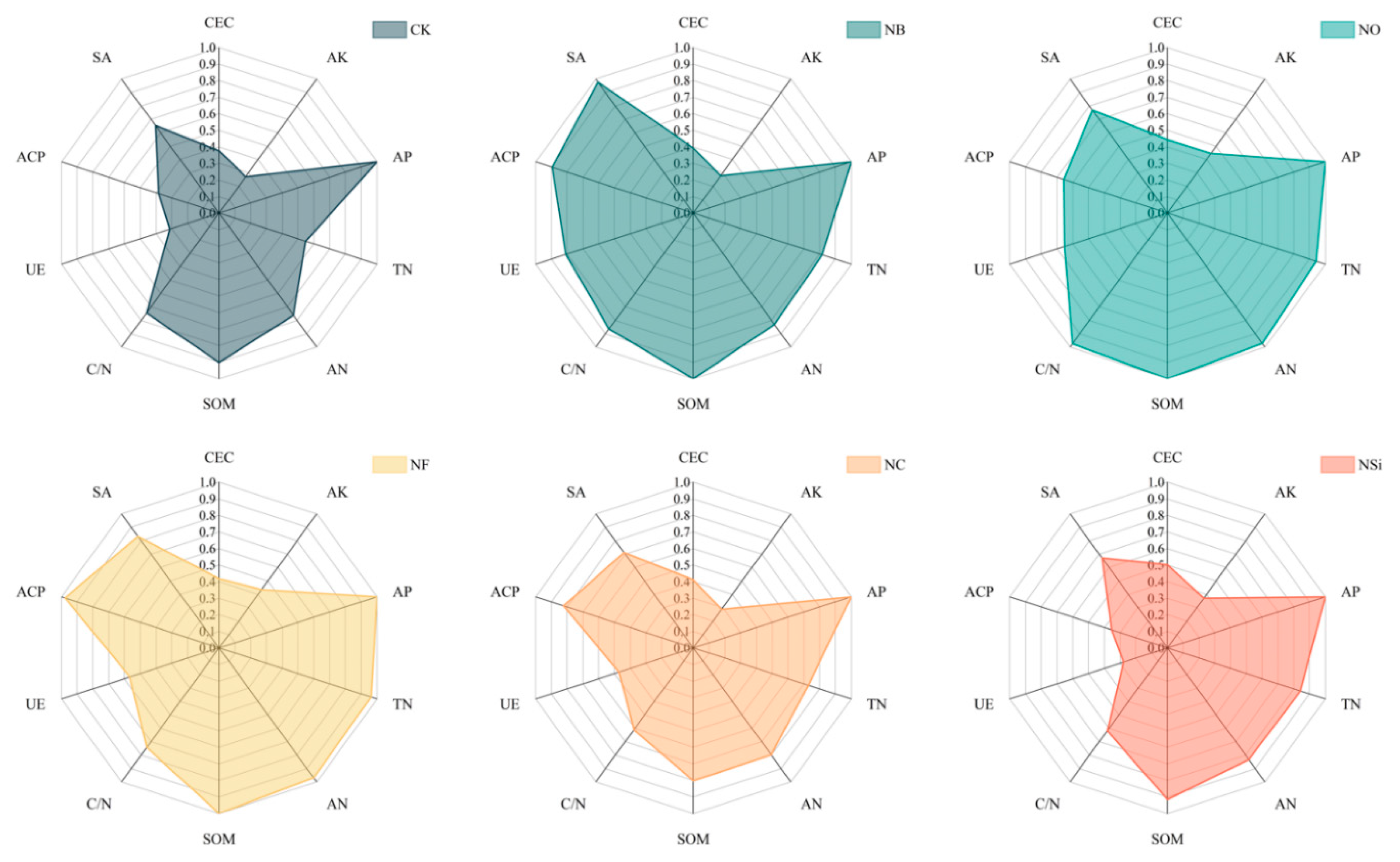
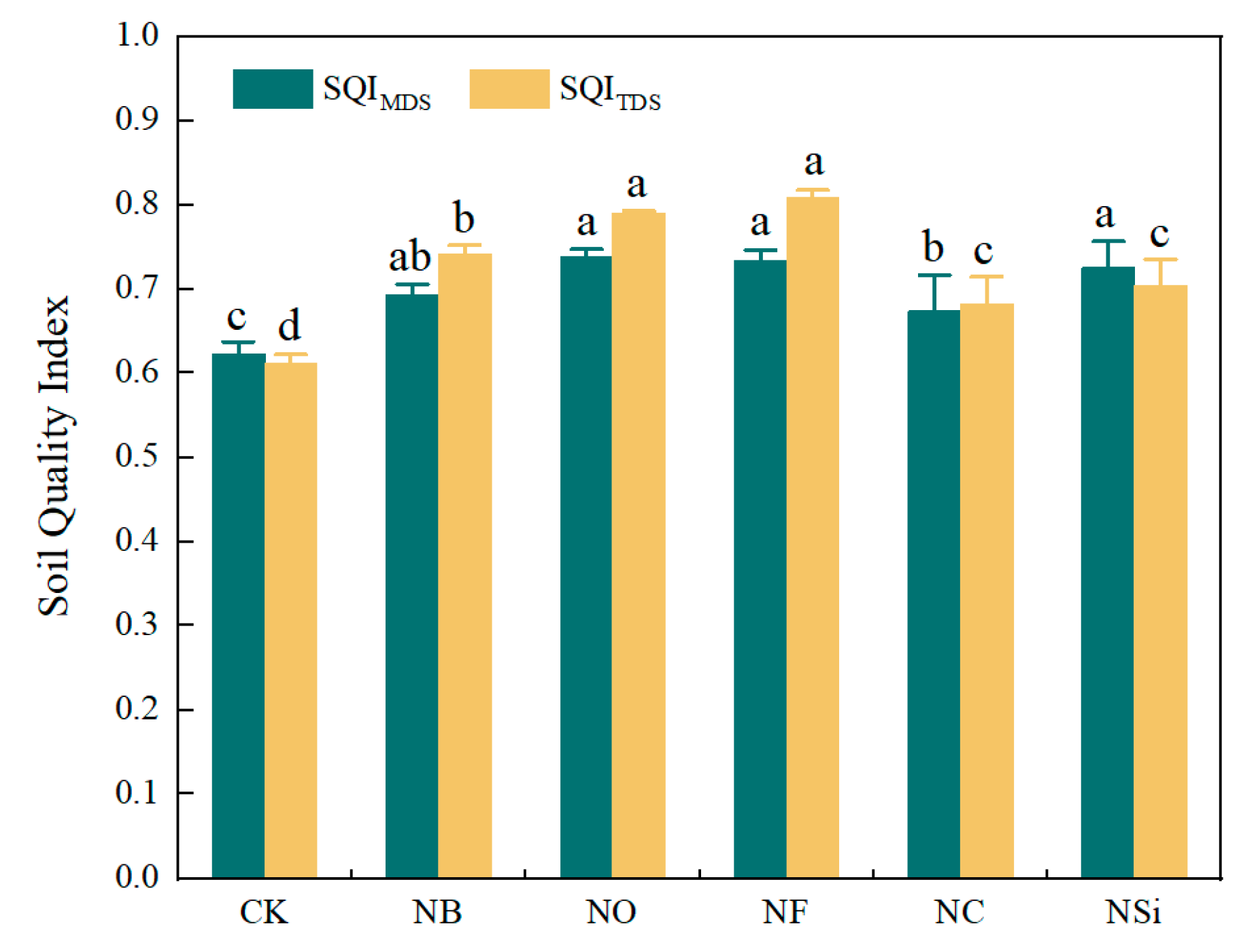
3.2. Effects of Different Treatments on SQI
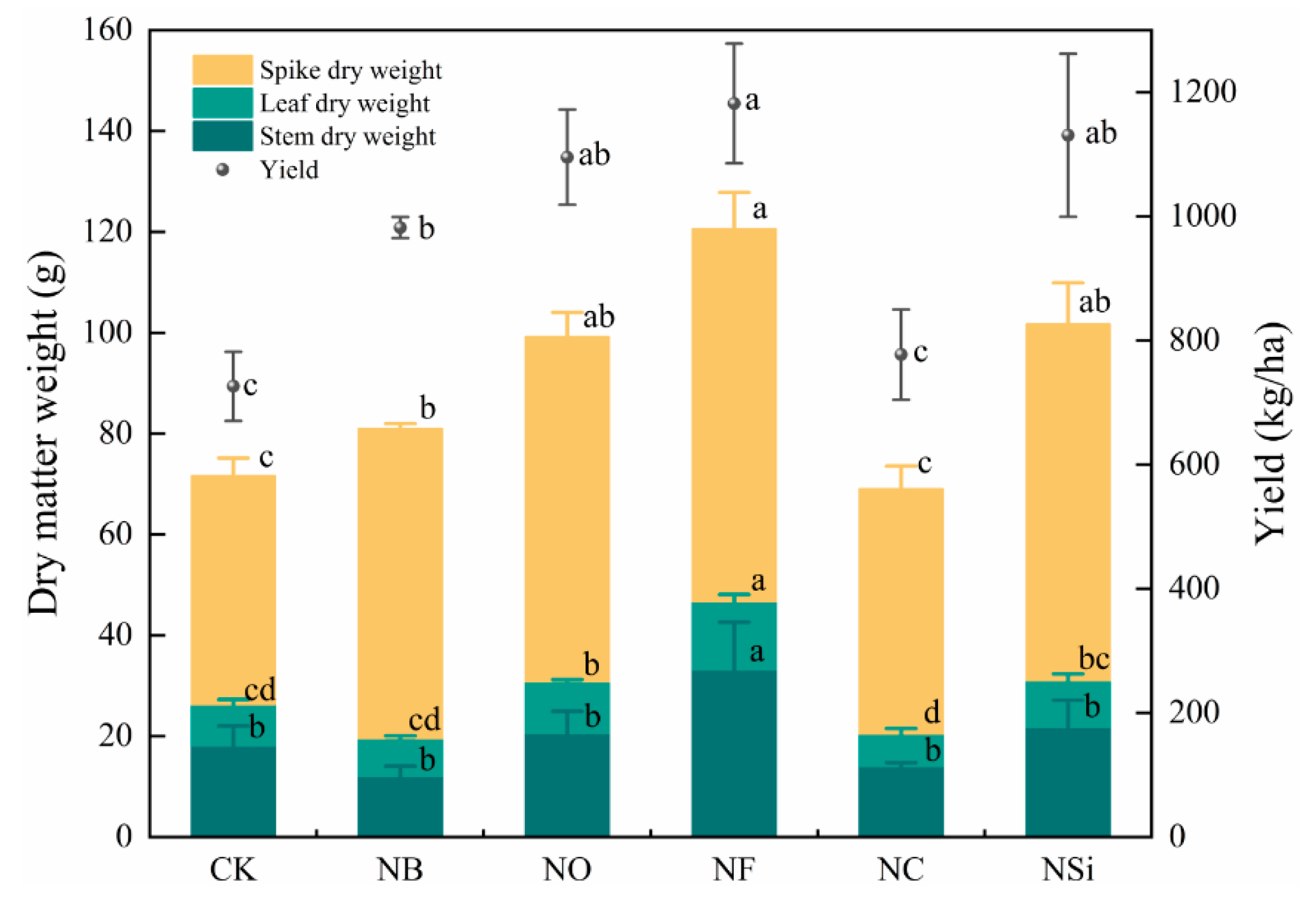
3.3. Effects of Different Treatments on Paddy Yield
4. Discussion
4.1. Effects of Chemical Fertilizer Combined with Different Soil Additives on Soil Properties of Plough Layer
4.2. Effects of Chemical Fertilizer Combined with Different Soil Additives on Soil Quality Evaluation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Zhang, W.; Wu, L.; Ren, Y.; Zhang, J.; Xu, M. Advance in indicator screening and methodologies of soil quality evaluation. Sci. Agric. Sin. 2021, 54, 3043–3056. [Google Scholar] [CrossRef]
- Smith, E.G.; Lerohl, M.; Messele, T.; Janzen, H.H. Soil Quality Attribute Time Paths: Optimal Levels and Values. J. Agric. Resour. Econ. 2000, 25, 307–324. [Google Scholar] [CrossRef]
- Kirschbaum, S.E.K.; Baeumner, A.J. A review of electrochemiluminescence (ECL) in and for microfluidic analytical devices. Anal. Bioanal. Chem. 2015, 407, 3911–3926. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Smith, P.; House, J.I.; Bustamante, M.; Harper, J.S.R.; Pan, G.; West, P.C.; Clark, J.M.; Adhya, T.; Rumpel, C.; Paustian, K.; et al. Global change pressures on soils from land use and management. Glob. Change Biol. 2016, 22, 1008–1028. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, L.; Mezzapesa, G.N.; Mondelli, D.; Miano, T. Mediterranean soil fertility management for economically feasible and environmentally sustainable production of organic tomatoes. Agrochimica 2020, 64, 107–120. [Google Scholar] [CrossRef]
- Meng, F.; Qiao, Y.; Wu, W.; Smith, P.; Scott, S. Environmental impacts and production performances of organic agriculture in China: A monetary valuation. J. Environ. Manag. 2017, 188, 49–57. [Google Scholar] [CrossRef]
- Tao, C.; Li, R.; Xiong, W.; Shen, Z.; Liu, S.; Wang, B.; Ruan, Y.; Geisen, S.; Shen, Q.; Kowalchuk, G.A. Bio-organic fertilizers stimulate indigenous soil Pseudomonas populations to enhance plant disease suppression. Microbiome 2020, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Kuila, D.; Ghosh, S. Aspects, problems and utilization of Arbuscular Mycorrhizal (AM) application as bio-fertilizer in sustainable agriculture. Curr. Res. Microb. Sci. 2022, 3, 100107. [Google Scholar] [CrossRef]
- Ditzler, C.A.; Tugel, A.J. Soil Quality Field Tools. Agron. J. 2002, 94, 33–38. [Google Scholar] [CrossRef]
- Zahedifar, M. Assessing alteration of soil quality, degradation, and resistance indices under different land uses through network and factor analysis. Catena 2023, 222, 106807. [Google Scholar] [CrossRef]
- Andrews, S.S.; Karlen, D.L.; Mitchell, J.P. A comparison of SQIing methods for vegetable production systems in Northern California. Agric. Ecosyst. Environ. 2002, 90, 25–45. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Xi, M.; Zhao, Q.; Lu, Q.; Jia, J. Soil quality assessment of coastal wetlands in the Yellow River Delta of China Based on the Minimum Data Set. Ecol. Indic. 2016, 66, 458–466. [Google Scholar] [CrossRef]
- Nabiollahi, K.; Golmohamadi, F.; Taghizadeh-Mehrjardi, R.; Kerry, R.; Davari, M. Assessing the effects of slope gradient and land use change on soil quality degradation through digital mapping of soil quality indices and soil loss rate. Geoderma 2018, 318, 16–28. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhong, X.; Miao, S.; Li, Q.; Lu, X. Evaluation Indicators of Soil Quality in plough layer of aeolian sandy land in northeast china based on Minimum Data Set. Res. Soil Water Conserv. 2019, 26, 132–138. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, G.; Jia, S.; Dong, J.; Chen, L.; Wang, S. Comprehensive evaluation of effects of fertilization on grassland quality index and soil properties in alpine steppe. Acta Pratac. Sin. 2020, 29, 85–93. [Google Scholar] [CrossRef]
- Gong, H.; Li, J.; Ma, J.; Li, F.; Ouyang, Z.; Gu, C. Effects of tillage practices and microbial agent applications on dry matter accumulation, yield and the soil microbial index of winter wheat in North China. Soil Tillage Res. 2018, 184, 235–242. [Google Scholar] [CrossRef]
- Li, Z.; Wei, X.; Zhu, Z.; Fang, Y.; Yuan, H.; Li, Y.; Zhu, Q.; Guo, X.; Wu, J.; Kuzyakov, Y.; et al. Organic fertilizers incorporation increased microbial necromass accumulation more than mineral fertilization in paddy soil via altering microbial traits. Appl. Soil. Ecol. 2024, 193, 105137. [Google Scholar] [CrossRef]
- Wang, L.; Ashraf, U.; Chang, C.; Abrar, M.; Cheng, X. Effects of Silicon and Phosphatic Fertilization on Rice Yield and Soil Fertility. J. Soil Sci. Plant Nutr. 2020, 20, 557–565. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.; Wu, G.; Chen, X.; Gruda, N.; Li, X.; Dong, J.; Duan, Z. Dose-Dependent Application of Straw-Derived Fulvic Acid on Yield and Quality of Tomato Plants Grown in a Greenhouse. Front. Plant Sci. 2021, 12, 736613. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, M.; Guo, X.; Wang, H.; Sui, B.; Zhao, L. Organic carbon content and humus composition after application aluminum sulfate and rice straw to soda saline-alkaline soil. Environ. Sci. Pollut. Res. 2019, 26, 13746–13754. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.P.; Yang, L.; Li, Z.W.; Liu, C.; Zheng, P.; Tong, D.; Chang, X.F.; Tang, C.; Xiao, H.B. Responses of microbial community composition and respiration to soil moisture in eroded soil. Appl. Soil Ecol. 2023, 181, 104662. [Google Scholar] [CrossRef]
- Wu, X.; Xu, H.; Liu, G.; Ma, X.; Mu, C.; Zhao, L. Bacterial communities in the upper soil layers in the permafrost regions on the Qinghai-Tibetan plateau. Appl. Soil Ecol. 2017, 120, 81–88. [Google Scholar] [CrossRef]
- Zhao, M.; Cong, J.; Cheng, J.; Qi, Q.; Sheng, Y.; Ning, D. Soil microbial community assembly and interactions are constrained by nitrogen and phosphorus in broadleaf forests of southern China. Forests 2020, 11, 285. [Google Scholar] [CrossRef]
- Yang, X.; Liu, H.L.; Huang, Y.Y.; Yang, H.Z.; Bei, M.R.; Lin, Q.H. Effect of application of organic–inorganic compound fertilizers on microbial communities and enzyme activities in tropical paddy soil. J. Plant Nutr. Fert. 2021, 27, 619–629. [Google Scholar] [CrossRef]
- Ge, G.F.; Li, Z.J.; Fan, F.L.; Chu, G.X.; Hou, Z.A.; Liang, Y.C. Soil biological activity and their seasonal variations in response to long-term application of organic and inorganic fertilizers. Plant Soil 2010, 326, 31–44. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Assay of urease activity in soils. Soil Boil. Biochem. 1972, 4, 479–487. [Google Scholar] [CrossRef]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline phosphatase: An overview. Indian J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef]
- Bo, H.; Li, Z.; Wang, W.; Zhang, R.; Wang, H.; Jin, D.; Xu, M.; Zhang, Q. Combining Organic and Inorganic Fertilization Enhances Soil Enzyme Activity, the Bacterial Community, and Molecular Ecological Network Complexity in Coal Mine Reclamation Areas. Agronomy 2024, 14, 1427. [Google Scholar] [CrossRef]
- Yap, B.W.; Sim, C.H. Comparisons of various types of normality tests. J. Stat. Comput. Simul. 2011, 81, 2141–2155. [Google Scholar] [CrossRef]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Thomson Brooks, Cole Publishing Co.: Pacific Grove, CA, USA, 1996; Available online: https://api.semanticscholar.org/CorpusID:121954573 (accessed on 31 July 2024).
- Ye, H.; Zhang, S.; Huang, Y.; Wang, S. Assessment of Surface Soil Fertility and Its Spatial Variability in Yanqing Basin, Beiing, China. Sci. Agric. Sin. 2013, 46, 3151–3160. [Google Scholar] [CrossRef]
- Qi, Y.; Darilek, J.L.; Huang, B.; Zhao, Y.; Sun, W.; Gu, Z. Evaluating soil quality indices in an agricultural region of Jiangsu Province, China. Geoderma 2009, 149, 325–334. [Google Scholar] [CrossRef]
- Quelal, M.O.; Yánez, Y.W.; Carrera, A.J. Bioindicators for Assessing Soil Quality in Ecuador’s Jun Jun Micro-Watershed. Agronomy 2024, 14, 1436. [Google Scholar] [CrossRef]
- Liebig, M.A.; Varvel, G.; Doran, J. A Simple Performance-Based Index for Assessing Multiple Agroecosystem Functions. Agron. J. 2001, 93, 313–318. [Google Scholar] [CrossRef]
- Rheinheimer, D.S.; Tiecher, T.; Gonzattoa, R.; Zafar, M.; Brunetto, G. Residual effect of surface-applied lime on soil acidity properties in a long-term experiment under no-till in a Southern Brazilian sandy Ultisol. Geoderma 2018, 313, 7–16. [Google Scholar] [CrossRef]
- Shi, L.; Zheng, W.; Lei, T.; Liu, X.; Hui, M. The Effect of Different Soil Additives on Soil Properties and on the Morphological and Physiological Characteristics of Chinese Cabbage. J. Soil Sci. Plant Nutr. 2021, 21, 1500–1510. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Liu, Z.; Tang, X.; Xiong, L.; Xia, W.; Wang, P.; Yuan, F.; Sun, G.; Li, Z.; et al. Evaluating soil fertility improvement effects of chemical fertilizer combined with organic fertilizers in a red paddy soil using the soil fertility index. J. Plant Nutr. Fert. 2021, 27, 777–790. [Google Scholar] [CrossRef]
- Chen, Q.; He, A.; Wang, W.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Comparisons of regeneration rate and yields performance between inbred and hybrid rice cultivars in a direct seeding rice-ratoon rice system in central China. Field Crop. Res. 2018, 223, 164–170. [Google Scholar] [CrossRef]
- Song, W.; Shu, A.; Liu, J.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S.; et al. Effects of long-term fertilization with different substitution ratios of organic fertilizer on paddy soil. Pedosphere 2022, 32, 637–648. [Google Scholar] [CrossRef]
| Bulk Density (g/cm3) | Porosity (%) | pH | Cation Exchange Capacity (cmol/kg) | Total Nitrogen (g/kg) | Carbon–Nitrogen Ratio (C/N) | Available Phosphorus (mg/kg) | Available Potassium (mg/kg) | Organic Matter (g/kg) |
|---|---|---|---|---|---|---|---|---|
| 1.29 | 51.32 | 6.84 | 12.30 | 1.23 | 16.71 | 16.09 | 41.48 | 28.81 |
| Additives | Manufacturer | Source | Main Components | Key Effect | References |
|---|---|---|---|---|---|
| Bacteria agent | Zhongke Chemical Products Co., Ltd., Zhengzhou, China | This product is produced using multi-layer liquid deep fermentation and modern biological preparation technology. It contains high-efficiency potassium-solubilizing gel-like Paenibacillus, disease-proof and bacteriostatic Bacillus subtilis, and antagonistic strains that are screened from common soil-borne diseases affecting pepper, cucumber, tomato, and fruit tree tea. | Bacillus subtilis, bacillus amyloliquefaciens, paenibacillus mucilaginosus | Repair soil biological environment | [17] |
| Alginate bio-organic fertilizer | Enbao Biotechnology Co., Ltd., China | This product is extracted from wild seaweed alginate and combined with beneficial bacteria. It undergoes dual-form fermentation, both solid and liquid, and is then processed with biological fermentation after decomposing acid organic fertilizer. | Alginate, organic matter, effective living bacteria | Improve soil fertility | [18] |
| Fulvic acid biomass nutrient solution | Qinheng Ecological Technology Co., Ltd., China | After pretreating crop residues and animal manure, the fulvic acid in these substances is dissolved in an alkaline solution and then acidified with sulfuric acid to obtain the final product. | Fulvic acid | Water and fertilizer conservation, improve soil nutrients availability | [20] |
| Acid soil conditioner | Maile Fertilizer Co., Ltd., China | Aluminum sulfate is prepared by reacting aluminum ore with sulfuric acid, then ground into particles. Additional trace elements are incorporated to complete the product. | Mineral matter | Regulate soil pH | [21] |
| Silicon-calcium-magnesium-potassium fertilizer | Akang Agricultural Technology Co., Ltd., China | Bio-extracted silicon-calcium is converted into inorganic complexed silicon-calcium through a double biochemical complexation process. | Effective silicon, effective calcium | Improve the resistance of crops | [19] |
| Treatments | Dosage | Usage |
|---|---|---|
| CK | 154 kg/hm2 of N, 82.9 kg/hm2 of P2O5 189.2, kg/hm2 of pure K2O | N (distributed as base fertilizer: tillering fertilizer: panicle fertilizer = 4:4:2), P2O5 (entirely applied as base fertilizer), K2O (base fertilizer: panicle fertilizer = 6:4) |
| NB | 154 kg/hm2 of N, 82.9 kg/hm2 of P2O5 189.2, kg/hm2 of pure K2O, 15 kg/hm2 of bacterial agent | N, P2O5, K2O are used in the same way as CK. Bacterial agent (diluted 50 times before irrigation pre-planting). |
| NO | 154 kg/hm2 of N, 82.9 kg/hm2 of P2O5 189.2, kg/hm2 of pure K2O, 1200 kg/hm2 of alginate bio-organic fertilizer | N, P2O5, K2O are used in the same way as CK. Alginate bio-organic fertilizer (applied pre-planting and thoroughly mixed with other base fertilizers, with an additional application of 1200 kg/hm2 during flowering and grain filling stages) |
| NF | 154 kg/hm2 of N, 82.9 kg/hm2 of P2O5 189.2, kg/hm2 of pure K2O, 750 kg/hm2 of fulvic acid biomass nutrient solution | N, P2O5, K2O are used in the same way as CK. Fulvic acid biomass nutrient solution (diluted 20 times before irrigation pre-planting) |
| NC | 154 kg/hm2 of N, 82.9 kg/hm2 of P2O5 189.2, kg/hm2 of pure K2O, 750 kg/hm2 of acid soil conditioner | N, P2O5, K2O are used in the same way as CK. Acid soil conditioner (applied pre-planting and mixed uniformly with other base fertilizers) |
| NSi | 154 kg/hm2 of N, 82.9 kg/hm2 of P2O5 189.2, kg/hm2 of pure K2O, 1200 kg/hm2 of silicon-calcium-magnesium-potassium fertilizer | N, P2O5, K2O are used in the same way as CK. Silicon-calcium-magnesium-potassium fertilizer (applied pre-planting and mixed evenly with other base fertilizers, with an additional application of 1200 kg/hm2 during flowering and grain filling stages). |
| Treatment | CK | NB | NO | NF | NC | NSi |
|---|---|---|---|---|---|---|
| BD (g/cm3) | 1.21 ± 0.07 a | 1.18 ± 0.15 a | 1.18 ± 0.01 a | 1.16 ± 0.06 a | 1.22 ± 0.07 a | 1.20 ±0.07 a |
| SP (%) | 54.46 ± 0.02 a | 55.40 ± 0.06 a | 55.56 ± 0.01 a | 56.17 ± 0.02 a | 54.02 ± 0.03 a | 54.75 ± 0.03 a |
| pH | 6.40 ± 0.24 b | 5.96 ± 0.18 c | 5.97 ± 0.19 c | 5.87 ± 0.28 cd | 5.60 ± 0.10 d | 6.80 ± 0.06 a |
| CEC (cmol/kg) | 9.61 ± 0.31 d | 9.90 ± 0.36 cd | 10.74 ± 0.19 b | 10.30 ± 0.08 bc | 10.19 ± 0.48 c | 11.70 ± 0.09 a |
| TN (g/kg) | 1.12 ± 0.04 c | 1.35 ± 0.06 ab | 1.45 ± 0.02 a | 1.47 ± 0.04 a | 1.27 ± 0.13 b | 1.37 ± 0.11 ab |
| AN (mg/kg) | 133.86 ± 2.64 c | 146.06 ± 3.73 b | 170.97 ± 4.56 a | 170.20 ± 2.72 a | 140.17 ± 5.75 bc | 146.44 ± 3.08 b |
| AP (mg/kg) | 17.61 ± 1.38 d | 23.06 ± 2.71 bc | 26.69 ± 0.41 ab | 28.25 ± 0.78 a | 19.53 ± 0.97 cd | 24.68 ± 3.83 ab |
| AK (mg/kg) | 52.72 ± 3.81 c | 53.70 ± 0.51 c | 76.06 ±3.03 a | 74.68 ± 4.00 a | 54.93 ± 2.31 c | 66.25 ± 2.21 b |
| SOM (g/kg) | 27.82 ± 0.56 cd | 38.70 ± 1.50 b | 47.13 ± 0.59 a | 36.36 ± 1.31 b | 25.60 ± 1.46 d | 28.12 ± 1.97 c |
| C/N | 14.37 ± 0.73 c | 16.69 ± 1.18 b | 18.84 ± 0.50 a | 14.34 ± 0.39 c | 11.79 ± 1.89 d | 11.92 ± 0.24 d |
| Variable | Maximum | Minimum | Range | Mean Value | Standard Deviation | The Coefficient of Variation (%) |
|---|---|---|---|---|---|---|
| SP | 61.85% | 51.31% | 10.54% | 55.06% | 0.03 | 4.89 |
| BD | 1.29 | 1.01 | 0.28 | 1.19 | 0.07 | 5.99 |
| pH | 6.85 | 5.54 | 1.31 | 6.10 | 0.43 | 7.10 |
| CEC | 11.80 | 9.26 | 2.54 | 10.40 | 0.74 | 7.07 |
| AP | 29.09 | 16.67 | 12.42 | 23.30 | 4.24 | 18.18 |
| AK | 79.34 | 49.12 | 30.22 | 63.06 | 10.36 | 16.43 |
| TN | 1.51 | 1.09 | 0.42 | 1.14 | 0.14 | 10.23 |
| AN | 175.67 | 131.57 | 44.10 | 151.28 | 15.06 | 9.95 |
| SOM | 47.48 | 23.98 | 23.50 | 33.95 | 7.87 | 23.17 |
| C/N | 19.30 | 10.04 | 9.26 | 14.66 | 2.70 | 18.45 |
| UE | 0.55 | 0.11 | 0.44 | 0.28 | 0.13 | 46.64 |
| ACP | 0.40 | 0.14 | 0.26 | 0.27 | 0.10 | 37.19 |
| CAT | 1.84 | 1.01 | 0.83 | 1.37 | 0.25 | 18.48 |
| SA | 86.86 | 54.18 | 32.68 | 66.81 | 10.23 | 15.31 |
| Item | Principal Component (PC) | Communality | Weight (%) | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Eigenvalues | 5.066 | 2.519 | 1.136 | ||
| Variance (%) | 50.660 | 25.186 | 11.357 | ||
| Cumulative contribution (%) | 50.660 | 75.846 | 87.203 | ||
| Factor loading | |||||
| CEC | 0.197 | 0.840 | 0.072 | 0.750 | 11.88 |
| AP | 0.857 | 0.373 | 0.143 | 0.894 | 17.12 |
| AK | 0.748 | 0.571 | −0.099 | 0.895 | 15.91 |
| TN | 0.836 | 0.320 | 0.334 | 0.914 | 17.59 |
| AN | 0.920 | 0.243 | −0.065 | 0.910 | 15.30 |
| SOM | 0.883 | −0.170 | −0.411 | 0.978 | 8.20 |
| C/N | 0.628 | −0.391 | −0.662 | 0.985 | 0.56 |
| UE | 0.572 | −0.609 | 0.067 | 0.703 | 2.51 |
| ACP | 0.550 | −0.520 | 0.563 | 0.891 | 6.50 |
| SA | 0.619 | −0.602 | 0.237 | 0.802 | 4.43 |
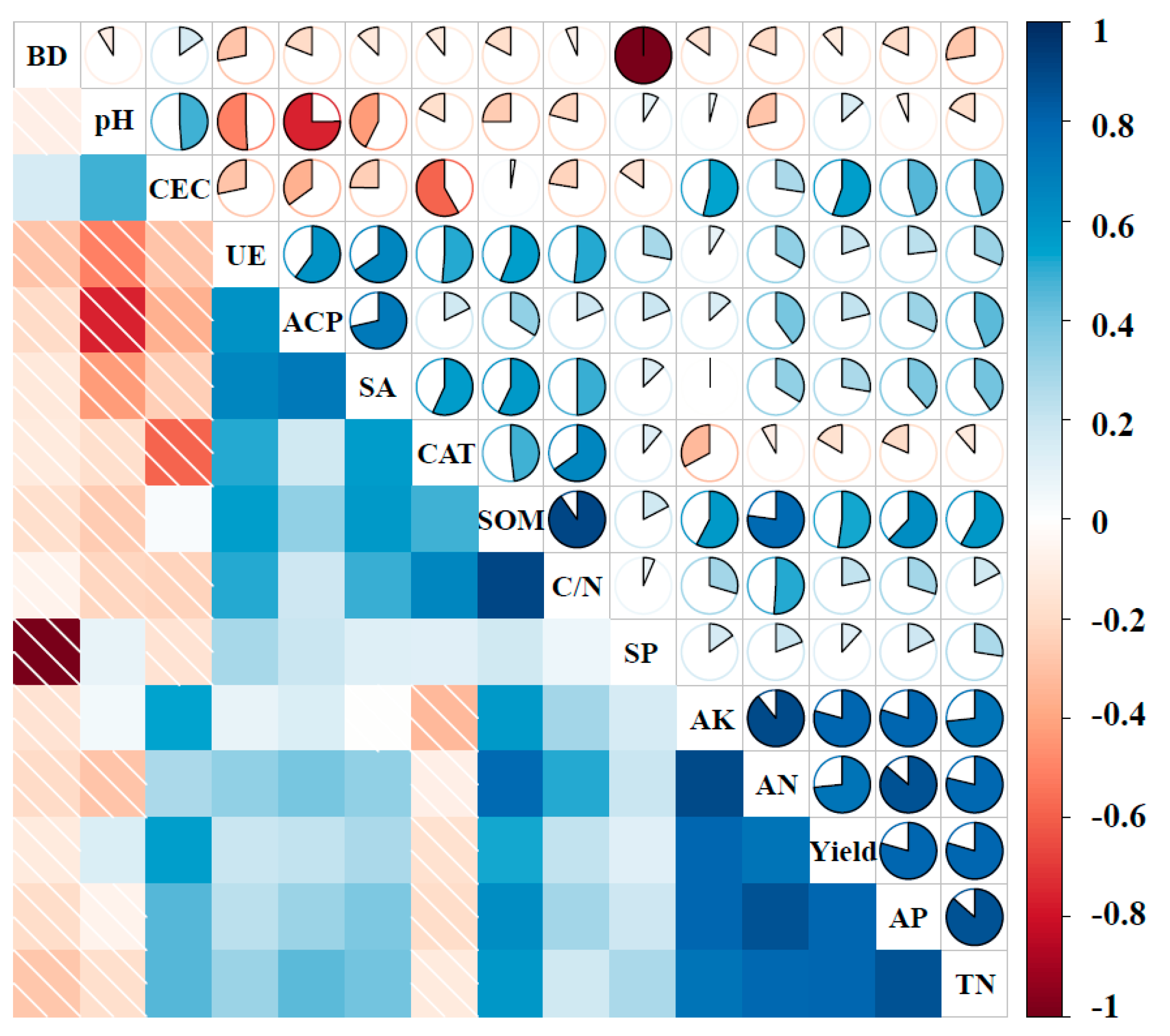
| PC1 Variables | CEC | AP | TN | AN | SOM | C/N |
|---|---|---|---|---|---|---|
| CEC | 1 | 0.455 | 0.459 | 0.275 | 0.026 | 0.224 |
| AP | 0.455 | 1 | 0.865 | 0.862 | 0.621 | 0.298 |
| TN | 0.459 | 0.865 | 1 | 0.788 | 0.580 | 0.176 |
| AN | 0.275 | 0.862 | 0.788 | 1 | 0.772 | 0.510 |
| SOM | 0.020 | 0.621 | 0.580 | 0.772 | 1 | 0.903 |
| C/N | 0.224 | 0.298 | 0.176 | 0.510 | 0.903 | 1 |
| Correlation sum | 2.433 | 4.101 | 3.868 | 4.207 | 3.902 | 3.111 |
| Correlation Coefficient | Yield | SQIMDS | SQITDS |
|---|---|---|---|
| yield | 1.000 | ||
| SQIMDS | 0.826 ** | 1.000 | |
| SQITDS | 0.789 ** | 0.880 ** | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Zhang, T.; Shi, H.; Zhang, X.; Wang, S.; Li, H. Evaluation of the Improvement Effect of Chemical Fertilizer Combined with Different Additives on Newly Built Paddy Soil. Agronomy 2024, 14, 1706. https://doi.org/10.3390/agronomy14081706
Wang N, Zhang T, Shi H, Zhang X, Wang S, Li H. Evaluation of the Improvement Effect of Chemical Fertilizer Combined with Different Additives on Newly Built Paddy Soil. Agronomy. 2024; 14(8):1706. https://doi.org/10.3390/agronomy14081706
Chicago/Turabian StyleWang, Nannan, Tibin Zhang, Hao Shi, Xianhua Zhang, Shiwen Wang, and Hongyi Li. 2024. "Evaluation of the Improvement Effect of Chemical Fertilizer Combined with Different Additives on Newly Built Paddy Soil" Agronomy 14, no. 8: 1706. https://doi.org/10.3390/agronomy14081706
APA StyleWang, N., Zhang, T., Shi, H., Zhang, X., Wang, S., & Li, H. (2024). Evaluation of the Improvement Effect of Chemical Fertilizer Combined with Different Additives on Newly Built Paddy Soil. Agronomy, 14(8), 1706. https://doi.org/10.3390/agronomy14081706







